Abstract
Background
Pure ground-glass opacity (GGO) on computed tomography (CT) is considered a diagnostic feature of noninvasive lung adenocarcinoma. However, pure GGO can sometimes be associated with invasive adenocarcinoma (IA). The purpose of this study was to determine the predictive factors for IA when pure GGO is present.
Methods
Between 2011 and 2014, 83 patients with persistent pure GGO on chest CT underwent surgical treatment for lung cancer. We compared the clinical, surgical, and pathological characteristics of non-IA with those of IA.
Results
A total of 66 patients (79.5%) were diagnosed with non-IA and 17 patients (20.5%) were diagnosed with IA. The mean axial diameter of the GGO lesions in IA was larger than that in non-IA (1.9 vs. 1.2 cm; P<0.001). The incidence of pleural retraction was higher in IA than in non-IA (76.5% vs. 15.2%; P<0.001). Multivariate logistic regression analysis identified GGO lesion size and the presence of pleural retraction as significant predictive factors for IA.
Conclusions
Both preoperative GGO lesion size on CT and the computed-tomography or operative finding of pleural retraction are predictive factors for IA. In patients with these findings, curative lobectomy is preferable to limited resection.
Keywords: Lung cancer, adenocarcinoma, ground-glass opacity (GGO), invasive adenocarcinoma (IA)
Introduction
Lung cancer is the leading cause of cancer death among men and the second leading cause among women worldwide (1). Recently, the use of chest computed tomography (CT) for lung-cancer screening and early-stage adenocarcinoma detection has increased (2), with a subsequent increase in the detection of pure ground-glass opacity (GGO) and part-solid GGO lesions. Several studies have shown that persistent GGO confers a high risk of malignancy (3,4), with most representing preinvasive adenocarcinoma. Many studies report a difference in prognosis for adenocarcinoma patients, depending on the extent of GGO (5-7).
In 2011, the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) proposed a new classification of adenocarcinoma (8), which has been adopted in the recently published fourth edition of the World Health Organization (WHO) Classification of Tumours of the Lung, Pleura, Thymus and Heart (9). According to this classification, adenocarcinoma can be divided into 3 broad groups: adenocarcinoma in situ (AIS); minimally invasive adenocarcinoma (MIA); and invasive adenocarcinoma (IA); the latter can be further divided by the predominant histologic pattern into acinar adenocarcinoma, papillary adenocarcinoma, micropapillary adenocarcinoma, solid adenocarcinoma, and lepidic adenocarcinoma. AIS is defined as an adenocarcinoma lesion less than 3 cm in diameter, consisting of a 100% lepidic pattern; MIA has a predominant lepidic pattern with an invasive component of less than 5 mm. Both AIS and MIA are considered non-IA with lepidic growth and often present as GGO on CT (10). When pure GGO is seen, the diagnosis is presumed to be AIS or MIA and tissue-sparing surgery (limited resection) is preferred.
In actual fact, not all pure GGO lesions are AIS or MIA (11). There have been many instances of pure GGO identified as IA on postoperative pathology (11-14). It stands to reason that patients with pure GGO who are at high risk for having IA should not undergo a planned limited resection. It would be very helpful in deciding on the optimal treatment plan if the likelihood of IA could be predicted among patients exhibiting pure GGO lesions.
The aim of this study was to identify how often lung cancers with pure GGO lesions are confirmed as IA and to determine the predictive factors. In addition, we planned to determine which histomorphologic patterns, in addition to lepidic, appear as pure GGO on CT imaging.
Methods
Patients
A retrospective chart review identified 1,023 patients at Seoul St. Mary’s Hospital in Korea who were diagnosed with non-small cell lung cancer and underwent surgical resection between January 2010 and October 2015. Of these, 83 patients had pure GGO lesions. The histology in all 83 patients was adenocarcinoma, classified according to the 2011 IASLC/ATS/ERS and the 2015 WHO classification systems (8,9). AIS and MIA were combined into a single non-IA group (AIS/MIA) and compared with the IA group. TNM staging was based on the seventh edition of the American Joint Committee on Cancer (AJCC) guidelines (15). When GGO was observed continuously for more than 3 months, the decision to proceed with surgery was made when the size of the GGO lesion was more than 1 cm, the size of the GGO lesion was less than 1 cm but was increasing, or when malignancy was confirmed by needle biopsy.
This study was approved by the institutional review board of Seoul St. Mary’s Hospital, The Catholic University of Korea.
Radiologic evaluation
CT scans were obtained at full inspiration. GGO was defined as a hazy increased opacity in the lung parenchyma with preservation of bronchial structures and vascular margins (16). GGO nodules were labeled “pure” or “part-solid” depending on the presence of a solid component: pure GGO did not have any solid component on either the lung-window setting or mediastinal setting. The GGO diameter was defined as the largest axial diameter of the lesion on the lung-window setting. When multiple pure GGO lesions were present, the largest was used for study purposes. Pleural retraction was defined as a retraction of adjacent pleura toward the nodule (17). The initial radiologic reports were rendered by certificated thoracic radiologists, with retrospective review of the radiologic records of all patients who were determined to have pure GGO by our study radiologists. All preoperative chest CT imaging was rechecked by 2 thoracic surgeons who were blinded to the surgical outcome.
Histologic evaluation
All pathology reports were rendered by certified pathologists and included tumor size, location, differentiation, pleural invasion, lymphatic invasion, vascular invasion, and mutation status. To describe the histomorphologic tumor patterns, the occupancy ratio of each growth pattern (lepidic, acinar, papillary, micropapillary, and solid) of the total tumor area was measured and recorded semiquantitatively, in 5% increments, according to the 2011 IASLC/ATS/ERS classification system and the 2015 WHO classification system (8,9).
Statistical analysis
Clinicopathological factors of AIS/MIA and IA were compared using either Student’s t-test or the Wilcoxon rank-sum test for continuous variables, and using the χ2 test or Fisher’s exact test for categorical variables. The optimal cutoff value for lesion size between AIS/MIA and IA was calculated by using a receiver operating characteristic (ROC) curve. Multivariate logistic regression was used to analyze predictive factors for IA with pure GGO. A P value of less than 0.05 was considered statistically significant.
Results
Of the 83 patients with adenocarcinoma and pure GGO, 66 patients (79.5%) were diagnosed with AIS/MIA and 17 patients (20.5%) were diagnosed with IA. The clinical characteristics of these 2 groups are listed in Table 1. The groups were similar in age, male-to-female ratio, and smoking history (P=0.790, P=0.844, P=0.221, respectively). There was no difference in the time elapsed between chest CT and surgery (P=0.280). There was no difference in the presence of underlying lung conditions, pulmonary function, serum carcinoembryonic antigen (CEA) levels (P=0.607), or maximum standardized uptake value (SUVmax) (P=0.087). Comparison of CT features revealed that most tumors were peripherally located. There was no difference in the involved lobes between groups (P=0.654), but there was a difference in mean GGO size (AIS/MIA, 1.2±0.5 cm; IA, 1.9±0.6 cm; P<0.001). The mean GGO lesion size in patients with MIA was larger than that in patients with AIS (1.4 vs. 1.1 cm; P=0.007), and the mean GGO size in patients with IA was larger than that in patients with MIA (1.9 vs. 1.4 cm; P=0.001). Pleural retraction was observed in patients with AIS, MIA, and IA (Figure 1), with a statistically significant difference between the AIS/MIA group [10 patients (15.2%)] and the AI group [13 patients (76.5%); P<0.001]. Even though there was no statistically significant difference between MIA and AIS patients, there was a tendency toward more pleural retraction in MIA (21.6% vs. 6.9%; P=0.098). Multiple GGO lesions were seen in 21.2% of AIS/MIA patients and 11.1% of AI patients; there was no statistical difference (P=0.379).
Table 1. Clinical characteristics of patients with pure ground-glass opacity nodules, according to pathologic classification.
| Variables | AIS/MIA | IA (n=17) | P valuea | ||
|---|---|---|---|---|---|
| AIS (n=29) | MIA (n=37) | Total (n=66) | |||
| Age (years) | 56.3±12.2 | 59.7±9.7 | 58.2±10.9 | 59.0±10.6 | 0.790 |
| Sex | 0.844 | ||||
| Male | 11 (37.9) | 14 (37.8) | 25 (37.9) | 6 (35.3) | |
| Female | 18 (62.1) | 23 (62.2) | 41 (62.1) | 11 (64.7) | |
| Smoking history | 0.221 | ||||
| Never | 22 (75.9) | 27 (73.0) | 49 (74.2) | 15 (88.2) | |
| Current or former | 7 (24.1) | 10 (27.0) | 17 (25.8) | 2 (11.8) | |
| Interval to surgery (days) | 13.3±13.0 | 17.2±16.5 | 15.5±15.1 | 11.2±11.4 | 0.280 |
| Pulmonary function | |||||
| FEV1 (%) | 92.7±15.5 | 93.7±16.6 | 93.3±16.0 | 97.1±16.8 | 0.416 |
| DLCO (%) | 82.3±13.4 | 86.3±14.8 | 84.6±14.3 | 90.2±14.2 | 0.181 |
| Serum CEA level (ng/mL) | 1.3±1.1 | 1.7±2.0 | 1.6±1.7 | 1.4±0.6 | 0.607 |
| SUVmax | 0.1±0.3 | 0.7±0.9 | 0.4±0.7 | 0.9±1.1 | 0.087 |
| Radiologic features | |||||
| Tumor location | 0.574 | ||||
| Central | 0 | 2 (5.4) | 2 (3.0) | 1 (5.9) | |
| Peripheral | 29 (100.0) | 35 (94.6) | 64 (97.0) | 16 (94.1) | |
| Involved lobe | 0.654 | ||||
| Right upper | 11 (37.9) | 16 (43.2) | 27 (40.9) | 5 (29.4) | |
| Right middle | 1 (3.4) | 3 (8.1) | 4 (6.1) | 0 | |
| Right lower | 6 (20.7) | 4 (10.8) | 10 (15.2) | 3 (17.6) | |
| Left upper | 6 (20.7) | 9 (24.3) | 15 (22.7) | 6 (35.3) | |
| Left lower | 5 (17.2) | 5 (13.5) | 10 (15.2) | 3 (17.6) | |
| GGO size | 1.1±0.4 | 1.4±0.5 | 1.2±0.5 | 1.9±0.6 | <0.001 |
| Pleural retraction | 2 (6.9) | 8 (21.6) | 10 (15.2) | 13 (76.5) | <0.001 |
| Multiple GGO lesions | 9 (31.0) | 5 (13.5) | 14 (21.2) | 2 (11.8) | 0.379 |
Data are shown as mean ± standard deviation or N (%). a, comparison between AIS/MIA and IA. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma; surgery delay, delay between CT to surgery; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; GGO, ground glass opacity.
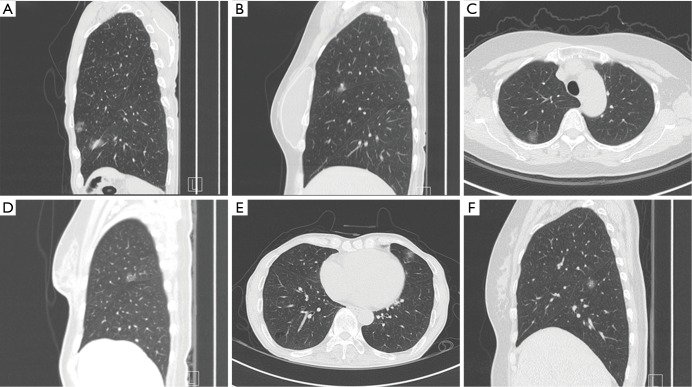
Figure 1.
Pure ground-glass opacity, with or without pleural retraction, on chest computed tomography. (A) Adenocarcinoma in situ (AIS) without pleural retraction; (B) AIS with pleural retraction; (C) minimally invasive adenocarcinoma (MIA) without pleural retraction; (D) MIA with pleural retraction; (E) invasive adenocarcinoma (IA) without pleural retraction; (F) IA with pleural retraction.
The surgical and pathological characteristics of AIS/MIA and IA are shown in Table 2. The rate of limited resection was 57.6% in AIS/MIA patients and 41.2% in IA patients; there was no statistical difference (P=0.463). However, there was a statistically significant difference between AIS and MIA patients in the rate of limited resection: 75.9% vs. 43.2%, respectively (P=0.01). Video-assisted thoracoscopic surgery was performed for most patients (AIS/MIA, 89.4%; IA, 88.2%) and there was no difference between groups in the rate of observed pleural adhesions (P=0.745). When pleural retraction was observed on chest CT, it was confirmed intraoperatively in all patients (Figure 2). There was no significant difference between pathological tumor size and GGO lesion size, with lesion size tending to increase as pathology progressed from AIS to MI to IA. The tumor grade was well differentiated in most AIS/MIA patients (98.5%) but significantly lower in IA patients (76.5%; P<0.001). There was no visceral pleural invasion seen in any group. Lymphatic invasion [2 patients (11.8%)] and vascular invasion [1 patient (5.9%)] was observed only in IA. There was no difference in the incidence of EGFR mutation and ALK mutation between groups.
Table 2. Surgical and pathological characteristics of patients with pure ground-glass opacity nodules, according to pathologic classification.
| Variables | AIS/MIA | IA | P valuea | ||
|---|---|---|---|---|---|
| AIS | MIA | Total | |||
| Surgical | |||||
| Operation type | 0.463 | ||||
| Wedge resection | 16 (55.2) | 8 (21.6) | 24 (36.4) | 4 (23.5) | |
| Segmentectomy | 6 (20.7) | 8 (21.6) | 14 (21.2) | 3 (17.7) | |
| Lobectomy | 7 (24.1) | 21 (56.8) | 28 (42.4) | 10 (58.8) | |
| VATS | 24 (82.8) | 35 (94.6) | 59 (89.4) | 15 (88.2) | 0.891 |
| Open thoracotomy | 5 (17.2) | 2 (5.4) | 7 (10.6) | 2 (11.8) | |
| Pleural adhesion | 8 (27.6) | 6 (16.2) | 14 (21.2) | 3 (17.6) | 0.745 |
| Pathological | |||||
| Tumor size (cm) | 1.0±0.4 | 1.3±0.5 | 1.2±0.5 | 1.8±0.6 | <0.001 |
| Grade | <0.001 | ||||
| Well differentiated | 29 (100.0) | 36 (97.3) | 65 (98.5) | 13 (76.5) | |
| Moderately differentiated | 0 | 0 | 0 | 4 (23.5) | |
| Poorly differentiated | 0 | 1 (2.7) | 1 (1.5) | 0 | |
| Visceral pleural invasion | 0 | 0 | 0 | 0 | |
| Lymphatic invasion | 0 | 0 | 0 | 2 (11.8) | 0.042 |
| Vascular invasion | 0 | 0 | 0 | 1 (5.9) | 0.051 |
| EGFR mutation | 9/14 (64.3) | 14/28 (50.0) | 23/42 (54.8) | 8/14 (57.1) | 0.877 |
| ALK mutation | 1/9 (11.1) | 0/25 (0) | 1/34 (2.9) | 0/13 (0) | 0.532 |
Data are shown as median ± standard deviation or N (%). a, comparison between AIS/MIA and IA. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma; VATS, video-assisted thoracoscopic surgery; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase.
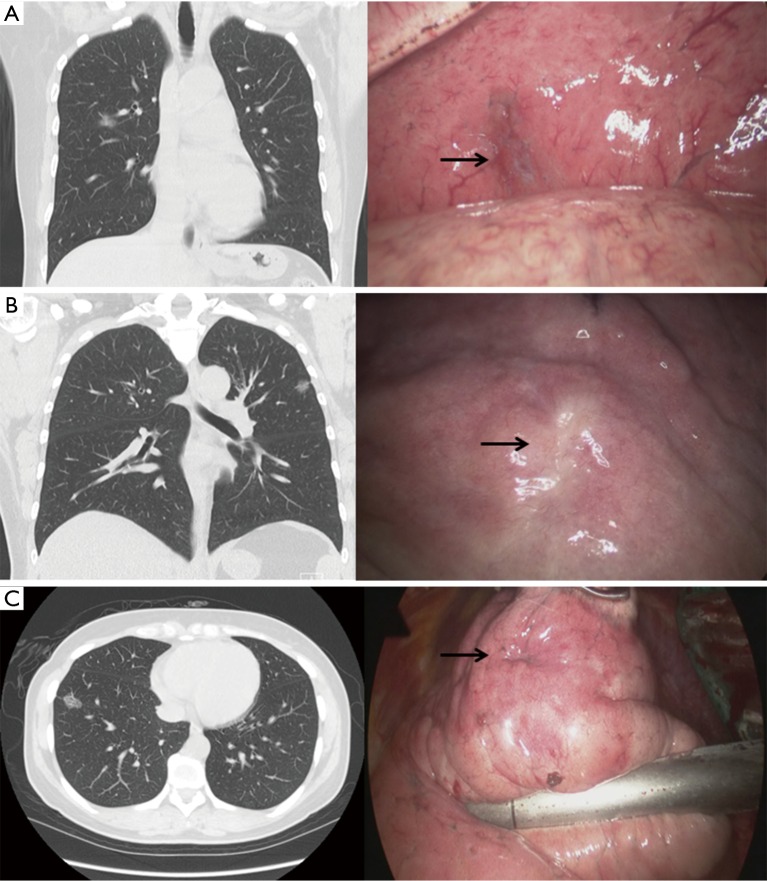
Figure 2.
Chest computed tomography, thoracoscopic view. (A) AIS with pleural retraction; (B) MIA with pleural retraction; (C) IA with pleural retraction. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma.
Logistic regression analysis was used to determine the predictive factors for IA that could be confirmed either before or during surgery (Table 3). The preoperative factors were patient age, sex, smoking history, interval to surgery, forced expiratory volume in 1 second (FEV1), diffusing capacity for carbon monoxide (DLCO), tumor location, GGO lesion size, the presence of pleural retraction, multiple GGO lesions, CEA value, and SUVmax. The intraoperative factors were the presence of pleural adhesion or pleural retraction. GGO size and pleural retraction had P values of less than 0.1 by univariate analysis. Both factors were confirmed to be significant predictive factors for IA by multivariate analysis [hazard ratio (HR) =9.016, P=0.007; HR =12.977, P<0.001, respectively).
Table 3. Predictive factors for invasive adenocarcinoma on logistic regression analysis.
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.007 | 0.957–1.060 | 0.787 |
| Sex (female) | 1.118 | 0.368–3.399 | 0.844 |
| Smoking history | 0.384 | 0.080–1.857 | 0.234 |
| Interval to surgery (days) | 0.976 | 0.935–1.020 | 0.281 |
| FEV1 (%) | 1.015 | 0.980–1.051 | 0.412 |
| DLCO (%) | 1.029 | 0.987–1.074 | 0.182 |
| Central location | 2.000 | 0.170–23.461 | 0.581 |
| GGO size | 9.584 | 2.870–31.999 | <0.001 |
| Pleural retraction | 18.200 | 4.925–67.259 | <0.001 |
| Multiple GGO | 0.495 | 0.101–2.426 | 0.386 |
| CEA | 0.890 | 0.572–1.385 | 0.606 |
| SUVmax | 1.802 | 0.880–3.693 | 0.108 |
| Pleural adhesion | 0.796 | 0.200–3.162 | 0.746 |
| Multivariate analysis | |||
| GGO size | 9.016 | 1.829–44.440 | 0.007 |
| Pleural retraction | 12.977 | 3.086–54.566 | <0.001 |
HR, hazard ratio; CI, confidence interval; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; GGO, ground glass opacity; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value.
A scatter plot was used to analyze the distribution of GGO lesion size in patients with IA (Figure 3): IA was not found with a GGO size of less than 1.0 cm. ROC analysis revealed that the area under the curve (AUC) for GGO size was 0.811 (95% CI, 0.703–0.919) (Figure 4). The optimal cutoff value for lesion size in differentiating AIS/MIA from IA was 1.5 cm, with a sensitivity of 76.5% and a specificity of 78.8%. GGO size larger than 1.5 cm accounted for 76.5% of IA patients (Table 4). Multivariate analysis confirmed that a GGO size ≥1.5 cm was a statistically significant predictive factor (HR =9.353; P=0.003) (Table 5). There were 13 patients with both GGO size ≥1.5 cm and pleural retraction: 11 were diagnosed with IA, with a sensitivity of 64.7%, a specificity of 97.0%, a positive predictive value of 84.6%, and a negative predictive value of 91.4%.
Figure 3.
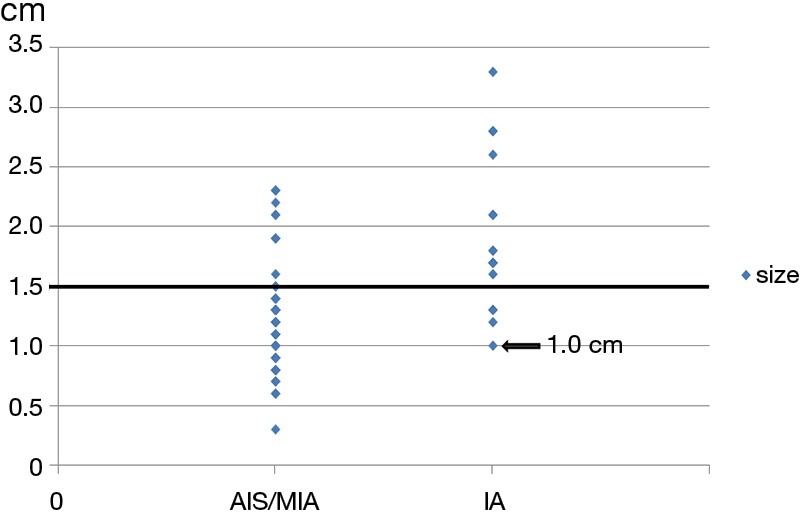
Scatter plot of tumor size and the presence of invasive adenocarcinoma. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma.
Figure 4.
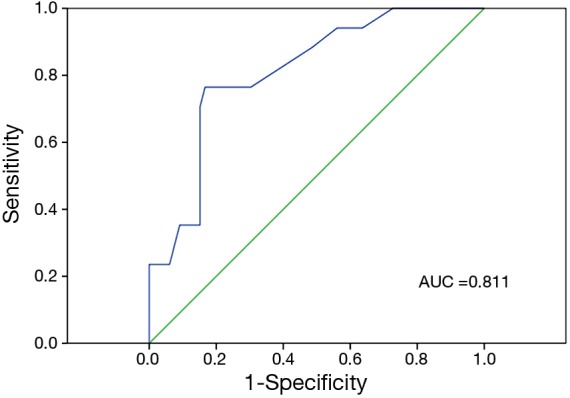
Receiver operating characteristic curve of ground-glass opacity lesion size.
Table 4. Incidence of ground-glass opacity size ≥1.5 in AIS/MIA and IA.
| GGO size | AIS/MIA | IA | P valuea | ||
|---|---|---|---|---|---|
| AIS | MIA | Total | |||
| <1.5 (%) | 25 (86.2) | 27 (73.0) | 52 (78.8) | 4 (23.5) | <0.001 |
| ≥1.5 (%) | 4 (13.8) | 10 (27.0) | 14 (21.2) | 13 (76.5) | |
a, comparison between AIS/MIA and IA. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma; GGO, ground glass opacity.
Table 5. Multivariate analysis on logistic regression analysis with GGO size ≥1.5 and pleural retraction.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| GGO size ≥1.5 | 9.353 | 2.187–39.999 | 0.003 |
| Pleural retraction | 14.550 | 3.442–61.500 | <0.001 |
GGO, ground glass opacity; HR, hazard ratio; CI, confidence interval.
The histomorphological growth patterns of AIS, MIA, and IA are shown in Table 6. Comparison of the mean occupancy rate of the tumor growth patterns revealed that all AIS, MIA, and IA lesions contained a great deal of the lepidic pattern (100%, 86.9%, and 40.3%, respectively). In MIA and IA, the acinar pattern and papillary pattern were observed primarily and the micropapillary pattern and solid pattern were not observed at all. When IA was classified by subtype, there were 8 patients with acinar adenocarcinoma, 3 with papillary adenocarcinoma, and 6 with lepidic adenocarcinoma.
Table 6. Mean occupancy rate of tumor histomorphologic growth pattern (%).
| Histomorphologic growth patterns | AIS | MIA | IA | P value |
|---|---|---|---|---|
| Lepidic pattern (%) | 100.0 | 86.9±10.1 | 40.3±15.2 | <0.001 |
| Acinar pattern (%) | 0 | 12.7±10.0 | 50.0±22.4 | <0.001 |
| Papillary pattern (%) | 0 | 0.2±0.9 | 12.3±19.6 | <0.001 |
| Micropapillary pattern (%) | 0 | 0 | 0 | |
| Solid pattern (%) | 0 | 0 | 0 |
Invasive adenocarcinoma (n=17); acinar predominant (n=8); papillary predominant (n=3); lepidic predominant (n=6). AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma.
Discussion
Lung adenocarcinoma has a variable prognosis due to its heterogeneity; therefore, it is important to differentiate a tumor with a favorable prognosis from a lesion with a gloomy prognosis. AIS and MIA are known to have 100% 5-year survival after surgical resection and are therefore known as favorable-prognosis tumors (18,19) and are often considered to be indications for limited resection (5,20,21). Lesions that demonstrate GGO on preoperative CT tend to represent a lepidic pattern without invasion, with a good possibility of being AIS or MIA in the case of pure GGO. However, all pure GGO lesions are not shown to be AIS or MIA on pathologic examination. In the present study, 20.5% of patients with pure GGO lesions were confirmed to have IA. Another study showed that 42.8% of patients with pure GGO had IA (11). Unfortunately, there is a good possibility of an invasive component to lesions that appear as part-solid GGO on CT (5,6,11), and anatomical resection has been recommended over lobectomy for treating these tumors. However, since a solid component to pure GGO is not visible on CT, it is important to determine other predictive factors for invasiveness.
We were not able to identify a predictive preoperative clinical characteristic for IA, but we did determine that GGO lesion size and the presence of pleural retraction were significant radiologic factors. GGO size was found to be similar to the pathological tumor size, and there was a significant difference in the mean GGO size between AIS/MIA and IA tumors, with a cutoff value of 1.5 cm conferring good sensitivity and specificity. In other studies, the size of pure GGO lesions proved to be a significant predictive factor for IA (12,13). While pleural retraction represents a pulling of the visceral pleura towards a pulmonary nodule, it may or may not be accompanied by a pleural tag. In all of our patients, pleural retraction observed on chest CT was also observed intraoperatively. Therefore, pleural retraction was both a preoperative and intraoperative predictive factor. Generally, pleural retraction has not been seen as a significant finding for discrimination between benign and malignant pulmonary nodules (22,23). However, a recent study reported that patients with IA and GGO lesions have a higher incidence of pleural retraction than those with AIS/MIA (17). In the present study, while pleural retraction could be observed in all patients with AIS, MIA, and IA, the incidence of pleural retraction was highest in IA. Especially since the incidence of pleural retraction tends to increase as disease progresses from AIS to MIA to IA, we theorize that as that the more invasive component of a GGO lesion increases, the incidence of pleural retraction is likely to increase. IA can be predicted more accurately if more than 2 categorical variables are used together. In this study, both positive and negative predictive values were high when both GGO size ≥1.5 cm and pleural retraction were positive. Since specificity was very high even though sensitivity was relatively low, it can be said that the possibility of AIS/MIA is very high in patients with pure GGO less than 1.5 cm and no pleural retraction.
According to the National Comprehensive Cancer Network (NCCN) guideline for lung-cancer screening (Version 1. 2016), chest CT is to be repeated if pure GGO is detected. When GGO persists, surgical excision can be performed if the lesion size is over 1 cm, the lesion is increasing in size, or excision is considered clinically necessary. At our institution, surgery is performed according to these guidelines. Lobectomy is performed when limited resection is not possible, but most patients are treated with limited resection and frozen section, with lobectomy performed if an invasive component is observed on frozen section. In this study, the rate of limited resection in both AIS/MIA and IA was about the same, around 50%. However, limited resection for AIS was significantly higher, at 75.9%, as invasive components were not detected on frozen section. Unfortunately, the accuracy of frozen section is not satisfactory (5,24). In addition, differentiation between MIA and IA is still vague, even if an invasive component is observed. Considering that the limited-resection rate is high in patients with AIS, frozen section may be helpful in determining the appropriate operation. But since the accuracy of intraoperative frozen section is still unclear, determining the appropriate treatment method will be easier if IA can be predicted more accurately. Even though limited resection is preferred for the treatment of pure GGO, it may not be able to be performed for all lesions. As observed in the present study, 20.5% of pure GGO lesions are ultimately diagnosed as IA, so the presence of pure GGO cannot be an indication for limited resection.
Generally, GGO is observed in lesions that demonstrate a lepidic growth pattern, although the acinar and papillary patterns can also be observed (11,25-28). In the present study, when we examined which patterns composed the invasive components of MIA and IA, all were acinar and papillary. Curiously, micropapillary and solid patterns were not observed at all. When classification was made according to IA subtype, acinar adenocarcinoma was the most predominant. Since the malignant potential of micropapillary and solid patterns is known to be the highest of the histologic types (18,29,30), IA presenting as pure GGO may have a better prognosis than other types of IA (11). Accordingly, since pure GGO has a good possibility of being IA with less malignant potential compared with solid- or part-solid GGO, a better prognosis may be anticipated even after limited resection if predictive factors are properly applied.
This study has several limitations. First, we used a retrospective study design. Second, we obtained the data from a single institution and the number of cases was relatively small. However, all data in this study are recent, since 2010, and since management was performed according to the same protocol, bias should be relatively low. Future studies with more patients may provide more accurate results. Third, our study was restricted to surgical patients. More accurate results might be obtained if pathologic analysis of pure-GGO patients who did not undergo surgery was made—a consideration which was not practically possible in this study; we still consider our results to be significant.
In conclusion, preoperative GGO size and the radiologic or intraoperative presence of pleural retraction are predictive factors for IA. In patients with these findings, lobectomy is preferable to limited resection. Using frozen section may help to identify IA intraoperatively. Future studies that collect data from larger sample sizes are needed to confirm these findings.
Acknowledgements
The manuscript has been edited by native English-speaking experts at BioMed Proofreading, LLC.
Ethical Statement: This study was approved by the institutional review board of Seoul St. Mary’s Hospital, The Catholic University of Korea.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1-19. 10.1007/978-3-319-24223-1_1 [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. 10.1148/radiol.2451061682 [DOI] [PubMed] [Google Scholar]
- 4.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. 10.2214/ajr.178.5.1781053 [DOI] [PubMed] [Google Scholar]
- 5.Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. 10.1053/j.semtcvs.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi M, Shigematsu Y, Ohta M, et al. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014;147:54-9. 10.1016/j.jtcvs.2013.08.058 [DOI] [PubMed] [Google Scholar]
- 7.Nitadori J, Bograd AJ, Morales EA, et al. Preoperative consolidation-to-tumor ratio and SUVmax stratify the risk of recurrence in patients undergoing limited resection for lung adenocarcinoma ≤2 cm. Ann Surg Oncol 2013;20:4282-8. 10.1245/s10434-013-3212-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 10.Lederlin M, Puderbach M, Muley T, et al. Correlation of radio- and histomorphological pattern of pulmonary adenocarcinoma. Eur Respir J 2013;41:943-51. 10.1183/09031936.00056612 [DOI] [PubMed] [Google Scholar]
- 11.Wilshire CL, Louie BE, Manning KA, et al. Radiologic evaluation of small lepidic adenocarcinomas to guide decision making in surgical resection. Ann Thorac Surg 2015;100:979-88. 10.1016/j.athoracsur.2015.04.030 [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Zhao SH, Gao J, et al. CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol 2015;25:2532-40. 10.1007/s00330-015-3637-z [DOI] [PubMed] [Google Scholar]
- 13.Liu LH, Liu M, Wei R, et al. CT findings of persistent pure ground glass opacity: can we predict the invasiveness? Asian Pac J Cancer Prev 2015;16:1925-8. 10.7314/APJCP.2015.16.5.1925 [DOI] [PubMed] [Google Scholar]
- 14.Son JY, Lee HY, Kim JH, et al. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol 2016;26:43-54. 10.1007/s00330-015-3816-y [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, et al, editors. AJCC cancer staging manual. 7th ed. New York: Springer, 2010. [Google Scholar]
- 16.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 17.Cohen JG, Reymond E, Lederlin M, et al. Differentiating pre- and minimally invasive from invasive adenocarcinoma using CT-features in persistent pulmonary part-solid nodules in Caucasian patients. Eur J Radiol 2015;84:738-44. 10.1016/j.ejrad.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 18.Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 2014;98:453-8. 10.1016/j.athoracsur.2014.04.108 [DOI] [PubMed] [Google Scholar]
- 19.Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. 10.1097/PAS.0000000000000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. 10.1016/j.jtcvs.2004.07.038 [DOI] [PubMed] [Google Scholar]
- 21.Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. 10.1016/j.athoracsur.2014.07.068 [DOI] [PubMed] [Google Scholar]
- 22.Hill CA. "Tail" signs associated with pulmonary lesions: critical reappraisal. AJR Am J Roentgenol 1982;139:311-6. 10.2214/ajr.139.2.311 [DOI] [PubMed] [Google Scholar]
- 23.Gruden JF. What is the significance of pleural tags? AJR Am J Roentgenol 1995;164:503-4. 10.2214/ajr.164.2.7840000 [DOI] [PubMed] [Google Scholar]
- 24.Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤ 3 cm: accuracy and interobserver agreement. Histopathology 2015;66:922-38. 10.1111/his.12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho H, Lee HY, Kim J, et al. Pure ground glass nodular adenocarcinomas: Are preoperative positron emission tomography/computed tomography and brain magnetic resonance imaging useful or necessary? J Thorac Cardiovasc Surg 2015;150:514-20. 10.1016/j.jtcvs.2015.06.024 [DOI] [PubMed] [Google Scholar]
- 26.Duann CW, Hung JJ, Hsu PK, et al. Surgical outcomes in lung cancer presenting as ground-glass opacities of 3 cm or less: a review of 5 years' experience. J Chin Med Assoc 2013;76:693-7. 10.1016/j.jcma.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 27.Sim HJ, Choi SH, Chae EJ, et al. Surgical management of pulmonary adenocarcinoma presenting as a pure ground-glass nodule. Eur J Cardiothorac Surg 2014;46:632-6; discussion 636. 10.1093/ejcts/ezu007 [DOI] [PubMed] [Google Scholar]
- 28.Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. 10.1378/chest.12-2987 [DOI] [PubMed] [Google Scholar]
- 29.Sasada S, Nakayama H, Miyata Y, et al. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann Thorac Surg 2015;99:956-60. 10.1016/j.athoracsur.2014.10.041 [DOI] [PubMed] [Google Scholar]
- 30.Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Oncol 2014;12:388. 10.1186/1477-7819-12-388 [DOI] [PMC free article] [PubMed] [Google Scholar]