Abstract
Background
Reports of comparison between robotic and thoracoscopic surgery for lung cancer are limited, we aimed to compare the perioperative outcomes of robotic and thoracoscopic anatomic pulmonary resection for lung cancer.
Methods
A total of 184 patients with lung cancer underwent anatomic pulmonary resection by robotics or thoracoscopy. A propensity-matched analysis with incorporated preoperative variables was used to compare the perioperative outcomes between the two procedures.
Results
Overall, 71 patients underwent robotic pulmonary resection, including 64 lobectomies and 7 segmentectomies, while 113 patients underwent thoracoscopic lobectomy and segmentectomy. Propensity match produced 69 pairs. The mean length of postoperative stay (7.6±4.6 vs. 6.4±2.6 d, P=0.078), chest tube duration (5.3±3.7 vs. 4.4±1.7 d, P=0.056), number of lymph nodes retrieved (17.9±6.9 vs. 17.4±7.0, P=0.660), stations of lymph nodes resected (7.4±1.6 vs. 7.6±1.7, P=0.563), operative blood loss (53.9±29.3 vs. 50.3±37.9 mL, P=0.531), morbidity rates (42.0% vs. 30.4%, P=0.157) were similar between the robotics and thoracoscopy. However, robotics was associated with higher cost ($12,067±1,610 vs. $8,328±1,004, P<0.001), and longer operative time (136±40 vs. 111±28 min, P<0.001).
Conclusions
Robotics seems to have higher hospital costs and longer operative time, without superior advantages in morbidity rates and oncologic efficiency. Further prospective randomized clinical trials were needed to validate both of its short- and long-term oncologic efficiency.
Keywords: Robotics, video-assisted thoracic surgery (VATS), lung cancer
Introduction
Video-assisted thoracic surgery (VATS), a minimally invasive approach with several advantages over thoracotomy for early stage lung cancer (1-5), its use is limited because of rigid instruments and poor 2-dimensional visualization. With technological innovations, robotic surgical systems appears to have several theoretical advantages over traditional VATS, including 3-dimensional field of view, improved greater dexterity due to more degrees of movement freedom of robotic arms and great comfort for the surgeon (6,7). Although retrospective studies are emerging comparing robotic and thoracoscopic pulmonary resections (8-11), the published papers are with conflicting results and have been limited to small case series, lobectomy or non-propensity scoring studies, in which patient selection bias would be inevitable. Therefore we are aimed to evaluate the perioperative outcomes of robotic and thoracoscopic lobectomy and segmentectomy for lung cancer by a propensity-matched analysis to reduce selection bias.
Methods
The institutional review board of First Affiliated Hospital of Zhejiang University approved this study and granted a waiver of the informed consent process because of the retrospective nature of the study. We perform robotic lobectomies based on the 4-incision technique established by Veronesi and associates (6), and segmentectomies refer to the 4-incision technique introduced by Pardolesi and colleagues (12). As for thoracoscopic lobectomy and segmentectomy, we use conventional 3-incision technique, a 4 cm incision at the anterior axillary line at the 4th or 5th intercostal space, a 30-degree thoracoscope was introduced via the 7th intercostal space in the midaxillary line, another 10 mm accessory incision was made at the tip of the scapula, pulmonary vessels and bronchus were transected with endoscopic staplers, while the specimen was removed by a plastic bag for pathological examination.
Since robotic pulmonary resection is a new technique for us, so we excluded the first 30 robotic cases, we included the records of all patients (N=184) treated from September 2014 to July 2015 in our center. The indication for robotic and thoracoscopic lobectomy were clinical T1–T3 disease, N0–N1, and absence of distant metastasis. While the eligibility criteria for segmentectomy were peripheral located cT1aN0M0 tumor. Propensity score matching was performed to compare the two different approaches for pulmonary resections.
The preoperative assessments in all cases of primary tumors included chest CT, standard hematology and blood chemistry, cardiologic examination, pulmonary function assessment and bronchoscopy. Positron emission tomography (PET)/CT was employed in selective cases, for patients do not employ PET/CT, additional diagnostic examinations including brain magnetic resonance imaging (MRI) or CT, abdominal ultrasonography and bone scanning were refer to exclude distant metastasis. Clinical data including age, sex, operative time, operative blood loss, complications, length of hospital stay, length of chest tube duration and tumor characteristics were collected. A panel of 19 cardiovascular, pulmonary, infectious, and intraoperative complications was selected based on a previous publication of outcomes after pulmonary lobectomy using HCUP (13). Surgical mortality was defined as death occurring during the same hospitalization or within 30 days after the operation. Histological type was established according to the World Health Organization classification of lung cancer. TNM stage was determined according to the American Joint Committee on Cancer staging system, 7th edition.
All patients except the ones with convert thoracotomies were one-to-one matched between the robotic and thoracoscopic lobectomy and segmentectomy groups on the basis of nearest estimated propensity score to minimize bias due to the nonrandom allocation of treatments among patients (14). A set of covariates including age, sex, tumor size, and operative procedure was selected to estimate the propensity score. The propensity score which were estimated using a logistic model summarizes the above features in a single variable that can be included in the analyses comparing perioperative outcomes across two groups.
Continuous data were expressed as mean ± SD and compared using the 2-tailed t-test. Comparisons of categorical data between the two groups were made by using the χ2 or Fisher-exact test. Statistical analysis was considered to be significant when the probability value was below 0.05. Propensity score matching was performed using the statistical package R, version 3.2.1 (The R Project for Statistical Computing, Vienna, Austria), while Statistical Package for the Social Science software (Version 17.0; SPSS Inc., Chicago, IL) was used for further data analysis.
Results
From September 2014 to July 2015, a total of 184 patients were enrolled for the analysis (Figure 1), and demographic data are presented in Table 1. Seventy-one underwent robotic surgery and 113 underwent thoracoscopic surgery. Conversion was performed in 7 patients, and these patients were excluded from further propensity-match analysis. Thoracoscopic pulmonary resections were successfully performed in 108 of the 113 patients, with conversion rate of 4.4%, which was close to the robotic surgery group with rate of 2.8% (P=0.874). There was one death due to cerebral infarction in thoracoscopic surgery group. As a result, 184 patients successfully underwent either robotic or thoracoscopic lobectomy (n=145) or segmentectomy (n=39).
Figure 1.
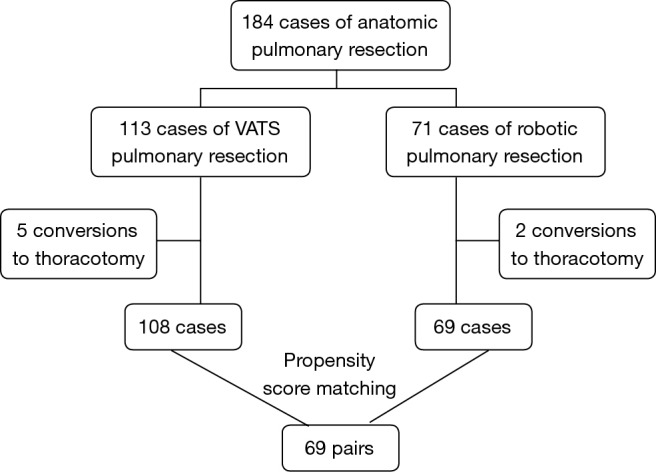
Flowchart summarizing propensity score matching analysis.
Table 1. Clinical characteristics of patients who underwent robotic or thoracoscopic pulmonary anatomic resection.
| Characteristics | All patients | Propensity-matched patients | |||||
|---|---|---|---|---|---|---|---|
| RATS (n=71) | VATS (n=113) | P | RATS (n=69) | VATS (n=69) | P | ||
| Age | 58.4±8.8 | 60.0±9.8 | 0.250 | 58.6±8.8 | 59.9±9.7 | 0.391 | |
| Male:female | 26:45 | 49:64 | 0.365 | 26:43 | 22:47 | 0.474 | |
| Operative procedure | 0.003 | 1 | |||||
| Lobectomy | 64 | 81 | 62 | 62 | |||
| Segmentectomy | 7 | 32 | 7 | 7 | |||
| Tumor size | 1.65±0.90 | 2.06±1.31 | 0.013 | 1.65±0.91 | 1.86±1.19 | 0.239 | |
| Pathologic stage | 0.691 | 0.991 | |||||
| IA | 65 | 87 | 65 | 58 | |||
| IB | 2 | 7 | 0 | 1 | |||
| IIA | 1 | 10 | 1 | 6 | |||
| IIIA | 3 | 9 | 3 | 4 | |||
Data are presented as mean ± SD or number. RATS, robotic-assisted thoracic surgery; VATS, video-assisted thoracic surgery.
The two study groups were well matched with respect to age, tumor size, sex, and operative procedures. The baseline characteristics of the 69 patients in each group are shown in Table 1. The corresponding 69 cases would be selected from thoracoscopic surgery group for one-to-one matching analysis. The mean age was 58.6 years in the robotic surgery group and 59.9 years in the thoracoscopic surgery group. The distribution of pathologic stages is similar in the 2 groups. The operative details are shown in Table 2.
Table 2. Operative outcomes of patients who underwent robotic or thoracoscopic pulmonary anatomic resection.
| Characteristics | RATS (n=69) | VATS (n=69) | P |
|---|---|---|---|
| Operative time* (min) | 136±40 | 111±28 | <0.001 |
| Blood loss* (mL) | 53.9±29.3 | 50.3±37.9 | 0.531 |
| LPS* (d) | 7.6±4.6 | 6.4±2.6 | 0.078 |
| Length of CT duration* (d) | 5.3±3.7 | 4.4±1.7 | 0.056 |
| Lymph node retrieved* | 17.9±6.9 | 17.4±7.0 | 0.660 |
| LN stations retrieved* | 7.4±1.6 | 7.6±1.7 | 0.563 |
| Upstaging rate (%) | 5.8 | 15.9 | 0.056 |
| Complication rate (%) | 42.0 | 30.4 | 0.157 |
| Cost (CNY) | 12,067±1,610 | 8,328±1,004 | <0.001 |
*, data are presented as mean ± SD. RATS, robotic-assisted thoracic surgery; VATS, video-assisted thoracic surgery; CT, chest tube; LPS, length of postoperative stay.
Analysis of the propensity-matched group for postoperative outcomes demonstrate that the robotic surgery group had a significant longer operative time (136 vs. 111 min, P<0.001), higher cost ($12,067 vs. $8,328, P<0.001). Robotic surgery seems to prolong chest tube duration (5.3 vs. 4.4 d, P=0.056) and length of postoperative stay (7.6 vs. 6.4 d, P=0.078), The rate of nodal upstaging for VATS resection appears to be superior to robotic resection (16.0% vs. 5.8%, P=0.055). No significant difference was found in numbers of dissected lymph nodes, stations of lymph nodes retrieved and complication rate.
Discussion
Accumulated evidence has shown that thoracoscopic pulmonary resection for lung cancer is associated with better perioperative outcomes and equivalent oncologic effects as open thoracotomy (1-5). The safety and the feasibility of robotic anatomic lung resection have been shown in several case series (6,12,15), robot is gaining in popularity as proponents claim additional benefits of improved ergonomics, three-dimensional optics, and 7-degree endowrist capabilities allowing more thorough lymph node dissection and simplifying operative procedure. However, limited and conflicting perioperative outcomes have been published by different case cohorts (8-11). In this early experience with robotic lung resection, since thoracoscopic surgery and robotic surgery are at opposite ends of the learning curve, we have shown no demonstrable advantages of robotic surgery exist over the mature thoracoscopic technique by the propensity-matched analysis, but with operative time prolonged and cost increased, as well as a trend of increasing length of postoperative stay. We consider longer operative time may due to a thoracic surgeon’s limited experience for robotic surgery, as was shown short learning curve for robotic surgery (12), we believe the outcomes would be improved for robotic surgery after more resections performed. One suggested robotic pulmonary resection may lead to a reduction in length of stay compared with VATS (9), some comparative studies in which similar lengths of stay were seen between VATS and robotic cases (10,11), however, our data suggest that robotic surgery seems to prolong length of both postoperative stay, we regard this phenomenon as the consequence of special caution being paid to robotic group because they are on initial attempt, which would make the surgeon to postpone the time of discharge to fully ensure safety.
Robotic seems a safe technique for both pulmonary lobectomy and segmentectomy, we found no difference in postoperative complications between the robotic and thoracoscopic groups (42.0% vs. 30.4%, P=0.157). No postoperative death was emerged in robotic group, there was one 30-day postoperative mortality in thoracoscopic cases.
One of the purported benefits of robotic lung resection is that the superior vision and stability will allow surgeons to perform extensive lymphadenectomy (6). Our data suggest that the ability to perform lymphadenectomy is similar between robotic and VATS cases in respect of both resected number of lymph nodes and lymph stations. Previous studies comparing mediastinal lymphadenectomy have demonstrated both equivalence between the two minimally invasive approaches (16) or favoring robotic approach because of higher upstaging rate (17). Although robotic technique is similar to traditional open thoracotomy which would be theoretically better for lymph node dissection, this lack of difference may reflect the most important determinant of lymph node dissection is whether the surgeon is dedicated to lymph node harvesting and systematic evaluation regardless of the technique (10,16).
Another important consideration is that robotic cases were associated with higher hospital costs, with an average incremental cost per case of $3,739, it was consistent with previous studies (8). High capital and running costs, limited availability of robotic systems, as we know less than 100 robotic systems were introduced in China. Only one company is currently producing robotic systems (Intuitive Surgical, Sunnyvale, CA) and entry of competitor companies should drive down costs. In addition, robotic surgery requires a dedicated operating team and an additional experienced surgeon standby at the table for emergency conditions, who are not generally required for standard VATS procedures, this cost is difficult to assess but needs to be considered (8).
This study has several limitations. First, the results were based on retrospective data from review of medical records, propensity matching reduced selection bias but do not eliminate it, prospective randomized studies may be warranted to further validate the application of robotic pulmonary resections. Second, the robotic data in our series includes initial experience, which would make its outcome worse. Third, our study lacked analysis of postoperative pain, and long-term survival outcomes. Last but not least, we compare the initial experience of robotics to the mature VATS lobectomy experience, although we excluded the first 30 cases, it still seems unequal to compare the two approaches, however, because of previous experience of open and thoracoscopic pulmonary resection, as well as the relative steep learning curve (18), we believe the gap of experience between the two approaches would be minimized but not eliminated.
In conclusion, initial experience with robotic lobectomy and segmentectomy resulted in no demonstrable advantages over the mature VATS technique by the propensity-matched analysis, including longer operative time, and increased cost. As more patients undergo robotic anatomic resection, and entry of competitor companies producing robotic instruments, it would be possible to reduce operative time and costs. Prospective randomized studies in experience centers may be warranted to further validate the application of robotic lobectomy and segmentectomy.
Acknowledgements
Funding: The study was supported by National Natural Science Foundation of China, with the grant identification number 31170720, and Department of Science and Technology of Zhejiang with identification number 2014C03032. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Ethical Statement: The institutional review board of First Affiliated Hospital of Zhejiang University approved this study and granted a waiver of the informed consent process because of the retrospective nature of the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. 10.1016/j.athoracsur.2005.07.078 [DOI] [PubMed] [Google Scholar]
- 2.Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. 10.1016/j.jtcvs.2009.11.059 [DOI] [PubMed] [Google Scholar]
- 3.Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. 10.1097/SLA.0b013e318265819c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witte B, Stenz C, Vahl CF, et al. Comparative intention-to-treat analysis of the video-assisted thoracoscopic surgery approach to pulmonary segmentectomy for lung carcinoma‡. Interact Cardiovasc Thorac Surg 2015;21:276-83. 10.1093/icvts/ivv143 [DOI] [PubMed] [Google Scholar]
- 5.Smith CB, Kale M, Mhango G, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol 2014;9:383-9. 10.1097/JTO.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 6.Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. 10.1016/j.jtcvs.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 7.Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. 10.1016/j.jtcvs.2011.10.055 [DOI] [PubMed] [Google Scholar]
- 8.Swanson SJ, Miller DL, McKenna RJ, Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. 10.1016/j.jtcvs.2013.09.046 [DOI] [PubMed] [Google Scholar]
- 9.Farivar AS, Cerfolio RJ, Vallières E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10-5. 10.1097/IMI.0000000000000043 [DOI] [PubMed] [Google Scholar]
- 10.Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. 10.1016/j.athoracsur.2012.01.067 [DOI] [PubMed] [Google Scholar]
- 11.Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. 10.1097/IMI.0b013e3182378b4c [DOI] [PubMed] [Google Scholar]
- 12.Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. 10.1016/j.athoracsur.2012.04.086 [DOI] [PubMed] [Google Scholar]
- 13.Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. 10.1093/ejcts/ezs428 [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757-63. 10.7326/0003-4819-127-8_Part_2-199710151-00064 [DOI] [PubMed] [Google Scholar]
- 15.Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. 10.1016/j.jtcvs.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 16.Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7. [DOI] [PubMed]
- 17.Lee BE, Shapiro M, Rutledge JR, et al. Nodal upstaging in robotic and video assisted thoracic surgery lobectomy for clinical N0 lung cancer. Ann Thorac Surg 2015;100:229-33; discussion 233-4. 10.1016/j.athoracsur.2015.03.109 [DOI] [PubMed] [Google Scholar]
- 18.Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. 10.1016/j.athoracsur.2009.04.039 [DOI] [PubMed] [Google Scholar]


