Abstract
Background
Visceral pleural invasion (VPI) had been demonstrated as an aggressive sign in non-small cell lung cancers (NSCLC). However, its incidence and clinical relevance in early lung cancer showing ground glass nodules (GGNs) has not been clarified.
Methods
All consecutive surgically treated patients with solitary GGNs between 2009 and 2013 were reviewed retrospectively. Inclusion criteria were defined as lesions ≤3 cm with pleura abutting on computed tomography (CT) scan and pathologically confirmed NSCLC.
Results
Out of 156 enrolled patients, 38 had pathologically confirmed VPI. The incidence of VPI was 41.5% (27/65) if the tumor diameter was larger than 2.0 cm and 14.3% (13/91) if diameter was smaller than 2.0 cm (P<0.001). Further, the incidence was 17.4% (12/69) in pure GGNs and 32.2% (28/87) in part-solid GGNs (P=0.040). The tumor size and the nodule nodule-pleural relationship were significant predictors of positive VPI. In cases with pleural indentation, attachment, and closeness, the incidence was 38.1%, 25.5%, and 5.3%, respectively (P=0.001). All cases were PL0 and PL1, with no PL2 cases observed.
Conclusions
Although VPI was visible in both pure/mix GGNs, it was more common in larger (>2 cm) GGNs. The radiographic findings of nodule abutment or a pleural tag did not reliably predict or exclude VPI. In patients with GGNs, a low rate of PL2 invasion may be observed.
Keywords: Non-small cell lung cancer (NSCLC), ground-glass nodules (GGN), visceral pleura invasion
Introduction
Visceral pleural invasion (VPI), defined as tumor extension beyond the elastic layer of viscera pleura, is one of the most important adverse prognostic factors in non-small cell lung cancers (NSCLC) (1). Osaki et al. (2) and Shimizu et al. (3,4) reported approximately 10–30% worse 5-year survival in tumors with VPI compared to those without VPI (45.6–49.8% in VPI group and 60.7–79.0% in non-VPI group). In the 7th edition of TNM classification for NSCLC, VPI increases the T staging factor of a tumor from T1 to T2 and upstages the tumor, even when ≤3 cm, from stage IA to stage IB (5).
On the other hand, due to the recent expansion in the availability of computed tomography (CT), early-stage lung cancer showing ground-glass nodules (GGNs) has become a major concern because of an increase in detection. Considering the “indolent” nature of GGNs, however, the significance of such an aggressive histological finding deserves further study. First, the volume doubling time was estimated as 813±375 days for pure GGNs and 457±260 days for part-solid GGNs (6). Second, the survivals were surprisingly good for the GGNs, with a 5-year survival rate higher than 90% (7). Therefore, an apparent controversy emerged between the indolent nature of GGN and aggressive behavior of VPI. Almost all prior studies on VPI had enrolled solid small nodules and focused on early-stage NSCLCs while information regarding VPI in GGNs remained unknown. Additionally, VPI is difficult to diagnose with CT, and radiologic features of GGN associated with VPI have yet to be elucidated. Hence, the present study aims to investigate the clinical, radiological, and pathological features of this special disease category.
Methods
The institutional review board (IRB) of Shanghai Pulmonary Hospital approved this study (IRB approval number: K15-130), and patients’ informed consent was waived because of the retrospective nature of the study design.
Patients
In total, 566 patients received radiological diagnosis as GGNs and underwent operations between January 2009 and December 2013. The inclusion criteria for the present study included solitary GGN smaller than 3.0 cm in the longest diameter; nodules with pleural indentation, pleural attachment (no visible space between the nodule and pleura), and pleural closeness (within 1.0 cm to the pleura); and pathologically confirmed NSCLCs. Patients who underwent neoadjuvant chemotherapy or radiotherapy or had a previous history of malignancy were excluded. Finally, 156 cases were enrolled in the present study.
CT imaging
Chest CT was performed using the following scanners: Somatom Definition AS (Siemens Medical Systems, Germany), Brilliance (Philips Medical Systems, Netherlands), and Lightspeed Ultra (GE Medical Systems, Milwaukee, Wis) with 120 kVp, 100–200 mAs, pitch of 0.875–1.5, and collimation of 1–2.5 mm. Images were reconstructed using a medium sharp reconstruction algorithm with a thickness of 1–2.5 mm. CT scans were collected from all patients in the supine position at full inspiration. All patients underwent a preoperative CT scan, and hilar or mediastinal nodes smaller than 1.0 cm in the shortest diameter were regarded as clinical N0. The preoperative CT images were photographed using a window level of −500 HU, a window width of 1,200 HU for lung windows, a level of 50 HU, and a width of 450 HU for mediastinal windows. Ground-glass component was defined as a hazy increase in attenuation not associated with obscured underlying lung structures in the lung window setting, whereas solid component was defined as an increase in attenuation that obscured the underlying structures. A “pure GGN” was defined as absence of solid parts while a “part-solid GGN” was defined as a tumor containing both solid and ground glass components (8). The consolidation/tumor ratio (CTR) was defined as the proportion of the maximum consolidation diameter divided by the maximum tumor diameter. The authors (Lilan Zhao, Junyan Zha, and Fangyu Zhou) independently evaluated the radiologic findings, including the attenuation of GGNs; the greatest diameter of the lesion and the solid component; and the nodule-pleural connection types presented as pleural indentation, pleural attachment, and pleural closeness. Pleural indentation was defined as tumor indentation of the visceral pleura on CT images at the lung window. Pleural attachment was defined as no visible space between the nodule and the visceral pleura on CT images at both the lung window and mediastinal window or tumor attachment to the interlobar pleura at the lung window. Pleural closeness was defined as tumor located within 1.0 cm of the pleura. Nodule pleural types were prioritized in descending order of complexity (from pleural indentation to pleural attachment and then pleural closeness) when more than one type was present. For example, we categorized pleural indentation when one patient had tumor located within 1.0 cm of the pleural that showed pleural indentation. Any differences were resolved by discussion and the results of consensus were documented.
Surgical procedures and pathology analysis
An intraoperative frozen section diagnosis was conducted to determine the pathologic types, which were classified into adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IA) based on the new IASLC/ATS/ERS classification criteria (9). If the diagnosis of IA was made, a lobectomy and systematic nodal dissection were recommended, including removal of stations 2, 4, and 7–12 on the right side and stations 4–12 on the left side (10). At least 3 mediastinal stations and more than 6 nodes were requested. For non-invasive adenocarcinoma (AIS, MIA), mediastinal lymph node dissection was not performed while lymph node involvement was unlikely in the preoperative evaluation. If hilar lymph node was negative on frozen section analysis, these patients were considered as the N0 stage. Limited resection (segmentectomy or wedge resection) was considered for some peripheral small lesions.
All resected specimens were formalin fixed and stained with hematoxylin and eosin (H&E) for final pathologic diagnosis. Two experienced lung pathologists interpreted all tissue sections. VPI was categorized into three groups by elastin staining (Victoria blue–van Gieson) (5). PL0 represented a tumor invading either the sub-pleural lung parenchyma or superficially the pleural connective tissue beneath the elastic layer. PL1 referred to a tumor that invades beyond the elastic layer without being exposed on the pleural surface. PL2 referred to a tumor that is exposed on the pleural surface, but it does not involve adjacent anatomic structures. PL0 was defined as without VPI, while PL1 and PL2 were both defined as VPI.
Follow-up
All patients were advised to re-visit 3–4 weeks after operations. The follow-up was scheduled every 3–4 months during the first 2 years and 6–12 months afterwards. Chest CT was suggested every 6–12 months. Information concerning survival status was also obtained by phone calls or emails. The follow-up for the present study was completed in April 2015.
Statistical analyses
Continuous variables, including age, CEA level, and tumor diameter, were transformed into categorical variables. The cut-off values were 60 for age, 3.5 μg/mL for CEA level, and 2.0 cm for tumor diameter. All categorical variables were presented as the numbers and percentages in each group and were compared by a Chi-square test or Fisher’s exact test. An overall Chi-square test for a 2×n table was constructed when comparisons involved more than 2 groups. A univariable analysis of the patient characteristics was compared between the VPI (−) group and VPI (+) group. Factors with a P value less than 0.05 in univariate analysis were included in the multivariate logistic regression model to detect any independent significant predictors. Considering the sample size and short-term follow-up data, an exploratory survival analysis was conducted to evaluate whether associations exist between VPI and overall survival in GGN patients. Survival rates were calculated using Kaplan-Meier survival plots and analyzed using the log-rank test. A P value of less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS software package (version 20.0).
Results
General information
Patient characteristics are shown in Table 1. Of the enrolled 156 patients, 56 were males and 100 females, with a median age of 59.2 (range, 30–81) years old. Lobectomy was performed in 133 patients while limited resections were performed in 23 patients [8 segmentectomies (including 2 VPI-positive GGNs) and 15 wedge resections (no VPI cases)]. The mean number of lymph nodes removed was 10.2. Histologic subtypes were 28 (17.9%) AIS, 49 (31.4%) MIA, 43 (27.6%) IA of lepidic predominant, 16 (10.3%) acinar predominant, 19 (12.2%) papillary predominant, and 1 (0.6%) mucinous variant. VPI was found in 40 (25.6%) of the total case series, all of which were at a PL1 level; thus, none were PL2 cases.
Table 1. General information of enrolled patients.
| Characteristics | Total (%) | VPI (−) PL0 (%) | VPI (+) PL1 (%) | P |
|---|---|---|---|---|
| Age | 0.585 | |||
| ≤60 years | 80 (51.3) | 58 (50.0) | 22 (55.0) | |
| >60 years | 76 (48.7) | 58 (50.0) | 18 (45.0) | |
| Gender | 0.806 | |||
| Male | 56 (35.9) | 41 (35.3) | 15 (37.5) | |
| Female | 100 (64.1) | 75 (64.7) | 25 (62.5) | |
| Smoking status | 0.900 | |||
| Smoker or ever smoker | 34 (21.8) | 25 (21.6) | 9 (22.5) | |
| Non-smoker | 122 (78.2) | 91 (78.4) | 31 (77.5) | |
| Preoperative CEA | 0.588 | |||
| ≤3.5 ìg/mL | 140 (89.7) | 105 (90.5) | 35 (87.5) | |
| >3.5 ìg/mL | 16 (10.3) | 11 (9.5) | 5 (12.5) | |
| GGN types | 0.036 | |||
| Pure | 69 (44.2) | 57 (49.1) | 12 (30.0) | |
| Part-solid | 87 (55.8) | 59 (50.9) | 28 (70.0) | |
| CTR | 0.040 | |||
| 0< CTR ≤50 | 45 (51.7) | 35 (59.3) | 10 (35.7) | |
| 50< CTR <100 | 42 (48.3) | 24 (40.7) | 18 (64.3) | |
| Tumor diameter | <0.001 | |||
| ≤2.0 cm | 91 (58.3) | 78 (67.2) | 13 (32.5) | |
| 2.0–3.0 cm | 65 (41.7) | 38 (32.8) | 27 (67.5) | |
| Air bronchogram sign | 0.112 | |||
| Positive | 69 (44.2) | 47 (40.5) | 18 (45.0) | |
| Negative | 87 (55.8) | 69 (59.5) | 22 (55.0) | |
| Pertaining pleura | 0.001 | |||
| Pleura indentation | 63 (40.4) | 39 (33.6) | 24 (60.0) | |
| Pleural attachment | 55 (35.3) | 41 (35.3) | 14 (35.0) | |
| Pleural closeness | 38 (24.4) | 36 (31.0) | 2 (5.0) | |
| Tumor position | 0.136 | |||
| Upper lobe | 105 (67.3) | 73 (62.9) | 32 (80.0) | |
| Middle lobe | 5 (3.2) | 4 (3.4) | 1 (2.5) | |
| Lower lobe | 46 (29.5) | 39 (33.6) | 7 (29.5) | |
| Tumor laterality | 0.706 | |||
| Left | 74 (47.4) | 54 (46.6) | 20 (50.0) | |
| Right | 82 (52.6) | 62 (53.4) | 20 (50.0) | |
| Histology | 0.179 | |||
| AIS | 28 (17.9) | 28 (24.1) | 0 (0.0) | |
| MIA | 49 (31.4) | 49 (42.2) | 0 (0.0) | |
| IA, mucinous variant | 1 (0.6) | 0 (0.0) | 1 (2.5) | |
| IA, lepidic predominant | 43 (27.6) | 25 (21.6) | 18 (45.0) | |
| IA, acinar predominant | 16 (10.3) | 5 (4.3) | 11 (27.5) | |
| IA, papillary predominant | 19 (12.2) | 9 (7.8) | 10 (25.0) | |
| Nodal status | 0.011 | |||
| N0 | 150 (96.2) | 114 (98.3) | 36 (90.0) | |
| N1 | 3 (1.9) | 0 (0.0) | 3 (7.5) | |
| N2 | 3 (1.9) | 2 (1.7) | 1 (2.5) |
VPI, visceral pleural invasion; CTR, consolidation/tumor ratio; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma.
Association of visceral pleural invasion (VPI) and radiologic parameters
Incidence of VPI was significantly higher (P=0.036) in part-solid GGNs (32.2%, 28/87) compared to pure GGNs (17.4%, 12/69) (Table 1). Moreover, according to the solid proportion, the VPI rate increased from 22.2% for 0<CTR≤0.5 to 42.9% for 0.5<CTR<1 (P=0.040). Additionally, VPI was observed more often in larger tumors (27/65, 41.5% if diameter 2–3 cm) compared to smaller tumors (13/91, 14.3% if smaller than 2 cm) (P<0.001). In the pleural indentation group, there were seemly more (38.1%, 24/63) VPI cases compared to the pleura attachment group (25.5%, 14/55) and pleural closeness group (5.3%, 2/38) (P=0.001).
Association of visceral pleural invasion (VPI) and pathologic parameters
Pathological variables included tumor histology and lymph node involvement. Among the 79 stage-IA invasive adenocarcinoma cases, 40 (50.6%) had VPI. The incidences of VPI were 41.9%, 68.8%, and 52.6% in lepidic predominant, acinar predominant, and papillary predominant stage-IA carcinomas, respectively (P=0.179). Lymph node metastasis was confirmed in six cases. The incidence of nodal involvement was also higher in the VPI (+) group (4/40 vs. 2/116, P=0.019).
Independent predictors of visceral pleural invasion (VPI)
Multivariate logistic regression analysis identified tumor size (P=0.016) and nodule-pleural relationship (P=0.038) as significant predictors of VPI while the GGN types did not significantly predict VPI (P=0.917) (Table 2).
Table 2. Multivariate analysis for predictors of VPI.
| Predictors | OR (95% CI) | P |
|---|---|---|
| GGN types | 0.917 | |
| Pure | 1.000 | |
| Part-solid | 0.947 (0.345–2.604) | |
| Tumor size | 0.016 | |
| ≤2.0 cm | 1.000 | |
| 2.0–3.0 cm | 3.229 (1.249–8.349) | |
| Lesion-pleura relationship | 0.038 | |
| Pleural indentation | 1.000 | |
| Pleural attachment | 0.672 (0.285–1.587) | |
| Pleural closeness | 0.134 (0.029–0.632) |
OR, odds ratio; CI, confidence interval.
Exploratory analysis of the prognostic impact of visceral pleural invasion (VPI)
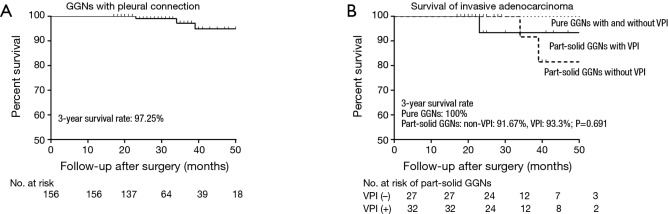
The mean follow-up time was 31.7 months (range, 17–78 months; SD =13.7 months). The survival curve of all patients is shown in Figure 1A. All AIS and MIA patients (n=77) survived, and all patients with pure GGN-represent IA (n=19) also survived. Out of the remaining 60 cases with IA histology and part-solid radiology, three did not survive due to distant metastasis. Two of the deceased cases had lymph node metastasis and only one of the three cases had VPI. Interestingly, no significant difference emerged in the 3-year survival rate between VPI (+) and VPI (−) groups (93.3% vs. 91.67%, P=0.691) (Figure 1B).
Figure 1.
Kaplan–Meier survival curves for patients. (A) Survival curve of ground-glass nodules (GGNs); (B) survival curves for invasive adenocarcinoma (IA) of radiological GGN appearance. No statistically significant difference between VPI (+) and VPI (−) groups emerged (P=0.691).
Discussion
Brewer first proposed VPI in 1977 as a poor prognostic factor in NSCLC patients (11). Subsequently, several later studies provided further additive evidence to support this theory (12,13). Five-year survival rates were reported to drop from 86% (for patients without VPI) to 62–70% (for patients with VPI) (14). Despite the recent emergence of lung cancer cases classified as GGNs, little has been known concerning the incidence and prognostic role of VPI in these part-solid or pure GGN lesions. Hence, the present study investigated related clinical, radiological, and pathological features of VPI in GGN-representing lung cancers as well as survival analysis.
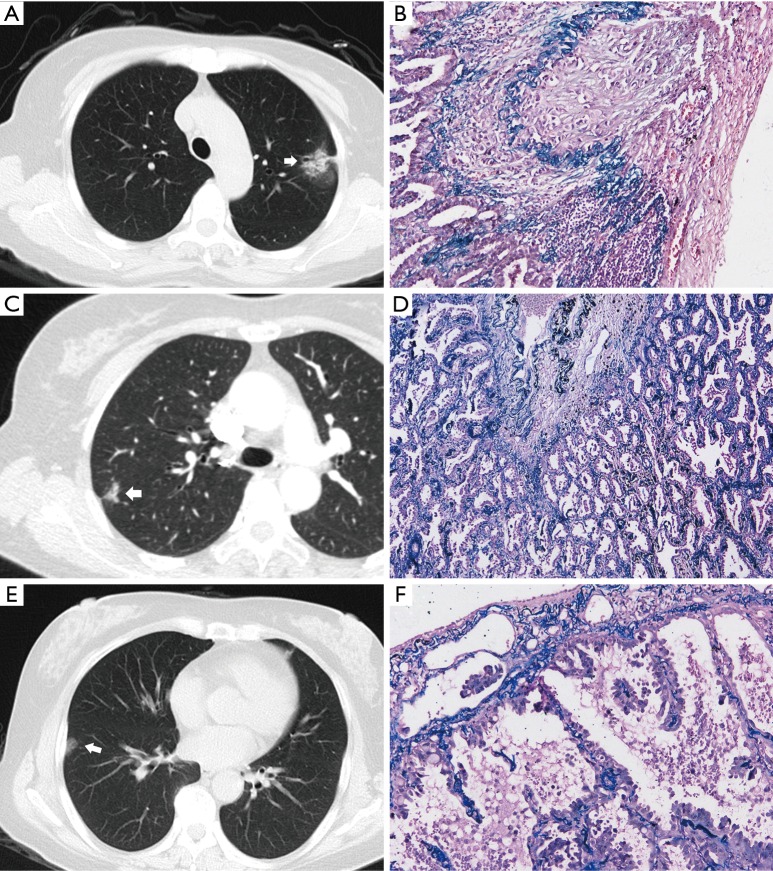
The overall incidence of VPI in NSCLC has been documented as 11.5–26.8% (4,15). Considering that VPI occurs only when tumor-pleura contact is direct, the exact incidence of the peripheral tumors has not been reported in previous studies. The data in the present study suggested again that VPI did not necessarily take place when there was pleural indentation or pleural attachment. In total, VPI occurred at a relatively high rate of about 25.6% when there was radiological pleural abutting of GGNs. Interestingly, only 38.1% of pleural indentations had VPI and 25.5% VPI had radiological pleural attachment while pleural closeness group showed a VPI rate of 5.3%. Therefore, the radiographic findings of nodule abutment or a pleural tag do not reliably predict or exclude VPI in peripheral nodules with ground-glass components (Figure 2A-F). Additionally, even though a visible space was seen between nodule and pleura, some VPI cases may still be present, as GGNs may show an indistinct boundary.
Figure 2.
GGNs with pleural indentation or pleura attachment and their corresponding pathologic manifestations. (A,B) A part-solid GGN with pleural indentation (arrow) showed typical VPI. The tumor was an invasive adenocarcinoma with lepidic predominant (elastic, 100×); (C,D) although radiologically similar to the above case, there was no tumor invasion into the visceral pleura despite being histologically lepidic predominant invasive adenocarcinoma and a radiologically part-solid GGN with pleural indentation (arrow) (elastic, 40×); (E,F) a pure GGN with simply pleural attachment (arrow) was also lepidic predominant invasive adenocarcinoma with prominent VPI (elastic, 100×).
Several candidate risk factors, including lesion-pleura relationship, tumor size, and GGN types, were found to predict VPI. Currently, CT plays a major role in the preoperative staging of NSCLC. The nodule-pleural relationship was a significant predictor of positive VPI. The pleural indentation (38.1%) and the pleura attachment (25.5%) could be sensitive predictors of VPI in GGN cases. Pleural indentation, or pleura tag, is a well-known radiological sign suggestive of pulmonary malignancy and possibly VP (16). Gallagher et al. (17) proposed the underlying histological changes that lead to pleural indentation as (I) prominent elastic reduplication and inflammatory infiltrate and (II) thick fibroblastic proliferation. Therefore, the presence of pleural indentation reflected only fibro-related traction of the pleura rather than VPI. Furthermore, Ebara et al. (18) also found a skirt-like 3-dimensional pleural pattern (accuracy, 77%) predicting pleural invasion. Regarding tumor size, larger tumors had a higher chance of developing peripherally; therefore, they were more likely to invade the visceral pleura. Manac’h et al. (15) showed that VPI was present in 10.4% of tumors smaller than 3 cm and in 19.6% of tumors between 3 and 5 cm and that it was significantly more frequent (P<0.001) in tumors larger than 5 cm in diameter (33%). The data from the present study revealed again that tumor size smaller than 2 cm and pleural indentation were statistically predictive of VPI. If substantiated in large prospective trials, this finding might assist the surgeons to plan the most complete lung cancer removal possible.
Furthermore, a part-solid appearance of GGNs has been a well-known factor in diagnosing their malignancy. Moreover, a part-solid component might be associated with degree of invasiveness. As suggested by Maeyashiki et al. (19), the maximum dimension of consolidation in part-solid GGNs was a significant predictor of lymph node positive status and poor prognosis. Shimizu and colleagues (4) suggested that tumors with VPI had usually moderate or worse differentiation and had a higher scar grade (tumors had fibroblastic tissue with amount of collagen fibers). In our case series, larger consolidation area was associated with higher rate of VPI. As the consolidation area in part-solid GGNs often comprises fibrous stroma and scar tissues, these findings suggested that tumor with VPI might occur more frequently in part-solid GGNs. Moreover, although pure GGNs were commonly taken as less aggressive (9), it was surprising that VPI was also frequently visible in adenocarcinomas of pure GGNs in our study, which was different from others (1).
Interestingly, PL2 invasion was not observed in our study. In our opinion, as a degree of invasiveness, PL2 was more likely to develop in late-stage tumors. As it takes time for the “indolent” GGNs to invade the surface of the visceral pleura, and the surgical intervention is usually conducted at the early stage, it is therefore uncommon to observe PL2. Adachi et al. (20) reported that in tumor smaller than 3 cm, the rate of PL1 was 16.7% while the rate of PL2 was 4.4%. In a large-scale investigation by Kawase et al. (21), the PL1 rate was about 12% and PL2 rate was 4%. All these studies showed that for tumor smaller than 3 cm, the rate of PL2 is low. However, one of their limitations is that they did not mention the rate of VPI extent (PL1, PL2) in patients with GGNs. Therefore, based on our results, it is reasonable to infer that GGNs have a low rate of PL2 invasion.
In the present study, the nodal metastatic rate was 10.0% (4/40) in the VPI (+) group, significantly higher (P=0.019) compared to 1.72% (2/116) in the VPI (−) group. Other studies, e.g., Shimizu and colleagues who reported that nodal metastasis occurred in about 40% VPI (+) cases and the 5-year survival rate was only about 50% for NSCLC tumors, supported our findings (4). About 30% of the patients with stage I lung adenocarcinoma show recurrence after complete resection, which indicates that certain patients may already have occult metastases. Several studies (22,23) ascribed worse survival to special tumor lymphatic drainage if there was VPI. These studies hypothesized that in VPI cases, the exfoliated tumor cells spread directly via pleural lymphatics to hilar and mediastinal lymph nodes.
Of interest, the presence of VPI was not a significant prognostic factor in GGN-representing adenocarcinomas based on the short-term result. Admittedly, this study was an exploratory survival analysis; thus, its conclusion would be more confirmative if long-term (5 years or more) survival information was available. Furthermore, only one study by Hattori et al. (1) yielded similar results. The authors examined only part-solid GGNs, reporting no significant survival differences [5-year survival 94.9% in VPI (−) and 85.6% in VPI (+) group, P=0.3798]. Whether the GGN-representing adenocarcinomas share the same T category, regardless of VPI presence, remains controversial. Adjuvant chemotherapy should be considered in patients with pathologic stage IB NSCLC, but it has not been used as a routine treatment in pathologic stage IA patients (24). From the treatment per se, upstaging VPI (+) patients for additional cytotoxic therapy (chemotherapy or radiation therapy) because of higher stage may expose them to unnecessary side effects from therapy that may not provide benefit (25). Therefore, a financial and hazard analysis may be conducted to compare those receiving adjuvant therapy with those not receiving the therapy. The adjuvant therapy associated complications, long-term outcomes (recurrence-free survival, overall survival and quality of life), and treatment costs could be analyzed. It could make a very convincing argument to consider whether GGN is a special circumstance for different staging classification.
Moreover, the role of VPI in the management of surgical strategy should be considered. From our point of view, no strong evidence or report exist to suggest that the choice between lobectomy and segmentectomy could be determined based solely on VPI. We could decide on the surgical strategy mainly according to the tumor size and tumor pathologic types. We could perform sublobar resection for AIS, while MIA and IA may require a more aggressive operation. The standard treatment for operable NSCLC is lobectomy with dissection of the ipsilateral hilar and mediastinal lymph nodes (26). For patients with GGO-predominant lesions, limited surgical resection that preserves lung parenchyma might be indicated. Many reports have been published on favorable prognoses after the limited resection of a GGO lesion. For example, 48 patients with lesions smaller than 2 cm with GGO proportions >50% survived without recurrence after partial resection in 33 patients and segmentectomy in 15 patients (27). Furthermore, intraoperative VPI confirmation may be needed for surgeons to guide surgical strategy. Intraoperative frozen pathology has a high concordance rate (about 85%) with final pathology. Precise diagnosis by intraoperative frozen section is an effective method to guide resection strategy for peripheral small-sized lung adenocarcinoma. We think that a radiologic VPI suspect could be determinant during the follow-up. A nodule with pleural indentation or pleural attachment showed a high rate of VPI. In our study, the rate was about 30%. Admittedly, the prognostic impact in GGNs has not been defined yet. However, in a similar study, Li et al. (28) examined 103 peripheral NSCLC patients with pleural indentation and reported that the sensitivity, specificity, and accuracy of pleural indentation sign in the diagnosis of pleural involvement were 83.3%, 76.1%, and 78.6%, respectively. The multivariate analysis showed that the 5-year survival rate of pleural indentation sign-positive patients was 46.5%, which was significantly lower compared to that of pleural indentation sign-negative patients (68.9%; P=0.044). Considering a radiologic VPI suspect may have a poor prognosis, a surgical intervention should be performed during the follow-up of a GGN.
A number of limitations in the present study need to be acknowledged. First, our retrospective study has several biases, like lack of random assignment, patient selection, as well as incomplete data acquisition. Second, the results of the prognostic effect of VPI should be interpreted with caution. It must be emphasized that GGN patients showed a relatively favorable survival (about 90% overall 5-year survival rate). Considering that the prognostic results are based on a short-term follow-up data with relatively small sample size, the survival analysis should be viewed as exploratory; thus, more data is needed to validate the results. Furthermore, we only showed overall survival rather than recurrence data, which was not available for 29% of the study population. If the recurrence pattern were presented, it would help understand the biology of GGNs. Finally, surgical techniques, including lobectomy and limited resection (segmental resection and wedge resection), have shown different survival effects (29). However, it is inappropriate to compare lobectomy procedures with limited resection in this cohort (only 23 sublobar resections). To consider the effects of confounding factors, other variables need to be assessed in relation to lung cancer outcomes in future analyses.
In conclusion, a GGN lesion may have VPI when larger than 2 cm with close pleura contact. Both pure and part-solid GGN lesions, if diagnosed as adenocarcinomas, may have microscopic VPI. The presence of VPI does not necessarily correlate with worse survival in GGN-representing adenocarcinomas, and an upgrade of the T category requires further verification.
Acknowledgements
Funding: This study was supported by the Shanghai Committee of Science and Technology (grants 15411968400 and 14411962600).
Ethical Statement: The institutional review board (IRB) of Shanghai Pulmonary Hospital approved this study (IRB approval number: K15-130), and patients’ informed consent was waived because of the retrospective nature of the study design.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hattori A, Suzuki K, Matsunaga T, et al. Visceral pleural invasion is not a significant prognostic factor in patients with a part-solid lung cancer. Ann Thorac Surg 2014;98:433-8. 10.1016/j.athoracsur.2014.04.084 [DOI] [PubMed] [Google Scholar]
- 2.Osaki T, Nagashima A, Yoshimatsu T, et al. Visceral pleural involvement in nonsmall cell lung cancer: prognostic significance. Ann Thorac Surg 2004;77:1769-73; discussion 1773. [DOI] [PubMed]
- 3.Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion classification in non-small cell lung cancer: a proposal on the basis of outcome assessment. J Thorac Cardiovasc Surg 2004;127:1574-8. 10.1016/j.jtcvs.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 4.Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;130:160-5. 10.1016/j.jtcvs.2004.11.021 [DOI] [PubMed] [Google Scholar]
- 5.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. 10.1259/bjr.73.876.11205667 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. 10.1097/JTO.0b013e31821038ab [DOI] [PubMed] [Google Scholar]
- 8.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. 10.1016/S0140-6736(99)06093-6 [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. 10.1016/j.ejcts.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 11.Brewer LA. Patterns of survival in lung cancer. Chest 1977;71:644-50. 10.1378/chest.71.5.644 [DOI] [PubMed] [Google Scholar]
- 12.Yoshida J, Nagai K, Asamura H, et al. Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol 2009;4:959-63. 10.1097/JTO.0b013e3181a85d5e [DOI] [PubMed] [Google Scholar]
- 13.Kang JH, Kim KD, Chung KY. Prognostic value of visceral pleura invasion in non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:865-9. 10.1016/S1010-7940(03)00119-2 [DOI] [PubMed] [Google Scholar]
- 14.Oyama M, Miyagi Maeshima A, Tochigi N, et al. Prognostic impact of pleural invasion in 1488 patients with surgically resected non-small cell lung carcinoma. Jpn J Clin Oncol 2013;43:540-6. 10.1093/jjco/hyt039 [DOI] [PubMed] [Google Scholar]
- 15.Manac'h D, Riquet M, Medioni J, et al. Visceral pleura invasion by non-small cell lung cancer: an underrated bad prognostic factor. Ann Thorac Surg 2001;71:1088-93. 10.1016/S0003-4975(00)02649-7 [DOI] [PubMed] [Google Scholar]
- 16.Shapiro R, Wilson GL, Yesner R, et al. A useful roentgen sign in the diagnosis of localized bronchioloalveolar carcinoma. Am J Roentgenol Radium Ther Nucl Med 1972;114:516-24. 10.2214/ajr.114.3.516 [DOI] [PubMed] [Google Scholar]
- 17.Gallagher B, Urbanski SJ. The significance of pleural elastica invasion by lung carcinomas. Hum Pathol 1990;21:512-7. 10.1016/0046-8177(90)90007-R [DOI] [PubMed] [Google Scholar]
- 18.Ebara K, Takashima S, Jiang B, et al. Pleural invasion by peripheral lung cancer: prediction with three-dimensional CT. Acad Radiol 2015;22:310-9. 10.1016/j.acra.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 19.Maeyashiki T, Suzuki K, Hattori A, et al. The size of consolidation on thin-section computed tomography is a better predictor of survival than the maximum tumour dimension in resectable lung cancer. Eur J Cardiothorac Surg 2013;43:915-8. 10.1093/ejcts/ezs516 [DOI] [PubMed] [Google Scholar]
- 20.Adachi H, Tsuboi M, Nishii T, et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;48:691-7; discussion 697. 10.1093/ejcts/ezu515 [DOI] [PubMed] [Google Scholar]
- 21.Kawase A, Yoshida J, Ishii G, et al. Visceral pleural invasion classification in non-small cell lung cancer. J Thorac Oncol 2010;5:1784-8. 10.1097/JTO.0b013e3181eedd9c [DOI] [PubMed] [Google Scholar]
- 22.Riquet M, Badoual C, Le Pimpec Barthes F, et al. Visceral pleura invasion and pleural lavage tumor cytology by lung cancer: a prospective appraisal. Ann Thorac Surg 2003;75:353-5. 10.1016/S0003-4975(02)04403-X [DOI] [PubMed] [Google Scholar]
- 23.Riquet M, Assouad J, Foucault C, et al. Visceral pleura invasion and lung cancer: further clarifications. Eur J Cardiothorac Surg 2004;25:471. 10.1016/j.ejcts.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 24.Tsuboi M, Ohira T, Saji H, et al. The present status of postoperative adjuvant chemotherapy for completely resected non-small cell lung cancer. Ann Thorac Cardiovasc Surg 2007;13:73-7. [PubMed] [Google Scholar]
- 25.Grønberg BH, Sundstrøm S, Kaasa S, et al. Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer 2010;46:2225-34. 10.1016/j.ejca.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. 10.1016/0003-4975(95)00537-U [DOI] [PubMed] [Google Scholar]
- 27.Kodama K, Higashiyama M, Takami K, et al. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg 2008;34:1068-74. 10.1016/j.ejcts.2008.07.044 [DOI] [PubMed] [Google Scholar]
- 28.Li M, Ito H, Wada H, et al. Pit-fall sign on computed tomography predicts pleural involvement and poor prognosis in non-small cell lung cancer. Lung Cancer 2004;46:349-55. 10.1016/j.lungcan.2004.05.017 [DOI] [PubMed] [Google Scholar]
- 29.Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. 10.1016/j.athoracsur.2011.05.091 [DOI] [PubMed] [Google Scholar]