Abstract
Background
Recently, surgical apgar score (SAS) has been reported to be strongly associated with major morbidity after major abdominal surgery. The aim of this study was to assess the value of esophagectomy SAS (eSAS) in predicting the risk of major morbidity after open esophagectomy in a high volume cancer center.
Methods
The data of all patients who admitted to intensive care unit (ICU) after open esophagectomy at Cancer Hospital of Chinese Academy of Medical Sciences & Peking Union Medical College from September 2008 through August 2010 was retrospectively collected and reviewed. Preoperative and perioperative variables were recorded and compared. The eSAS was calculated as the sum of the points of EBL, lowest MAP and lowest HR for each patient. Patients were divided into high-risk (below the cutoff) and low-risk (above the cutoff) eSAS groups according to the cutoff score with optimal accuracy of eSAS for major morbidity. Univariable and multivariable regression analysis were used to define risk factors of the occurrence of major morbidity.
Results
Of 189 patients, 110 patients developed major morbidities (58.2%) and 30-day operative mortality was 5.8% (11/189). There were 156 high risk patients (eSAS ≤7) and 33 low risk (eSAS >7) patients. Univariable analysis demonstrated that forced expiratory volume in one second of predicted (FEV1%) ≤78% (44% vs. 61%, P=0.024), McKeown approach (22.7% vs. 7.6%, P=0.011), duration of operation longer than 230 minutes, intraoperative estimated blood loss (347±263 vs. 500±510 mL, P=0.015) and eSAS ≤7 (62.2% vs. 90.0%, P=0.001) were predictive of major morbidity. Multivariable analysis demonstrated that FEV1% ≤78% (OR, 2.493; 95% CI, 1.279–4.858, P=0.007) and eSAS ≤7 (OR, 2.810; 95% CI, 1.105–7.144; P=0.030) were independent predictors of major morbidity after esophagectomy. Compared with patients who had eSAS >7, patients who had eSAS ≤7 had longer hospital length of stay (25.39±14.36 vs. 32.22±22.66 days, P=0.030). However, there were no significant differences in ICU length of stay, duration of mechanical ventilation, ICU death, 30-day death rate and in-hospital death rate between high risk and low risk patients.
Conclusions
The eSAS score is predictive of major morbidity, and lower eSAS is associated with longer hospital length of stay in esophageal cancer patients after open esophagectomy.
Keywords: Esophagectomy, esophageal cancer, esophagectomy surgical apgar score (eSAS), major morbidity
Introduction
It is estimated that 455,800 new esophageal cancer cases and 400,200 deaths occurred in 2012 worldwide (1). According to statistics of esophageal cancer in China, the number of new esophageal cancer cases and death were 286,700 and 210,900 in 2012 respectively, being the half of those in the world (2). Surgery remains the main treatment modality for resectable carcinoma of the esophagus (3). However, esophagectomy for esophageal cancer is a complex procedure which carries high risk of morbidity rate of 24% (4) and a mortality rate of 2% to 5.6%, respectively (4-8).
Preoperative variables including age, forced expiratory volume in one second (FEV1), medical comorbidities have been identified and validated as risk factors for the development of major morbidity after esophagectomy (4,8-10). Moreover, major morbidity negatively impacts the long-term survival in patients who underwent esophagectomy after neoadjuvant treatment for locally advanced adenocarcinoma (11). Therefore, defining preoperative risk factors is important for clinicians to define whether patients will tolerate the anesthesia and pass through perioperative period safely.
In addition to preoperative predictors, intraoperative factors have drawn more attentions recently in the risk prediction of postoperative morbidity. Gawande et al. developed the surgical apgar score (SAS) based on three variables during operation in 2007 (12). The SAS was a 10-point scoring system which was based on a patient’s estimated amount of blood loss, lowest heart rate, and lowest mean arterial pressure during general or vascular operations, and the score was significantly associated with major complications or death within 30 days. Subsequent validated studies demonstrated that the SAS was effective in identify patients who are at risk of developing major complications after they underwent general, orthopaedic, gynecologic, obstetric, urologic, and vascular surgeries (13,14).
Recently, Janowak and Eto reported their experiences of usefulness of the SAS in predicting the risk of major morbidity after esophagectomy (15,16). They concluded that the SAS was strongly associated with 30-day major morbidity after esophagectomy. However, in Janowak study, the proportion of open esophagectomy was only 48%, and the other half patients underwent hybrid or total minimally invasive esophagectomy, which led to fewer EBL with a median volume of 200 mL (15). While in Eto study, all patients underwent three incisional esophagectomy which led to more EBL with a median volume of 500–600 mL (16). Based on above results, we postulated that different cutoff value of EBL derived from different surgical procedures adopted, hence different SAS cutoff score. Therefore, the purpose of our study was to investigate a modified esophagectomy SAS, in which the EBL, one of the three components of SAS, was based on cutoff value in patients who underwent open esophagectomy mainly by Sweet approach.
Methods
The data of all patients who admitted to intensive care unit (ICU) after esophagectomy at Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College from September 2008 through August 2010 was collected and reviewed. Patients’ data included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, smoking history, preoperative forced expiratory volume in 1 second of predicted (FEV1%), comorbidities and administration of neoadjuvant chemotherapy, and/or radiotherapy. Comorbidities included history of hypertension, coronary heart disease, diabetic mellitus, and chronic obstructive pulmonary disease. Postoperative variables included 30-day major morbidity rate, ICU length of stay, ICU death, hospital LOS, 30-day death and in-hospital death.
Intraoperative data including surgical approach adopted, duration of operation, estimated blood loss (EBL), lowest mean arterial pressure (MAP), and lowest heart rate (HR) were recorded. Surgical approaches included Sweet (left thoracoabdominal incision), open Ivor Lewis and open McKeown. Intraoperative data were collected from handwritten anesthesia records. The methodology described by Gawande was used to assign points for lowest MAP and lowest HR (12). However, we used a modified range of EBL. The EBL cutoff points were based on quartile values of EBL and the median EBL was 300 mL (range, 50–4,800 mL; interquartile range, 200–600 mL) in our cohort (Table 1). The eSAS was calculated as the sum of the points of EBL, lowest MAP and lowest HR for each patient (Table 1). FEV1% and duration of operation were divided into two groups according to cutoff value defined as the median value of all patients respectively.
Table 1. The esophagectomy surgical apgar score.
| Variables | 0 points | 1 points | 2 points | 3 points | 4 points |
|---|---|---|---|---|---|
| EBL (mL) | >600 | 301–600 | 201–300 | ≤200 | – |
| Lowest MAP (mmHg) | <40 | 40–54 | 55–69 | ≥70 | – |
| Lowest HR (beats/min) | >85 | 76–85 | 66–75 | 56–65 | ≤55 |
EBL, estimated blood loss; MAP, mean arterial pressure; HR, heart rate.
Institutional Review Board of Cancer Hospital of Chinese Academy of Medical Sciences approved the study and patients’ consent was waived owning to the observational nature of this study.
The primary outcome of our study was 30-day major morbidity. Morbidity was evaluated according to the thoracic morbidity and mortality (TM&M) classification system (17). Complications meeting the definition for class III and class IV were categorized as major morbidity. Class III complications includes those which require intervention with or without general anesthesia such as surgical drainage of anastomotic leak, ligation of ruptured thoracic duct. Class IV complications includes single or multiple organ dysfunctions such as respiratory insufficiency in need of mechanical ventilation, shock, and acute kidney injury. All patients with 30-day death were recorded as having major morbidity. Other outcomes include ICU LOS, duration of mechanical ventilation, ICU death, hospital LOS, 30-day death and in-hospital death. Pathological staging was performed using the American Joint Committee on Cancer (AJCC) Cancer Staging Handbook (7th edition) (18).
Statistics
Statistical analyses were carried out using SPSS software for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation and compared respectively using Student’s t-test. Categorical variables were reported as absolute numbers (frequency percentages) and analyzed using χ2 test. The area under the receiver operating characteristic curve (AUROC) was used to evaluate the ability of eSAS to discriminate between patients who developed postoperative major morbidity or not. Patients were divided into high-risk (below the cutoff) and low-risk (above the cutoff) eSAS groups according to the cutoff score with optimal accuracy of eSAS for major morbidity. Univariable and multivariable regression analysis were used to define risk factors of the occurrence of major morbidity. A two-tailed P value <0.05 was considered statistically significant.
Results
Patient characteristics
Of all 189 patients who underwent esophagectomy for esophageal cancer, 161 patients (85.2%) were male, and the median age was 64.55±9.61 years. Mean FEV1% was (76.85±10.86) %, and mean duration of operation was 252±87 min. Median ICU LOS was 3 days. Of 189 patients who underwent open esophagectomy, 154 patients (81.5%) were through Sweet approach, 31 patients (16.4%) through open McKeown approach, and 4 patients (2.1%) through open Ivor Lewis approach. No patients with minimally invasive esophagectomy were admitted to ICU during this period. Histological classification was squamous cell carcinomas in 141 patients (74.6%), adenocarcinomas in 45 patients (23.8%), and small cell carcinomas of esophagus in 3 patients (1.6%). Distribution of eSAS is displayed in Figure 1. Other preoperative and intraoperative variables are displayed in Tables 2,3.
Figure 1.
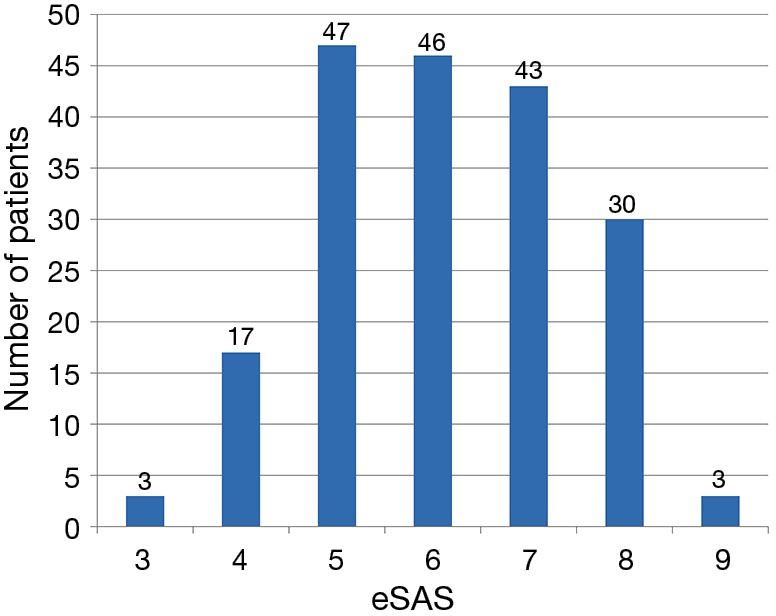
Distribution of eSAS in 189 esophageal cancer patients after esophagectomy. eSAS, esophagectomy surgical apgar score.
Table 2. Preoperative and postoperative variables of patients who developed major morbidity or not.
| Clinical characteristics | Major morbidity (n=110) | No or minor morbidity (n=79) | P value |
|---|---|---|---|
| Age (years) | 0.140 | ||
| ≤65 | 54 (49.1) | 30 (38.0) | |
| >65 | 56 (50.9) | 49 (62.0) | |
| Male gender (%) | 92 (83.6) | 69 (87.3) | 0.538 |
| ASA class (%) | 0.461 | ||
| I–II | 60 (54.5) | 38 (48.1) | |
| III–IV | 50 (45.5) | 41 (51.9) | |
| BMI (kg/m2) | 24.07±4.21 | 23.72±3.50 | 0.555 |
| FEV1% (%) | 0.024 | ||
| ≤78 | 64 (61.0) | 33 (44.0) | |
| >78 | 41 (39.0) | 42 (56.0) | |
| Smoking history (%) | 70 (63.6) | 54 (68.4) | 0.537 |
| Hypertension (%) | 41 (37.3) | 37 (46.8) | 0.231 |
| Coronary heart diseases (%) | 11 (10.1) | 9 (11.4) | 0.814 |
| Diabetic mellitus (%) | 11 (10.1) | 12 (15.2) | 0.367 |
| COPD (%) | 3 (2.7) | 1 (1.3) | 0.641 |
| Neoadjuvant therapy (%) | 6 (5.5) | 1 (1.3) | 0.242 |
| AJCC staging (%) | 0.272 | ||
| Stage 0–1 | 7 (6.4) | 8 (10.1) | |
| Stage 2 | 45 (40.9) | 24 (30.4) | |
| Stage 3–4 | 58 (52.7) | 47 (59.5) |
ASA, American Society of Anesthesiologists; FEV1, forced expiratory volume in 1 second of predicted; BMI, body mass index; COPD, chronic obstructive pulmonary disease; AJCC, American Joint Committee on Cancer.
Table 3. Intraoperative variables of patients who developed major morbidity or not.
| Clinical characteristics | Major morbidity (n=110) | No or minor morbidity (n=79) | P value |
|---|---|---|---|
| Surgical approach (%) | 0.011 | ||
| Sweet | 84 (76.4) | 70 (88.6) | |
| Open McKeown | 25 (22.7) | 6 (7.6) | |
| Open Ivor Lewis | 1 (0.9) | 3 (3.8) | |
| Duration of operation (min) | 0.027 | ||
| ≤230 | 48 (43.6) | 48 (60.8) | |
| >230 | 62 (56.4) | 31 (39.2) | |
| EBL (mL) | 500±510 | 347±263 | 0.015 |
| Lowest MAP (mmHg) | 84.30±9.50 | 86.10±7.53 | 0.164 |
| Lowest HR (/min) | 75.58±8.69 | 77.06±10.76 | 0.367 |
| The eSAS (%) | 0.001 | ||
| ≤7 | 99 (90.0) | 57 (62.2) | |
| >7 | 11 (10.0) | 22 (27.8) |
EBL, estimated blood loss; MAP, mean arterial pressure; HR, heart rate; eSAS, esophagectomy surgical apgar score.
Patients were divided into low risk (eSAS score >7) and high risk groups (eSAS score ≤7) according to the cutoff value determined by the AUROC of eSAS to discriminate whether patients who developed postoperative major morbidity or not. The AUROC of eSAS in patients was displayed in Figure 2. Of 189 patients, 110 patients developed major morbidities (58.2%), 15 patients developed minor morbidities, and 64 patients recovered uneventful (Table 4). The most common major morbidity was respiratory insufficiency (n=91, 82.7%), followed by anastomotic leak (n=30, 27.3%), and pulmonary infection combined with respiratory insufficiency (n=15, 13.6%). Thirty-day operative mortality rate was 5.8% (n=11) and in-hospital death rate was 6.9% (n=13).
Figure 2.
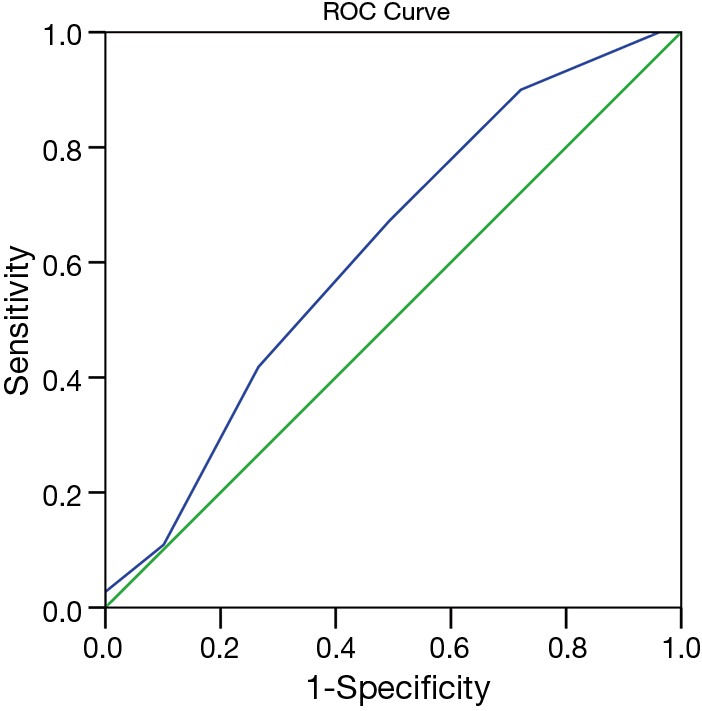
Receiver operator characteristic curve for eSAS and major morbidities. Area uder the curve =0.620±0.042 (P=0.005; 95% CI, 0.538 to 0.703). eSAS, esophagectomy surgical apgar score; CI, confidential interval.
Table 4. Major complications after esophagectomy (n=110).
| Grade | Complication | Number (%) |
|---|---|---|
| III a | Esophageal leak | 7 (6.4) |
| Pleural fluid | 1 (0.9) | |
| Thoracic wound dehiscence | 1 (0.9) | |
| Intrathoracic abscess | 3 (2.7) | |
| III b | Reoperation because of bleeding | 3 (2.7) |
| Chyle leak | 2 (1.8) | |
| IV a | Respiratory insufficiency | 52 (47.3) |
| Esophageal leak with respiratory insufficiency | 23 (20.9) | |
| Acute renal failure | 1 (0.9) | |
| Pneumonia with respiratory insufficiency | 15 (13.6) | |
| Pulmonary embolus | 1 (0.9) | |
| IV b | Respiratory insufficiency and acute renal failure | 1 (0.9) |
Univariable and multivariable analysis
Univariable analysis including preoperative and intraoperative variables demonstrated that compared with patients who developed minor or no morbidity, patients who developed major morbidity had low preoperative pulmonary function with FEV1% ≤78% (44% vs. 61%, P=0.024), underwent more open McKeown approaches (22.7% vs. 7.6%, P=0.011), experienced longer operation more than 230 minutes (39.2% vs. 56.4%, P=0.027), had more EBL (347±263 vs. 500±510 mL, P=0.015) and had more eSAS ≤7 (62.2% vs. 90.0%, P=0.001). While age, sex, ASA class, BMI, history of smoking, comorbidities, neoadjuvant chemotherapy and/or radiotherapy, AJCC stage, intraoperative lowest MAP, and lowest HR were not predictive the occurrence of postoperative major morbidity.
Multivariable analysis demonstrated that FEV1% ≤78% (OR, 2.493; 95% CI, 1.279–4.858, P=0.007) and eSAS ≤7 (OR, 2.810; 95% CI, 1.105–7.144; P=0.030) were independent predictors of major morbidity after esophagectomy (Table 5).
Table 5. Multivariable analysis of predictors of major morbidity after open esophagectomy.
| Clinical characteristics | Adjusted OR (95% CI) | P value |
|---|---|---|
| FEV1 ≤78% | 2.493 (1.279–4.858) | 0.007 |
| The eSAS ≤7 | 2.810 (1.105–7.144) | 0.030 |
| Duration of operation >230 min | 1.413 (0.670–2.979) | 0.363 |
| EBL | 1.001 (0.999–1.002) | 0.362 |
| Surgical approach | ||
| Open Mckeown approach | 2.671 (0.916–7.792) | 0.072 |
| Open Ivor Lewis approach | 0.288 (0.025–3.359) | 0.321 |
| Sweet approach | reference |
OR, odds ratio; CI, confidential interval; EV1, forced expiratory volume in 1 second of predicted; eSAS, esophagectomy surgical apgar score ; EBL, estimated blood loss.
Compared with patients who had eSAS >7, patients who had eSAS ≤7 had longer hospital length of stay (25.39±14.36 vs. 32.22±22.66 days, P=0.030). However, there were no significant differences in ICU length of stay, duration of mechanical ventilation, ICU death rate, 30-day death rate and in-hospital death rate between high risk and low risk patients (Table 6).
Table 6. Perioperative outcomes of patients who had eSAS >7 and ≤7.
| eSAS >7 (n=33) | eSAS ≤7 (n=156) | P value | |
|---|---|---|---|
| ICU length of stay (d) | 3.79±4.54 | 5.52±5.87 | 0.112 |
| Hospital length of stay (d) | 25.39±14.36 | 32.22±22.66 | 0.030 |
| Duration of mechanical ventilation (d) | 1.30±3.34 | 2.92±5.34 | 0.096 |
| ICU death (%) | 1 (3.0) | 2 (1.3) | 0.465 |
| 30-day death (%) | 0 | 11 (7.1) | 0.217 |
| In-hospital death (%) | 1 (3.0) | 12 (7.7) | 0.472 |
eSAS, esophagectomy surgical apgar score; ICU, intensive care unit.
Discussion
In this study, we found that eSAS is an independent predictor of major morbidity after esophagectomy. Moreover, patients who had eSAS ≤7 had longer hospital length of stay than patients who had eSAS >7.
Prediction of postoperative morbidity is challenging for clinicians. Lagarde and colleagues developed a risk model including 6 preoperative factors using data of 663 patients and validated the model in 95 patients (19). Two subsequent studies validated the risk score of Lagarde. However, the risk score was moderately good in predicting the risk of postoperative morbidity with area under the curve of 0.64–0.71 in these two studies (13,14). On the other hand, the occurrence of postoperative morbidity was not only related to preoperative factors, intraoperative and postoperative management also played important roles in the development of postoperative morbidity. Minimally invasive esophagectomy has been reported to be associated with decreased major complication rate compared with open esophagectomy (20). Postoperative fluid management was also important as positive fluid balance was predictive of pulmonary complications in patients after esophagectomy (21). Therefore, in addition to preoperative predictors (22), ntraoperative physiologic variables resulting from intraoperative management and postoperative management may also play important roles in the prediction of risk of major morbidity.
Recently, studies demonstrated that the SAS developed by Gawande in 2007 was effective in identify patients who are at risk of developing major complications after several types of operations (13,14). In 2015, Janowak applied the concept of SAS to esophagectomy and concluded that the eSAS was predictive of postoperative morbidity using data of 168 esophagectomy patients (15). Eto et al. validated the SAS in 399 esophageal cancer patients and concluded that SAS was useful in predicting the development of postoperative morbidities after esophagectomy for esophageal cancer (16). In our study, we assessed the eSAS in 189 patients after esophagectomy who were in need of intensive care, and concluded that the eSAS score is predictive of major morbidity after esophagectomy. Therefore, the eSAS was both important for thoracic surgeons and intensivists to predict the risk of major morbidity for patients after esophagectomy.
The eSAS score was consisted of three variables: EBL, lowest MAP and lowest HR. Intraoperative blood loss may occur first, followed by low MAP and elevated HR. As inadequate systemic perfusion resulted from significant EBL, tachycardia and hypotension were physiologic responses to hypoperfusion. In Eto and our studies, greater EBL was associated with major morbidity, but no significant association was found in Janowak study (15,16). The mechanism needs further studying.
Janowak et al. demonstrated that the eSAS ≤6 was a strong predictor of postoperative major complications in multivariable analysis (15). Eto et al. validated the SAS in esophageal cancer patients and concluded that a SAS <5 was found to be an independent risk factor for major morbidities (16). In our study, we found that eSAS ≤7 is an independent predictor of major morbidity after esophagectomy. Different cut-off values for eSAS may be due to different points assigned for EBL. The values assigned for lowest MAP and lowest HR was same in Janowak and Eto and our study. However, in Janowak and Eto study, the value to assign 0 point was >300 and >1,000 mL, respectively, while it was >600 mL in our study (15,16). Different approaches/techniques may lead to different cut-off value of EBL, hence leading to different SAS cutoff value. There are transhiatal esophagectomy, open/minimally invasive Ivor Lewis esophagectomy (ILE), and open/minimally invasive McKeown esophagectomy (MKE) in Janowak study (15). In our study, techniques included Sweet, open ILE and open MKE. While all patients underwent three-incision esophagectomy in Eto study (16). Based on above results, we concluded that different cutoff value of EBL derived from different surgical procedures. Therefore, caution is needed when using the eSAS to predicting the risk of morbidity after esophagectomy, and type of esophagectomy should be taken into account. Our score applies to patients undergoing open esophagectomy through Sweet approach, and Eto score applies to patients undergoing open three-incisional esophagectomy, while Janowak score applies to patients undergoing open/minimally invasive esophagectomy.
Patients who had eSAS ≤7 had longer hospital length of stay than patients who had eSAS >7. More rate of morbidity in patients who had eSAS ≤7 may account for the results. Early intervention was independently associated with decreased in-hospital mortality rate in critically ill cancer patients (23). Theoretically, intensive care for patients after esophagectomy who were at high risk may timely detect and intervene the major morbidity, which may lower in-hospital death and shorten hospital length of stay. However, prospective studies are needed to assess the value of eSAS in predicting the risk of major morbidity, and whether intensive care of high risk patients based on eSAS could lower the morbidity and shorten hospital LOS in patients after esophagectomy.
In our study, only seven patients received neoadjuvant therapy. Several meta-analyses demonstrated survival advantage of neoadjuvant treatment over surgery alone for resectable esophageal adenocarcinoma (24-26). However, there were limited data regarding the survival advantage of neoadjuvant treatment over surgery alone for resectable esophageal squamous cell carcinoma. Therefore, the rate of neoadjuvant treatment for locally advanced esophageal carcinoma was only 20% in our hospital (27).
There are several limitations in our study. The first limitation of our study comes from the retrospective nature, and relatively small sample. Further large prospective studies are needed to determine the role of eSAS in predicting the occurrence of major morbidity. Second, the results are from single medical center, which need to be validated in multicenter trial. Last, our study included only open Sweet esophagectomy, the results may not be applicable to other approaches including transhiatal or minimally invasive esophagectomy.
In conclusion, the eSAS score is predictive of major morbidity after open esophagectomy. Low eSAS is associated with longer hospital LOS.
Acknowledgements
This study was carried out in the Department of Intensive Care Unit, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Ethical Statement: Institutional Review Board of Cancer Hospital of Chinese Academy of Medical Sciences approved the study and patients’ consent was waived owning to the observational nature of this study.
Footnotes
Conflict of Interest: The authors have no conflict of interest to declared .
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. 10.1056/NEJMra1314530 [DOI] [PubMed] [Google Scholar]
- 4.Wright CD, Kucharczuk JC, O'Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg 2009;137:587-95. 10.1016/j.jtcvs.2008.11.042 [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Yang Y, Yan W, et al. Postoperative 30-day mortality may underestimate the risk of esophagectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2015;18:897-900. [PubMed] [Google Scholar]
- 6.Dikken JL, van Sandick JW, Allum WH, et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg 2013;100:83-94. 10.1002/bjs.8966 [DOI] [PubMed] [Google Scholar]
- 7.Smith RC, Creighton N, Lord RV, et al. Survival, mortality and morbidity outcomes after oesophagogastric cancer surgery in New South Wales, 2001-2008. Med J Aust 2014;200:408-13. 10.5694/mja13.11182 [DOI] [PubMed] [Google Scholar]
- 8.D'Journo XB, Berbis J, Jougon J, et al. External validation of a risk score in the prediction of the mortality after esophagectomy for cancer. Dis Esophagus 2016. [Epub ahead of print]. 10.1111/dote.12447 [DOI] [PubMed] [Google Scholar]
- 9.Ferguson MK, Celauro AD, Prachand V. Assessment of a scoring system for predicting complications after esophagectomy. Dis Esophagus 2011;24:510-5. 10.1111/j.1442-2050.2011.01185.x [DOI] [PubMed] [Google Scholar]
- 10.Grotenhuis BA, van Hagen P, Reitsma JB, et al. Validation of a nomogram predicting complications after esophagectomy for cancer. Ann Thorac Surg 2010;90:920-5. 10.1016/j.athoracsur.2010.06.024 [DOI] [PubMed] [Google Scholar]
- 11.Luc G, Durand M, Chiche L, et al. Major post-operative complications predict long-term survival after esophagectomy in patients with adenocarcinoma of the esophagus. World J Surg 2015;39:216-22. 10.1007/s00268-014-2754-1 [DOI] [PubMed] [Google Scholar]
- 12.Gawande AA, Kwaan MR, Regenbogen SE, et al. An Apgar score for surgery. J Am Coll Surg 2007;204:201-8. 10.1016/j.jamcollsurg.2006.11.011 [DOI] [PubMed] [Google Scholar]
- 13.Regenbogen SE, Ehrenfeld JM, Lipsitz SR, et al. Utility of the surgical apgar score: validation in 4119 patients. Arch Surg 2009;144:30-6; discussion 37. 10.1001/archsurg.2008.504 [DOI] [PubMed] [Google Scholar]
- 14.Haynes AB, Regenbogen SE, Weiser TG, et al. Surgical outcome measurement for a global patient population: validation of the Surgical Apgar Score in 8 countries. Surgery 2011;149:519-24. 10.1016/j.surg.2010.10.019 [DOI] [PubMed] [Google Scholar]
- 15.Janowak CF, Blasberg JD, Taylor L, et al. The Surgical Apgar Score in esophagectomy. J Thorac Cardiovasc Surg 2015;150:806-12. 10.1016/j.jtcvs.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 16.Eto K, Yoshida N, Iwatsuki M, et al. Surgical Apgar Score Predicted Postoperative Morbidity After Esophagectomy for Esophageal Cancer. World J Surg 2016;40:1145-51. 10.1007/s00268-016-3425-1 [DOI] [PubMed] [Google Scholar]
- 17.Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. 10.1016/j.athoracsur.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 18.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4. [DOI] [PubMed] [Google Scholar]
- 19.Lagarde SM, Reitsma JB, Maris AK, et al. Preoperative prediction of the occurrence and severity of complications after esophagectomy for cancer with use of a nomogram. Ann Thorac Surg 2008;85:1938-45. 10.1016/j.athoracsur.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 20.Palazzo F, Rosato EL, Chaudhary A, et al. Minimally invasive esophagectomy provides significant survival advantage compared with open or hybrid esophagectomy for patients with cancers of the esophagus and gastroesophageal junction. J Am Coll Surg 2015;220:672-9. 10.1016/j.jamcollsurg.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 21.Xing X, Gao Y, Wang H, et al. Correlation of fluid balance and postoperative pulmonary complications in patients after esophagectomy for cancer. J Thorac Dis 2015;7:1986-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg 2011;91:1494-1500; discussion 1500-1. 10.1016/j.athoracsur.2010.12.036 [DOI] [PubMed] [Google Scholar]
- 23.Song JU, Suh GY, Park HY, et al. Early intervention on the outcomes in critically ill cancer patients admitted to intensive care units. Intensive Care Med 2012;38:1505-13. 10.1007/s00134-012-2594-0 [DOI] [PubMed] [Google Scholar]
- 24.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer 2013;49:3149-58. 10.1016/j.ejca.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 25.Kumagai K, Rouvelas I, Tsai JA, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg 2014;101:321-38. 10.1002/bjs.9418 [DOI] [PubMed] [Google Scholar]
- 26.Kumagai K, Rouvelas I, Tsai JA, et al. Survival benefit and additional value of preoperative chemoradiotherapy in resectable gastric and gastro-oesophageal junction cancer: a direct and adjusted indirect comparison meta-analysis. Eur J Surg Oncol 2015;41:282-94. 10.1016/j.ejso.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 27.Mu JW, Gao SG, Xue Q, et al. Updated experiences with minimally invasive McKeown esophagectomy for esophageal cancer. World J Gastroenterol 2015;21:12873-81. 10.3748/wjg.v21.i45.12873 [DOI] [PMC free article] [PubMed] [Google Scholar]


