Abstract
Background
Decortication for thoracic empyema is associated with significant blood loss and prolonged postoperative air leak. We sought to assess the potential application of an irrigated-tip radiofrequency (RF) sealing device, in an attempt to reduce this morbidity.
Methods
Data for all patients undergoing open decortication (OD) for stage II thoracic empyema, using either conventional approach or facilitated by use of the Aquamantys® device, at a single thoracic surgical unit between April 2010 and July 2014, were retrospectively analysed. Unpaired t-test and Fisher’s exact test were used for statistical analysis.
Results
Thirty-three patients, aged 54±15 years (mean ± SD), and with a Charlson comorbidity index of 2.5±1.9 were included. Preoperative and intraoperative characteristics, including surgical time, were similar in the conventional and Aquamantys® groups. Patients in the Aquamantys group were less likely to require red cell transfusion (9/22 vs. 10/11 patients, P=0.024) and received lower volume transfusions [0.0 (2.0) vs. 3.0 (1.6) units (median, IQR), P<0.0001]; chest drain duration was shorter [3.0 (1.0) vs. 6.5 (6.8) days, P=0.006], as was length of postoperative hospital stay [6.0 (8.7) vs. 10.0 (4.6) days, P=0.031]. There was no demonstrable difference in mortality.
Conclusions
Our data indicates that the use of irrigated tip RF ablation is safe and effective in improving pneumostasis and haemostasis in decortication for thoracic empyema; and that this translates to morbidity and logistical benefit.
Keywords: Decortication, empyema, radiofrequency (RF), Aquamantys, pneumostasis
Introduction
Thoracic empyema is a triphasic inflammatory process occurring within the pleural space, comprising exudative phase (stage I), fibropurulent phase (stage II) and organising phase with granulation tissue formation (stage III). In the last phase, the chronically inflamed granulation tissue may cause contraction of the affected hemithorax and ipsilateral shift of the mediastinum (1).
The British Thoracic Society (BTS) Pleural Disease Guideline 2010 recommends chest tube drainage, antibiotics and fibrinolytic drugs for the treatment of early stage empyema (stage I–II). In the absence of resolution with conservative management within a time frame that is considered reasonable, arbitrarily set at six weeks, there is a general consensus for surgical intervention in patients deemed to be sufficiently fit (2).
Decortication can successfully treat both early and chronic empyema, aiming to eliminate the source of sepsis, whilst releasing the trapped lung and allowing its re-expansion (3). It has furthermore been demonstrated that video-assisted thoracoscopic surgery (VATS) is a valuable alternative to open decortication (OD); achieving equivalent empyema remission rates, whilst reducing overall morbidity and mortality (4,5).
Major thoracic surgical procedures carry significant risk of morbidity; particularly significant haemorrhage, and high-volume or prolonged air leak, with associated prolonged length of hospital stay. Several strategies have been proposed in an attempt to minimize the extent and duration of parenchymal air leak and bleeding. Lung sealants have shown mixed success in reducing air leaks (6). Haemostatic agents may be considered in an attempt to control diffuse bleeding (7).
The Aquamantys® system (Medtronic, Minneapolis, MN, USA) is a single-use portable handheld device with a saline-irrigated tip and an adjustable radiofrequency (RF) energy generator system with integrated pump. It was launched commercially in its current form in March 2006, and sells for GBP 290 per disposable unit.
The combination of RF energy and saline provides haemostatic sealing of tissues, without charring or carbonisation. It has been widely adopted in a number of other surgical specialties, including orthopaedic, hepatobiliary and neurosurgery (8-10). Reports in the literature of the use of the Aquamantys® system in a thoracic surgical setting are limited and deal with older, lower-energy models of the device that proved inefficient at sealing lung tissue (11,12).
We sought to assess the potential application of this technology in adult patients undergoing OD for stage III post-pneumonic empyema. The primary outcome measures included red cell transfusion requirements as a marker of clinically-significant bleeding, and chest tube duration as a surrogate for air leak duration. Secondary end-points included the need for critical care admission, length of hospital stay, complication rates and mortality.
Methods
Clinical element
Data for all patients undergoing OD for stage III thoracic empyema at Papworth Hospital, Cambridge, UK between April 2010 and July 2014 were collected from electronic and paper-based clinical documentation, and retrospectively analysed.
Patients with additional lung resection, VATS approach, stage I and II post pneumonic empyema and aetiology other than post pneumonic empyema were excluded.
The patients were divided into two groups, according to whether or not the Aquamantys® system was utilised in an attempt to improve haemostasis and pneumostasis.
Clinical data was obtained in anonymised format through the Clinical Audit department at Papworth Hospital, Cambridge, UK.
Statistical analyses
Unpaired student t-test and Pearson’s Chi-squared test were used for statistical analyses of parametric continuous and categorical data respectively. Significance was defined as a P value of <0.05.
Histopathological analysis
Lung tissues biopsies were taken from a single patient in each group, and examined by a single consultant pathologist for comparison.
Results
Baseline characteristics
Thirty-three patients, 66.6% (n=22) male, were included in the study. Mean (SD) age was 54.0 (14.9) years (range, 20 to 81), with a Charlson comorbidity index of 2.5 (1.9). None of the patients were noted to have bleeding diatheses. Twenty-two patients (66.6%) presented with right sided disease, with a causative organism being isolated in 18 (54.5%). Sixteen patients (48.5%) underwent OD out of hours.
Preoperative characteristics
There was no difference in age, gender, comorbidity status, sidedness or preoperative haemoglobin between the conventional and Aquamantys® groups. Details are presented in Table 1.
Table 1. Preoperative characteristics: no differences are noted in demographics, premorbid state or nature of presenting pathology between the two groups.
| Variable | Conventional (n=11) | Aquamantys (n=22) | P value |
|---|---|---|---|
| Demographics | |||
| Age (years)* | 55.5 (18.5) | 60.0 (21.3) | 0.476 |
| Male sex^ | 6 (50%) | 16 (76.2%) | 0.125 |
| Premorbid state | |||
| Charlson comorbidity index* | 2.0 (1.0) | 2.0 (3.3) | 0.443 |
| Preoperative Hb (g/dL)* | 10.9 (1.8) | 10.5 (1.6) | 0.111 |
| Pathology | |||
| Right-sided^ | 7 (58.3%) | 15 (71.4%) | 0.443 |
| Organism isolated^ | 8 (66.7%) | 10 (47.6%) | 0.291 |
*, non-parametric continuous data is presented as medians (interquartile range); ^, dichotomous data is presented as number (percentage).
Intraoperative details
There was no difference between groups for incidence of procedures performed out of hours, operative time, or use of surgical sealant. Intraoperative blood loss data was not available for the conventional group. Details are presented in Table 2.
Table 2. Operative details: no difference is noted between the two groups, in terms of operative time or the use of surgical sealant.
| Variable | Conventional (n=11) | Aquamantys (n=22) | P value |
|---|---|---|---|
| Operated out-of-hours^ | 4 (33.3%) | 12 (57.1%) | 0.188 |
| Operative time (minutes)* | 95.5 (54.2) | 115.0 (48.7) | 0.120 |
| Surgical sealant^ | 2 (16.7%) | 6 (28.6%) | 0.443 |
| Intraoperative blood loss (mL)* | – | 300.0 (492.5) | – |
*, non-parametric continuous data is presented as medians (interquartile range); ^, dichotomous data is presented as number (percentage).
Postoperative outcomes
Patients treated with the Aquamantys® system experienced a lower drop between their pre- and post-operative haemoglobin levels, received lower volumes of blood transfusion, and were less likely to be transfused at all during their postoperative course. Chest drain duration was shorter for the Aquamantys® group, as the incidence of critical care admission and the length of hospital stay. There was no difference in the number pulmonary and non-pulmonary complications, and hospital mortality. Details are presented in Table 3.
Table 3. Postoperative outcomes: patients in the Aquamantys® group experienced less bleeding, a shorter duration of postoperative air leak, and a shorter length of critical care and hospital stay.
| Variable | Conventional (n=11) | Aquamantys (n=22) | P value |
|---|---|---|---|
| Bleeding and transfusion | |||
| Postoperative Hb (g/dL)* | 8.4 (2.3) | 9.2 (1.5) | 0.143 |
| Drop in Hb (g/dL)* | 2.0 (2.7) | 1.5 (1.0) | 0.049 |
| Patients transfused PRCs^ | 10 (83.3%) | 9 (42.9%) | 0.024 |
| Units PRCs transfused (n/pt)* | 3.0 (1.6) | 0.0 (2.0) | 0.0003 |
| Chest drains | |||
| Chest drains in situ (days)* | 6.5 (6.8) | 3.0 (1.0) | 0.0006 |
| Discharged with drain^ | 2 (16.7%) | 1 (4.8%) | 0.253 |
| Complications | |||
| Pulmonary complications^ | 2 (16.7%) | 4 (19.0%) | 0.865 |
| Non-pulmonary complications^ | 1 (8.3%) | 5 (23.4%) | 0.268 |
| Critical care admission^ | 5 (41.7%) | 1 (4.8%) | 0.008 |
| Post-operative stay (days)* | 10.0 (4.6) | 6.0 (8.7) | 0.029 |
| Mortality^ | 0 (0%) | 1 (4.8%) | 0.443 |
*, non-parametric continuous data is presented as medians (interquartile range); ^, dichotomous data is presented as number (percentage).
Histopathology
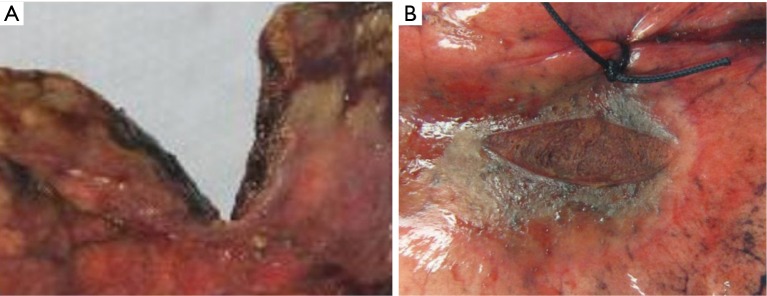
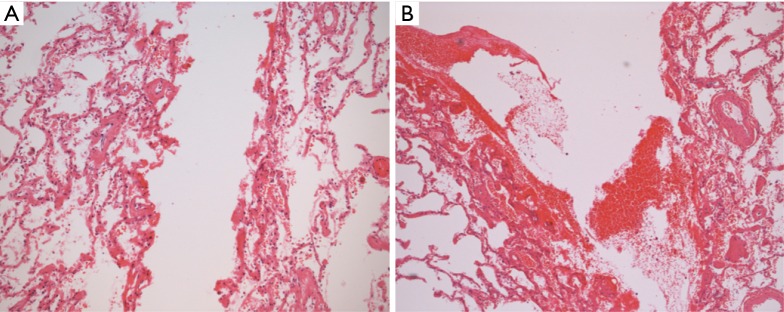
Gross examination revealed charred and friable tissue at the site of the diathermy lesion, whilst the parenchyma treated with Aquamantys was characterised by coagulation but minimal charring (Figure 1). At microscopy, it was noted that coagulation was limited to a depth of approximately 0.5 mm in the diathermy specimen, being almost double that at the Aquamantys lesion, reaching a depth in excess of 0.9 mm at the perimeter (Figure 2).
Figure 1.
Macroscopic images of (A) conventional diathermy, and (B) Aquamantys® lesions on lung parenchyma.
Figure 2.
Microscopic of (A) conventional diathermy, and (B) Aquamantys® lesions on lung parenchyma (magnification, 100×).
Discussion
In the fibropurulent phase of thoracic empyema, purulent infective fluid and fibrin deposition create multiple loculations. Late in the natural history, specifically the organising phase, granulation of the fibrous capsule limits lung expansion and causes progressive restriction of the hemithorax (13-16).
Surgical management of thoracic empyema ranges from minimally invasive (chest tube drainage in association with medical therapy, VATS debridement or VATS decortication) to open procedures such as OD, open window thoracostomy (OWT) or thoracomyoplasty. These latter more invasive procedures are used secondarily for recurring or complicated empyema.
The Aquamantys® system is widely used during orthopaedic joint replacement, spinal surgery and solid organ resection (17-19); and with good effect, including reduced transfusion requirements, post-operative drainage (17,18) and post-operative incidence of haemarthrosis, as well as a reduction in pain scores following joint prosthesis surgery. It has been proven also to reduce blood loss and improve visualization during spinal surgery (8,14,19). A recent report of its application in brain tumour resection has demonstrated similar benefits in terms of reducing blood loss.
Reports of its application in the field of thoracic surgery are limited. Yim et al. reported early experience with two early forms of this device involving a monopolar floating ball and bipolar sealing forceps, both continuously irrigated with saline. They reported deceased thermal spread but similar depths of coagulative necrosis with the floating ball when compared to bipolar sealing forceps; whilst noting minimal complications and largely similar outcomes between groups. No comparison was made with standard diathermy; with the primary endpoint being the declaration of safety of such devices in principle for use in non-anatomical lung resection (11). Ambrogi et al. made use of the floating ball device to achieve cold coagulation of blebs in spontaneous pneumothorax, with good clinical effect but no comparative data (12).
The Aquamantys® system induces a heat-driven denaturation of the collagen triple helix that occurs when thermally susceptible intramolecular crosslinks along the length of the molecule begin to uncouple at around 70 °C. As the intramolecular crosslinks break and the intermolecular crosslinks hold, the long molecular chains shorten and take on a more random orientation. In blood vessels and other biological tubes, the collagen strands shorten in parallel with the dominant direction of fibre orientation, which causes a corresponding swelling in a perpendicular direction. This concurrent shortening and swelling can permanently occlude vessels of up to 5 mm in diameter, in our experience.
In practice, this device is invaluable in controlling focal bleeding. However, it may prove to be equally useful in stemming bleeding from a larger raw area, such as the chest wall following extra-pleural mobilisation after induction treatment, or the lung following complicated fissural dissection. In contrast, our experience with conventional electrosurgery demonstrates frequent charring achieving only partial and often transitory haemostasis.
This technology also holds significant potential for the future of intraoperative air-leak management. Traditional diathermy often produces a friable eschar that frequently fractures, allowing recurrence of bleeding and air leakage in the early postoperative period. In our experience, the Aquamantys® produces a thin and pliant eschar that is well adhered to the deeper tissues and less likely to fracture, as demonstrated on histological analysis. Based on our own intra-operative observations, there seems to be an immediate sealing of the lung that is at least as effective as synthetic spray-on sealant. Furthermore, it is to be expected that the demonstrable increased depth and consistency of coagulation in the Aquamantys lesion would correlate with improved pneumostasis and haemostasis.
Subjectively, the immediate coagulation over a wide surface area, together with the lack of smoke generated also greatly assists in facilitating intraoperative visualization.
Whilst the use of this device in OD has been suggested by Kaufman (20), no previous clinical data to date exists that demonstrates its efficacy.
Our clinical results from 33 consecutive patients demonstrate a good safety profile for this new application of the device. We demonstrate a significant reduction in blood loss and transfusion requirements in the postoperative period. We have also demonstrated a reduction in chest drain duration, as a surrogate for duration and magnitude of postoperative air leak. We have also noted a significant decline in hospital length of stay by 4 days, with the implied health and economic benefits.
The authors propose that this device offers a double effect: superior haemostasis and pneumostasis, thus reducing morbidity on two fronts. It is likely to be cost-effective, particularly in a practice already making use of synthetic topical haemostatic products and polymeric sealants, and in view of the hastened postoperative recovery. Further investigation into this field is warranted to further define the role of what is demonstrably a promising technology.
Study limitations
The authors recognize the various limitations of this study. Particularly, the non-randomised retrospective nature and small cohort of patients, the potential for differences in surgical approach, the lack of protocolised drain management, and the absence of long-term follow-up data.
Conclusions
The use of irrigated tip RF ablation appears to be safe, and is associated with a reduction in blood loss and transfusion requirements, a shorter duration of air leak and subsequent earlier removal of chest drainage systems, and an earlier postoperative discharge.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Molnar TF. Current surgical treatment of thoracic empyema in adults. Eur J Cardiothorac Surg 2007;32:422-30. 10.1016/j.ejcts.2007.05.028 [DOI] [PubMed] [Google Scholar]
- 2.Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii41-53. 10.1136/thx.2010.137000 [DOI] [PubMed] [Google Scholar]
- 3.Zahid I, Routledge T, Billè A, et al. What is the best treatment of postpneumonectomy empyema? Interact Cardiovasc Thorac Surg 2011;12:260-4. 10.1510/icvts.2010.254706 [DOI] [PubMed] [Google Scholar]
- 4.Chambers A, Routledge T, Dunning J, et al. Is video-assisted thoracoscopic surgical decortication superior to open surgery in the management of adults with primary empyema? Interact Cardiovasc Thorac Surg 2010;11:171-7. 10.1510/icvts.2010.240408 [DOI] [PubMed] [Google Scholar]
- 5.Zahid I, Nagendran M, Routledge T, et al. Comparison of video-assisted thoracoscopic surgery and open surgery in the management of primary empyema. Curr Opin Pulm Med 2011;17:255-9. 10.1097/MCP.0b013e3283473ffe [DOI] [PubMed] [Google Scholar]
- 6.Anger WH, Jr. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. AORN J 2010;92:351-2. 10.1016/j.aorn.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 7.Dell'Amore A, Caroli G, Nizar A, et al. Can topical application of tranexamic acid reduce blood loss in thoracic surgery? A prospective randomised double blind investigation. Heart Lung Circ 2012;21:706-10. 10.1016/j.hlc.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 8.Frank SM, Wasey JO, Dwyer IM, et al. Radiofrequency bipolar hemostatic sealer reduces blood loss, transfusion requirements, and cost for patients undergoing multilevel spinal fusion surgery: a case control study. J Orthop Surg Res 2014;9:50. 10.1186/s13018-014-0050-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currò G, Lazzara S, Barbera A, et al. The Aquamantys® system as alternative for parenchymal division and hemostasis in liver resection for hepatocellular carcinoma: a preliminary study. Eur Rev Med Pharmacol Sci 2014;18:2-5. [PubMed] [Google Scholar]
- 10.Grasso G, Giambartino F, Iacopino DG. Hemostasis in brain tumor surgery using the Aquamantys system. Med Sci Monit 2014;20:538-43. 10.12659/MSM.890583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yim AP, Rendina EA, Hazelrigg SR, et al. A new technological approach to nonanatomical pulmonary resection: saline enhanced thermal sealing. Ann Thorac Surg 2002;74:1671-6. 10.1016/S0003-4975(02)03901-2 [DOI] [PubMed] [Google Scholar]
- 12.Ambrogi MC, Melfi F, Duranti L, et al. Cold coagulation of blebs and bullae in the spontaneous pneumothorax: a new procedure alternative to endostapler resection. Eur J Cardiothorac Surg 2008;34:911-3. 10.1016/j.ejcts.2008.06.046 [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society Management of nontuberculous empyema: a statement of the subcommittee on surgery. Am Rev Respir Dis 1962;935-6. [Google Scholar]
- 14.Moores DW. Management of acute empyema. Chest 1992;102:1316-7. 10.1378/chest.102.5.1316 [DOI] [PubMed] [Google Scholar]
- 15.Petrakis IE, Kogerakis NE, Drositis IE, et al. Video-assisted thoracoscopic surgery for thoracic empyema: primarily, or after fibrinolytic therapy failure? Am J Surg 2004;187:471-4. 10.1016/j.amjsurg.2003.12.048 [DOI] [PubMed] [Google Scholar]
- 16.Mutsaers SE, Prele CM, Brody AR, et al. Pathogenesis of pleural fibrosis. Respirology 2004;9:428-40. 10.1111/j.1440-1843.2004.00633.x [DOI] [PubMed] [Google Scholar]
- 17.Marulanda GA, Ragland PS, Seyler TM, et al. Reductions in blood loss with use of a bipolar sealer for hemostasis in primary total knee arthroplasty. Surg Technol Int 2005;14:281-6. [PubMed] [Google Scholar]
- 18.Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty 2007;22:82-5. 10.1016/j.arth.2007.02.018 [DOI] [PubMed] [Google Scholar]
- 19.Marulanda GA, Ulrich SD, Seyler TM, et al. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices 2008;5:125-31. 10.1586/17434440.5.2.125 [DOI] [PubMed] [Google Scholar]
- 20.Kaufman AJ, Flores RM. Technique of Pleurectomy and Decortication. Oper Tech Thorac Cardiovasc Surg 2010;15:294-306. 10.1053/j.optechstcvs.2010.12.002 [DOI] [Google Scholar]