Abstract
Using three examples drawn from animal systems, I advance the hypothesis that major transitions in multicellular evolution often involved the constitution of new cell-based materials with unprecedented morphogenetic capabilities. I term the materials and formative processes that arise when highly evolved cells are incorporated into mesoscale matter ‘biogeneric’, to reflect their commonality with, and distinctiveness from, the organizational properties of non-living materials. The first transition arose by the innovation of classical cell-adhesive cadherins with transmembrane linkage to the cytoskeleton and the appearance of the morphogen Wnt, transforming some ancestral unicellular holozoans into ‘liquid tissues’, and thereby originating the metazoans. The second transition involved the new capabilities, within a basal metazoan population, of producing a mechanically stable basal lamina, and of planar cell polarization. This gave rise to the eumetazoans, initially diploblastic (two-layered) forms, and then with the addition of extracellular matrices promoting epithelial–mesenchymal transformation, three-layered triploblasts. The last example is the fin-to-limb transition. Here, the components of a molecular network that promoted the development of species-idiosyncratic endoskeletal elements in gnathostome ancestors are proposed to have evolved to a dynamical regime in which they constituted a Turing-type reaction–diffusion system capable of organizing the stereotypical arrays of elements of lobe-finned fish and tetrapods. The contrasting implications of the biogeneric materials-based and neo-Darwinian perspectives for understanding major evolutionary transitions are discussed.
This article is part of the themed issue ‘The major synthetic evolutionary transitions’.
Keywords: morphological evolution, liquid tissues, diploblasty, triploblasty, tetrapod limb, reaction–diffusion mechanism
1. Introduction
The animals (Metazoa) are one of several dozen independently arising groups of multicellular organisms [1]. They emerged more than 600 million years ago (Ma) among cells belonging to a broader phylogenetic group, Holozoa, which also includes some present-day unicellular and transiently colonial forms. Comparative analysis of extant genomes indicates that the ancestral holozoan cells must have contained many thousands of genes [2], all of which were subject to mutation, with consequent variations in organismal phenotypes.
It is therefore curious that once multicellularity had been achieved in this group, animal body plans and organs came to exhibit a fairly limited number of stereotypic structural motifs—body layers and cavities, tubes and lobes, tandemly arranged repetitive elements, ensheathing exoskeletons, articulated endoskeleton and so forth [3,4]. These motifs arise during embryogenesis and are developmental outcomes of recurrent arrangements of embryonic tissues—non-mixing cell masses (‘germ layers’), lumens, tissue elongation with polarity (i.e. with structural or biochemical differences from one end to the other), segmentation, external or internal skeletogenic matrices and appendages that embody some of the same arrangements. With all the variability afforded by the collective genetic complement of animal cells, why is all animal life characterized by such a restricted set of forms and patterns?
It is the purpose of this article to show that within the Metazoa, what we recognize as ‘major transitions’ of animal form corresponded to explicit enhancements in the capability of cell collectives to generate novel classes of morphological motifs. Specifically, changes in the material properties of cell clusters at various stages of metazoan evolution made the clusters susceptible to morphogenetic and patterning processes based on mesoscale physical effects that were inoperative at prior stages of evolution [3,5]. These material changes, in turn, depended on the repurposing of genes present in ancestral unicellular organisms owing to the changed scale of the multicellular state, or acquisition of new genes, the products of which enabled the cell clusters and tissue masses to mobilize these new mesoscale effects.
The types of material changes considered here include (i) the transformation of cell aggregates into ‘liquid tissues’, that is materials which, like non-living liquids, have well-defined cohesivity and surface tension, and similarly to their non-living counterparts are capable of phase separating from other such materials; (ii) the consequences within the multicellular-context of polarization of surface properties and shapes of cells, which lead to the formation of interior spaces within, and elongation and other reshapings of cell masses, corresponding in many respects to the formation of micelles and liquid crystals by physically and geometrically polarized molecules and polymers; (iii) the outcomes of deposition of materials (extracellular matrices, ECM) by liquid tissues' subunit cells that induce solidification, spreading and disaggregation, similarly to the alloying, wetting and emulsification phase behaviours and transitions seen in non-living liquids interacting with non-liquid materials; (iv) symmetry breaking and pattern formation owing to the coupling between compositional changes in a tissue and molecular transport through it, corresponding to the reaction–diffusion effects seen in chemically reactive, permeable liquids. Materials exhibiting such properties (though not typically all at once) also exist outside biology, where they are known as ‘soft matter’ [6] and ‘excitable media’ [7,8].
For each of the listed phenomena, the physical effects can be described as ‘generic’ insofar as their dynamics and morphological consequences are similar (more than simply analogous) to those of non-living systems [9]. Because the subunits of liquid tissues are, however, living eukaryotic cells that are the products of several billion years of evolution prior to the emergence of the metazoans, I have termed the respective developmental processes ‘biogeneric’ [10]. The goal of this paper is twofold: first, to show how the organismal forms and patterns produced by biogeneric processes typically reflect those of the corresponding generic ones, and second, to indicate how, in metazoan cell clusters and tissues, single-cell biology becomes subordinated to mesoscale effects, so that morphological development and its evolution can be understood to a large extent by focusing on the biogeneric effects operating at the multicellular level.
According to this interpretation, when a biological material with new morphogenetic potential arose, the result was not simply a marginally different variant of a previously evolved structure (as in the conventional Darwinian narrative), or even the generation of a unique morphological novelty, but rather an array of forms that the new biogeneric material could manifest in concert with processes that were already present in progenitor cell clusters before the transition. The role of natural selection in this scenario, then, is not to build up morphological phenotypes gradually by preserving the better adapted outcomes of genes of small effect, but rather to cull among the often sharply different morphotypes generated by a newly emergent set of ‘physico-genetic’ [11] processes.
Many of the genes that came to be involved in multicellular biogeneric effects pre-existed the metazoans, and others that may have evolved gradually exerted their effects on morphology or pattern in an abrupt fashion owing to the frequently nonlinear properties of mesoscale physical effects. Thus, a straightforward relationship between genotype and phenotype does not generally hold in this picture. Moreover, as will be seen, some key genes (e.g. Wnt, Vang/Stbm, peroxidasin) appear in the inferred history of the animals with no precedent (at least under the current taxon sampling) either in holozoans or other eukaryotic or prokaryotic groups, further confounding the reconstruction of early evolutionary lineages.
While a variety of morphological motifs are compatible with multicellular life, not every new one represents, or arises along with, a major transition. Each of the emergent biogeneric properties described here, however, set the stage for new types of bodies and organs, including opportunities to enhance and refine physiological capabilities and to occupy novel ecological niches. For example, aggregation of unicellular organisms is not a major transition, but the constitution of liquid tissues, which affords the possibility of evolving multilayered and hollow embryos, is. Alteration of the physical state of tissues by secretion and deposition of ECMs qualitatively extends even further morphological variation and the capacity to construct novel two- and three-layered body plans and organ forms, with exo- and endoskeletons. Finally, in a major transition in the jawed vertebrates, the skeletal nodules, rods and plates of fins engendered the stereotypical proximodistally increasing array of elements of tetrapod limbs only when the earlier-evolved skeletogenic activators came under the control of one or another biogeneric reaction–diffusion mechanism.
2. ‘Liquid tissues’ and the first animals
Animal embryos attain multicellularity when cells that are clonally derived from a zygote—a fertilized egg—remain attached to one another by one or more members of the cadherin family of integral membrane proteins. (The name ‘cadherin’ reflects the fact that these proteins require sufficient levels of extracellular calcium ions for them to serve as cell adhesion molecules (CAMs) [12]). The cell-adhesive factors that mediate cell aggregation in non-metazoan holozoans, however, differ from those used by animal embryos. Monosiga brevicollis and Salpingoeca rosetta, members of Choanoflagellata (the non-metazoan holozoans most closely related to the animals), contain several genes that specify proteins called protocadherins [13]. These proteins, which are also found in metazoans, have cadherin-type extracellular domains that mediate homophilic cell–cell adhesion, but they lack the cytoplasmic domains of other (‘classical’) cadherins [12], exclusive to metazoans, that link the cell surface to cytoskeletal actin filaments. While M. brevicollis is unicellular, S. rosetta exhibits colonial forms but produces them by a mechanism that differs from the cadherin-based ones of metazoan embryos: retention of cytoplasmic bridges after division [14,15]. Capsaspora owczarzaki, a filasterean (the sister group to animals and choanoflagellates), also has multicellular life cycle stage [16,17], but cell–cell attachment in this group appears to be independent of cadherins, possibly mediated instead by the integrins [18], a different class of integral membrane proteins.
Morphogenesis of early-stage animal embryos depends on cells being independently mobile and capable of slipping past one another while remaining attached to each other collectively. In contrast to a non-living liquid's molecules that translocate by randomly directed Brownian motion, cells in liquid tissues translocate by the analogous effect of uncorrelated cytoskeleton-driven fluctuations of their surfaces, which lead (in the absence of tissue gradients) to random locomotion [19]. The cell masses or layers from which body plans and organs develop thus behave formally like liquids [5,20]. Tissues composed of cells that are attached via cytoplasmic bridges (as in S. rosetta) or (as is possibly the case for C. owczarzaki) via integrins (which typically bind to secreted extracellular matrix (ECM) materials), would lack the fluidity that accompanies transient contacts between cellular subunits. The emergence of liquid tissues, based on repurposing of the protocadherins in a common ancestor of Filasterea + Choanoflagellata + Metazoa, seems to have been a foundational step in the origination of the animals (figure 1, top two rows).
Figure 1.
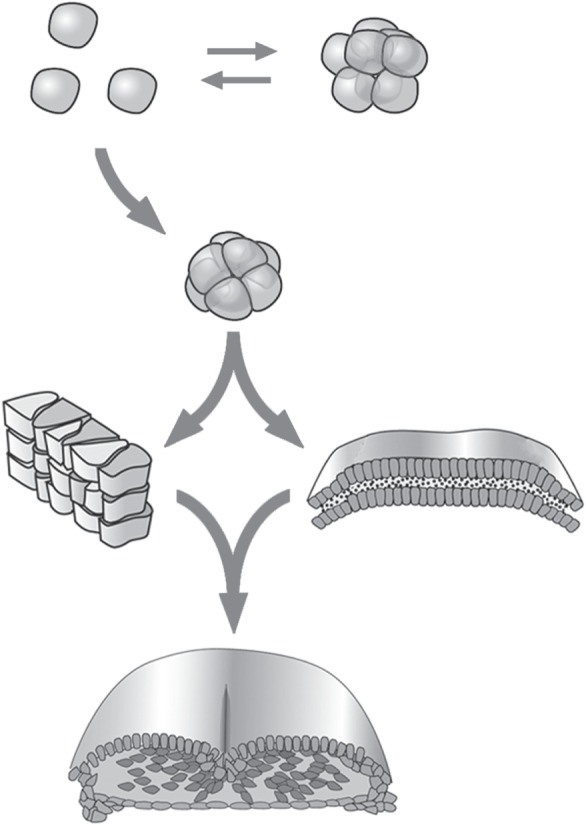
Major morphological transitions in the early history of the animals mediated by the emergence of novel biogeneric material properties. Ancestral unicellular holozoans (top row) had adhesive protocadherins and other cell surface molecules that permitted some species to form transient colonies. Certain subpopulations of these cells acquired DNA sequences specifying both Wnt, a secreted protein that induces A/B polarization, and the cytoskeleton-binding domain of the animal-specific classical cadherins. Together, these molecular novelties acted in cell aggregates to coordinate cell–cell adhesion with intracellular mechanics, constituting these clusters as cohesive ‘liquid tissues’ (second row), with the capacity to form lumens and undergo transient multilayering, forming body plans with features of present-day sponges and placozoans. Among these early emerging metazoans, some further acquired the capacity for tissue reshaping, such as elongation by convergent extension (third row, left), based on the PCP pathway elicited also by Wnt, but using novel mediators such as Vang/Stbm. Some of these organisms also acquired the ability to form stably layered sheet-like tissues (third row, right) and hence epithelial appendages, due to the presence of the novel enzyme peroxidasin which cross-links type IV collagen into a stiff, flexible basal lamina (stippling). Both PCP and a basal lamina are present in all extant eumetazoans, the morphologically simplest of which, the ctenophores and the cnidarians, are referred to together as ‘diploblasts,’ although their phylogenetic affinity is obscure. In some diploblastic forms, novel extracellular matrix molecules (galectins, TSR superfamily proteins, fibronectin) were acquired that elicited epithelial–mesenchymal transformation (EMT) in one or another of the two basic tissue layers during development, leading to an intermediate layer, the mesoblast, constituting the resulting animals as ‘triploblasts’ (fourth row; a developing bird embryo is represented). All of these triploblastic forms are bilaterally symmetric during at least part of their life cycles, possibly owing to the geometrical constraints of three-layered development, leading them to also be referred to as ‘bilaterians’. The phylogenetic affinities among many triploblastic groups and their genealogical relationships to extant diploblasts are unclear. A generalization that can be derived from this perspective is that certain major animal body plan categories have unambiguous morphological hallmarks that can be directly attributed to the material properties of their constituent tissues. These material properties, in turn, often depend straightforwardly on the consequences of the mobilization of new physical forces and effects by the products of novel genes [3,4].
This repurposing of ancestral cadherins may have depended in part on a change in the external environment, e.g. an increase in the concentration of calcium ions [21–23] which would have converted proteins that evolved in association with single-cell functions (substratum attachment, prey capture) into ones that mediated homophilic attachment. But while, as mentioned, animal phyla retain genes that specify protocadherins, all of them, from the early-branching Placozoa and Porifera (sponges) through the more complex eumetazoans (diploblasts and triploblasts), also have classical cadherins. The evolution of these proteins required the molecular innovation of a cytoplasmic domain (not encoded in the genome of M. brevicollis [24,25]) that enables them to interact with cytoskeletal linking elements including β-catenin and vinculin [12]. Cell–cell adhesive strength in present-day tissues is controlled by the density of cadherin extracellular domains on the adjacent cells, as well as by the tension of the submembrane cell cortex (mediated by the actin cytoskeleton) which affects the extent of contact between the adjoining cell surfaces [26]. The synergy between these effects afforded by classical cadherins defines the liquid state peculiar to developing animals and by inference, their evolutionary prototypes.
When liquids are immiscible (oil and water, for example), sharp interfaces form between them, with contours shaped by their relative cohesivity. A mixed suspension of droplets will sort out into distinct phases. Similar phase-separation effects are responsible for the establishment of the two or three germ layers of animal embryos, and randomized mixtures of cells from different germ layers will also sort out. While cohesivity of physical liquids depends on attractive or repulsive molecular interactions, the cohesivity of tissues depends not only on the affinity or repulsion of cell surface molecules, but also on internal cellular properties, including cortical tension, mentioned above. This active aspect of liquid tissue behaviour is not analogous to any property of non-living liquids [27–29], making these materials biogeneric rather than making simply generic.
The morphological consequences of the constitution of liquid tissues show that this event was indeed a major transition in the history of life. It is likely that some of the simple cell clusters seen in the Precambrian Ediacaran deposits beginning about 630 Ma and identified as ‘embryos’ [30] were ‘droplets’ of such liquids. However, these fossil beds also contain sheet-like and hollow spherical forms [31], and budding and segmented tubes [32]. This suggests that the subunits of these liquid tissues—the cells—exhibited polarity, in the sense of having different properties on different regions of their surfaces.
When amphiphilic polymers (e.g. ones that are charged at one end and hydrophobic at the other) are present in aqueous media, they tend to form liposomes or micelles [33]. The molecules self-assemble in an energetically favourable configuration in which they come to surround a fluid-filled interior space. Analogously, a mass of cells polarized with respect to the surface expression of adhesive proteins will spontaneously self-organize, so that the less adhesive regions will enclose a lumen [34,35]. The forces that bring about this topological change are the same differential interfacial tension effects, described above, that cause a cell aggregate of lower cohesivity to surround a more cohesive liquid or cell aggregate [5].
The kind of polarity that enables cells to organize tissues with interior spaces (cysts, tubes) is called apicobasal (A/B) polarity. This cellular feature predated metazoan evolution. In brewers yeast, a member of Opisthokonta, a phylogenetic group that contains the holozoans as well as the fungi, the cytoplasmic protein Mo25 is essential for elongating growth and regulation of cell division [36]. This protein also exists in animals, where it mediates A/B-related cell shape changes during embryogenesis [37]. In regulating cell polarity, Mo25 operates in conjunction with the enzyme Lkb1 [38], which not only has a homologue in brewers yeast, but also in the more distantly related social amoebae Dictyostelium discoideum, where it is also involved in morphogenesis [39].
While ancestral unicellular organisms were capable of undergoing A/B polarization employing the same molecular toolkit that drives this process in animal cells, all extant metazoans have in addition the important distinction of containing one or more genes for the secreted protein Wnt. This factor induces the activities of both Mo25 and Lkb1, and because it is a diffusible morphogen, leads to a coordinated polarization response across a cell cluster or local tissue domain. Wnt is found only in Metazoa; there are no homologues of it in other sequenced groups. (This is in contrast to another conserved morphogen of the metazoans, hedgehog, which has deep roots in the unicellular eukaryotes [40].) There were very probably a series of evolutionary steps (in whichever direction) between the constitution of metazoan liquid tissues (defined by the presence of classical cadherins) and the appearance of Wnt, but this cannot be discerned from the analysis of any present-day genomes or the fossil record.
Because, as noted, liquid tissues can phase-separate by regional differences in cohesivity, and this can vary with internal cell states, adjoining portions of a cell mass can become immiscible if the density of cadherins on cell surfaces, or cortical tension, are modulated in a spatially dependent fashion by a molecular gradient, a phenomenon readily produced by a variety of biogeneric processes [5]. In fact, all known animals, from the early-branching placozoans and sponges, through the diploblasts, and the triploblasts, are at least transiently multilayered at one or another stage of their life cycles.
The reinforcement of such layering occurs by additional mechanisms, some of which are more limited in their phyletic distribution. One mechanism of reinforcement is dynamical, involving the Notch pathway found in every animal phylum with the exception of Placozoa (based on the single representative, Trichoplax adhaerens). The other is structural, involving a mechanically stable planar ECM. This second mechanism is absent in both the placozoan and most sponges, but present in all other animal groups. It was associated with a second major transition in the evolution of life forms and is the subject of §3.
The Notch signalling pathway is the main means by which a cell exerts a direct effect on a second cell with which it is in contact, forcing the latter to take on an alternative differentiated state. Notch is a cell surface receptor for a class of other integral membrane proteins that act as its ligands: Delta, Serrate (or Jagged) and Lag2 (the DSL-class proteins) [41]. Activation of Notch results in the translocation of an intracellular fragment of it to the nucleus, where it acts as a transcriptional co-regulator of certain transcription factors, changing them from repressors to activators. A variety of transcription factors come under Notch's influence, in a wide range of developing tissues. Thus, the pathway does not determine the specific fate of a cell, but rather causes the cell to choose one of two of its potential fates.
DNA sequences encoding portions of metazoan Notch receptors are present in M. brevicollis, although not in the same gene [25]. Once the full pathway was assembled in the ancestors of sponges (Delta is present in all metazoans including the placozoan [42]), it enabled (by enforcing alternative gene expression states in adjoining cells with a common genome), the regulated coexistence of multiple cell types within a common tissue mass. These states potentially include adhesive differences, which even though established in a local fine-grained fashion by the Notch–DSL lateral inhibition mechanism, will lead to sorting out into distinct tissue layers by virtue of the biogeneric liquid behaviours described above.
One additional consequence of the constitution of embryonic tissues as biogeneric liquids was their capability to behave like liquid crystals. While not seen in sponges or the placozoan, which lack the enabling gene products, this is a characteristic morphogenetic mode of all diploblastic and triploblastic phyla, where it is associated with body, appendage and organ elongation (see §3). The default shape of a cluster of cells, like that of a droplet of a typical liquid, is spherical (or if attached to a substratum, hemispherical), owing to surface tension and the shape isotropy of the constituent cells or molecules. For certain liquid crystal-forming polymers or anisotropic nanoparticles, however, the resting shape of the droplet becomes elongated in one direction [43,44]. Cells are similarly capable of becoming anisotropic in shape, an effect known as planar cell polarization ((PCP); figure 1, third row). PCP is elicited in animal embryos by Wnt, but (in contrast to A/B polarization) acting through other (‘non-canonical’) cytoplasmic intermediates such as the membrane protein Van Gogh/Strabismus (Vang/Stbm) [45], which is only present in the eumetazoans. Cells oriented by PCP can align and intercalate with one another, leading to a narrowing of the tissue mass in the direction of intercalation accompanied by elongation in the orthogonal direction [46]. This effect is seen in germ-band extension in insects such as Drosophila [47] and convergent extension in vertebrates, each of which establishes the elongated body axis during gastrulation [48], and a variety of other morphogenetic processes [49]. Although this mode of behaviour of liquid tissues is a novel property that enhances the morphogenetic capabilities of the organisms that exhibit it, it does not appear to define a major evolutionary transition in its own right. However, all extant organisms exhibiting PCP also have true epithelia and vice versa, for reasons currently not understood, and the emergence of the group that contained both features was indeed a newly complex form of life.
3. Extracellular matrices, diploblasty and triploblasty
The development of phylum-specific body plans is initiated in eumetazoan embryos by the formation of adjoining but non-mixing germ layers: two in diploblasts such as ctenophores and cnidarians, and three in triploblasts such as arthropods, chordates and most other animal phyla.1 The early-branching metazoans either do not exhibit a layered organization or exhibit ones that are not stably so. The placozoan T. adhaerens is a flattened gel-filled sac. Although the dorsal cells are of a different type from the ventral cells, with still another type sparsely scattered in the intervening matrix [53], the sac itself is unilaminar. Most sponge species (those of the demosponge class) have several cell types that reside in a soft ECM, the mesohyl, and organize in a transient fashion to define labyrinthine spaces and channels. They lack the apposed cell layers, including a true epithelium, of eumetazoans [54]. (The homoscleromorph sponges, a minority class, provide a partial exception to this [54]).
The formation of a sharply defined second layer was the foundation of the first major evolutionary transition in animal life following the constitution of liquid tissues. It is the basis of ‘gastrulation’ in present-day diploblastic and triploblastic forms [55]. Multilayering provides a platform for a profusion of body plans and occurs in several different manners with varying degrees of stability, each dependent on phase-separation behaviours of the liquid tissue state: in topologically solid embryos, (i) an internal mass of cells can acquire greater cohesivity than the surrounding layer and pull away from it (‘delamination’), or (ii) a less cohesive cell mass comes to envelop a more cohesive one (‘epiboly’), or (iii) curls in on itself over the latter (‘involution’); in embryos with interior spaces (which can form spontaneously by cells with A/B polarity), (iv) inward folding of the surface layer (‘invagination’), or (v) migration of a subset of surface cells (‘ingression’) can give rise to a new layer.
Diploblastic ancestors appeared first, giving rise, probably via independent lineages [56], to present-day cnidarians and ctenophores. They have ‘basal laminae’, organized planar ECMs that underlie and enable the formation of cell sheets (figure 1, third row). An essential component of the basal lamina is type IV collagen. Although T. adhaerens [42] and some sponges [57] express portions of this protein, they either lack the enzyme peroxidasin, which in eumetazoans converts certain amino acid residues of type IV collagen into sulfilimine cross-links, or their type IV-like collagen domains lack the residues themselves [58]. Recent work indicates that the evolutionary confluence of the enzyme and its protein substrates, which produced a mechanically stable planar scaffold, was a key step in the innovation of true epithelia and diploblasty, and essential to organogenesis [58]. While the presence of a stiff basal lamina renders epithelia elastic when undergoing out-of-plane deformations, the cells themselves retain in-plane fluidity, at least during developmental stages, accounting for the unique morphogenetic properties of these tissues [59].
Triploblasts are thought to have arisen from diploblastic ancestors [60]. One of the two germ layers (in present-day forms usually the endoderm) outpockets, separates or disaggregates into a third germ layer (figure 1, fourth row). The evolution of the disaggregating mode, which is seen in chordates, arthropods and many other phyla, appears to have occurred via the addition of molecules to the mesoglea that promoted ’epithelial–mesenchymal transformation‘ (EMT) [61] of the endodermal tissues, followed by cell ingression into this middle zone during gastrulation [62]. Some proteins (e.g. fibronectin and tenascin in chordates) that promote formation of a third, mesodermal layer are phylum-specific innovations [63], whereas others have deeper roots in triploblast evolution. For example, the thrombospondin type 1 repeat (TSR) superfamily of proteins has members throughout the animal phyla and in unicellular holozoans [64], but none in prokaryotes, yeast or plants [65]. Functions of some TSR proteins include stimulation of migration; others mediate adhesion or breakdown of the ECM. Papilin and ADAMTR, two TSR proteins, have reciprocal roles in Drosophila gastrulation [66], and other members of the superfamily have complementary functions in early nematode development [67].
Galectins, endogenous carbohydrate-binding proteins present in most metazoan phyla [68] and some multicellular fungi (mushrooms) [69], but in neither unicellular holozoans nor in yeast, may have been involved in the origination of the triploblastic body plan. Interference with galectins leads to the loss of the primitive streak in the early-stage chicken embryo [70], and exogenous administration or degradation of the sugar ligand for a putative endogenous sea urchin lectin dramatically perturbs gastrulation in that organism [71]. Like multilayering, lumen formation and body elongation, triploblasty may thus have arisen (perhaps more than once) by the mobilization of physical organizational effects by a small number of pre-existing gene products (and perhaps a few novel ones), acting in new contexts.
The specific biogeneric liquid character of the cell clusters of primordial metazoans and of early-stage animal embryos is undermined in mesenchymal tissues. Although mesenchymes and connective tissues may retain some fluidity and malleability during development, their material properties are dominated instead by the compositional and organizational properties of their ECMs. At later stages of development, the patterning and morphogenesis of exo- and endoskeletal structures are driven by physical determinants different or additional to those pertaining to liquid tissues (see §4).
As mentioned, the PCP pathway is exclusively found in the eumetazoans among the present-day phyla, although there is no apparent connection at the molecular level between this pathway and the presence of a basal lamina. Because planar polarization of cells facilitates intercalation and convergent extension in layered tissues, elongated bodies and appendages are found in essentially all eumetazoans. In triploblasts, the interpolation of a middle layer is typically associated (possibly for purely mechanical reasons) with a flattening of the elongating body, resulting in a bilaterally symmetric form, which is sometimes manifested only at embryonic or larval stages. The triploblasts are thus coincident with the bilaterians.
With triploblasty the consequences of the major evolutionary transition first marked by the formation of distinct germ layers in diploblasts were realized in a dramatic fashion. Triploblasts comprise the majority of animal phyla and have more complex body plans than diploblasts. This complexity is built on the topological separation of an internal tubular primordium from the body wall, which then became the locus of specialized structures: digestive, cardiovascular, pulmonary, urogenital and other organs. The interaction between the in-plane fluid epithelium, with its elastic, planar substratum and the typically mesenchymal mesoderm, with ECM of varying consistency, from watery to stiff, between its loosely packed cells, enabled a range of morphological motifs not possible with either type of tissue in isolation. The rise of triploblasty from diploblastic ancestors thus constitutes a major evolutionary transition.
4. Reaction–diffusion processes in living tissues: the fin-to-limb transition
The final major evolutionary step considered here is the fin-to-limb transition of the jawed vertebrates (gnathostomes). This is the process that led to the evolutionary appearance in an ancestral fish of the characteristic limbs of tetrapods. As in the emergence of Metazoa and of the eumetazoans, the liquid tissue properties of developing animal tissues were in play, but in this case not in the generation of morphological motifs of the main body but in those of the paired appendages. Furthermore, the developing tissue mass in this case participates in the developmental process and its evolutionary manifestations not simply as a viscoelastic material, but as a chemically permeable and reactive, and mechanically compressible ‘excitable medium’.
The body plans of jawed vertebrates are characterized by paired appendages, fins or limbs. These appendages (which were secondarily lost in snakes and some other species) are shaped, as epithelial–mesenchymal extensions of the body wall, by liquid and elastic tissue properties and processes similar to those that generate the triploblastic body [72,73]. However, in addition, they contain various arrangements of ‘endoskeletal’ elements—nodules, plates, parallel rods—that arise from cartilaginous primordia and are typically replaced by bone during late development, in addition to skeletal structures that arise independently of cartilage (figure 2).
Figure 2.
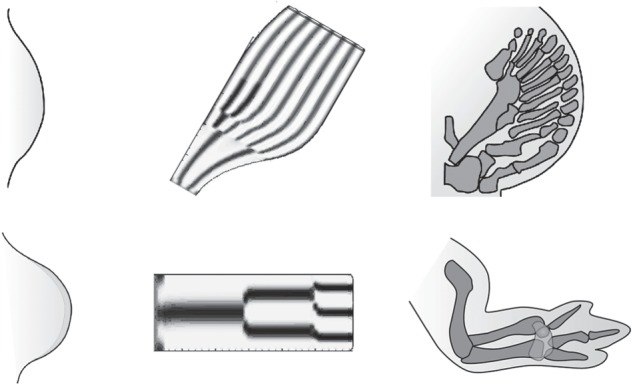
Gnathostome paired appendage development and hypothesized Turing mechanism for skeletal pattern formation. Early-stage embryonic limb buds (left column) and late-stage embryonic skeletons (right column) are shown for two gnathostomes, the Japanese catshark, a cartilaginous fish (top row) and the chicken, a tetrapod (bottom row). Morphogenetic toolkit molecules have been shown experimentally to interact in Turing-type reaction–diffusion networks in the limb bud mesenchyme of several tetrapod species, where they mediate patterned skeletogenesis. This suggests that ancestral fin tissues may have contained primitive versions of such networks that became ‘tuned’ over the course of evolution to generate the stereotypical limb skeletons of tetrapods. The middle column shows the results of computer simulations of a reaction–diffusion network with parameter choices showing, in principle, that the distinctive patterns of distantly related gnathostomes can be generated from a single patterning mechanism with no change in network topology [74].
In the sarcopterygians, which include lobe-finned fish such as the coelacanth, as well as all extant tetrapods and their direct ancestors, the appendicular endoskeleton typically exhibits increasing numbers of elements along the proximodistal (body wall-to-digit-tip) axis. In the tetrapods themselves, the pattern is more narrowly stereotypical, usually with an arithmetic increase in the number of parallel elements. A single element (the humerus or femur, referred to generically as the ‘stylopod’) is attached directly to the body, typically followed by two elements (the radius and ulna, or tibia and fibula: the ‘zeugopod’) and a species- or limb type-characteristic number of wrist elements followed by fingers or toes (the ‘autopod’) [75].
The tetrapod limb skeleton is now recognized as being patterned by a mechanism related to the chemical reaction–diffusion process described by the mathematician Alan Turing [76,77] (figure 2). Turing's analysis demonstrated that a balance of positive and negative feedbacks in an open chemical system, when coupled with differences in the rates of diffusion of the key reactive molecules, could lead (counterintuitively), to stable, non-uniform concentration patterns, often exhibiting periodicities [78]. Later versions of this model [79–81] indicated how both ‘reaction’ and ‘diffusion’ could be realized biogenerically.
Several Turing-type mechanisms have been advanced for the tetrapod skeletal patterning networks. In the mouse, the spatio-temporal expression of the cartilage master transcription factor Sox9 in vitro and in vivo depends on its dynamical interaction with the two toolkit morphogens Bmp2 and Wnt (the ‘BSW network’) [82]. In the chicken, galectin-1a and galectin-8, two members of the galectin family of carbohydrate-binding proteins that may have also mediated some forms of triploblasty (see §3), form a multiscale skeletal pattern formation network [83,84]. The extent to which the various tetrapod classes share these two reaction–diffusion patterning mechanisms is as yet unknown.
The ancestry of the galectin-based limb patterning network suggests an intriguing scenario for the fin-to-limb transition. A skeletogenic paralog of galectin-1, which (as galectin-1a) is the component of the avian reaction–diffusion network that mediates limb chondrogenesis [83], first appeared in basal gnathostomes and its structure has been conserved by purifying selection in all descendant clades [85]. This is consistent with the fins of all jawed vertebrates containing endoskeletal elements [75]. Galectin-8, the network component that blocks the cartilage-inductive effect of galectin-1, can be traced back to invertebrate chordates. Its three-dimensional structure (which determines its inhibitory function in the two-galectin network) was retained by purifying selection throughout the sarcopterygians, but not in ray-finned fish. In the sarcopterygians, moreover, the galectin-8 gene acquired new cis-regulatory motifs with binding sites for what were later limb-associated transcription factors [86]. Although these data do not permit strong inferences regarding the fin-to-limb transition, the following scenario is consistent with the available information: (i) a galectin-1–galectin-8 regulatory network in cartilaginous fish and their ancestors are/were capable of generating the repeated parallel cartilage elements and plates in the fins of those species; (ii) the common ancestor of those fish with the ray-finned fish and sarcopterygians diverged by protein- and gene-regulatory element evolution to produce a galectin-1–galectin-8 network in the former (ray-finned) group that generates fin cartilaginous elements less regularly arrayed and less consistent from species to species than in the cartilaginous fish, and in the latter (sarcopterygian) group, the propensity to produce small numbers of parallel elements in a highly controlled fashion [83,84,86].
In summary, this major transition late in animal evolution, like the early ones described in the previous sections, was based on the constitution of a tissue with new biogeneric properties. In this case, the novelty was a reaction–diffusion–adhesion network with narrowly stereotypical patterning outcomes, which contrasted with the loosely organized networks specifying the more variable species-idiosyncratic limb endoskeletons of ancestral and divergent modern groups.
5. Conclusion
Using three examples, I have put forward the general hypothesis that certain major transitions in the evolution of the animals were based on the constitution of new biogeneric materials and their associated dynamical processes. I have focused on (i) the emergence of the animals, which involved the origination, from populations of unicellular holozoans, of ‘liquid tissues’; (ii) the addition of planar and interstitial ECMs to some ancestral metazoan lineages, thus giving rise to diploblastic and triploblastic animals; and (iii) the establishment within a liquid-tissue appendage (the ancestral gnathostome fin) of a reaction–diffusion system based on a pair of galectin-family carbohydrate-binding proteins, which generates patterns typically consisting of arrays of small numbers of parallel endoskeletal elements.
Each of these transitions occurred with a degree of complexity not entirely captured by these summary descriptions. Regarding the emergence of the liquid-tissue state, what was involved was not simply the aggregation of previously free-living holozoan cells into cohesive clusters with randomly perambulating subunits. Such assemblages would indeed have some liquid characteristics, but not the biogeneric liquid properties found in all developing animal tissues. What these latter tissues embody in addition are classical cadherins that link cell–cell attachment to the cytoskeleton, thus making adhesion and its modulation dynamical processes, dependent on the cells' internal states. All animals also produce a morphogen, Wnt, which regulates (via the cytoskeleton) the distribution of cell surface molecules, thus enabling cells to self-organize into liquid tissues with interior lumens. The molecular evolutionary steps leading to these defining features of animals, and the order of their appearance in ancestral forms, are obscure.
Regarding the origins of the defining characteristics of eumetazoans—the ECMs of epithelial basal membranes and mesenchymal interstitiums, respectively—there are some hints in the genomes of extant unicellular holozoans (and thus their inferred common ancestors with the animals) of sequences specifying domains that eventually came to be found in laminin, collagens and fibronectin, though without key metazoan functional signatures [25,64]. The appearance in certain metazoan lineages of the type IV collagen cross-linking enzyme peroxidasin was constitutive of the out-of-plane elasticity of epithelia, and consequently of morphogenesis [58]. Analogous acquisitions and transformations of ancient secreted proteins enabled the formation of the invasion-receptive and EMT-promoting ECMs conducive to a third germ layer. Concomitant with a basal lamina, all eumetazoans have the capability of undergoing PCP and, consequently, tissue reshaping mediated by the non-canonical, Van/Stbm-dependent branch of the Wnt pathway. Because, as noted, there is no evident functional or molecular connection between these two features, their association suggests that there may have been a bottleneck at the origination of the eumetazoans. Whichever appeared first, the basal lamina or the PCP pathway, the second may have been the ‘killer app’ that permitted the novel lineage to persist and move forward. In contrast, the existing evidence on the mechanisms and enabling ECMs of the triploblastic state is consistent with polyphyly of this anatomical arrangement. There is currently little independent evidence to evaluate this possibility, however.
Another interesting, but conceptually complicating, phylogenetic coincidence is the presence in all eumetazoan phyla except echinoderms, but not in sponges or the placozoan, of pannexins [87]. The gap junctions mediated by this family of proteins (which are unrelated to the connexins, found only in chordates) convert cell clusters with the foundational liquid properties of all animal tissues into yet another type of biogeneric material, one which is electrically integrated, with a qualitatively new suite of regulative and regenerative capabilities for promoting the stability of its morphological attributes [88,89].
With respect to the tetrapod endoskeleton, the formation of its elements involve not only liquid-tissue effects, but also reaction–diffusion instabilities, local increases in cell density (‘precartilage condensation’), and tissue solidification (chondrogenesis and osteogenesis). Much remains to be learned about this system, including whether the pattern-forming mechanism is embodied in the same gene regulatory network (e.g. the BSW versus two-galectin networks) in all tetrapods, and how the developing limb's gradients of Hox transcription factors modulate the Turing-type system to generate elements that are biologically customized rather than the uniform stripes dictated by purely generic reaction–diffusion mechanisms [90,91]. What seems clear, however, is that the range of skeletal patterns seen throughout sarcopterygian evolution and in experimental and mutational variants in present-day tetrapods is consistent with determination by a Turing-type system under different parameter regimes [74].
This latter observation, in fact, pertains to each of the examples discussed in this paper. Once a novel biogeneric material has been constituted, either as a result of a mutation in a predecessor material's components that leads to a qualitative change in its physical properties [92], by horizontal gene transfer bringing in a new ingredient [93], or as a side-effect of gradual evolution for something else [94], it will immediately entail a characteristic, and in some cases unprecedented, set of morphogenetic possibilities. Highly disparate phenotypic outcomes of a developmental system containing this new material can be adjacent to one another in the system's dynamical space [95], implying that one form can change into another with no intermediates. Subsequent evolutionary steps in the relevant lineages may thus be decoupled from the commonly assumed gradual selection for adaptive advantage. Instead, previously non-existent ecological niches may have simultaneously arisen with the abrupt appearance of new biological entities [96], generating new ways of life along with the novel phenotypes.
Consider the transformations in animal evolution described here: (i) unicellular holozoans giving rise to multicellular liquid tissues; (ii) basal metazoans giving rise to hollow, elongated, epithelial appendage-bearing diploblasts; (iii) diploblasts giving rise to transiently or constitutively bilaterally symmetric, complex-organ-bearing triploblasts; (iv) gnathostome fins with species-idiosyncratic arrangements of endoskeletal elements giving rise to tetrapod limbs with stereotypical arrays of small but proximodistally increasing numbers of elements. Each of these can be understood in terms of the novel morphogenetic capabilities of newly constituted biogeneric materials. In each case, moreover, the morphological novelties afforded by these partially unprecedented developmental systems led to the occupation of new ecological niches without superseding the organismal types whose development was based on earlier-evolving material properties. (In particular, the biosphere still contains non-triploblastic metazoans, as well as non-tetrapod gnathostomes.) This is very different from the widely accepted view that holds that natural selection, operating on marginally fitter forms over many repeated cycles, is the primary driver of macroevolutionary change.
As the reality of evolution came to be recognized in the nineteenth century, hierarchical schemes for the ‘grades’ of organisms relative to their morphological complexity (primitive to advanced) were propounded, such as Haeckel's ‘Gastrea’ theory based on his recapitulationism, embodied in his famous ‘tree of life’ diagram [97,98]. The rise of a strong theoretical commitment in the twentieth century to the function-related opportunism of natural selection led evolutionists such as Stephen Jay Gould to reject the hierarchical view of the history of life as a remnant of the pre-Enlightenment ‘great chain of being’, and to favour instead a ramifying bush-like representation of the relationships among organisms [99]. Taken together with the comparable age of all extant lineages, the neo-Darwinian tenet that evolution never ceases transforming phenotypes has made references to ‘primitive’ and ‘advanced’ characters unfashionable, if not outright taboo.
Notwithstanding these insights, the implication of the analysis presented in this paper is that, at least concerning the early evolution of the animals and certain of their organs, the hierarchical picture contained an important truth (see also [100]). However long the evolutionary history of sponges has been, for example, their bodies bear the signatures of a more primitive form of biogenetic matter than the bodies of jellyfish. Similarly, in successive steps of the hierarchy, with jellyfish versus arthropods, and salmon fins versus bat wings. By focusing on how the conserved toolkit genes of multicellular organisms actually mobilize physical effects to drive morphological development, rather than (as per the standard narrative) as indifferent determinants whose mutations can bring about any arbitrary outcome, it is possible to formulate a theory of the major transitions of morphological evolution that has explanatory power and causal coherence.
Endnote
The validity of the terms ‘diploblast’ and ‘triploblast’ has been questioned owing to ambiguities in defining these terms, as well as problems of excluding all types of sponges from the former category, or possibly including some types of cnidarians in the latter [50]. This issue is also tied up with uncertainties in the cladistics of early-branching groups (the two mentioned, as well as ctenophores [51,52]). The physico-genetic perspective adopted here favours the use of structural descriptors (lumens, segments, number of layers) rather than those based on functional features (e.g. presence of muscle or nerves) or symmetries (e.g. radiolarian, bilaterian). These descriptors acquire precision when they are tied (as done here) to specific toolkit genes and their functions. While the presence or absence of the respective genes may not map exactly to generally accepted clade assignments, they may themselves prove helpful in the resolution of controversial phylogenetic branching topologies.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Nielsen C. 2012. Animal evolution: interrelationships of the living phyla, 3rd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Torruella G, Derelle R, Paps J, Lang BF, Roger AJ, Shalchian-Tabrizi K, Ruiz-Trillo I. 2011. Phylogenetic relationships within the Opisthokonta based on phylogenomic analyses of conserved single-copy protein domains. Mol. Biol. Evol. 29, 531–544. ( 10.1093/molbev/msr185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman SA, Bhat R. 2008. Dynamical patterning modules: physico-genetic determinants of morphological development and evolution. Phys. Biol. 5, 15008 ( 10.1088/1478-3975/5/1/015008) [DOI] [PubMed] [Google Scholar]
- 4.Newman SA, Bhat R. 2009. Dynamical patterning modules: a ‘pattern language’ for development and evolution of multicellular form. Int. J. Dev. Biol. 53, 693–705. ( 10.1387/ijdb.072481sn) [DOI] [PubMed] [Google Scholar]
- 5.Forgacs G, Newman SA. 2005. Biological physics of the developing embryo. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.de Gennes PG. 1992. Soft matter. Science 256, 495–497. ( 10.1126/science.256.5056.495) [DOI] [PubMed] [Google Scholar]
- 7.Mikhailov AS. 1990. Foundations of synergetics I. Berlin, Germany: Springer. [Google Scholar]
- 8.Levine H, Ben-Jacob E. 2004. Physical schemata underlying biological pattern formation-examples, issues and strategies. Phys. Biol. 1, P14–P22. ( 10.1088/1478-3967/1/2/P01) [DOI] [PubMed] [Google Scholar]
- 9.Newman SA, Comper WD. 1990. ‘Generic’ physical mechanisms of morphogenesis and pattern formation. Development 110, 1–18. [DOI] [PubMed] [Google Scholar]
- 10.Newman SA. 2014. Physico-genetics of morphogenesis: the hybrid nature of developmental mechanisms. In Towards a theory of development (eds Minelli A, Pradeu T), pp. 95–113. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Newman SA. 2012. Physico-genetic determinants in the evolution of development. Science 338, 217–219. ( 10.1126/science.1222003) [DOI] [PubMed] [Google Scholar]
- 12.Halbleib JM, Nelson WJ. 2006. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 20, 3199–3214. ( 10.1101/gad.1486806) [DOI] [PubMed] [Google Scholar]
- 13.Nichols SA, Roberts BW, Richter DJ, Fairclough SR, King N. 2012. Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/β-catenin complex. Proc. Natl Acad. Sci. USA 109, 13 046–13 051. ( 10.1073/pnas.1120685109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayel MJ, Alegado RA, Fairclough SR, Levin TC, Nichols SA, McDonald K, King N. 2011. Cell differentiation and morphogenesis in the colony-forming choanoflagellate Salpingoeca rosetta. Dev. Biol. 357, 73–82. ( 10.1016/j.ydbio.2011.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayel MJ, King N. 2014. Prey capture and phagocytosis in the choanoflagellate Salpingoeca rosetta. PLoS ONE 9, e95577 ( 10.1371/journal.pone.0095577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebé-Pedrós A, Irimia M, Del Campo J, Parra-Acero H, Russ C, Nusbaum C, Blencowe BJ, Ruiz-Trillo I. 2013. Regulated aggregative multicellularity in a close unicellular relative of metazoa. Elife 2, e01287 ( 10.7554/eLife.01287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Trillo I. 2016. What are the genomes of premetazoan lineages telling us about the origin of Metazoa? In Multicellularity (eds Niklas KJ, Newman SA), pp. 171–184. Cambridge, MA: MIT Press. [Google Scholar]
- 18.Sebé-Pedrós A, Róger AJ, Lang FB, King N, Ruiz-Trillo I. 2010. Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc. Natl Acad. Sci. USA 107, 10 142–10 147. ( 10.1073/pnas.1002257107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghi N, Lowndes M, Maruthamuthu V, Gardel ML, Nelson WJ. 2010. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc. Natl Acad. Sci. USA 107, 13 324–13 329. ( 10.1073/pnas.1002662107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg MS, Poole TJ. 1982. Liquid behavior of embryonic tissues. In Cell behavior (eds Bellairs R, Curtis ASG), pp. 583–607. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 21.Fernàndez-Busquets X, Körnig A, Bucior I, Burger MM, Anselmetti D. 2009. Self-recognition and Ca2+-dependent carbohydrate-carbohydrate cell adhesion provide clues to the Cambrian explosion. Mol. Biol. Evol. 26, 2551–2561. ( 10.1093/molbev/msp170) [DOI] [PubMed] [Google Scholar]
- 22.Kazmierczak J, Kempe S. 2004. Calcium build-up in the Precambrian sea: a major promoter in the evolution of eukaryotic life. In Origins (ed. Seckbach J.), pp. 329–345. Dordrecht, The Netherlands: Kluwer. [Google Scholar]
- 23.Petrychenko OY, Peryt TM, Chechel EI. 2005. Early Cambrian seawater chemistry from fluid inclusions in halite from Siberian evaporites. Chem. Geol. 219, 149–161. ( 10.1016/j.chemgeo.2005.02.003) [DOI] [Google Scholar]
- 24.Abedin M, King N. 2008. The premetazoan ancestry of cadherins. Science 319, 946–948. ( 10.1126/science.1151084) [DOI] [PubMed] [Google Scholar]
- 25.King N, et al. 2008. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451, 783–788. ( 10.1038/nature06617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maître JL, Berthoumieux H, Krens SF, Salbreux G, Jülicher F, Paluch E, Heisenberg CP. 2012. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338, 253–256. ( 10.1126/science.1225399) [DOI] [PubMed] [Google Scholar]
- 27.Amack JD, Manning ML. 2012. Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science 338, 212–215. ( 10.1126/science.1223953) [DOI] [PubMed] [Google Scholar]
- 28.Brodland GW. 2002. The differential interfacial tension hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J. Biomech. Eng. 124, 188–197. ( 10.1115/1.1449491) [DOI] [PubMed] [Google Scholar]
- 29.Krieg M, Arboleda-Estudillo Y, Puech P-H, Käfer J, Graner F, Muller DJ, Heisenberg CP. 2008. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429–436. ( 10.1038/ncb1705) [DOI] [PubMed] [Google Scholar]
- 30.Hagadorn JW, et al. 2006. Cellular and subcellular structure of neoproterozoic animal embryos. Science 314, 291–294. ( 10.1126/science.1133129) [DOI] [PubMed] [Google Scholar]
- 31.Yin L, Zhu M, Knoll AH, Yuan X, Zhang J, Hu J. 2007. Doushantuo embryos preserved inside diapause egg cysts. Nature 446, 661–663. ( 10.1038/nature05682) [DOI] [PubMed] [Google Scholar]
- 32.Droser ML, Gehling JG. 2008. Synchronous aggregate growth in an abundant new Ediacaran tubular organism. Science 319, 1660–1662. ( 10.1126/science.1152595) [DOI] [PubMed] [Google Scholar]
- 33.Zana R. 2005. Dynamics of surfactant self-assemblies: micelles, microemulsions, vesicles, and lyotropic phases. Boca Raton, FL: Taylor & Francis/CRC Press. [Google Scholar]
- 34.Tsarfaty I, Resau JH, Rulong S, Keydar I, Faletto DL, Vande Woude GF. 1992. The met proto-oncogene receptor and lumen formation. Science 257, 1258–1261. ( 10.1126/science.1387731) [DOI] [PubMed] [Google Scholar]
- 35.Tsarfaty I, Rong S, Resau JH, Rulong S, da Silva PP, Vande Woude GF. 1994. The Met proto-oncogene mesenchymal to epithelial cell conversion. Science 263, 98–101. ( 10.1126/science.7505952) [DOI] [PubMed] [Google Scholar]
- 36.Mendoza M, Redemann S, Brunner D. 2005. The fission yeast MO25 protein functions in polar growth and cell separation. Eur. J. Cell Biol. 84, 915–926. ( 10.1016/j.ejcb.2005.09.013) [DOI] [PubMed] [Google Scholar]
- 37.Chien S-C, Brinkmann E-M, Teuliere J, Garriga G. 2013. Caenorhabditis elegans PIG-1/MELK acts in a conserved PAR-4/LKB1 polarity pathway to promote asymmetric neuroblast divisions. Genetics 193, 897–909. ( 10.1534/genetics.112.148106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baas AF, Smit L, Clevers H. 2004. LKB1 tumor suppressor protein: PARtaker in cell polarity. Trends Cell Biol. 14, 312–319. ( 10.1016/j.tcb.2004.04.001) [DOI] [PubMed] [Google Scholar]
- 39.Veeranki S, Hwang SH, Sun T, Kim B, Kim L. 2011. LKB1 regulates development and the stress response in Dictyostelium. Dev. Biol. 360, 351–357. ( 10.1016/j.ydbio.2011.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bürglin TR. 2008. Evolution of hedgehog and hedgehog-related genes, their origin from Hog proteins in ancestral eukaryotes and discovery of a novel Hint motif. BMC Genomics 9, 127 ( 10.1186/1471-2164-9-127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehebauer M, Hayward P, Martinez Arias A. 2006. Notch, a universal arbiter of cell fate decisions. Science 314, 1414–1415. ( 10.1126/science.1134042) [DOI] [PubMed] [Google Scholar]
- 42.Srivastava M, et al. 2008. The Trichoplax genome and the nature of placozoans. Nature 454, 955–960. ( 10.1038/nature07191) [DOI] [PubMed] [Google Scholar]
- 43.Croll AB, Massa MV, Matsen MW, Dalnoki-Veress K. 2006. Droplet shape of an anisotropic liquid. Phys. Rev. Lett. 97, 204502 ( 10.1103/PhysRevLett.97.204502) [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Huck WT, Clarke SM, Tajbakhsh AR, Terentjev EM. 2005. Shape-memory nanoparticles from inherently non-spherical polymer colloids. Nat. Mater. 4, 486–490. ( 10.1038/nmat1389) [DOI] [PubMed] [Google Scholar]
- 45.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. 2006. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220–224. ( 10.1038/nature04375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zajac M, Jones GL, Glazier JA. 2003. Simulating convergent extension by way of anisotropic differential adhesion. J. Theor. Biol. 222, 247–259. ( 10.1016/S0022-5193(03)00033-X) [DOI] [PubMed] [Google Scholar]
- 47.Irvine KD, Wieschaus E. 1994. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120, 827–841. [DOI] [PubMed] [Google Scholar]
- 48.Keller R. 2002. Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298, 1950–1954. ( 10.1126/science.1079478) [DOI] [PubMed] [Google Scholar]
- 49.Torban E, Iliescu A, Gros P. 2012. An expanding role of Vangl proteins in embryonic development. Curr. Top. Dev. Biol. 101, 237–261. ( 10.1016/B978-0-12-394592-1.00005-3) [DOI] [PubMed] [Google Scholar]
- 50.Dunn CW, Leys SP, Haddock SH. 2015. The hidden biology of sponges and ctenophores. Trends Ecol. Evol. 30, 282–291. ( 10.1016/j.tree.2015.03.003) [DOI] [PubMed] [Google Scholar]
- 51.Whelan NV, Kocot KM, Halanych KM. 2015. Employing phylogenomics to resolve the relationships among cnidarians, ctenophores, sponges, placozoans, and bilaterians. Integr. Comp. Biol. 55, 1084–1095. ( 10.1093/icb/icv037) [DOI] [PubMed] [Google Scholar]
- 52.Pisani D, Pett W, Dohrmann M, Feuda R, Rota-Stabelli O, Philippe H, Lartillot N, Wörheide G. 2015. Genomic data do not support comb jellies as the sister group to all other animals. Proc. Natl Acad. Sci. USA 112, 15 402–15 407. ( 10.1073/pnas.1518127112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith CL, Varoqueaux F, Kittelmann M, Azzam RN, Cooper B, Winters CA, Eitel M, Fasshauer D, Reese TS. 2014. Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Curr. Biol. 24, 1565–1572. ( 10.1016/j.cub.2014.05.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Degnan BM, et al. 2015. Porifera. In Evolutionary developmental biology of invertebrates (ed. Wanninger A.), pp. 65–106. Vienna, Austria: Springer. [Google Scholar]
- 55.Gilbert SF. 2010. Developmental biology, 9th edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 56.Jékely G, Paps J, Nielsen C. 2015. The phylogenetic position of ctenophores and the origin(s) of nervous systems. EvoDevo 6, 1 ( 10.1186/2041-9139-6-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boute N, Exposito JY, Boury-Esnault N, Vacelet J, Noro N, Miyazaki K, Yoshizato K, Garrone R. 1996. Type IV collagen in sponges, the missing link in basement membrane ubiquity. Biol. Cell 88, 37–44. ( 10.1016/S0248-4900(97)86829-3) [DOI] [PubMed] [Google Scholar]
- 58.Fidler AL, et al. 2014. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl Acad. Sci. USA 111, 331–336. ( 10.1073/pnas.1318499111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittenthal JE, Mazo RM. 1983. A model for shape generation by strain and cell-cell adhesion in the epithelium of an arthropod leg segment. J. Theor. Biol. 100, 443–483. ( 10.1016/0022-5193(83)90441-1) [DOI] [PubMed] [Google Scholar]
- 60.Burton PM. 2008. Insights from diploblasts; the evolution of mesoderm and muscle. J. Exp. Zool. B, Mol. Dev. Evol. 310, 5–14. ( 10.1002/jez.b.21150) [DOI] [PubMed] [Google Scholar]
- 61.Savagner P. 2015. Epithelial-mesenchymal transitions: from cell plasticity to concept elasticity. Curr. Top. Dev. Biol. 112, 273–300. ( 10.1016/bs.ctdb.2014.11.021) [DOI] [PubMed] [Google Scholar]
- 62.Lim J, Thiery JP. 2012. Epithelial–mesenchymal transitions: insights from development. Development 139, 3471–3486. ( 10.1242/dev.071209) [DOI] [PubMed] [Google Scholar]
- 63.Adams JC, Chiquet-Ehrismann R, Tucker RP. 2015. The evolution of tenascins and fibronectin. Cell. Adh. Migr. 9, 22–33. ( 10.4161/19336918.2014.970030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams F, Tew HA, Paul CE, Adams JC. 2014. The predicted secretomes of Monosiga brevicollis and Capsaspora owczarzaki, close unicellular relatives of metazoans, reveal new insights into the evolution of the metazoan extracellular matrix. Matrix Biol. 37, 60–68. ( 10.1016/j.matbio.2014.02.002) [DOI] [PubMed] [Google Scholar]
- 65.Tucker RP. 2004. The thrombospondin type 1 repeat superfamily. Int. J. Biochem. Cell Biol. 36, 969–974. ( 10.1016/j.biocel.2003.12.011) [DOI] [PubMed] [Google Scholar]
- 66.Kramerova IA, Kramerov AA, Fessler JH. 2003. Alternative splicing of papilin and the diversity of Drosophila extracellular matrix during embryonic morphogenesis. Dev. Dyn. 226, 634–642. ( 10.1002/dvdy.10265) [DOI] [PubMed] [Google Scholar]
- 67.Kawano T, Zheng H, Merz DC, Kohara Y, Tamai KK, Nishiwaki K, Culotti JG. 2009. C. elegans mig-6 encodes papilin isoforms that affect distinct aspects of DTC migration, and interacts genetically with mig-17 and collagen IV. Development 136, 1433–1442. ( 10.1242/dev.028472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaltner H, Gabius HJ. 2012. A toolbox of lectins for translating the sugar code: the galectin network in phylogenesis and tumors. Histol. Histopathol. 27, 397–416. [DOI] [PubMed] [Google Scholar]
- 69.Luan R, Liang Y, Chen Y, Liu H, Jiang S, Che T, Wong B, Sun H. 2010. Opposing developmental functions of Agrocybe aegerita galectin (AAL) during mycelia differentiation. Fungal Biol. 114, 599–608. ( 10.1016/j.funbio.2010.05.001) [DOI] [PubMed] [Google Scholar]
- 70.Jeeva J, Zalik SE. 1996. Hapten inhibitors of the endogenous galactose binding lectins and anti-lectin antibodies inhibit primitive streak formation in the early chick embryo. Glycobiology 6, 517–526. ( 10.1093/glycob/6.5.517) [DOI] [PubMed] [Google Scholar]
- 71.Liang J, Aleksanyan H, Metzenberg S, Oppenheimer SB. 2015. Involvement of L(−)-rhamnose in sea urchin gastrulation. Part II: α-L-Rhamnosidase. Zygote 24, 371–377. ( 10.1017/S0967199415000283) [DOI] [PubMed] [Google Scholar]
- 72.Damon BJ, Mezentseva NV, Kumaratilake JS, Forgacs G, Newman SA. 2008. Limb bud and flank mesoderm have distinct ‘physical phenotypes’ that may contribute to limb budding. Dev. Biol. 321, 319–330. ( 10.1016/j.ydbio.2008.06.018) [DOI] [PubMed] [Google Scholar]
- 73.Hopyan S, Sharpe J, Yang Y. 2011. Budding behaviors: growth of the limb as a model of morphogenesis. Dev. Dyn. 240, 1054–1062. ( 10.1002/dvdy.22601) [DOI] [PubMed] [Google Scholar]
- 74.Zhu J, Zhang YT, Alber MS, Newman SA. 2010. Bare bones pattern formation: a core regulatory network in varying geometries reproduces major features of vertebrate limb development and evolution. PLoS ONE 5, e10892 ( 10.1371/journal.pone.0010892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clack JA. 2012. Gaining ground: the origin and evolution of tetrapods, 2nd edn Bloomington, IN: Indiana University Press. [Google Scholar]
- 76.Zhang Y-T, Alber MS, Newman SA. 2013. Mathematical modeling of vertebrate limb development. Math. Biosci. 243, 1–17. ( 10.1016/j.mbs.2012.11.003) [DOI] [PubMed] [Google Scholar]
- 77.Cooper KL. 2015. Self-organization in the limb: a Turing mechanism for digit development. Curr. Opin. Genet. Dev. 32, 92–97. ( 10.1016/j.gde.2015.02.001) [DOI] [PubMed] [Google Scholar]
- 78.Turing AM. 1952. The chemical basis of morphogenesis. Phil. Trans. R. Soc. Lond. B 237, 37–72. ( 10.1098/rstb.1952.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gierer A, Meinhardt H. 1972. A theory of biological pattern formation. Kybernetik 12, 30–39. ( 10.1007/BF00289234) [DOI] [PubMed] [Google Scholar]
- 80.Newman SA, Frisch HL. 1979. Dynamics of skeletal pattern formation in developing chick limb. Science 205, 662–668. ( 10.1126/science.462174) [DOI] [PubMed] [Google Scholar]
- 81.Hentschel HG, Glimm T, Glazier JA, Newman SA. 2004. Dynamical mechanisms for skeletal pattern formation in the vertebrate limb. Proc. R. Soc. Lond. B 271, 1713–1722. ( 10.1098/rspb.2004.2772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raspopovic J, Marcon L, Russo L, Sharpe J. 2014. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science 345, 566–570. ( 10.1126/science.1252960) [DOI] [PubMed] [Google Scholar]
- 83.Bhat R, Lerea KM, Peng H, Kaltner H, Gabius H-J, Newman SA. 2011. A regulatory network of two galectins mediates the earliest steps of avian limb skeletal morphogenesis. BMC Dev. Biol. 11, 6 ( 10.1186/1471-213X-11-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glimm T, Bhat R, Newman SA. 2014. Modeling the morphodynamic galectin patterning network of the developing avian limb skeleton. J. Theor. Biol. 346, 86–108. ( 10.1016/j.jtbi.2013.12.004) [DOI] [PubMed] [Google Scholar]
- 85.Bhat R, Chakraborty M, Mian IS, Newman SA. 2014. Structural divergence in vertebrate phylogeny of a duplicated prototype galectin. Genome Biol. Evol. 6, 2721–2730. ( 10.1093/gbe/evu215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhat R, Chakraborty M, Glimm T, Stewart TA, Newman SA. In press. Deep phylogenomics of a tandem-repeat galectin regulating appendicular skeletal pattern formation: Galectin-8 and the origination of the tetrapod limb skeleton. BMC Evol. Biol. [DOI] [PMC free article] [PubMed]
- 87.Abascal F, Zardoya R. 2013. Evolutionary analyses of gap junction protein families. Biochim. Biophys. Acta 1828, 4–14. ( 10.1016/j.bbamem.2012.02.007) [DOI] [PubMed] [Google Scholar]
- 88.Levin M. 2014. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 25, 3835–3850. ( 10.1091/mbc.E13-12-0708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levin M, Stevenson CG. 2012. Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering. Annu. Rev. Biomed. Eng. 14, 295–323. ( 10.1146/annurev-bioeng-071811-150114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheth R, Marcon L, Bastida MF, Junco M, Quintana L, Dahn R, Kmita M, Sharpe J, Ros MA. 2012. Hox genes regulate digit patterning by controlling the wavelength of a Turing-type mechanism. Science 338, 1476–1480. ( 10.1126/science.1226804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Čapek D, Metscher BD, Müller GB. 2014. Thumbs down: a molecular-morphogenetic approach to avian digit homology. J. Exp. Zool. B Mol. Dev. Evol. 322, 1–12. ( 10.1002/jez.b.22545) [DOI] [PubMed] [Google Scholar]
- 92.Halper J, Kjaer M. 2014. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 802, 31–47. ( 10.1007/978-94-007-7893-1_3) [DOI] [PubMed] [Google Scholar]
- 93.Boto L. 2014. Horizontal gene transfer in the acquisition of novel traits by metazoans. Proc. R. Soc. B 281, 20132450 ( 10.1098/rspb.2013.2450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Müller GB. 1990. Developmental mechanisms at the origin of morphological novelty: a side-effect hypothesis. In Evolutionary Innovations (ed. Nitecki M.), pp. 99–130. Chicago, IL: University of Chicago Press. [Google Scholar]
- 95.Salazar-Ciudad I. 2010. Morphological evolution and embryonic developmental diversity in metazoa. Development 137, 531–539. ( 10.1242/dev.045229) [DOI] [PubMed] [Google Scholar]
- 96.Gilbert SF, Bosch TC, Ledón-Rettig C. 2015. Eco-Evo-Devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat. Rev. Genet. 16, 611–622. ( 10.1038/nrg3982) [DOI] [PubMed] [Google Scholar]
- 97.Rasmussen N. 1991. The decline of recapitulationism in early twentieth-century biology: disciplinary conflict and consensus on the battleground of theory. J. Hist. Biol. 24, 51–89. ( 10.1007/BF00130474) [DOI] [Google Scholar]
- 98.Amundson R. 2005. The changing role of the embryo in evolutionary thought: roots of evo-devo. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 99.Gould SJ. 2002. The structure of evolutionary theory. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 100.Diogo R, Ziermann JM, Linde-Medina M. 2015. Is evolutionary biology becoming too politically correct? A reflection on the scala naturae, phylogenetically basal clades, anatomically plesiomorphic taxa, and ‘lower’ animals. Biol. Rev. Camb. Philos. Soc. 90, 502–521. ( 10.1111/brv.12121) [DOI] [PubMed] [Google Scholar]


