Abstract
Active mutations of the EGFR gene have been proved to predict the activity of EGFR-TKI. The most common mutations are the exon 19 deletion and exon 21 point mutation, both of which are sensitive to EGFR-TKI. However, rare EGFR mutations or complex mutations still exist, and data of which are scarce and controversial. Their response to EGFR-TKI remains uncertain. We presented a patient diagnosed with stage IV lung adenocarcinoma who was found to have the EGFR mutation in exon 19 (L747P) before any treatment. The disease progressed 2 months after the chemotherapy containing cisplatin and pemetrexed, and erlotinib was administered, but there was no response found. This EGFR-TKI naïve patient failed to achieve the desired effect with the therapy of EGFR-TKI. L747P may be associated with primary resistance to EGFR-TKI in this case.
Keywords: EGFR 19 mutation, adenocarcinoma, lung
Introduction
It has been reported that patients with non-small cell lung cancer (NSCLC) that harbor activating mutations of the EGFR gene may be sensitive to the EGFR-TKI therapy which has been proved to improve response and survival rate, therefore, clinical screening for EGFR gene has been widely performed for patients with NSCLC. EGFR mutation testing usually focuses on common mutations like the exon 19 deletion and exon 21 point mutation, both of which are sensitive to EGFR-TKI. However, beyond the identification of classic EGFR mutations, there are still rare or complex mutations which may or may not benefit from EGFR-TKI therapy. Up to now, we have little knowledge of rare or complex mutations, and the results in this field remain limited and controversial. Studies with large numbers of patients harboring these mutations need to be performed (1).
Case presentation
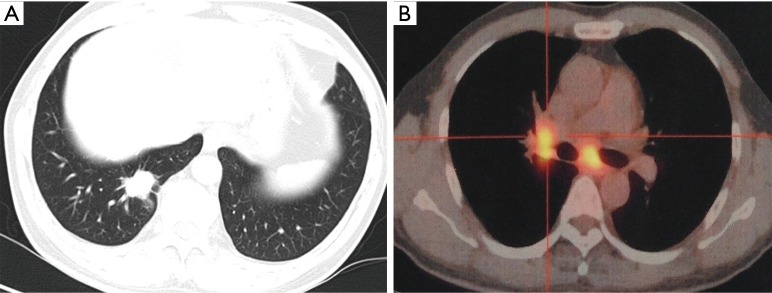
A 61-year-old Chinese male with a smoking history of 30 years (12 pack-years) began to cough associated with a large number of white sticky sputum since August 2014. CT scan of the chest showed that the right lower lobe consisted of a nodule (2.5 cm × 1.8 cm) (Figure 1A) and the mediastinal lymph nodes along with the right hilar lymph nodes enlarged. PET-CT showed that both the nodule in the right lung and the enlarged lymph nodes were related with high SUV (Figure 1B), and multiple bone metastases were revealed. After a percutaneous lung biopsy, the pathological diagnosis demonstrated that the nodular lesion was lung adenocarcinoma of the right lower lobe (invasive tiny papillary carcinoma) (Figure 2). The tumor markers, CEA, CA125, CYFRA211 and NSE elevated with values of 14.59 ng/mL, 58.96 U/mL, 6.48 ng/mL and 21.73 ng/mL, respectively. The patient was finally diagnosed with stage IV lung adenocarcinoma for multiple vertebra and rib metastases.
Figure 1.
The medical image materials of the lungs on the first time the patient was diagnosed. (A) The computerized tomographic scan showed that the right lower lobe consisted of a nodule (2.5 cm × 1.8 cm); (B) the PET-CT image showed that the mediastinal lymph nodes and the right hilar lymph nodes enlarged and both were related with high SUV.
Figure 2.
Histology of the primary tumor—an invasive tiny papillary adenocarcinoma (HE, A: 40×; B: 100×).
Genetic testing was performed using a paraffin slice from the tissue specimen by means of first-generation sequencing technique (Sanger) for the detection of EGFR gene 18, 19, 21, K-ras gene, B-raf gene, ALK gene, ROS1 gene. As shown in Figure 3, L747P was found in exon 19.
Figure 3.
Gene sequencing results of the primary tumor. It showed the EGFR mutation L747P in exon 19.
A platinum doublet was chosen as a first-line therapy according to the existing treatment protocol in 2009. Six cycles of combination chemotherapy containing cisplatin and pemetrexed was administered at 3-week intervals. The CT scan of the chest indicated that the lesion in the right lower lobe remained almost the same as before. The tumor markers as mentioned above returned to the base-line value, except NSE with values of 21.1 ng/mL out of normal range. The patient was judged as having a stable disease. After 2-month observation, the patient began to cough again with the right chest pain. The CT scan of the chest suggested that multiple small nodules could be seen in both lung fields (Figure 4A). The levels of the tumor markers increased significantly. The disease progressed obviously, and EGFR-TKI was chosen as a second-line therapy. He received the erlotinib therapy at a dose of 150 mg/d administered orally for 1 month. After that, the CT scan of the chest showed that much more metastatic nodules were found in both lungs (Figure 4B). The concentration of the tumor markers continued to rise rapidly. Obviously, the disease did not stop progressing, and erlotinib failed to achieve the desired response.
Figure 4.
Computerized tomographic scan of the lungs before and after erlotinib was treated. Metastatic nodules in (B) were much more than those in (A).
In order to study whether there were some changes of gene mutation after using chemotherapy and EGFR-TKI, we performed genetic testing again using a whole blood sample by means of next-generation sequencing (NGS) technique (ctDNA detection method). As shown in Figure 5, L747P missense mutation was found in EGFR exon 19, and the mutation abundance was 79.50%. This gene sequencing was consistent with the first-generation gene sequencing result in the primary tumor tissue.
Figure 5.
Gene sequencing (using whole blood as sample by means of ctDNA detection method) results of the tumor after 1-month treatment with erlotinib. It showed the EGFR mutation L747P in exon 19.
Discussion
Previous studies have shown that exon 19 deletion and exon 21 point mutation of EGFR are sensitive to EGFR-TKI in patients with advanced NSCLC. Most of the deletions in exon 19 encompass the amino acids from codons L747-750 (LRE fragment) which activate EGFR and respond to EGFR-TKI, while the other deletions which do not involve any of the LRE fragment are reported to have a worse response (2). This may be because that the LRE deletion mutation can significantly change the conformation of a protein in this region from the initial to the “active dimer” state and can keep the state longer (3).
Recently, some mixed insertion/substitutions are found in exon 19. A few patients with the L858R-EGFR-activating mutation that acquired the secondary L747S mutation after treatment to gefitinib and became resistant to gefitinib subsequently have been reported. Further researches have shown that a dose escalation of gefitinib or a switch to erlotinib, both ways increase a dose of reversible TKI, would lead to beneficial clinical effects in these L858R-L747S-mutant patients (4,5). The mechanisms of these secondary mutations resistant to EGFR-TKI may be associated with the BIM up-regulation and the mitochondrial apoptosis pathway. As the L858R-L747S attenuates both the up-regulation of BIM and apoptosis, a dose escalation of reversible TKI would change the situation of TKI resistance (6).
Intense researches in NSCLC have identified two major mechanisms of resistance to EGFR-TKI: secondary resistance mutations and “oncogene kinase switch” systems (7). The EGFR T790M mutation as the most common secondary resistance mutation is the first identified mechanism of acquired resistance to EGFR-TKIs. Other secondary mutations (D761Y, L747S) seem to be rare (4-6,8).
We presented a patient diagnosed with stage IV lung adenocarcinoma who was found to have the EGFR mutation in exon 19 (L747P). After the first-line chemotherapy, the patient was judged as having a stable disease. Two months later, the disease progressed and the erlotinib was chosen as the second line therapy. However, the disease did not stop progressing. In recent studies in China, two patients with lung adenocarcinoma harboring L747P mutation before any treatment were found to be resistant to EGFR-TKI (9,10). Besides, a recent study in Japan showed that L747S mutation associated with EGFR-TKI resistance was detected in some NSCLC patients, none of whom had ever received EGFR-TKI (11). In our study, we performsed next-generation sequencing (NGS) by means of ctDNA detection method and L747P missense mutation was found in exon 19 of EGFR. We indicated that the patient failed to achieve the desired effect after using erlotinib because of harboring L747P mutation in exon 19 of EGFR.
Conclusions
We reported a patient with advanced NSCLC who had the EGFR mutation L747P in exon 19. This EGFR-TKI naïve patient failed to achieve the desired effect with the therapy of TKI. L747P may be associated with primary resistance. Because data are limited and results may vary, the mechanism of the L747P mutation resistance to EGFR-TKI needs further investigation.
Acknowledgements
The authors would like to thank Guangzhou Burning Rock Clinical Lab Co., Ltd for the next-generation sequencing using whole blood as sample by means of ctDNA detection method.
Funding: This work was supported by Jiangsu Provincial Special Program of Medical Science (BL2012023), and Clinical Medical Center of Suzhou.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Karachaliou N, Molina-Vila MA, Rosell R. The impact of rare EGFR mutations on the treatment response of patients with non-small cell lung cancer. Expert Rev Respir Med 2015;9:241-4. 10.1586/17476348.2015.1046439 [DOI] [PubMed] [Google Scholar]
- 2.Chung KP, Wu SG, Wu JY, et al. Clinical outcomes in non-small cell lung cancers harboring different exon 19 deletions in EGFR. Clin Cancer Res 2012;18:3470-7. 10.1158/1078-0432.CCR-11-2353 [DOI] [PubMed] [Google Scholar]
- 3.Tsigelny IF, Wheler JJ, Greenberg JP, et al. Molecular determinants of drug-specific sensitivity for epidermal growth factor receptor (EGFR) exon 19 and 20 mutants in non-small cell lung cancer. Oncotarget 2015;6:6029-39. 10.18632/oncotarget.3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa DB, Schumer ST, Tenen DG, et al. Differential responses to erlotinib in epidermal growth factor receptor (EGFR)-mutated lung cancers with acquired resistance to gefitinib carrying the L747S or T790M secondary mutations. J Clin Oncol 2008;26:1182-4; author reply 1184-6. 10.1200/JCO.2007.14.9039 [DOI] [PubMed] [Google Scholar]
- 5.Costa DB, Nguyen KS, Cho BC, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res 2008;14:7060-7. 10.1158/1078-0432.CCR-08-1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med 2007;4:1669-79; discussion 1680. [DOI] [PMC free article] [PubMed]
- 7.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 2009;10:281-9. 10.3816/CLC.2009.n.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res 2006;12:6494-501. 10.1158/1078-0432.CCR-06-1570 [DOI] [PubMed] [Google Scholar]
- 9.Yu G, Xie X, Sun D, et al. EGFR mutation L747P led to gefitinib resistance and accelerated liver metastases in a Chinese patient with lung adenocarcinoma. Int J Clin Exp Pathol 2015;8:8603-6. [PMC free article] [PubMed] [Google Scholar]
- 10.Yi S, Zhuang Y, Zhou J, et al. A comparison of epidermal growth factor receptor mutation testing methods in different tissue types in non-small cell lung cancer. Int J Mol Med 2014;34:464-74. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi F, Fukuchi K, Yamazaki Y, et al. Acquired resistance L747S mutation in an epidermal growth factor receptor-tyrosine kinase inhibitor-naïve patient: A report of three cases. Oncol Lett 2014;7:357-360. [DOI] [PMC free article] [PubMed] [Google Scholar]