Abstract
Background
To assess and evaluate the prognostic value of the 7th edition of the Union for International Cancer Control–American Joint Committee on Cancer (UICC-AJCC) tumor, node, metastasis (TNM) staging system for Chinese patients with esophageal cancer in comparison with the 6th edition.
Methods
A retrospective review was performed on 766 consecutive esophageal cancer patients treated with esophagectomy between 2008 and 2012. Patients were staged according to the 6th and 7th editions for esophageal cancer respectively. Survival was calculated by the Kaplan-Meier method, and multivariate analysis was performed using Cox regression model.
Results
Overall 3-year survival rate was 59.5%. There were significant differences in 3-year survival rates among T stages both according to the 6th edition and the 7th edition (P<0.001). According to the 7th edition, the 3-year survival rates of N0 (75.4%), N1 (65.2%), N2 (39.7%) and N3 (27.3%) patients were significant differences (P<0.001). Kaplan-Meier curve revealed a good discriminatory ability from stage I to IV, except for stage IB, IIA and IIB in the 7th edition staging system. Based on the 7th edition, the degree of differentiation, tumor length and tumor location were not independent prognostic factors on multivariate analysis. The multivariate analyses suggested that pT-, pN-, pTNM-category were all the independent prognostic factors based on the 6th and 7th edition staging system.
Conclusions
The 7th edition of AJCC TNM staging system of esophageal cancer should discriminate pT2–3N0M0 (stage IB, IIA and IIB) better when considering the esophageal squamous cell cancer patients. Therefore, to improve and optimize the AJCC TNM classification for Chinese patients with esophageal cancer, more considerations about the value of tumor grade and tumor location in pT2–3N0M0 esophageal squamous cell cancer should be taken in the next new TNM staging system.
Keywords: Esophageal cancer, esophagectomy, staging, prognosis, survival
Introduction
Esophageal cancer is ranked as one of the most common cancers worldwide, and the seventh most common cause of cancer related death in the United States (1). According to European Society for Medical Oncology (ESMO) clinical practice guidelines for esophageal cancer, surgical treatment remains the prime treatment protocol for esophageal cancer and surgery alone is regarded as standard treatment only in carefully selected operable patients with localized esophageal squamous cell carcinomas (ESCC, T1–2N0–3M0) (2). Therefore, tumor staging is of vital importance for patients’ treatment protocol. Accurate staging can help oncologists to predict prognosis, such as recurrence and survival, and help to make the most appropriate therapy strategy. Union for International Cancer Control–American Joint Committee on Cancer (UICC-AJCC) tumor, node, metastasis (TNM) staging system for esophageal cancer has been used worldwide to predict the prognosis, to minimize inappropriate treatment, and allowing for the communications conveniently among different institutions.
Previous studies have shown the implication of the 7th UICC-AJCC TNM staging system in a better prognostic stratification for patients’ overall survival (OS) in comparison with the 6th edition (3-5). However, most studies were from western countries. In addition, the 7th edition for esophageal cancer was based on data of Western populations collected from 13 institutions (Asia, 2; Europe, 2; North America, 9). However, only 25% of the analysis database was collected from Asian, and 60% of esophageal cancers were esophageal adenocarcinoma (EAC) and ESCC accounts for only 40% (6,7). In sharp contrast, squamous cell carcinoma is the dominant histopathologic type of esophageal cancer in East Asia, especially in China, while adenocarcinoma has been increasing rapidly in western countries (8,9). Therefore, the 7th edition of AJCC TNM staging system remains questionable for Asian countries, further validation about ESCC is needed and necessary. To evaluate this issue, we retrospectively analysis the clinicopathologic data of 766 ESCC patients who received surgery, compared and assessed the prognostic value of the 6th and 7th AJCC TNM staging systems.
Methods
We retrospectively gathered information on 850 esophageal cancer patients who underwent resection at Zhongshan Hospital, Changzhen Hospital, Second Military Medical University, Jiangsu Cancer Hospital between August 2008 and December 2012. Institutional Research Ethics Board approval was obtained, and the study protocol was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). All patients submitted written informed consent before inclusion in the study. Inclusion criteria were a history of esophageal squamous cell cancer, receiving resection without neoadjuvant therapy, and metastasis-free according to the 7th edition for esophageal cancer. Patients who died within 30 days after surgery, lost during the follow-up, had distant metastasis or had a baseline history of cancer were excluded. Among the 850 patients, 84 patients were excluded from the study according to the exclusion criteria, leaving 766 ESCC patients to be analyzed retrospectively. The clinicopathologic features including demographic, tumor location, length, degree of differentiation, invasion depth of tumor (T category), and the information of lymph node metastasis (N category) were all recorded.
All patients were restaged according to the 6th and 7th editions of the AJCC staging systems. In the 7th edition, T1 was subclassified into T1a and T1b, T4 was subclassified into T4a (tumor invades pleura, pericardium, diaphragm or adjacent peritoneum) and T4b (tumor invades other adjacent structures such as aorta, vertebral body or trachea). The 7th edition redefined the N categories into three subclasses according to the number of positive lymph nodes, as follows: N1, one or two positive lymph nodes; N2, three to six positive lymph nodes; and N3, seven or more positive lymph nodes. Radical resection plus regional lymph node dissection was given to all patients without distant metastasis. For the 7th edition, grade (G) was used by pathologists to express and assess qualitatively differentiation from well-differentiated (G1), moderately-differentiated (G2), and poorly-differentiated (G3) to least differentiated (G4).
Preoperative evaluation and staging methods
Pretreatment evaluation consisted of patient’s history, physical examinations, complete blood count, liver function tests, studies of tumor markers, computed tomography (CT) scans from the neck to the upper abdomen, or supraclavicular and abdominal ultrasonography, esophagogastroduodenoscopy, endoscopic ultrasound (EUS), barium esophagography. No routine positron emission tomography (PET)-CT was performed. PET-CT was suggested when CT scans were ambiguity, which may help to assess the T- and N-category of the tumor. Some cervical nodal metastases were proven by fine needle aspiration. According to the above results, we evaluated the disease in the round and ultimately decided which type of surgical approach should be chosen.
Treatment
Transthoracic surgery was approach either through two-field lymphadenectomy or three-field lymphadenectomy. Different types of surgical approach mainly depended on the tumor location and lymph node metastasis. Patients with cancer in the upper or middle thoracic esophagus, Ivor Lewis approach or McKeown approach was recommended. Patients with cancer in the lower thoracic esophagus and suspicious superior mediastinal lymph node metastasis, Ivor Lewis approach was recommended. Patients with cancer in the lower thoracic esophagus and without superior mediastinal lymph node metastasis, sweet approach was recommended. For the accuracy of clinical N-staging did not exceed 80%, some patients were found to have different N scores before and after operation (2). Patients who had palliative resection (R1 or R2 resection) needed postoperative treatments. The main chemotherapy regiment consisted of 5-fluorouracil and cisplatin or nedaplatin. For radiotherapy regiment, patients underwent in general 2 Gy/day for 5 days per week, to a total radiation dose of 54 Gy.
Follow-up
All of the patients with esophageal cancer were followed until January 15, 2014. Median follow-up period for surviving patients was 38.9 mo (range, 13.7–66.8 mo), and the follow-up rate was 100%. The follow-up information was obtained primarily from the planned outpatient clinic visits and by telephone interview. Survival status, disease progression, and reasons for death were recorded. Surviving patients were followed at regular intervals at the outpatient clinic until 5 years after surgery. Outpatient clinic visits encompassed history taking, physical examination, blood chemistry analysis, image examination.
Statistical analysis
OS was defined as the time between date of operation and date of death or last follow-up. The χ2 test was applied to compare the baseline characteristics. Variables in the analysis included age, gender, tumor differentiation, tumor length, tumor location, pathologic T-, N-, TNM-category. Survival curves were plotted by the Kaplan-Meier method, and differences between curves were assessed by the log rank test. Multivariate analysis was performed using the Cox regression model. P≤0.05 was considered significant. All statistical analyses were performed using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients characteristics
There were 624 male and 142 female patients (male vs. female =4.4:1), with a median age of 51.4 years (range, 25–77 years) included in the study. Clinicopathological characteristics and OS rates were summarized Table 1. All of the 766 patients were esophageal squamous cell cancer. Most patients had tumor located in the mid-esophagus and lower esophagus. Among of 766 patients, 52 patients (6.8%) had a tumor located in the upper esophagus, whereas for 502 patients (65.5%) and 212 patients (27.7%) with the tumor were situated in the mid-esophagus and lower esophagus, respectively. According to the 7th edition, 195 patients were regarded as G1, 342 were G2, 229 were G3 and G4. Of all the patients, a total of 359 lesions were N0, 135 were N1, 184 were N2, and 88 were N3.
Table 1. Demographics and univariate survival analysis results.
| Characteristic | Number (%) | 3-y OS (%) | P value |
|---|---|---|---|
| Age (years) | 0.813 | ||
| <65 | 576 (75.2) | 61.2 | |
| ≥65 | 190 (24.8) | 60.3 | |
| Gender | 0.042 | ||
| Male | 624 (81.5) | 58.8 | |
| Female | 142 (18.5) | 68.3 | |
| Location | 0.075 | ||
| Upper third | 52 (6.8) | 62.7 | |
| Middle third | 502 (65.5) | 56.2 | |
| Lower third | 212 (27.7) | 53.6 | |
| Grade | 0.000 | ||
| Well-moderately differentiated (G1–G2) | 537 (70.1) | 67.1 | |
| Poorly-least differentiated (G3–G4) | 229 (29.9) | 49.6 | |
| Tumor length (cm) | 0.062 | ||
| ≤3 | 242 (31.5) | 66.1 | |
| 3–5 | 341 (44.5) | 62.5 | |
| >5 | 183 (24.0) | 49.3 |
OS, overall survival.
Survival analysis of patients with esophageal cancer
The 3-year survival rate of all the patients was 59.5%. In univariate analysis, the following clinicopathological characteristics were significantly associated with OS after surgery in the 766 patients: gender, degree of cell differentiation, pathological (p)T-, pN-, pTNM-category. Univariate analysis indicated that age, tumor histology, length, and location did not significantly influence survival. In multivariate analysis, only pT-, pN-, and pTNM-category were independent factors affecting 3-year survival rate. Whereas, the tumor length, gender, degree of cell differentiation were not significant prognostic factors.
Staging esophageal cancer patients according to the 6th and 7th editions of UICC-AJCC TNM staging system
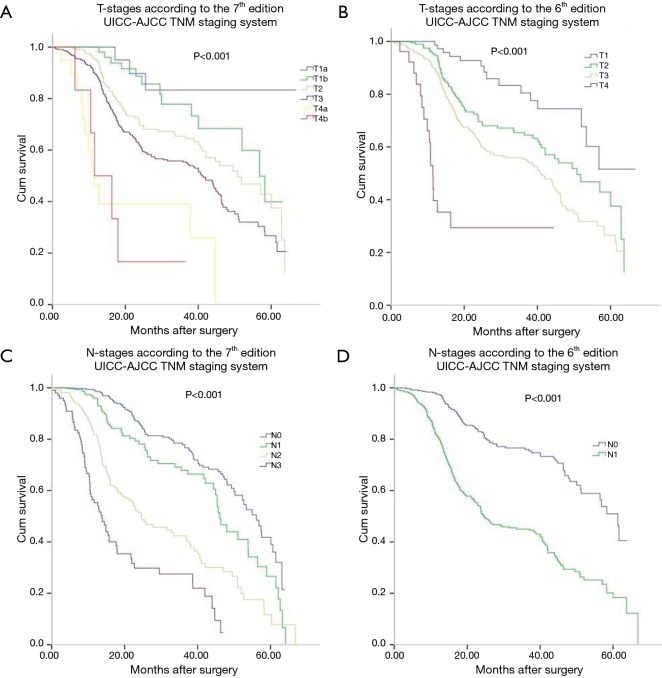
Table 2 lists the detail distribution of patient classifications according to the 6th and 7th of AJCC TNM staging systems. Based on the classification of esophageal cancer according to 7th edition staging system, significant differences were found in 3-year survival rate between patients with different T and N categories. The 3-year survival curves in the 7th edition showed a stepwise decrease with increase in T and N scores. The 3-year survival rate of T1a, T1b, T2, T3, T4a, and T4b diseases was 85.7%, 78.4%, 67.2%, 54.4%, 39.1%, and 18.5%, respectively. The 3-year survival rate of N0, N1, N2, and N3 diseases was 75.4%, 65.2%, 39.7%, and 27.3%, respectively.
Table 2. Comparison of 3-year OS rates among different stages according to the 6th and the 7th UICC-AJCC TNM staging systems.
| Variable | 6th edition | 7th edition | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Total (n) | Survival (n) | 3-y OS (%) | Total (n) | Survival (n) | 3-y OS (%) | |||
| T stage | 0.000 | |||||||
| Tis | 0 | 0 | – | – | ||||
| T1 | 72 | 59 | 81.9 | – | – | – | ||
| T1a | – | 21 | 18 | 85.7 | ||||
| T1b | – | 51 | 40 | 78.4 | ||||
| T2 | 195 | 131 | 67.2 | 195 | 131 | 67.2 | ||
| T3 | 480 | 261 | 54.4 | 480 | 261 | 54.4 | ||
| T4 | 19 | 5 | 26.3 | – | – | – | ||
| T4a | – | 13 | 5 | 38.5 | ||||
| T4b | – | 6 | 1 | 16.7 | ||||
| N stage | 0.000 | |||||||
| N0 | 359 | 271 | 75.5 | 359 | 271 | 75.5 | ||
| N1 | 407 | 185 | 45.5 | 135 | 88 | 65.2 | ||
| N2 | – | 184 | 73 | 39.7 | ||||
| N3 | – | 88 | 24 | 27.3 | ||||
| M stage | 0.000 | |||||||
| M0 | 686 | 433 | 63.1 | 766 | 456 | 59.5 | ||
| M1a | 80 | 23 | 28.8 | |||||
UICC-AJCC, Union for International Cancer Control–American Joint Committee on Cancer; TNM, tumor, node, metastasis; OS, overall survival.
Survival curves of each T and N categories were analyzed in Figure 1. Three-year survival rates of each T and N categories were measured and compared. The 3-year survival curves of N categories according to the 7th edition were well segregated. Eighty patients with M1a disease (non-regional lymph node metastasis) according to the 6th edition were reclassified as M0 disease according to the 7th edition. T-, N- and M-category in the 6th edition had significant impact on prognosis as well as in the 7th edition. M categories were restaged into M0, M1a and M1b in the 6th edition, and 80 patients with M1a stage in the 6th edition was restaged as N categories in the 7th edition. These 80 patients had cervical or celiac node metastases rather than distant organ metastases. The 3-year survival rate of M1a was 28.8% according to the 6th edition.
Figure 1.
Kaplan-Meier survival curves for patients stratified by tumor (T), node (N) classifications. T staging in the 7th edition staging system (A), T staging in the 6th edition staging system (B), N staging in the 7th edition staging system (C), N staging in the 6th edition staging system (D). UICC-AJCC, Union for International Cancer Control–American Joint Committee on Cancer; TNM, tumor, node, metastasis.
Comparison of 6th and 7th editions of UICC-AJCC TNM staging system
As shown in Table 3, according to the 7th edition, 22 patients with stage IIA who were assessed according to the 6th edition criteria were reclassified as stage IB. All the patients with stage IV disease who were assessed according to the 6th edition criteria were reclassified as stage IIB, IIIA, IIIB, and IIIC in the 7th edition.
Table 3. Staging esophageal cancer patients according to the 6th and 7th editions UICC-AJCC TNM staging systems.
| Stage | 6th edition | 7th edition | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Total (n) | Survival (n) | 3-y OS (%) | Total (n) | Survival (n) | 3-y OS (%) | |||
| I | 44 | 36 | 81.8 | 66 | – | – | ||
| IA | – | – | – | 14 | 12 | 85.7 | ||
| IB | – | – | – | 52 | 37 | 71.1 | ||
| II | 385 | 324 | 0.142 | |||||
| IIA | 281 | 216 | 76.9 | 117 | 96 | 82.1 | ||
| IIB | 104 | 69 | 66.3 | 207 | 159 | 76.8 | ||
| III | 257 | 112 | 43.6 | 376 | – | – | 0.000 | |
| IIIA | – | – | – | 185 | 99 | 53.5 | ||
| IIIB | – | – | – | 83 | 27 | 32.5 | ||
| IIIC | – | – | – | 88 | 26 | 29.5 | ||
| IV | 80 | 23 | 28.8 | |||||
| Total | 766 | 456 | 59.5 | 766 | 456 | 59.5 | ||
UICC-AJCC, Union for International Cancer Control–American Joint Committee on Cancer; TNM, tumor, node, metastasis; OS, overall survival.
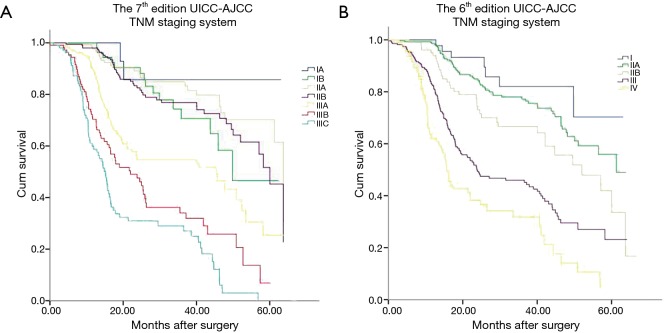
The Kaplan-Meier curves of esophageal cancer patients based on the 6th and 7th edition of AJCC TNM staging systems were depicted in Figure 2. Kaplan-Meier plot showed a good discriminatory ability from stage I to III, excepting for stage IB, IIA and IIB in the 7th edition staging system. According to the 7th edition staging system, there was no significant difference between the 3-year survival of stage IB and II (P=0.142, Table 3). The 3-year OS rate for stage IB, IIA, and IIB were 71.1%, 82.1% and 76.8%, respectively, whose survival curves were overlapped (Figure 2A). Significant differences of the 3-year survival were showed between stage III and I, stage III and II (P=0.000, P=0.000).
Figure 2.
Kaplan-Meier survival curves for patients stratified by the 7th edition staging system (A) and 6th edition staging system (B). UICC-AJCC, Union for International Cancer Control–American Joint Committee on Cancer; TNM, tumor, node, metastasis.
Discussion
The UICC-AJCC TNM staging system is important to predict prognosis and to make treatment strategies. Previous studies based on the data mainly from EAC patients, concluded that the 7th edition AJCC TNM staging system was a better model for predicting outcomes (5,10). However, in comparison with EAC, patients with ESCC has worse prognosis and a distinct pattern of lymphatic spread, and are susceptible to spread locally rather than systemically (11,12). In this study, we compared the performance of the 6th and the 7th AJCC TNM staging systems through the prognosis of 766 ESCC patients with resection, which can reflect the clinicopathologic features of Chinese patients with esophageal cancer better.
T classification is one of essential pieces of information for accurate staging. From a surgical point of view, T4a in the 7th edition was reclassified into stage III in the 7th edition and could receive curative surgery. However, T4b was associated with dismal prognosis and was impossible to receive a radical resection. In accordance with previous studies (4,5,13-15), we also found that T categories, according to the 6th edition and the 7th edition, were significantly related with prognosis both in univariate and multivariate analysis (P<0.05). In line with previous study (16), we found that T1a patients (85.7%) had a higher 3-year survival rate than T1b patients (78.4%). This could be due to the different risk of nodes metastases in pT1a and pT1b tumors (0–1.8% vs. 17.5–21%) (16,17). Our results demonstrated that significant discrepancy of 3-year survival were seen among T3 (54.4%), T4a (39.1%) and T4b (18.5%). Both in EAC and ESCC patients, the differentiation among pT3, pT4a and pT4b also showed statistically significant differences in prognosis (P<0.001) (5,13,18). Thus, the 7th edition for T stratification provided a better prognostic power, and discriminated effectively for patients with esophageal cancer with resection.
Stage I, II, III and IV diseases were also reclassified in the 7th AJCC TNM staging system. In the 7th edition, tumor grade and location for staging pT2–3N0M0 (stage IB, IIA and IIB) ESCC were added. Patients with pT2–3N0M0, classified as stage IIA according to the 6th edition stage system, could be reclassified as stage IB, IIA, or IIB according to 7th edition. In our analysis, more than 22 patients with stage IB were reclassified as in the 7th TNM staging system, compared to the 6th TNM staging system. However, we found that the 3-year survival curves for stage IB, IIA, and IIB were overlapped. The 3-year survival for stage IIA (82.1%) and IIB (76.8%) were even better than stage IB (71.1%) surprisingly. Identify with our results, Situ et al. retrospectively analyzed 317 patients with postoperative pathologic T2N0M0 who underwent esophagectomy. He found that for pT2N0M0 ESCC, the 7th edition did not provide a more distinguishable prediction of prognosis compared with the 6th edition (19). In terms of the T category, based on 85 ESCC patients with T2 tumors who underwent esophagectomy, Guo et al. concluded that T2 tumors could be subclassified further into T2a and T2b categories, patients with different T2 categories might have different prognoses (P=0.045), but the tumor grade, location, length were not significantly associated with the survival of patients with T2 tumors (20). In our study, tumor grade, location, length were not prognostic factors both in univariate and multivariate analysis. Therefore, in line with above studies, we also suggest that the 7th edition of AJCC TNM staging system do not provide accurately prognostic ability for pT2-3N0M0 ESCC patients. Larger sample sizes and longer follow-up need to further confirm the influence of tumor grade and location on the survival of ESCC patients with pT2-3N0M0 tumors.
The regional lymph nodes in the 7th edition, irrespective of the site of the primary tumor, are those in the esophageal drainage area including celiac axis nodes and paraesophageal nodes in the neck but not supraclavicular nodes (2). In our data, M1a in the 6th edition was restaged as N categories according to the 7th edition. The patients with stage IV disease according to the 6th edition were reclassified as stage IIB, IIIA, IIIB, and IIIC according to the 7th edition. It might explain the significant difference in the 3-year survival between M1a and M1b according to the 6th edition. Hence, more patients can receive radical resection according to the 7th edition compared with the 6th edition, which may prolong the survival for these patients.
Compared with the 6th edition, the 7th edition was more complicated, and the most significant distinctions were adding the histological type, differentiation, tumor location besides for the T- and N-category. As for the tumor grade and location, the results were still inconsistent (5,14,21,22). Agree with the views of our results, a 292 patient-based study also showed grade of differentiation of esophageal cancer as a prognostic factor in univariate analysis but not in multivariate analysis (22). Based on analysis of 392 ESCC patients who underwent primary surgical resection, Hsu et al. did not find tumor grade and location to be significant prognostic factors in their database (5). Although many studies showed that survival improves in the lower third location esophageal cancer, these studies mainly consisted of a large portion of EAC, and the results may not reflect the exact impact of tumor location on ESCC prognosis (23-25). Likewise, subgroup analyses by surgical approach (open thoracotomy vs. thoracoscopy) and cancer stage (stage I, II, III, and IV) did not reveal any significant prognostic impact of tumor location (21). Identify with the above studies, we found that tumor grade, location, length were not significantly associated with the prognosis of patients. As for all the patients in our study were ESCC patients, we may indicate that tumor location for the prognosis of ESCC patients might be not as important as for the prognosis of EAC patients.
Some deficiencies in the present study were that our study was a retrospective review, and some patients without R0 resection underwent chemotherapy or radiotherapy after resection. However, the treatment strategies for the majority of patients were uniformity. All the analysis data were from ESCC patients, which can reflect the clinicopathologic features of Chinese patients with esophageal cancer better..
Conclusions
Both the 6th and the 7th edition of UICC-AJCC TNM staging systems can predict the outcome of patients with esophageal cancer who treated with resection. The 7th edition can predict the prognosis precisely, and give a better guidance to clinical treatment strategies for patients with esophageal squamous cell cancer, excepting for the ESCC patients with pT2–3N0M0 tumors after radical resection. Therefore, more considerations about this shortage should be taken in the next new TNM stage, to improve and perfect the UICC-AJCC TNM classification for patients with esophageal squamous cell cancer.
Acknowledgements
None.
Ethical Statement: The study was approved by Institutional Research Ethics Board and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Stahl M, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi51-6. 10.1093/annonc/mdt342 [DOI] [PubMed] [Google Scholar]
- 3.Lagarde SM, Franssen SJ, van Werven JR, et al. Patient preferences for the disclosure of prognosis after esophagectomy for cancer with curative intent. Ann Surg Oncol 2008;15:3289-98. 10.1245/s10434-008-0068-y [DOI] [PubMed] [Google Scholar]
- 4.Talsma K, van Hagen P, Grotenhuis BA, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol 2012;19:2142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu PK, Wu YC, Chou TY, et al. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg 2010;89:1024-31. [DOI] [PubMed] [Google Scholar]
- 6.Rusch VW, Rice TW, Crowley J, et al. The seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals: the new era of data-driven revisions. J Thorac Cardiovasc Surg 2010;139:819-21. [DOI] [PubMed] [Google Scholar]
- 7.Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. 10.1111/j.1442-2050.2008.00901.x [DOI] [PubMed] [Google Scholar]
- 8.Yokobori T, Kuwano H. Molecular biological review of esophageal cancer. Kyobu Geka 2013;66:73-84. [PubMed] [Google Scholar]
- 9.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol 2009;24:729-35. 10.1111/j.1440-1746.2009.05824.x [DOI] [PubMed] [Google Scholar]
- 10.Strong VE, D'Amico TA, Kleinberg L, et al. Impact of the 7th Edition AJCC staging classification on the NCCN clinical practice guidelines in oncology for gastric and esophageal cancers. J Natl Compr Canc Netw 2013;11:60-6. [DOI] [PubMed] [Google Scholar]
- 11.Bollschweiler E, Schröder W, Hölscher AH, et al. Preoperative risk analysis in patients with adenocarcinoma or squamous cell carcinoma of the oesophagus. Br J Surg 2000;87:1106-10. 10.1046/j.1365-2168.2000.01474.x [DOI] [PubMed] [Google Scholar]
- 12.Mariette C, Finzi L, Piessen G, et al. Esophageal carcinoma: prognostic differences between squamous cell carcinoma and adenocarcinoma. World J Surg 2005;29:39-45. 10.1007/s00268-004-7542-x [DOI] [PubMed] [Google Scholar]
- 13.Yam PC, Tong D, Law S. Comparisons of sixth and seventh edition of the American Joint Cancer Committee staging systems for esophageal cancer. Ann Surg Oncol 2014;21:583-8. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Wu SG, Gao JM, et al. Impact of esophageal cancer staging on overall survival and disease-free survival based on the 2010 AJCC classification by lymph nodes. J Radiat Res 2013;54:307-14. 10.1093/jrr/rrs096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gertler R, Stein HJ, Loos M, et al. How to classify adenocarcinomas of the esophagogastric junction: as esophageal or gastric cancer? Am J Surg Pathol 2011;35:1512-22. 10.1097/PAS.0b013e3182294764 [DOI] [PubMed] [Google Scholar]
- 16.Chen SB, Weng HR, Wang G, et al. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: evaluation of the seventh edition of the American Joint Committee on Cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol 2013;8:495-501. [DOI] [PubMed] [Google Scholar]
- 17.Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010;210:418-27. 10.1016/j.jamcollsurg.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Jiang Y, Yu X, et al. Analysis of new N-category on prognosis of oesophageal cancer with positive lymph nodes in a Chinese population. Radiol Oncol 2013;47:63-70. 10.2478/v10019-012-0039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Situ D, Wang J, Lin P, et al. Do tumor location and grade affect survival in pT2N0M0 esophageal squamous cell carcinoma? J Thorac Cardiovasc Surg 2013;146:45-51. 10.1016/j.jtcvs.2013.01.034 [DOI] [PubMed] [Google Scholar]
- 20.Guo W, Xiao HL, Ma Z, et al. Should stage T2 esophageal squamous cell carcinoma be subclassified? Ann Surg Oncol 2014;21:2540-5. 10.1245/s10434-014-3636-3 [DOI] [PubMed] [Google Scholar]
- 21.Takeno S, Takahashi Y, Hashimoto T, et al. Is the prognostic impact of tumor location in patients with surgically resected esophageal squamous cell carcinoma affected by surgical approach? Eur Surg Res 2013;51:91-8. 10.1159/000355680 [DOI] [PubMed] [Google Scholar]
- 22.Wijnhoven BP, Tran KT, Esterman A, et al. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg 2007;245:717-25. 10.1097/01.sla.0000251703.35919.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies AR, Sandhu H, Pillai A, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. Br J Surg 2014;101:511-7. 10.1002/bjs.9456 [DOI] [PubMed] [Google Scholar]
- 24.Costa P, Esteves R, Lages P, et al. Esophageal cancer: surgical strategies. Acta Med Port 2014;27:593-600. 10.20344/amp.5159 [DOI] [PubMed] [Google Scholar]
- 25.Delpisheh A, Veisani Y, Sayehmiri K, et al. Esophageal carcinoma: long-term survival in consecutive series of patients through a retrospective cohort study. Gastroenterol Hepatol Bed Bench 2014;7:101-7. [PMC free article] [PubMed] [Google Scholar]