Abstract
Background
we conducted this systematic meta-analysis to determine the association between chronic obstructive pulmonary disease (COPD) and risk of bronchopleural fistula (BPF) in patients undergoing lung cancer surgery.
Methods
Literature retrieval was performed in PubMed, Embase and the Web of Science to identify the full-text articles that met our eligibility criteria. Odds ratio (OR) with 95% confidence interval (CI) served as the summarized statistics. Q-test and I2-statistic were used to evaluate the level of heterogeneity. Sensitivity analysis was performed to further examine the stability of pooled OR. Publication bias was detected by both Begg’s test and Egger’s test.
Results
Eight retrospective observational studies were included into this meta-analysis. The overall summarized OR was 2.03 (95% CI: 1.44–2.86; P<0.001), revealing that COPD was significantly associated with the risk of BPF after lung cancer surgery. In subgroup analysis, the relationship between COPD and BPF occurrence remained statistically prominent in the subgroups stratified by statistical analysis (univariate analysis, OR: 1.91; 95% CI: 1.35–2.69; P<0.001; multivariate analysis, OR: 3.18; 95% CI: 1.95–5.19; P<0.001), operative modes (pneumonectomy, OR: 2.11; 95% CI: 1.15–3.87; P=0.016) and in non-Asian populations (OR: 2.36; 95% CI: 1.18–4.73; P=0.016). No significant impact of COPD on BPF risk was observed in Asian patients (OR: 1.48; 95% CI: 0.85–2.57; P=0.16). No significant heterogeneity or publication bias was discovered across the included studies.
Conclusions
Our meta-analysis indicates that COPD can significantly predispose to BPF formation in patients undergoing lung cancer surgery. Because some limitations still exist in this meta-analysis, our findings should be further verified and modified in the future.
Keywords: Bronchopleural fistula (BPF), chronic obstructive pulmonary disease (COPD), lung cancer surgery, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is generally considered as a fatal disease resulting in low cardiopulmonary reserve, and it can reduce the operable opportunity largely in thorax and upper abdomen (1,2). Meanwhile, COPD is also the most crucial comorbidity in lung cancer patients, with the prevalence of 40% to 70% (3). A latest evidence-based report has indicated that COPD is a strong predictor for the poor prognosis of lung cancer (4). Most recent evidences suggest that an increased risk of postoperative pulmonary complications is the major cause of morbidity in lung cancer patients with COPD (1,2,5). However, the impact of COPD on each type of postoperative complications still remains controversial because of the inconsistent results derived from different studies (2,6).
Among the major complications after lung cancer surgery, bronchopleural fistula (BPF) is a particularly severe one because of the devastating leakage from airways into the pleural space, causing the mortality rate from 18% to 50% in hospital (7). Currently, surgical procedures remain the leading causes of BPF. Some other parameters, including neo-adjuvant induction therapy (NIT), incomplete resection and mechanical ventilation, have also been widely studied and accepted as significant risk factors of BPF (8-11). As an important preoperative factor, COPD may predispose lung cancer patients to bronchial stump leakage. However, its roles in BPF development are still not well-defined. All of the available investigations evaluating the impact of COPD on BPF occurrence have not yet been systematically reviewed.
Therefore, we conducted this systematic review and meta-analysis to determine whether concomitant COPD was significantly associated with the increased risk of BPF in patients undergoing lung cancer surgery.
Methods
Protocol
No protocol had been previously published for this review. Patients’ consent or ethical approval is not required in a systematic review and meta-analysis. We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (12). The additional PRISMA 2009 checklist is prepared as the Table S1.
Search strategy
The literature retrieval for this meta-analysis ranged from January 15, 2016 to January 17, 2016. A comprehensive literature search was performed by three researchers (J Huang, XD Zhou and L Tian) in PubMed, EMBASE (via Ovid interface) and the Web of Science (via the campus network of Sichuan University), to identify the full-text articles published up to January 15, 2016 that met our eligibility criteria. No language limitation was imposed during the retrieval.
Consulting the search details in previous meta-analyses resolving the clinical problems correlated with COPD, two search strings were combined with four key words and two Boolean Operators (“AND” and “OR”) to search the online databases (13-15). These key words are listed as follows: (I) “bronchopleural fistula” and “bronchial fistula”; (II) “chronic obstructive pulmonary disease” and “COPD”. The complete search details in each database are outlined in the Table S2.
In addition, the reference list in each article was also manually screened for identification of possibly included studies with no duplication.
Inclusion and exclusion criteria
We formulated the following inclusion and exclusion criteria to determine the eligible studies included into meta-analysis.
Inclusion criteria: (I) the target disease is lung cancer; (II) a BPF developed from the surgical procedures instead of the spontaneous diseases; (III) COPD status is independently analyzed as one possible parameter in original articles; (IV) the demographics associated with the BPF formation in COPD patients are available; (V) any statistic derived from multivariate analysis or univariate analysis, including the odds ratio (OR), relative risk (RR) or hazard ratio (HR) with corresponding 95% confidence interval (CI), is validly reported in original literatures.
Exclusion criteria: (I) the following articles are directly excluded: case reports or series, reviews, letters and conference abstracts; (II) BPF occurrence is not clearly described; (III) lung transplantation is not considered in this meta-analysis.
Quality assessment
Newcastle-Ottawa Scale (NOS) was employed to estimate the quality level of original non-randomized studies (16). Three perspectives including selection, comparability and exposure were considered for a semi-quantitative estimation. The “star system” with a maximum of nine stars was used as the assessment tool. After grading all of the included studies, we regarded 8–9 stars as a good quality, 6–7 stars as a medium quality, and lower than 6 stars as a poor quality.
Data collection
We designed a Microsoft Excel sheet to collect the following key information from each study: (I) publication data including authors, publication years and languages; (II) experimental data including study design, study period, patients’ origins, operative modes and the onsets of BPF; (III) demographic data including age, sample sizes and the number of patients with COPD and postoperative BPF; (IV) statistical data including any reported statistic, extraction of incorporative statistics, statistical analysis methods and researchers’ attitude. In addition, the adjusted confounding factors would be recorded if a multivariate analysis using logistic regression or Cox proportional hazards model was performed in the original articles.
Statistical analysis
In general, the incidence of BPF was far lower than 20% (7). No evidence revealing any significant difference between OR and RR was observed, and the risk of overestimating the impact of COPD on BPF occurrence could be greatly avoided (17). Therefore, we finally applied OR with 95% CI as the appropriate summarized statistics. Incorporating the multivariate OR outcomes into quantitative synthesis was our first priority. However, if multivariate analysis was not performed, we could also extrapolate the univariate OR with 95% CI from published demographic data. Remarkably, a significant relationship between COPD and increased BPF risk could be proved when the pooled OR with 95% CI was more than 1.
We used Q-test and I2-statistic to determine the heterogeneity level within this meta-analysis. Fine heterogeneity was defined by I2<40% and P>0.1, and the standard fixed-effect model test (Mantel-Haenszel method) would be applied for integrations of ORs. Otherwise, the random-effect model test (DerSimonian and Laird method) would be considered when high heterogeneity was revealed by I2≥40% or P≤0.1 (18).
For additional analysis, we performed a sensitivity analysis to further evaluate the stability of the summarized estimates. We removed the study which was identified to be associated with the increased heterogeneity and repeated a meta-analysis of the remaining studies for adjustments. The robustness of our meta-analysis would be confirmed if no substantial variation was identified between the adjusted estimates and primary estimates (19).
Moreover, both Begg’s test and Egger’s test were used to detect the potential publication bias existed across the included studies. Its presence could be suggested by the symmetry of funnel plot conducted by Begg’s test, in which log ORs were plotted against their standard errors (SEs) (20). The significant bias could also be confirmed by Egger’s P value <0.05.
Finally, we declared that all of the above statistical analyses were accomplished by STATA 12.0 (STATA Corporation, College Station, TX).
Results
The selection of included studies
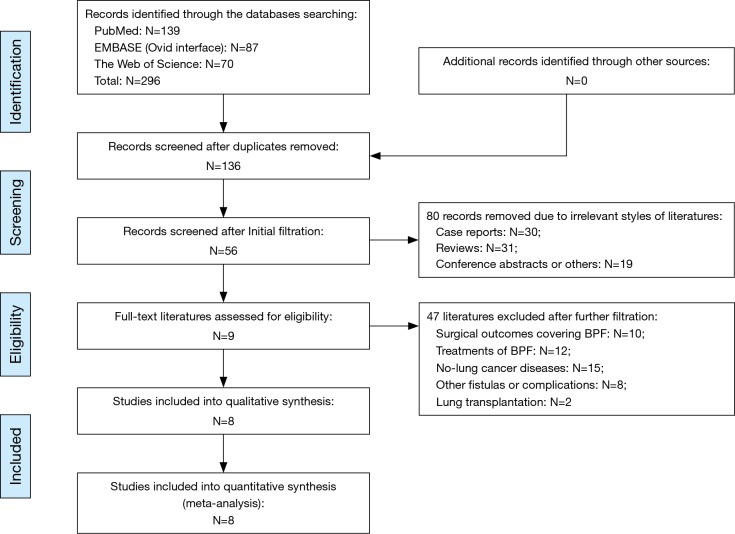
The complete details for literature retrieval were shown as a PRISMA flow diagram (Figure 1). Primary retrieval identified a total of 296 citations of publications, including 139 citations in PubMed, 87 citations in Embase and 70 citations in the Web of Science. There were 136 of them entered into the initial filtration based on screening their titles and abstracts after excluding 160 duplicates. A total of 80 irrelevant unqualified article styles were immediately excluded after initial filtration, including 30 case reports, 31 reviews and 19 conference abstracts. The further filtration was continued by reading through the full-text of the remaining 56 articles. Then, after excluding 47 items which focused on other topics, nine studies (2,6,21-27) were considered for possible eligibility of our meta-analysis (Figure 1). However, we excluded one of them out of the qualitative synthesis due to the scarcity of extractable data of lung cancer from various pulmonary diseases (27). Finally, the remaining eight studies met all of the eligibility criteria and were included into our meta-analysis (2,6,21-26).
Figure 1.
PRISMA flow diagram of the literature retrieval. BPF, bronchopleural fistula; PRISMA, preferred reporting items for systematic reviews and meta-analysis.
The quality level of included studies
Two researchers (SJ Li and J Liu) were assigned to grade all of the included studies. The complete details for estimations are tabulated as Table S3. Finally, we identified that the mean NOS score of these studies was 8.0 (range, 7–9), suggesting a generally good quality level (Table 1).
Table 1. Basic characteristics of the included studies.
| Authors [year] | Language | Patients’ origin | Study design | Study period | No. of samples | Mean age (years) | Operative modes | Onset, days (mean, range) | NOS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | COPD | BPF | PN | LB | Others | ||||||||
| Algar et al. [2001] (21) | English | Spain | ROS | 1986–1997 | 242 | 127 | 13 | 60.0 | ✓ | ✗ | ✗ | 11.0±8.0 | 8 |
| Hu et al. [2013] (22) | English | China | ROS | 1995–2012 | 684 | 240 | 30 | 57.0 | ✓ | ✗ | ✗ | NI | 8 |
| Lindner et al. [2010] (23) | English | Germany | ROS | 2000–2007 | 243 | 103 | 13 | 62.2 | ✓ | ✗ | ✗ | NI | 8 |
| Panagopoulos et al. [2009] (24) | English | Greece | ROS | 1999–2005 | 221 | 28 | 5 | 62.4 | ✓ | ✗ | ✗ | 26.6 [7–70] | 8 |
| Sekine et al. [2002] (2) | English | USA | ROS | 1992–1997 | 244 | 78 | 5 | 64.1 | ✓ | ✓ | ✓ | NI | 7 |
| Sekine et al. [2013] (6) | English | Japan | ROS | 1990–2005 | 1461 | 363 | 27 | 63.9 | ✓ | ✓ | ✓ | NI | 7 |
| Stolz et al. [2014] (25) | English | Czech | ROS | 1998–2012 | 329 | 63 | 12 | 55.8 | ✓ | ✗ | ✗ | 9.5 [2–140] | 9 |
| Yena et al. [2006] (26) | French | France | ROS | 1989–2003 | 725 | 125 | 58 | 61.0 | ✓ | ✗ | ✗ | NI | 9 |
BPF, bronchopleural fistula; COPD, chronic obstructive pulmonary disease; LB, lobectomy; NI, no information; NOS, Newcastle-Ottawa Scale; PN, pneumonectomy; ROS, retrospective observational study.
The basic characteristics of included studies
Baseline characteristics for the eight included studies are shown in Table 1. All of these studies belong to retrospective observational studies, which were published between 2001 and 2014 (2,6,21-26). A total of 4,149 lung cancer patients were enrolled from 1986 to 2012, including 1,461 patients from Japan (6), 684 patients from China (22) and 2,004 patients from Europe and North America (2,21,23-26). The operations performed on these patients contained pneumonectomy (2,570/4,149, ratio =61.9%) (2,6,21-26), lobectomy (1,491/4,149, ratio =36.0%) and other operative modes (88/4,149, ratio =2.1%) (2,6). COPD was diagnosed in 1,127 surgical patients (ratio =27.2%) but its severity was not reported in most of the included studies (2,21-26). Overall, postoperative BPF was diagnosed in 163 patients by both endoscopic inspection and clinical manifestation, and its incidence was 3.9% in this meta-analysis (2,6,21-26). Besides, the interval between primary operations and BPF formation varied a lot across the included studies and their details are summarized in Table 1.
The statistical characteristics of included studies
Most of the eight included studies performed both univariate analysis and multivariate analysis to identify the significant risk factors of BPF (21-26). However, only three of them reported the multivariate statistics revealing the correlation between COPD and BPF risk, including OR with 95% CI in two studies (25,26) and β value with SE in one study (21). Complete demographic details could be extracted from seven studies and extrapolated for univariate OR outcomes (2, 6,21-24,26). Only one study reported by Stolz et al. (25) did not give the demographics but published both multivariate and univariate OR with 95% CI directly. Therefore, the majority of the data incorporated into our meta-analysis was based on univariate analysis. The detailed 2×2 cross-table of demographics and OR results in each included study are outlined in Table 2.
Table 2. Statistical characteristics of the included studies1.
| Authors [year] | BPF | COPD | OR with 95% CI | P value | Extraction | Analysis | Attitude | Adjusted confounding factors (by multivariate analysis) | |
|---|---|---|---|---|---|---|---|---|---|
| COPD (+) | COPD (−) | ||||||||
| Algar et al. [2001] (21) | BPF (+) | 11 (8.7%) | 2 (1.7%) | 7.95 (1.25–50.79) | 0.0283 | Reported | Multivariate | Positive | Age, smoking, BMI, routine blood indexes, steroid use, PFT, albumin, NT, operative modes, sides and time, bronchial closure and coverage, MV |
| BPF (−) | 116 | 113 | |||||||
| Hu et al. [2013] (22) | BPF (+) | 14 (5.8%) | 16 (3.6%) | 1.66 (0.79–3.46) | 0.17 | DDE | Univariate | Negative | NI |
| BPF (−) | 226 | 428 | |||||||
| Lindner et al. [2010] (23) | BPF (+) | 4 (3.9%) | 9 (6.4%) | 0.59 (0.18–1.97) | 0.64 | DDE | Univariate | Negative | NI |
| BPF (−) | 99 | 131 | |||||||
| Panagopoulos et al. [2009] (24) | BPF (+) | 0 (0.0%) | 5 (2.6%) | 0.60 (0.03–11.05) | 1.0 | DDE | Univariate | Negative | NI |
| BPF (−) | 28 | 186 | |||||||
| Sekine et al. [2002] (2) | BPF (+) | 3 (3.8%) | 2 (1.2%) | 3.28 (0.54–20.04) | 0.17 | DDE | Univariate | Negative | NI |
| BPF (−) | 75 | 164 | |||||||
| Sekine et al. [2013] (6) | BPF (+) | 8 (2.2%) | 19 (1.7%) | 1.28 (0.56–2.95) | 0.56 | DDE | Univariate | Negative | NI |
| BPF (−) | 355 | 1079 | |||||||
| Stolz et al. [2014] (25) | BPF (+) | Not available | 4.10 (1.60–9.50) | 0.065 | Reported | Multivariate | Negative | Age, gender, BMI, smoking, NT, coronary artery diseases, hypertension, diabetes, operative modes and sides | |
| BPF (−) | |||||||||
| Yena et al. [2006] (26) | BPF (+) | 19 (15.2%) | 39 (6.5%) | 2.55 (1.38–4.72) | 0.003 | Reported | Multivariate | Positive | Age, gender, smoking, PFT, previous malignancy, diabetes, operative modes, sides and time, bronchial closure and reinforcement |
| BPF (−) | 106 | 561 | |||||||
1, numbers in parentheses indicate the incidence of BPF in patients with COPD and without COPD. BMI, body mass index; BPF, bronchopleural fistula; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DDE, demographic data extrapolated; MV, mechanical ventilation; NI, no information; NT, neo-adjuvant therapy; OR, odds ratio; PFT, pulmonary function test.
Overall analysis
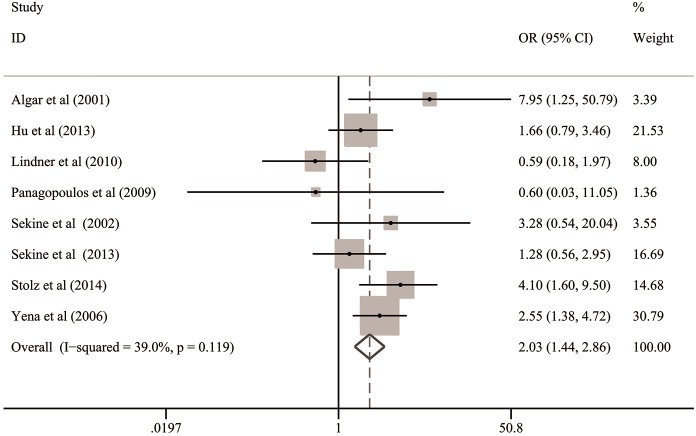
On the basis of quantitative integrations of eligible statistics from all the included studies, the pooled OR was 2.03 (95% CI: 1.44–2.86; P<0.001; Table 3 and Figure 2) with low heterogeneity (I2=39.0%, P=0.12), suggesting that COPD was significantly associated with the risk of BPF in patients undergoing lung cancer surgery.
Table 3. Meta-analysis of the association between COPD and risk of BPF after lung cancer surgery.
| Groups of outcomes | N | Enrolled samples | Heterogeneity (I2, P) | Model | OR with 95% CI | P value | Publication bias | Conclusion | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | COPD | BPF | Begg (P) | Egger (P) | |||||||
| Overall | 8 | 4,149 | 1,127 | 163 | 39.0%, 0.12 | Fixed | 2.03 (1.44–2.86) | <0.001 | 0.90 | 0.89 | Significant |
| Statistical analysis1 | |||||||||||
| Univariate analysis | 8 | 4,149 | 1,127 | 163 | 20.9%, 0.26 | Fixed | 1.91 (1.35–2.69) | <0.001 | 0.90 | 0.79 | Significant |
| Multivariate analysis | 3 | 1,296 | 315 | 83 | 0.0%, 0.42 | Fixed | 3.18 (1.95–5.19) | <0.001 | 0.30 | 0.19 | Significant |
| Operative modes | |||||||||||
| Pneumonectomy | 6 | 2,444 | 686 | 131 | 49.4%, 0.08 | Random | 2.11 (1.15–3.87) | 0.016 | 0.71 | 0.76 | Significant |
| Lobectomy | Given up because of the scarcity of available data | ||||||||||
| The origins of patients | |||||||||||
| Asian | 2 | 2,145 | 603 | 57 | 0.0%, 0.65 | Fixed | 1.48 (0.85–2.57) | 0.16 | 1.0 | NI | Not significant |
| Non-Asian | 6 | 2,004 | 524 | 106 | 45.8%, 0.10 | Random | 2.36 (1.18–4.73) | 0.016 | 1.0 | 0.77 | Significant |
1, all the included studies reported demographics or statistics derived from univariate analysis. The OR with 95% CI from multivariate analysis was reported in Ref (16,20,21). BPF, bronchopleural fistula; CI, confidence interval; COPD, chronic obstructive pulmonary disease; N, reference count; NI, no information; OR, odds ratio.
Figure 2.
Overall analysis for the association between COPD and risk of BPF in patients undergoing lung cancer surgery. BPF, bronchopleural fistula; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Subgroup analysis
To further evaluate the impact of COPD on postoperative BPF, we classified all cases into several subgroups according to the methods of statistical analysis, operative modes and the origins of patients. Their results are summarized in Table 3.
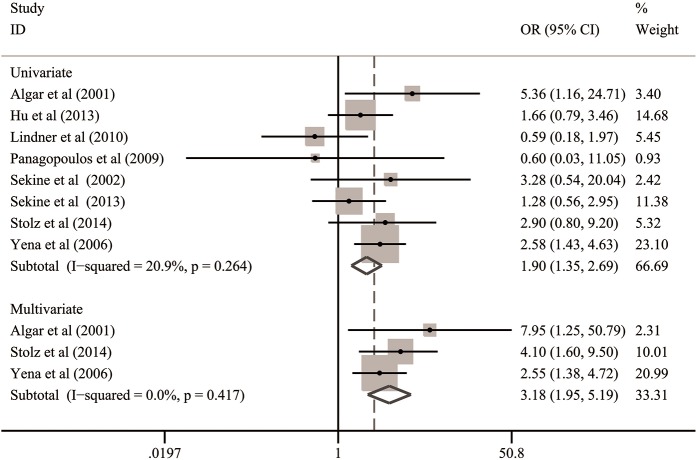
In the subgroups stratified by statistical analysis, all the included studies could provide univariate OR outcomes but only three of them published multivariate OR outcomes. Therefore, we incorporated the univariate data from eight studies (2,6,21-26) and multivariate data from three studies (21,25,26), respectively. Finally, both the summarized data based on univariate analysis (OR: 1.91; 95% CI: 1.35–2.69; P<0.001) and multivariate analysis (OR: 3.18; 95% CI: 1.95–5.19; P<0.001) indicated that COPD could significantly predispose to BPF formation after lung cancer surgery (Table 3 and Figure 3).
Figure 3.
Subgroup analysis for the association between COPD and risk of BPF in the subgroups stratified by statistical analysis. BPF, bronchopleural fistula; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
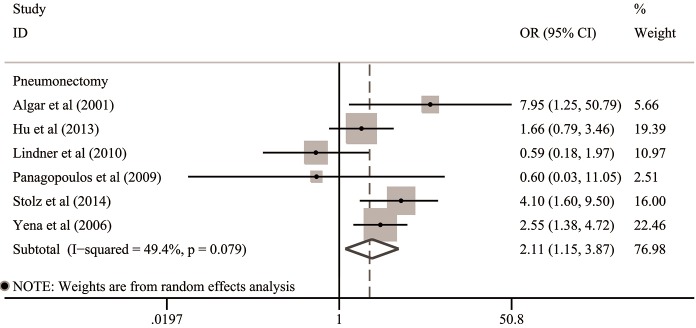
For operative modes, the clinical data of 2,444 patients undergoing pneumonectomy from six included studies were available for subgroup analysis (21-26). The integrated OR of these six studies was 2.11 (95% CI: 1.15–3.87; P=0.016), indicating that COPD was significantly associated with the increased risk of post-pneumonectomy BPF (Table 3 and Figure 4). The remaining two studies enrolled a total of 1,705 lung cancer patients undergoing variety of resections but analyzed them as a whole (2,6). Thus, we gave up a further assessment on the association between COPD and post-lobectomy BPF because of the scarcity of available details.
Figure 4.
Subgroup analysis for the association between COPD and risk of BPF in the subgroups stratified by operative modes. BPF, bronchopleural fistula; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
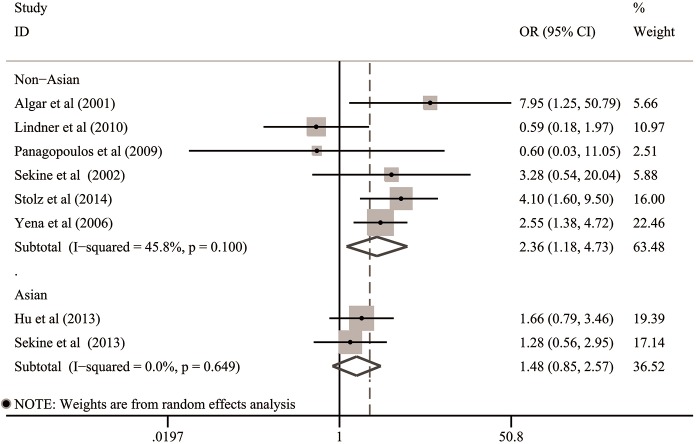
In the subgroups stratified by the origins of patients, no evidence revealing any significant relationship between COPD and BPF risk was observed in Asian populations (6,22) (OR: 1.48; 95% CI: 0.85–2.57; P=0.16). However, the summarized estimates integrating the clinical data of 2,004 European and North-American patients from six studies (2,21,23-26) suggested that postoperative BPF occurred more frequently in non-Asian patients with COPD (OR: 2.36; 95% CI: 1.18–4.73; P=0.016) (Table 3 and Figure 5).
Figure 5.
Subgroup analysis for the association between COPD and risk of BPF in the subgroups stratified by the origins of patients. BPF, bronchopleural fistula; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
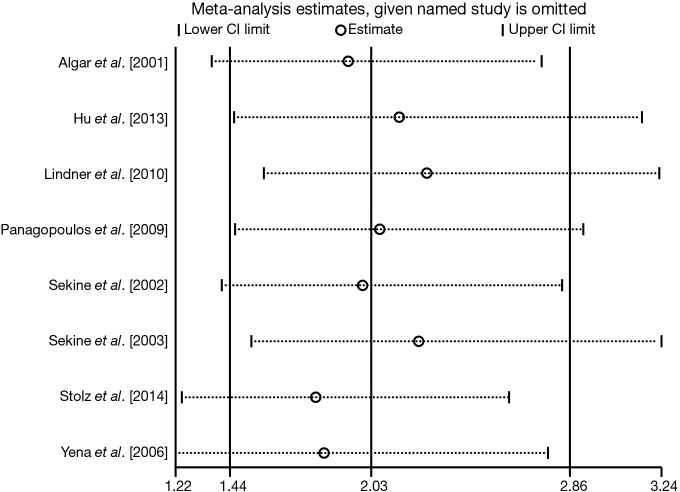
Sensitivity analysis
The forest plot derived from sensitivity analysis was shown as Figure 6. None of the individual OR statistics was out of the estimated ranges by visual inspection, and no substantial variation interfering the primary summarized OR was observed. Therefore, the leave-one-out method and further adjustments of heterogeneity were no longer necessary. The strong robustness of our meta-analysis was thus confirmed.
Figure 6.
Sensitivity analysis for the association between COPD and risk of BPF in patients undergoing lung cancer surgery. BPF, bronchopleural fistula; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Besides, the stability of integrated outcomes from each of the subgroups was also examined by sensitivity analysis. No significant variation was observed between the primary pooled OR and adjusted pooled OR in all of the subgroups, including the multivariate data group, univariate data group, pneumonectomy group, Asian populations and non-Asian populations. Complete details for each adjusted OR with 95% CI were not shown. Finally, the strong robustness of the summarized outcomes derived from subgroup analysis was also confirmed.
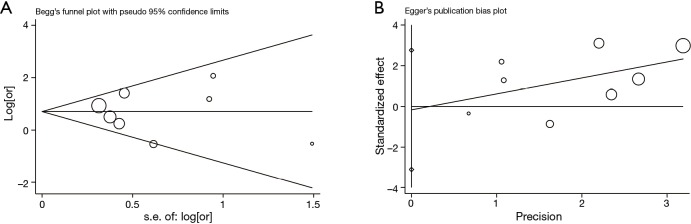
Publication bias
The funnel plots conducted by Begg’s test and Egger’s test were shown as Figure 7A,B. No evidence for significant publication bias was detected within this meta-analysis by visually inspecting the symmetry of Figure 7A and estimating the Egger’s P value (P=0.89). These tests were also performed in each subgroup and no significant bias was finally revealed (Table 3).
Figure 7.
Publication bias for the association between COPD and risk of BPF in patients undergoing lung cancer surgery detected by (A) Begg’s test and (B) Egger’s test. BPF, bronchopleural fistula; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Discussion
According to the measurement criteria, the predicted forced expiratory volume in one second (FEV1) ≤70% and the ratio of FEV1/forced vital capacity (FVC) ≤70%, indicate the diagnosis of COPD (28). Heavy cigarette smoking, genetic predisposition and environmental exposure significantly increase the risk of COPD, especially in patients with lung cancer (2,4). Surgical attack further decreases the already limited respiratory reserve and causes more postoperative complications in lung cancer patients with COPD. Remarkably, postoperative pneumonia, hypoxemia and pneumothorax seem to occur more frequently among the major complications (2). The reduced area of gas exchange and inadequate distance from alveolar surface to capillary endothelium may be the possible mechanisms resulting in adverse effects on hemoglobin concentration (2,6). However, a consensus on the relationship between COPD and risk of BPF has not been achieved because of some controversial results reported in previous studies (2,6,22-25). When pooling these studies together, we got an initial impression that a little higher incidence of postoperative BPF was commonly identified in patients with COPD. Therefore, we speculated that the negative effects of small sample size might carry some biases on analyzing the clinical outcomes with statistical significance.
Meta-analysis is a well-established statistical method integrating the appropriate data from homogeneous studies to draw global conclusions (9,11,29,30). By applying this evidence-based method to a larger number of samples enrolled from the included studies, the integrated statistics may help to verify the real impact of COPD on BPF risk. To the best of our knowledge, our study is the first meta-analysis to systematically evaluate the association between COPD and BPF occurrence after lung cancer surgery. In this meta-analysis, we integrated the currently available evidences quantitatively, which led to the final conclusion that COPD was significantly associated with the risk of BPF in patients undergoing lung cancer surgery. Meanwhile, we identified that the relationship between COPD and risk of BPF still remained statistically prominent in the subgroups stratified by statistical analysis, operative modes and in non-Asian populations. However, the validity of our summarized outcomes should be seriously evaluated in clinical practice because of the following two potential bias risks from the statistical sources.
On the one hand, no randomized controlled trial (RCT) or prospective study investigating the risk factors of BPF was identified during the literature retrieval. All of the included studies were retrospective observational studies, resulting in a large decline of evidence level in our meta-analysis. That may be because of the following two aspects of inherent limitations for this issue to be addressed. Firstly, thoracic surgeons usually make more efforts on buttressing the bronchial stumps in patients considered of high BPF risk, such as those undergoing pneumonectomy, NIT or steroid therapy (8,11). An adequately balanced comparison between the groups of patients with different baseline risks was explicitly unfeasible. Secondly, it seems that COPD cannot be regarded as an intervention which is validly mandated by researchers for an operable RCT or prospective study. Therefore, there is no high-quality RCT or prospective study resolving this pending question until now. Because of the retrospective nature of all the included studies, some insufficiently eliminated confounding factors may bring unavoidable interferences on pooling the accurate results.
On the other hand, univariate analysis is usually used to evaluate some possible clinical variables preliminarily, and a subsequent multivariate analysis is used to clarify their statistical significances on serving as the independent risk factors of BPF (21-26). However, the majority of the included studies in our meta-analysis just published the multivariate statistics for some significant parameters rather than all the analyzed events. A subgroup analysis enrolling three available OR outcomes derived from multivariate analysis revealed a significant relationship between COPD and risk of BPF. However, considering the poor sensitivity of both Begg’s test and Egger’s test on pooling far fewer than 20 studies, we doubted that potential publication bias might exist in the subgroup analysis based on multivariate data, although no evidence was currently detected (20).
In this meta-analysis, the majority of incorporative data was based on univariate analysis, without sufficient eliminations of confounding factors (2,6,22-24). We realized that the validity and accuracy of the present pooled analysis might be slightly attenuated by the following four major confounding factors that should be especially considered.
Firstly, we had performed a subgroup analysis on the patients undergoing pneumonectomy separately but failed to further assess the potential bias caused by right anatomic side because of the scarcity of extractable data. In addition, NIT was generally encouraged for locally advanced lung cancer but a much higher rate of BPF was frequently observed in patients undergoing NIT and followed by pneumonectomy (7,8,22). Therefore, the application of NIT should also be judiciously evaluated in surgical patients with COPD. However, none of the eight included studies published the complete records of patients receiving NIT. We were unable to extract the valid data about the prevalence of COPD and BPF in patients treated with NIT. Thus, we gave up a further subgroup analysis stratified by NIT acceptance.
Secondly, systematic steroid therapy was generally encouraged to be applied on the patients with stable COPD. Evidences from previously published RCTs and prospective studies indicated that the long-term treatment with inhaled corticosteroids in COPD exacerbations could shorten recovery time, improve pulmonary function and arterial hypoxemia, and reduce the risk of early relapse, treatment failure and the length of hospitalization (28). However, steroid treatment was also proved to be a significant risk factor of BPF (7). Its possible mechanisms may be related to the secondary pulmonary infections and hypoalbuminemia after applying long-term and high-dose corticosteroids. In our meta-analysis, only one study (21) has reported the potential impact of steroid therapy on BPF risk after pneumonectomy. The scarcity of relevant data in most of the included studies led to the insufficient eliminations of bias risks in steroid use (2,6,22-26).
Thirdly, Sekine et al. (6) suggested that surgical intervention might lead to respiratory failure in lung cancer patients with COPD because pulmonary resections could further decrease the already limited lung functions and cause hypoventilation, hypoxia, hypercapnia and the retention of secretions. Prolonged mechanical ventilation was urgently required to sustain the vital respiratory signs of surgical patients with COPD. However, a recent systematic review indicated that most of the current evidences revealed a significant relationship between postoperative mechanical ventilation and the occurrence of BPF (10). Continuous barotrauma on the bronchial stump caused by prolonged ventilation predisposed to the development of BPF. Its impact on BPF risk could be easily confused by the presence of COPD. Unfortunately, we failed to perform a formally statistical analysis to evaluate this confounding factor, because no detail of ventilation and BPF presence in patients with COPD was extractable from the included studies. Therefore, the validity of the integrated results should be judiciously considered in clinical practice.
Finally, the severity of COPD was not clearly described in most of the included studies (2,21-26). Only one study reported by Sekine et al. (6) analyzed the impact of different degrees of COPD severity on surgical outcomes in 1,461 lung cancer patients from Japan, and a significantly increased risk of BPF was revealed in patients with severe COPD but not in those with moderate or mild COPD. In general, COPD goes along with lung cancer quite frequently, but its severity varies greatly. The concept of COPD consists of the percentage of predicted FEV1 and ratio of FEV1/FVC but does not involve any assessment for pulmonary diffusing capacity, which is represented by diffusion capacity for carbon monoxide of the lung (DLCO). Therefore, DLCO may be a potential confounder causing a few biases within the pooled analysis. However, any one of above basic pulmonary function values has not yet been proved as an independent risk factor of BPF in the previously published studies (7). On the basis of such concerns, a scoring collaboration of some basic pulmonary function indices, such as FEV1, FVC and DLCO, may have more discriminative power on the occurrence of BPF, although no convincing evidence is currently reported. As Toufektzian et al. (10) identified, performing pulmonary resections in patients with infectious conditions may be more likely to result in BPF. Thus, we supposed that chronic inflammation of bronchial mucosa in patients with severe COPD could cause large adverse effects on bronchial stump healing. The ratio of severe COPD varied across different studies and might bring large bias risks on pooling valid data. However, a further subgroup analysis was not feasible because no enough detail on COPD severity was published in the current studies. More studies enrolling a large number of patients with explicit degrees of COPD are required for performing a much more detailed updated meta-analysis in the future.
In addition, a new finding from subgroup analysis aroused our interests and indicated that the relationship between COPD and risk of BPF might have potential ethnic differences. BPF seemed like to appear more frequently in COPD patients from non-Asian nations. But no evidence revealing any significant impact of COPD on BPF risk was observed in Asian populations (6,22). The prevalence, susceptibility and burden of COPD vary by ethnic groups, environments and socio-economic status (28,31). However, new evidences reveal no racial difference in the main clinical characteristics of COPD, including pulmonary function impairment, gas exchange and exercise performance (31). Bhopal et al. (32) investigated the ethnic inequalities on health status and care in non-cancer respiratory diseases and COPD. In their study, both the risks of respiratory hospitalization and death from any COPD disorder seemed to be lower in Chinese, Indian and Pakistani patients than in European white races (32). These discoveries might suggest that the prevalence of COPD was lower, or less comorbidity appeared in non-White groups. However, we found that none of the current studies could interpret our discoveries directly. We suspected that only two included studies might not be enough to demonstrate a statistically prominent relationship between COPD and BPF occurrence in Asian patients, although a tendency towards the increased risk of BPF was observed in this subgroup. Therefore, this finding need further affirmations and modifications in the future.
Regarding the current preventive and therapeutic regimens, the key to BPF management is prevention (33). Therefore, it will be most crucial for thoracic surgeons to recognize the patients considered of high BPF risk correctly and buttress the bronchial stumps strongly before closing chest. Moreover, as Hu et al. (22) suggested, some important strategies during the perioperative period, such as parenteral alimentation, oxygen therapy and strengthened antibiotic therapy, can also reduce the prevalence of BPF. The therapeutic options for BPF range from extensive surgical procedures to conservative bronchoscopic approaches (34). In the last few decades, bronchoscopic occlusion of BPF has been increasingly promoted in clinical practice because a secondary operation can be avoided. The current bronchoscopic techniques, including expandable stent implantation, fibrin glue adhesion, submucosal injection of polidocanol and amplatzer device closure, have been proved to represent a safe and effective alternative for treating small and early BPFs compared to surgical interventions (35-40). However, the efficacy of bronchoscopic management for large and late BPFs still remains a debate according to the current investigations because re-operations seem to be required in most of the patients undergoing endoscopic closure of large fistulas (41). Recently, Petrella et al. (42) introduced a novel minimally invasive technique by transplanting the stem cells under bronchoscopy to cure BPF in an animal model, and a great efficacy of bronchoscopic transplantation has been discovered in their experiment. This novel technique has begun to be applied in clinical practice but not yet been proved as a feasible alternative for BPF closure because of the scarcity of large-scale trials (43). Therefore, further clinical trials with large sample size are required to provide more convincing evidence revealing the efficacy of bronchoscopic occlusion of BPF in the future.
Limitations
Finally, several major limitations existed in this meta-analysis should be acknowledged. First, no RCT or prospective study was included into our meta-analysis because of some intrinsic limitations of the pending issue itself. The evidence level of our meta-analysis was attenuated by including only eight retrospective observational studies with a total of 4,149 patients with lung cancer. Second, the OR with 95% CI incorporated into quantitative synthesis were mainly originated from univariate analysis. Thus, some insufficiently eliminated confounders might cause some adverse effects on the validity of our meta-analysis. Third, we gave up a further assessment for BPF development in patients with different degrees of COPD severity because of the scarcity of available data from the current studies. Four, more additional works might be identified and included into our meta-analysis if searching other native electronic databases in non-English languages. Finally, the eight studies included into our meta-analysis came from different countries. Thoracic surgeons should judiciously consider the validity of our discoveries in the clinical settings of their own nations.
Conclusions
In conclusion, our meta-analysis has demonstrated that COPD can significantly predispose to BPF formation in patients undergoing lung cancer surgery. The relationship between COPD and risk of BPF remains statistically significant in the subgroups stratified by statistical analysis, operative modes and in non-Asian populations. No significant impact of COPD on BPF formation was observed in Asian patients. Because some limitations still exist in this meta-analysis, our findings need to be further verified and modified in the future.
Acknowledgements
Special thanks to the statistical analysis contribution from the statistician, Miss. Jing Liu, from the Institution of Medical Statistics, West China School of Public Health, Sichuan University, Chengdu, China.
Funding: This study was supported by the Foundation of Science and Technology support plan Department of Sichuan Province (2014SZ0148 and 2015SZ0158).
Table S1. PRISMA 2009 checklist.
| Section/topic | # | Checklist item | Reported on page # | |
|---|---|---|---|---|
| Title | ||||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 | |
| Abstract | ||||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 1 | |
| Introduction | ||||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 2 | |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 2 | |
| Methods | ||||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | 2 | |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 2 | |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 2 | |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 2, Table S2 | |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 2, 4 (Figure 1) | |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 3 | |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 3 | |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 2, 3 | |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 3 | |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 3 | |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 3 | |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 3 | |
| Results | ||||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 4 (Figure 1), Table S2 | |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 4–6 | |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 3, 4, Table S3 | |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (I) simple summary data for each intervention group (II) effect estimates and confidence intervals, ideally with a forest plot | 5–9 (Figures 2,3,4,5) | |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | 5, 7 | |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | 7, 8, 10 (Figure 7) | |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g., sensitivity or subgroup analyses, meta-regression (see Item 16)] | 5–9 (Figures 3,4,5,6) | |
| Discussion | ||||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 8–12 | |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 12 | |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 12 | |
| Funding | ||||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 12, 13 | |
From: Moher D et al. (12). #, number.
Table S2. Summary of the electronic literature search.
| Searches | Search details | Items found |
|---|---|---|
| PubMed search strategy | ||
| #1 | Search ((bronchopleural[All Fields] AND (“fistula”[MeSH Terms] OR “fistula”[All Fields])) OR (“bronchial fistula”[MeSH Terms] OR (“bronchial”[All Fields] AND “fistula”[All Fields]) OR “bronchial fistula”[All Fields])) AND (“pulmonary disease, chronic obstructive”[MeSH Terms] OR (“pulmonary”[All Fields] AND “disease”[All Fields] AND “chronic”[All Fields] AND “obstructive”[All Fields]) OR “chronic obstructive pulmonary disease”[All Fields] OR (“chronic”[All Fields] AND “obstructive”[All Fields] AND “pulmonary”[All Fields] AND “disease”[All Fields])) | 68 |
| #2 | Search ((bronchopleural[All Fields] AND (“fistula”[MeSH Terms] OR “fistula”[All Fields])) OR (“bronchial fistula”[MeSH Terms] OR (“bronchial”[All Fields] AND “fistula”[All Fields]) OR “bronchial fistula”[All Fields])) AND (“pulmonary disease, chronic obstructive”[MeSH Terms] OR (“pulmonary”[All Fields] AND “disease”[All Fields] AND “chronic”[All Fields] AND “obstructive”[All Fields]) OR “chronic obstructive pulmonary disease”[All Fields] OR “copd”[All Fields]) | 71 |
| EMBASE (via Ovid interface) search strategy | ||
| #1 | Search ((bronchopleural fistula or bronchial fistula) and chronic obstructive pulmonary disease).af. | 42 |
| #2 | Search ((bronchopleural fistula or bronchial fistula) and COPD).af. | 45 |
| The Web of Science (via campus network of Sichuan University) search strategy | ||
| #1 | Search TS = ((bronchopleural fistula OR bronchial fistula) AND chronic obstructive pulmonary disease) | 49 |
| #2 | Search TS = ((bronchopleural fistula OR bronchial fistula) AND COPD) | 21 |
Table S3. NOS scoring records of the included studies.
| Authors [year] | Selection | Comparability | Exposure | Total score | ||
|---|---|---|---|---|---|---|
| Assessment of outcome | Follow-up long enough for outcomes | Adequacy of follow-up of cohorts | ||||
| Algar et al. [2001] (21) | 4 | 2 | 1 | 0 | 1 | 8 |
| Hu et al. [2013] (22) | 4 | 1 | 1 | 1 | 1 | 8 |
| Lindner et al. [2010] (23) | 4 | 1 | 1 | 1 | 1 | 8 |
| Panagopoulos et al. [2009] (24) | 4 | 1 | 1 | 1 | 1 | 8 |
| Sekine et al. [2002] (2) | 4 | 1 | 1 | 0 | 1 | 7 |
| Sekine et al. [2013] (6) | 4 | 1 | 1 | 0 | 1 | 7 |
| Stolz et al. [2014] (25) | 4 | 2 | 1 | 1 | 1 | 9 |
| Yena et al. [2006] (26) | 4 | 2 | 1 | 1 | 1 | 9 |
NOS, Newcastle-Ottawa Scale.
Ethical Statement: Patients’ consent or ethical approval is not required in a systematic review and meta-analysis.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kroenke K, Lawrence VA, Theroux JF, et al. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest 1993;104:1445-51. 10.1378/chest.104.5.1445 [DOI] [PubMed] [Google Scholar]
- 2.Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002;37:95-101. 10.1016/S0169-5002(02)00014-4 [DOI] [PubMed] [Google Scholar]
- 3.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. 10.1183/09031936.00144208 [DOI] [PubMed] [Google Scholar]
- 4.Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2016;21:269-79. 10.1111/resp.12661 [DOI] [PubMed] [Google Scholar]
- 5.Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. 10.1016/j.athoracsur.2005.11.048 [DOI] [PubMed] [Google Scholar]
- 6.Sekine Y, Suzuki H, Yamada Y, et al. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac Cardiovasc Surg 2013;61:124-30. [DOI] [PubMed] [Google Scholar]
- 7.Shekar K, Foot C, Fraser J, et al. Bronchopleural fistula: an update for intensivists. J Crit Care 2010;25:47-55. 10.1016/j.jcrc.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 8.Di Maio M, Perrone F, Deschamps C, et al. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196-200. 10.1093/ejcts/ezu381 [DOI] [PubMed] [Google Scholar]
- 9.Li S, Fan J, Zhou J, et al. Residual disease at the bronchial stump is positively associated with the risk of bronchoplerual fistula in patients undergoing lung cancer surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;22:327-35. 10.1093/icvts/ivv327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toufektzian L, Patris V, Sepsas E, et al. Does postoperative mechanical ventilation predispose to bronchopleural fistula formation in patients undergoing pneumonectomy? Interact Cardiovasc Thorac Surg 2015;21:379-82. 10.1093/icvts/ivv149 [DOI] [PubMed] [Google Scholar]
- 11.Li S, Fan J, Liu J, et al. Neoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: a systematic meta-analysis of 14 912 patients. Jpn J Clin Oncol 2016. [Epub ahead of print]. 10.1093/jjco/hyw037 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Cai X, Shi X, et al. Chronic Obstructive Pulmonary Disease as a Risk Factor for Cognitive Dysfunction: A Meta-Analysis of Current Studies. J Alzheimers Dis 2016;52:101-11. 10.3233/JAD-150735 [DOI] [PubMed] [Google Scholar]
- 14.Liao YB, He ZX, Zhao ZG, et al. The relationship between chronic obstructive pulmonary disease and transcatheter aortic valve implantation-A systematic review and meta-analysis. Catheter Cardiovasc Interv 2016;87 Suppl 1:570-8. 10.1002/ccd.26443 [DOI] [PubMed] [Google Scholar]
- 15.Cao C, Wu Y, Xu Z, et al. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: a systematic review and meta-analysis of observational research. Sci Rep 2015;5:16461. 10.1038/srep16461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available online: www.cochrane-handbook.org
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 21.Algar FJ, Alvarez A, Aranda JL, et al. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg 2001;72:1662-7. 10.1016/S0003-4975(01)03096-X [DOI] [PubMed] [Google Scholar]
- 22.Hu XF, Duan L, Jiang GN, et al. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 2013;96:419-24. 10.1016/j.athoracsur.2013.04.050 [DOI] [PubMed] [Google Scholar]
- 23.Lindner M, Hapfelmeier A, Morresi-Hauf A, et al. Bronchial stump coverage and postpneumonectomy bronchopleural fistula. Asian Cardiovasc Thorac Ann 2010;18:443-9. 10.1177/0218492310380574 [DOI] [PubMed] [Google Scholar]
- 24.Panagopoulos ND, Apostolakis E, Koletsis E, et al. Low incidence of bronchopleural fistula after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2009;9:571-5. 10.1510/icvts.2009.203646 [DOI] [PubMed] [Google Scholar]
- 25.Stolz AJ, Harustiak T, Simonek J, et al. Pneumonectomy for non-small cell lung cancer: predictors of early mortality and morbidity. Acta Chir Belg 2014;114:25-30. [PubMed] [Google Scholar]
- 26.Yena S, Doddoli C, Doumbia S, et al. Bronchial fistula postpneumonectomy: predictive factors. Ann Chir 2006;131:22-6. 10.1016/j.anchir.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 27.Birdas TJ, Morad MH, Okereke IC, et al. Risk factors for bronchopleural fistula after right pneumonectomy: does eliminating the stump diverticulum provide protection? Ann Surg Oncol 2012;19:1336-42. 10.1245/s10434-011-2119-z [DOI] [PubMed] [Google Scholar]
- 28.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 29.Li SJ, Chen DL, Zhang WB, et al. Prognostic value of stromal decorin expression in patients with breast cancer: a meta-analysis. J Thorac Dis 2015;7:1939-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li SJ, Fan J, Zhou J, et al. Diabetes Mellitus and Risk of Bronchopleural Fistula After Pulmonary Resections: A Meta-Analysis. Ann Thorac Surg 2016;102:328-39. 10.1016/j.athoracsur.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick dP, Dransfield MT. Racial and sex differences in chronic obstructive pulmonary disease susceptibility, diagnosis, and treatment. Curr Opin Pulm Med 2009;15:100-4. 10.1097/MCP.0b013e3283232825 [DOI] [PubMed] [Google Scholar]
- 32.Bhopal R, Steiner MF, Cezard G, et al. Risk of respiratory hospitalization and death, readmission and subsequent mortality: scottish health and ethnicity linkage study. Eur J Public Health 2015;25:769-74. 10.1093/eurpub/ckv064 [DOI] [PubMed] [Google Scholar]
- 33.Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001;13:3-7. 10.1053/stcs.2001.22493 [DOI] [PubMed] [Google Scholar]
- 34.Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. 10.1378/chest.128.6.3955 [DOI] [PubMed] [Google Scholar]
- 35.Hollaus PH, Lax F, Janakiev D, et al. Endoscopic treatment of postoperative bronchopleural fistula: experience with 45 cases. Ann Thorac Surg 1998;66:923-7. 10.1016/S0003-4975(98)00589-X [DOI] [PubMed] [Google Scholar]
- 36.Varoli F, Roviaro G, Grignani F, et al. Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg 1998;65:807-9. 10.1016/S0003-4975(97)01427-6 [DOI] [PubMed] [Google Scholar]
- 37.Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013;146:575-9. 10.1016/j.jtcvs.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 38.Stratakos G, Zuccatosta L, Porfyridis I, et al. Silver nitrate through flexible bronchoscope in the treatment of bronchopleural fistulae. J Thorac Cardiovasc Surg 2009;138:603-7. 10.1016/j.jtcvs.2008.10.054 [DOI] [PubMed] [Google Scholar]
- 39.Fruchter O, El Raouf BA, Abdel-Rahman N, et al. Efficacy of bronchoscopic closure of a bronchopleural fistula with amplatzer devices: long-term follow-up. Respiration 2014;87:227-33. 10.1159/000357074 [DOI] [PubMed] [Google Scholar]
- 40.Klotz LV, Gesierich W, Schott-Hildebrand S, et al. Endobronchial closure of bronchopleural fistula using Amplatzer device. J Thorac Dis 2015;7:1478-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West D, Togo A, Kirk AJ. Are bronchoscopic approaches to post-pneumonectomy bronchopleural fistula an effective alternative to repeat thoracotomy? Interact Cardiovasc Thorac Surg 2007;6:547-50. 10.1510/icvts.2007.159319 [DOI] [PubMed] [Google Scholar]
- 42.Petrella F, Toffalorio F, Brizzola S, et al. Stem cell transplantation effectively occludes bronchopleural fistula in an animal model. Ann Thorac Surg 2014;97:480-3. 10.1016/j.athoracsur.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 43.Díaz-Agero Álvarez PJ, Bellido-Reyes YA, Sánchez-Girón JG, et al. Novel bronchoscopic treatment for bronchopleural fistula using adipose-derived stromal cells. Cytotherapy 2016;18:36-40. 10.1016/j.jcyt.2015.10.003 [DOI] [PubMed] [Google Scholar]