Abstract
Evolution is marked by well-defined events involving profound innovations that are known as ‘major evolutionary transitions'. They involve the integration of autonomous elements into a new, higher-level organization whereby the former isolated units interact in novel ways, losing their original autonomy. All major transitions, which include the origin of life, cells, multicellular systems, societies or language (among other examples), took place millions of years ago. Are these transitions unique, rare events? Have they instead universal traits that make them almost inevitable when the right pieces are in place? Are there general laws of evolutionary innovation? In order to approach this problem under a novel perspective, we argue that a parallel class of evolutionary transitions can be explored involving the use of artificial evolutionary experiments where alternative paths to innovation can be explored. These ‘synthetic’ transitions include, for example, the artificial evolution of multicellular systems or the emergence of language in evolved communicating robots. These alternative scenarios could help us to understand the underlying laws that predate the rise of major innovations and the possibility for general laws of evolved complexity. Several key examples and theoretical approaches are summarized and future challenges are outlined.
This article is part of the themed issue ‘The major synthetic evolutionary transitions’.
Keywords: major transitions, artificial life, synthetic biology, evolutionary robotics, phase transitions
1. Introduction
An old fascination of scientists, engineers and philosophers alike has to do with the possibility (and the consequences) of imitating life through the creation and analysis of mechanical agents. The mechanical automata that were developed during the eighteenth century in Europe aimed at replicating some of the features and behaviours observed in their biological counterparts [1]. The origin of these man-made artefacts has to be found in the development of sophisticated and reliable clocks and the development of gears and networks of interacting parts that could generate complicated classes of motions beyond a purely regular, predictable cycle. Long before the terms software or program were even formulated, automata builders where using ways of programming their creations in such a way that they could display a diverse range of behaviours. One of the masterpieces in the history of automata is Jaquet Droz's mechanical writer, which is still operational and that was capable of writing, using a feather, a variety of given sentences, including Descartes' famous saying ‘I think, therefore I am’. The era of mechanical marvels made a long-lasting mark [2].
Mechanical automata were one of the wonders of the enlightenment, shaping some of the crucial questions (such as the mind–body problem) that would became the subject of a very active scientific enquiry 100 years later. Some engineers extended the clock's technology, while others, like Jacques de Vaucanson, tried to imitate life itself. In both cases, the question raised over and over again (and nowadays) was obvious: could we build living systems? Is it possible to create a living machine? Could such a machine think? Without a real understanding of the nature of biology, answering these questions was impossible at the time. The first step in this direction was Darwin's theory of evolution by natural selection, which became a cornerstone of modern thought and the first theory of biological complexity. For the first time, a coherent picture of the origins of living organization was established, providing an elegant and powerful framework where many pieces fell into place.
Is the Darwinian picture enough to explain the complexity of our biosphere and its origins? It seems obvious that we do need to include natural selection theory as an essential piece of any theory of evolution. However, as pointed by several authors, the structure of Neodarwinian thinking is grounded in the dynamics of alleles, individuals and populations, thus implicitly assuming that the entities under consideration are already in place [3]. To explain the origin of novelties (or, following DeVries, the arrival of the fittest [4]) an extended theory of biological organization is required. The pioneering work of Maynard Smith & Sztáthmary [5] provided a well-defined set of case studies associated with what they called major transitions of evolution, each one dealing with a fundamental qualitative transition towards new forms of organization involving cooperation and information processing. In this classification, some regularities are common to all transitions, including a reduced replicative potential associated with the agents composing the new entity (such as multicellular versus single cell), the emergence of division of labour and the presence of new forms of dealing with information at different scales in the hierarchy.
After more than two decades of its original formulation [5], the concept of major evolutionary transition (MET) still needs to be formally defined in a satisfactory way. On the one hand, some authors have elaborated different lists of candidate transitions that were not included in the original list [6–11]. Different lists have been introduced by different authors and some of them also contain transitions (such as the emergence of the nervous system, see [12]) that are neither directly affecting DNA nor the genetic system but instead affect the computational or cognitive nature of the underlying agents. These include not only the emergence of cognitive agents but also the emergence of consciousness [7].
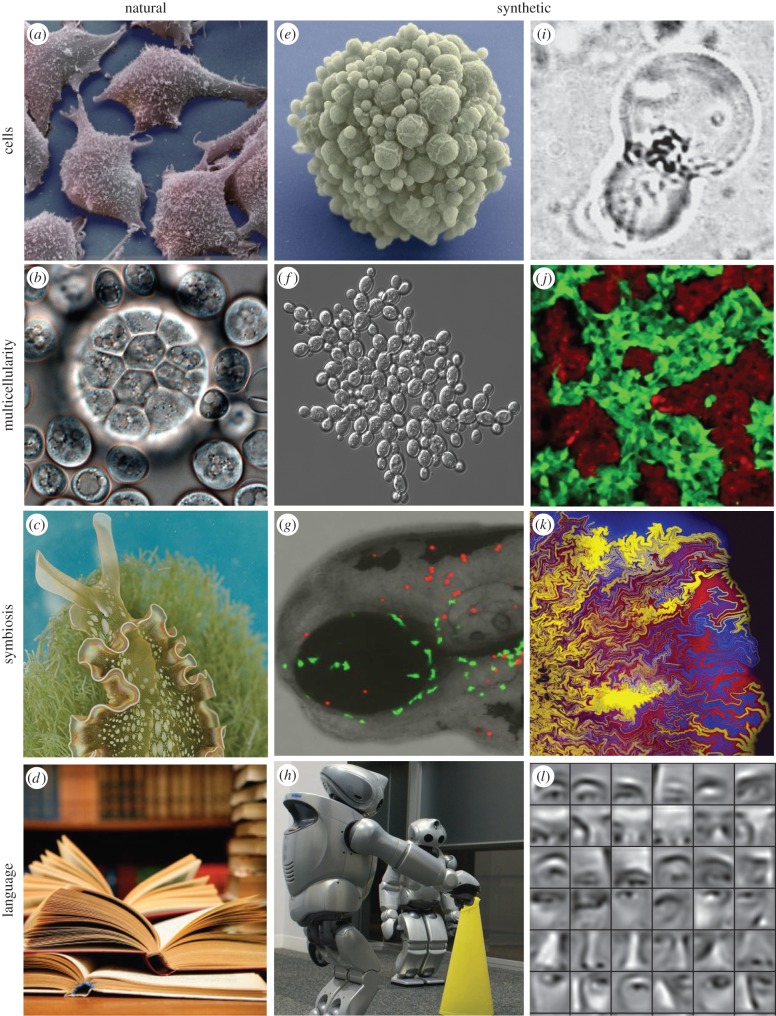
The evolution of life in our planet is a single experiment. We have no alternative planets where to look at and thus some generalizations concerning the nature and probability of METs might seem rather difficult to achieve. However, we do have some alternatives and these ‘synthetic’ approaches provided by synthetic biology, artificial life and evolutionary robotics [13]. These alternative approaches to biological evolution involve different ways of obtaining—by artificial or ‘synthetic’ means—transitions between different levels of complexity in adaptive systems, if alive or not. This theme issue aims to explore the major transitions under this novel perspective. The search for an artificial cell, the conditions for achieving multicellularity out of single-celled species, the ‘order for free’ contained in the physics of tissue and organ formation and engineering novel cooperation ties between non-cooperating species or the spontaneous emergence of proto-grammars in evolving robots are some examples (figure 1). All these systems incorporate artificial means of crossing the boundaries between complexity levels without following a ‘natural’ pathway. Instead, they can actually be grounded—partially or totally—in a design framework.
Figure 1.
Natural versus synthetic transitions. In the left column (a–d), four instances of observable types of evolutionary novelties are shown. From top to bottom, cells, multicellular systems, a symbiotic association between photosynthetic algae and a sea slug and language, as illustrated by written texts. The two columns on the right illustrate some examples of synthetic counterparts of these examples. These include synthetic cells using a genome reduction strategy (e) or a bottom-up protocell approach (i), evolved (f) and designed (j) multicellular systems, engineered cooperation (g–k) as well as evolved communicating robots and (l) artificial neural networks capable of pattern recognition and language processing.
A good reason to look for a list of major synthetic transitions (MSTs) is that it might be the case that they share profound commonalities with METs. If that were the case, then it would make sense to consider the possibility of building a theory of evolutionary novelty. Moreover, if both types of systems exhibit common mechanisms of transition, such convergence would indicate that how life evolves and the way in which novel forms of organization emerge might be strongly constrained [14], thus leaving little room for contingency [15]. What kind of general theoretical context framework would be appropriate to address this? In an updated version of the major transitions, Szathmáry [16] pointed to the theory of phase transitions as a potentially useful framework to define and characterize METs. Phase transitions have been a major domain of study within statistical physics. We should not forget that it is precisely the presence of sharp qualitative changes that defines a transition point separating two qualitatively different phases. These are associated with fundamentally different types of structural and dynamical organization and not surprisingly the conceptual framework has been widely explored [17–20]. A specially important feature of phase transitions is the existence of universal behaviour: very different systems share the same quantitative properties at the transition points. Moreover, an accurate theory of these transitions can be obtained using extremely simple models of the underlying system, thus indicating that the exact details of the components and their interactions might not play a crucial role.
The ambition of our proposal is high, because it seeks the exploration of a whole new area where a great synthesis of ideas and disciplines will be required. The contributions of this theme issue cover some of the most interesting problems related to the nature of METs and their synthetic counterparts. These contributions cover several scales and different methodological approximations, from systems chemistry and experimental evolution to artificial life and artificial intelligence. A tentative classification of these contributions would include these basic categories: (i) molecular and protocellular replicators, (ii) multicellular systems, (iii) cognitive and communicating agents, and (iv) technological transitions. In the following sections, I will briefly introduce some key problems in each of these domains. The last section will outline future challenges and potential directions towards a new synthesis regarding major evolutionary transitions as a class of phase transition phenomena.
2. Replicators, viruses and protocells
The origins of early replicators and the first protocellular systems are necessarily closer to physics and systems chemistry than any other MET. Evolved, autonomous entities capable of replicating or reproducing [5] and being the subject of selection must have been to some extent capable of moving beyond non-living chemistry to a novel form of out-of-equilibrium organization that is able to self-sustain itself and experience Darwinian evolution. A large literature exists involving the experimental recreation of primitive atmospheres and related geochemical scenarios [21]. Some substantial progress has taken place in relation to our understanding of the diverse range of molecular species that may have been available before life emerged and the potential reaction networks involved. In this context, computational models allow us to systematically explore the landscape of preconditions that might have predated the chemical space favourable to life in different contexts [22].
In order to develop complex sets of replicating molecules, mechanisms of amplification are needed. Several formal approaches provide a powerful theoretical ground for autocatalytic sets of molecules as early ways of breaking the stationary states of prebiotic mixtures, leading to self-sustained sets of cooperating molecules [23–26]. These catalytic systems define the systems’ prebiotic instance of a new level of complexity resulting from the integration of simpler agents into a new entity. The theory of molecular hypercycles in particular [27–29] has been very influential in the definition of MET. As discussed in [30] by Peter Schuster, they also help us to study the role played by resources as limiting factors for METs. Interestingly, although it has been possible to create synthetic molecules displaying self-replicating properties, they are either based on the biological template-based mechanism or involve homogeneous polymers unable to store information or allow for selection–mutation mechanisms to operate [13].
The rise of cooperative systems forming catalytic sets might have been threatened by the inevitable appearance of parasitic components [31]. The transition to a protocellular system would provide an efficient way of helping reactions to occur easily while protecting the system against parasites. There is no general theory showing the inevitability of parasites, but we can conjecture that they are one of those inevitable outcomes of evolved systems [32]. Nowadays we know that viruses, the most common class of parasite, are also the most abundant entity of ecological systems and play a crucial role, from disease spreading to global climate. But, as discussed here by Eugene Kooning [33], they have also played a key role in the MET and are an excellent target to design experimental evolution [33]. Mathematical models as well as comparative genomic analyses support the picture that multicellularity prevented the collapse induced by parasites in a single-cell, host–parasite association. Evolutionary transitions can also be experimentally approached by taking advantage of the enormous evolutionary potential of RNA viruses, as shown by Santiago Elena [34] by means of several well-defined case studies including the origin of a new virus, the incorporation or loss of genes, the origination of segmented genomes or even the emergence of cooperative interactions among different viruses during co-infection.
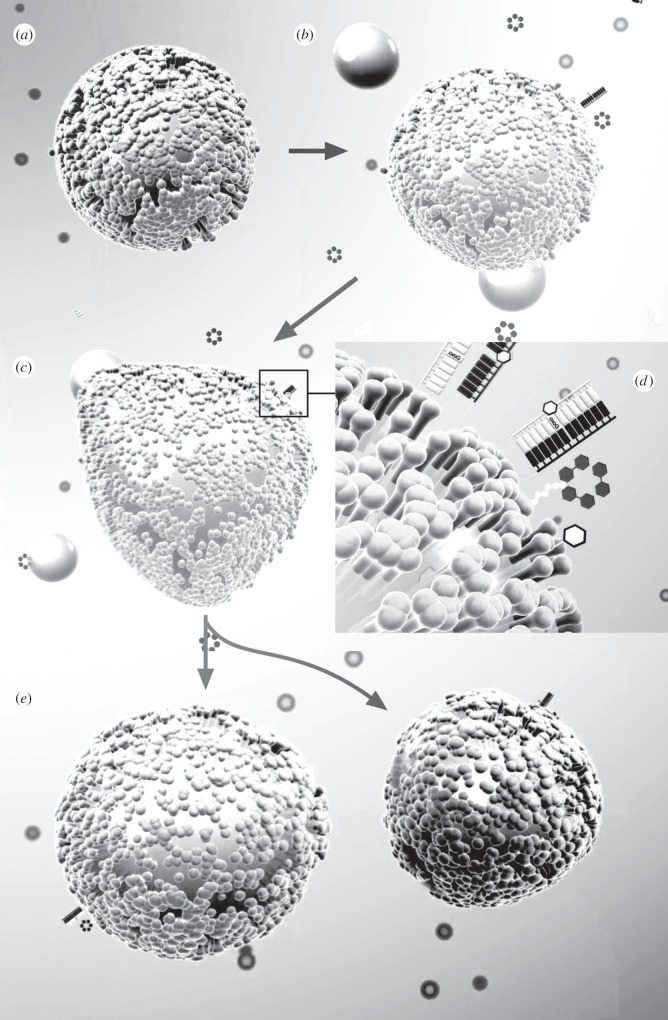
In relation to synthetic cells, different attempts have been made towards the construction of either reduced genomes capable of maintaining a cell cycle or minimal macromolecular constructs involving three coupled systems, namely a compartment, a metabolic system and some (even minimal) class of information-carrying molecules [35–39]. The apparently simple design has been shown to be more difficult to implement than expected and the synthetic approximations to this MET have incorporated some components that are not found in the current cellular structures. An example of a minimal synthetic protocell is reviewed by Steen Rasmussen and co-workers [40] and the basic cell cycle in summarized in figure 2. The system starts from a lipid aggregate (a) that grows by the addition of droplets of membrane precursors (b–c). Information molecules interact with metabolic units and all reactions occur on the surface (d) instead within the aggregate's bulk. Once the aggregate grows beyond a given threshold size, it is expected to replicate [41,42]. Other synthetic mechanisms of vesicle or micelle division have been proposed [43–45] but experimental support for this type of protocell cycle is still missing, and successful growth-division cycles rely on external driving mechanisms [13].
Figure 2.
Artificial life simulation of a realistic protocell cycle incorporating a compartment, a minimal (single-reaction) metabolism and an information molecule (an eight-base string). Here, the initial aggregate (a) is feeded with lipid precursor droplets (yellow spheres) that coalesce with the initial micelle (b) and are then transformed into new surfactant molecules (c). Information-carrying molecules and precursors are also supplied and get attached to the droplet surface (d), where they can also replicate through a mechanism of template replication. The coupled reactions lead to growth and division (e). Computer renderings courtesy of Rayn Norkus, Bruce Damer and Steen Rasmussen.
3. Transitions to multicellularity
One of the best known METs is the transition to multicellularity, which took place independently at least 25 times [46–49]. Multicellular forms of organization have successfully proven the advantages of division of labour as a way of overcoming environmental constraints. The widespread presence of multicellular organisms and their disparate evolutionary origins suggest that the selective conditions that pervade this MET must be common. Both theoretical and experimental evidence supports this view and experimental evolution of multicellular systems [50–54] shows how stable multicellular systems capable of undergoing a growth-and-break life cycle can be obtained under artificial conditions. As shown here by Eric Libby and co-workers [55], these synthetic multicellular systems are stabilized by ‘ratchets’, i.e. stabilization barriers that block the route back to individuality.
A key component for achieving functionally stable multicellular structures is a physical embodiment. Any relevant model of the evolution of multicellular organisms should actually take into account the role of generic physical mechanisms associated with cell–cell interactions and their consequences [56]. By generic, we mean mechanisms and processes not associated with complex regulatory processes, i.e. physical constraints involving gravity, adhesion or diffusion. Such fundamental constraints have been incorporated into new models of MET where some form of development is required. As discussed by Stuart Newman [57], transitions to multicellularity might have involved the constitution of new cell-based materials with novel morphogenetic properties. Such ‘biogeneric’ processes and materials might have predated the rise of metazoans and include different seemingly universal steps, from the use of adhesion molecules, the emergence of cell polarization and extracellular matrices to reaction-diffusion mechanisms. The picture that emerges from these studies is the possibility of a rich, but ultimately limited repertoire of generative rules.
A crucial requirement for a fully fledged multicellular system endowed with a developmental programme is a germ-soma segregation among cell types [5]. How can this be achieved in a synthetic or artificially evolved context? This goal has not yet been achieved but once again theoretical models suggest some potential requirements that must be met. Lachmann & Libby [58], for example, show that such a requirement is achieved in an asexual scenario starting with some class of primitive multicellular system with no germline–soma separation. If a dual transmission involving both genetic and epigenetic changes is used, it can be shown that these two levels of information transmission lead to the next generation [58]. The general conditions used in this model are likely to be universal and useful to understand other METs.
4. Cognitive systems
The original list of METs did not include the emergence of cognitive agents and non-genetic forms of information transmission, with the exception of human language. Even the problem of the emergence of eusociality, where the superorganism necessarily implies a higher-order processing of information, was treated in terms of a problem of clonality and gene-based social cohesion. But, information and computation are as relevant as mass and energy when dealing with complex life. As pointed out by John Hopfield [59], computation is what makes a difference between physics and biology. Physical systems can exhibit growth and spatio-temporal patterns of change, but they do not adapt to external signals by processing them. Computation is also a major topic within synthetic biology [60–62]. The presence of decision-making circuits pervades all levels of organization, from microorganisms to brains, and has inspired synthetic alternatives [63]. Several types of neural structures can be identified within the long road from cells equipped with simple sensors to fully fledged brains [64]. The road to complex cognitive agents is paved with several transition events, including language and consciousness.
One of the most celebrated achievements of evolutionary robotics is the evolution of synthetic proto-grammars in embodied communicating agents. Artificial intelligence pioneer Luc Steels demonstrated that shared communication among embodied robots can result in the evolution of a set of grammatical rules ([65]; see also [66,67])]. As discussed in [68], there are actually several transitions that can be described affecting different stages of synthetic language evolution. Here too, robots and other types of embodied agents capable of learning and communication are a unique opportunity to approach the likelihood of evolving a complex language and understanding, perhaps, what makes human language unique.
A different but related MET is associated with the emergence of a rather unique property of complex cognitive systems: consciousness [69,70]. The subjective nature of conscious experience makes it a special case among METs. The scientific study of consciousness became a reality after Francis Crick & Christof Koch [71] introduced the idea of neural correlates of consciousness. This and other conceptual approaches provided a much required framework to explore the nature and origins of this property that seems to be shared, at different levels, by evolved biological systems [72]. One of the key questions is the origin and potential selective advantage of consciousness, and whether or not it can be experienced by a machine. Synthetic systems, particularly within the field of robotic agents, indicate that some key preconditions, such as mirror recognition, can already be obtained under appropriate conditions [73,74]. Achieving a synthetic consciousness is a big challenge and will require a proper combination of quantitative measures associated with brain connectomes [75,76], along with the development of synthetic artefacts (Edelman). In this direction, Vershure's work on distributed adaptive control theory offers a promising avenue to integrate our current picture of the mammalian brain and embodied synthetic agents [77,78]. The ongoing development of convolutional neural networks is also offering new avenues to understand synthetic forms of cognition and their relationship with brain complexity [79,80].
5. Technological systems
The list of synthetic transitions would not be complete unless we incorporate technological innovation as part of our synthesis. Technological change and the evolution of artefacts give an additional opportunity of testing the existence of universal laws pervading the emergence of novelties. Both remarkable similarities as well as disanalogies can be found when comparing technological change and biological evolution [81]. Sometimes, the similarities can be understood in terms of generative rules and mechanisms that are common to both natural and artificial evolution. In some cases, the similarities call for a deeper explanation beyond biology and technology. Computer viruses, for example, are man-made artefacts and have been designed to overcome security barriers also designed by engineers [82–84]. And yet, one major innovation in the story of computer viruses has to do with the ‘invention’ of a source of variability that we can identify with mutations in real viruses. But these are not real random mutations. They are part of the programming and only affect part of the code, with no phenotypic (or deleterious) effects. However, on an abstract level the two ways of evolving strategies to escape the code-specific recognition developed either through immune responses or anti-virus software [13].
Although the evolution of technology has been only marginally represented in most evolutionary views, we should not forget that technology offers a rather good fossil record at different scales, and this is particularly true for information technology (IT) both within hardware and software designs. As discussed by Sergi Valverde [85], we can study the time-dependent traits developed through the history of IT since the 1950s using novel techniques from complex networks theory. These methods reveal punctuated patterns of evolution marked by bursts in diversification. These are associated with innovations (both in hardware and software) and the method used to uncover the underlying phylogenetic trees is grounded on a simple topological approximation [86] that largely ignores most of the fine-grained information contained by each invention. This result encourages us to think that a ‘tree of technology’ might eventually be characterized and fully compared with the tree of life.
Among the similarities found between technological and biological evolution, there are good examples of convergent designs [81] resulting from selective forces and engineering design principles. An example is given by the common organization of neutral fitness landscapes associated with Boolean computing networks [87] when compared with RNA folding landscapes. Another example is provided by the scaling laws resulting from wiring optimization of very large scale integrated (VLSI) circuits (figure 3a) as well as cortical maps (figure 3b) under strong packing constraints [88]. Convergence of designs has also been reported from the study of brain circuits in the visual cortex, which obey the same basic architecture of parallel computer vision [89]. The close relationship between integrated circuits and neural systems is provided by the so-called Rent's rule, which defines a power law in networks that exhibit hierarchical modularity. But there is more than convergence. In a paper by Melanie Moses and co-workers [90], a theoretical framework is developed to study the outcome of optimal circuit designs where both energy dissipation and delivery times are minimized under a Pareto optimal framework. The study reveals that common scaling laws are shared by both metabolic rates in living organisms and computer chips that have been experiencing microarchitecture evolution. An implication of this work is that a major technological transition might have taken place with the emergence of distributed multi-core systems in a way that reminds us of the transition to multicellularity.
Figure 3.
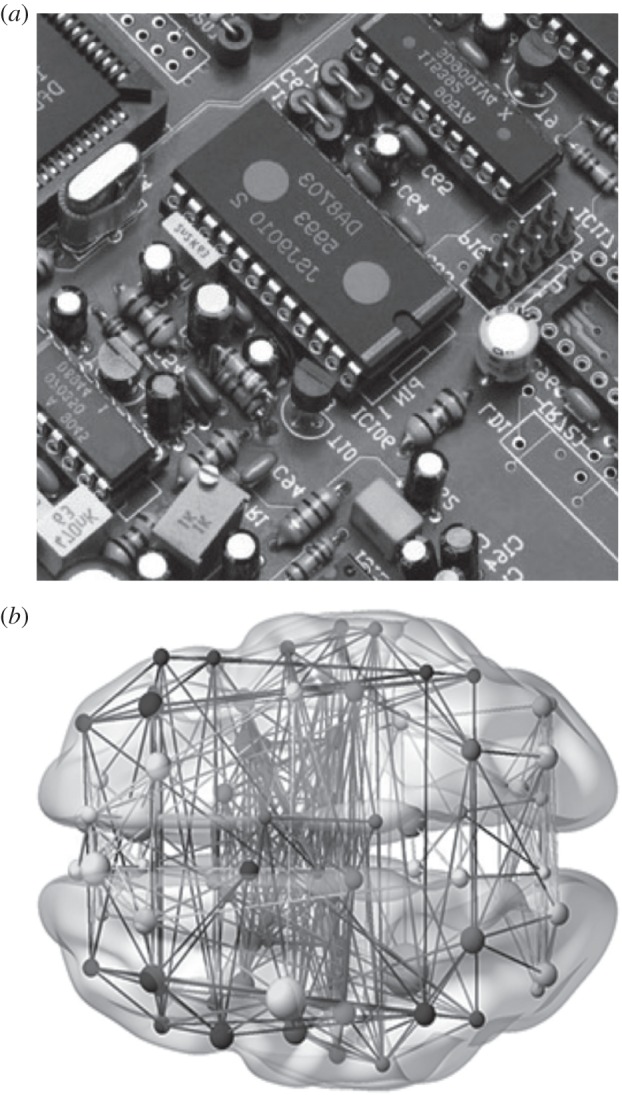
Electronic circuits (a) are the iconic representations of standard computing machines. Brains (b) and computer circuits have been often compared as implementations of hardware systems capable of performing computations. The analogy has some realistic elements (such as common laws for minimization of wiring costs) and obvious drawbacks.
6. Discussion
Synthetic transitions, along with technological evolution, define a much needed set of case studies where universal patterns of innovation might be at work. The biosphere as we know it is a single evolution experiment and all major transitions have occurred in the more or less remote past to which we have no access other than indirect evidence. A potentially rich source of understanding of the tempo and mode of MET is given by our current potential of creating, designing or evolving ‘synthetic’ alternatives using artificial means provided by synthetic biology, evolving robotic agents and neural networks or artificial life systems. Together with powerful mathematical models, the nature of innovations resulting from these synthetic alternatives can shed light onto the nature of METs, their contingency or inevitability and how likely are they to occur.
In order to develop a true theory of evolutionary innovation, we will require novel theoretical approximations. We do not have yet a thermodynamic theory of molecular evolution and we are just starting to make some progress in defining a thermodynamic cell cycle [91]. Similarly, despite the common use of information theory in biology and evolution, much work is needed in particular to address the presence of multiple scales of organization. A strong candidate in this direction is space state compression, which explicitly addresses the hierarchy of complexity levels displayed by both natural and engineered systems [92].
Along with statistical physics, which has been shown to be a specially powerful framework to address phase transitions, we might need to search for extended theories incorporating the generative rules underlying most major transitions. Language, for example, can be approached by means of a phase transition and information theory formalism, but the theory falls short in considering and incorporating the existence of a syntax. As most complex evolved systems include some ‘grammar’ providing the source for open-ended evolution, such an extended formalism seems very necessary. A major (conceptual) transition might also be needed in order to achieve this highly ambitious goal.
Acknowledgements
I thank my colleagues at the Complex Systems Laboratory and the Santa Fe Institute with whom I have shared ideas about the origins of complexity over many years. Special thanks to B. Corominas-Murtra, M. Gell-Mann, S. Kauffman, S. Manrubia, L. Seoane, E. Smith, J. West, M. Moses and S. Forrest for discussions related to the topics addressed in this paper. I also thank all the participants of the Santa Fe Institute working group on ‘Synthetic Major Transitions’ where this theme issue started to take shape.
Competing interests
I declare I have no competing interests.
Funding
This work has been supported by the Generalitat de Catalunya (AGAUR), by an ERC Advanced Grant (SYNCOM) Number 294294 from the EU seventh framework program (SYNCOM), by the Botín Foundation by Banco Santander through its Santander Universities Global Division, a MINECO FIS2015-67616 fellowship and by the Santa Fe Institute.
References
- 1.Wood G. 2003. Living dolls: a magical history of the quest for mechanical life. New York, NY: Faber. [Google Scholar]
- 2.Langton CG. 1986. Studying artificial life with cellular automata. Physica D 22, 120–149. ( 10.1016/0167-2789(86)90237-X) [DOI] [Google Scholar]
- 3.Fontana W, Buss LW. 1994. What would be conserved if ‘the tape were played twice’? Proc. Natl Acad. Sci. USA 91, 757–761. ( 10.1073/pnas.91.2.757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vries H. 1904. Species and varieties: their origin by mutation. London, UK: Open court publishing Company. [Google Scholar]
- 5.Maynard-Smith J, Szathmáry E. 1995. The major transitions in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Schuster P. 1996. How does complexity arise in evolution: Nature's recipe for mastering scarcity, abundance, and unpredictability Complexity 2, 22–30. ( 10.1002/(SICI)1099-0526(199609/10)2:1%3C22::AID-CPLX6%3E3.0.CO;2-H) [DOI] [Google Scholar]
- 7.Lane N. 2009. Life ascending: the ten great inventions of evolution. London, UK: Profile. [Google Scholar]
- 8.Niklas KJ. 1997. The evolutionary biology of plants. Chicago, IL: University of Chicago Press. [Google Scholar]
- 9.Morowitz H. 2004. The emergence of everything. How the world became complex. New York, NY: Oxford University Press. [Google Scholar]
- 10.Koonin E. 2007. The biological big bang model for the major transitions in evolution. Biol. Dir. 2, 21 ( 10.1186/1745-6150-2-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Duve C. 2005. Singularities: landmarks on the pathways of life. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Jablonka E, Lamb MJ. 2006. The evolution of information in the major transitions. J. Theor. Biol. 239, 236–246. ( 10.1016/j.jtbi.2005.08.038) [DOI] [PubMed] [Google Scholar]
- 13.Solé R. 2016. Synthetic transitions: towards a new synthesis. Phil. Trans. R. Soc. B 371, 20150438 ( 10.1098/rstb.2015.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conway Morris S. 2003. Life‘s solution: inevitable humans in a lonely universe Chicago, IL: Cambridge University Press. [Google Scholar]
- 15.Gould SJ. 1989. Wonderful life. New York, NY: Penguin. [Google Scholar]
- 16.Szathmáry E. 2015. Toward major evolutionary transitions theory 2.0. Proc. Natl Acad. Sci. USA 112, 10 104–10 111. ( 10.1073/pnas.1421398112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanley HE. 1987. Introduction to phase transitions and critical phenomena. Oxford, UK: Oxford University Press. [Google Scholar]
- 18.Solé RV, Manrubia S, Luque B, Delgado J, Bascompte J. 1996. Phase transitions in complex systems. Complexity 1, 13–26. ( 10.1002/cplx.6130010405) [DOI] [Google Scholar]
- 19.Solé RV. 2011. Phase transitions. Princeton, NJ: Princeton University Press. [Google Scholar]
- 20.Goldenfeld N, Woese C. 2011. Life is physics: evolution as a collective phenomenon far from equilibrium. Annu. Rev. Condens. Matter Phys. 2, 375–399. ( 10.1146/annurev-conmatphys-062910-140509) [DOI] [Google Scholar]
- 21.Smith E, Morowitz H. 2016. The origin and nature of life on earth: the emergence of the fourth geosphere. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Ruiz-Mirazo K, Briones C, de la Escosura A. 2013. Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 114, 285–366. ( 10.1021/cr2004844) [DOI] [PubMed] [Google Scholar]
- 23.Kauffman SA. 1986. Autocatalytic sets of proteins. J. Theor. Biol. 119, 1–24. ( 10.1016/S0022-5193(86)80047-9) [DOI] [PubMed] [Google Scholar]
- 24.Hordjik W, Steel M. 2013. A formal model of autocatalytic sets emerging in an RNA replicator system. J. Sys. Chem. 4, e3 ( 10.1186/1759-2208-4-3) [DOI] [Google Scholar]
- 25.Virgo N, Ikegami T, McGregor S. 2016. Complex autocatalysis in simple chemistries. Artif. Life 22, 138–152. ( 10.1162/ARTL_a_00195) [DOI] [PubMed] [Google Scholar]
- 26.Peretó J. 2012. Out of fuzzy chemistry: from prebiotic chemistry to metabolic networks. Chem. Soc. Rev. 41, 5394–5403. ( 10.1039/c2cs35054h) [DOI] [PubMed] [Google Scholar]
- 27.Eigen M, Schuster P. 1979. The hypercycle: a principle of natural self-organization. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 28.Smith JM. 1979. Hypercycles and the origin of life. Nature 280, 445–446. ( 10.1038/280445a0) [DOI] [PubMed] [Google Scholar]
- 29.Szathmáry E. 2006. The origin of replicators and reproducers. Phil. Trans. R. Soc. B 361, 1761–1776. ( 10.1098/rstb.2006.1912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuster P. 2016. Some mechanistic requirements for major transitions. Phil. Trans. R. Soc. B 371, 20150439 ( 10.1098/rstb.2015.0439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boerlijst MC, Hogeweg P. 1991. Spiral wave structure in pre-biotic evolution: hypercycles stable against parasites. Physica D 48, 17–28. ( 10.1016/0167-2789(91)90049-F) [DOI] [Google Scholar]
- 32.Hogeweg P, Takeuchi N. 2003. Multilevel selection in models of prebiotic evolution: compartments and spatial self-organization. Orig. Life Evol. Biosph. 33, 375–403. ( 10.1023/A:1025754907141) [DOI] [PubMed] [Google Scholar]
- 33.Koonin EV. 2016. Viruses and mobile elements as drivers of evolutionary transitions. Phil. Trans. R. Soc. B 371, 20150442 ( 10.1098/rstb.2015.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elena SF. 2016. Evolutionary transitions during RNA virus experimental evolution. Phil. Trans. R. Soc. B 371, 20150441 ( 10.1098/rstb.2015.0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deamer DW. 2011. First life. Oakland, CA: University of California Press. [Google Scholar]
- 36.Rasmussen S, Bedau MA, Chen L, Deamer D, Krakauer DC, Packard NH, Stadler PF (eds). 2008. Protocells: bridging nonliving and living matter. Cambridge, MA: MIT Press. [Google Scholar]
- 37.Solé RV, Munteanu A, Rodriguez-Caso C, Macia J. 2007. Synthetic protocell biology: from reproduction to computation. Phil. Trans. R. Soc. B 362, 1727–1739. ( 10.1098/rstb.2007.2065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luisi PL. 2006. The emergence of life: from chemical origins to synthetic biology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 39.Gánti T. 2003. The principles of life. Oxford, UK: Oxford University Press. [Google Scholar]
- 40.Rasmussen S, Constantinescu A, Svaneborg C. 2016. Generating minimal living systems from non-living materials and increasing their evolutionary abilities. Phil. Trans. R. Soc. B 371, 20150440 ( 10.1098/rstb.2015.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fellermann H, Rasmussen S, Ziock HJ, Solé RV. 2007. Life cycle of a minimal protocell: a dissipative particle dynamics study. Artif. Life 13, 319–345. ( 10.1162/artl.2007.13.4.319) [DOI] [PubMed] [Google Scholar]
- 42.Fellermann H, Solé RV. 2007. Minimal model of self-replicating nanocells: a physically embodied information-free scenario. Phil. Trans. R. Soc. B 362, 1803–1811. ( 10.1098/rstb.2007.2072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurihara K, Tamura M, Shohda KI, Toyota T, Suzuki K, Sugawara T. 2011. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem. 3, 775–781. ( 10.1038/nchem.1127) [DOI] [PubMed] [Google Scholar]
- 44.Ichihashi N, Usui K, Kazuta Y, Sunami T, Matsuura T, Yomo T. 2013. Darwinian evolution in a translation-coupled RNA replication system within a cell-like compartment. Nat. Commun. 4, 2494 ( 10.1038/ncomms3494) [DOI] [PubMed] [Google Scholar]
- 45.Takakura K, Toyota T, Sugawara T. 2003. A novel system of self-reproducing giant vesicles. J. Am. Chem. Soc. 125, 8134–8140. ( 10.1021/ja029379a) [DOI] [PubMed] [Google Scholar]
- 46.Knoll AH. 2011. The multiple origins of complex multicellularity. Annu. Rev. Earth Planet. Sci. 39, 217–239. ( 10.1146/annurev.earth.031208.100209) [DOI] [Google Scholar]
- 47.Ruiz-Trillo I, Nedelcu AM (eds). 2015. Evolutionary transitions to multicellular life: principles and mechanisms. Berlin, Germany: Springer. [Google Scholar]
- 48.Bonner JT. 2001. First signals: the evolution of multicellular development. Princeton, NJ: Princeton University Press. [Google Scholar]
- 49.Michod RE, Roze D. 1997. Transitions in individuality. Proc. R. Soc. Lond. B 264, 853–857. ( 10.1098/rspb.1997.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratcliff WC, Denison RF, Borrello M, Travisano M. 2012. Experimental evolution of multicellularity. Proc. Natl Acad. Sci. USA 109, 1595–1600. ( 10.1073/pnas.1115323109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maharbiz MM. 2012. Synthetic multicellularity. Trends Cell Biol . 22, 617–623. ( 10.1016/j.tcb.2012.09.002) [DOI] [PubMed] [Google Scholar]
- 52.Libby E, Rainey PB. 2013. A conceptual framework for the evolutionary origins of multicellularity. Phys. Biol. 10, 035001 ( 10.1088/1478-3975/10/3/035001) [DOI] [PubMed] [Google Scholar]
- 53.Duran-Nebreda S, Solé R. 2015. Emergence of multicellularity in a model of cell growth, death and aggregation under size-dependent selection. J. R. Soc. Interface 12, 20140982 ( 10.1098/rsif.2014.0982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Libby E, Ratcliff W, Travisano M, Kerr B. 2014. Geometry shapes evolution of early multicellularity. PLoS Comput. Biol. 10, e1003803 ( 10.1371/journal.pcbi.1003803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Libby E, Conlin PL, Kerr B, Ratcliff WC. 2016. Stabilizing multicellularity through ratcheting. Phil. Trans. R. Soc. B 371, 20150444 ( 10.1098/rstb.2015.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newman SA, Bhat R. 2009. Dynamical patterning modules: a ‘pattern language’ for development and evolution of multicellular form. Int. J. Dev. Biol. 53, 693 ( 10.1387/ijdb.072481sn) [DOI] [PubMed] [Google Scholar]
- 57.Newman SA. 2016. ‘Biogeneric’ developmental processes: drivers of major transitions in animal evolution. Phil. Trans. R. Soc. B 371, 20150443 ( 10.1098/rstb.2015.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lachmann M, Libby E. 2016. Epigenetic inheritance systems contribute to the evolution of a germline. Phil. Trans. R. Soc. B 371, 20150445 ( 10.1098/rstb.2015.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopfield JJ. 1994. Physics, computation, and why biology looks so different. J. Theor. Biol. 171, 53–60. ( 10.1006/jtbi.1994.1211) [DOI] [Google Scholar]
- 60.Benenson Y. 2012. Biomolecular computing systems: principles, progress and potential. Nat. Rev. Gen. 13, 455–468. ( 10.1038/nrg3197) [DOI] [PubMed] [Google Scholar]
- 61.Regot S, et al. 2011. Distributed biological computation with multicellular engineered networks. Nature 469, 207–211. ( 10.1038/nature09679) [DOI] [PubMed] [Google Scholar]
- 62.Macía J, Posas F, Solé R. 2012. Distributed computation: the new wave of synthetic biology devices. Trends Biotech. 30, 342–349. ( 10.1016/j.tibtech.2012.03.006) [DOI] [PubMed] [Google Scholar]
- 63.Solé R, Amor DR, Duran-Nebreda S, Conde-Pueyo N, Carbonell-Ballestero M, Montañez R. 2016. Synthetic collective intelligence. Biosystems. ( 10.1016/j.biosystems.2016.01.002) [DOI] [PubMed] [Google Scholar]
- 64.Rose S. 2005. The future of the brain: the promise and perils of tomorrow‘s neuroscience New York, NY: Oxford University Press. [Google Scholar]
- 65.Steels L. 2003. Evolving grounded communication for robots. Trends Cogn. Sci. 7, 308–312. ( 10.1016/S1364-6613(03)00129-3) [DOI] [PubMed] [Google Scholar]
- 66.Cangelosi A. 2010. Grounding language in action and perception: from cognitive agents to humanoid robots. Phys. Life Rev. 7, 139–151. ( 10.1016/j.plrev.2010.02.001) [DOI] [PubMed] [Google Scholar]
- 67.Nolfi S, Mirolli M (eds). 2009. Evolution of communication and language in embodied agents. Berlin, Germany: Springer. [Google Scholar]
- 68.Steels L. 2016. Agent-based models for the emergence and evolution of grammar. Phil. Trans. R. Soc. B 371, 20150447 ( 10.1098/rstb.2015.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dennett DC. 1993. Consciousness explained. London, UK: Penguin. [Google Scholar]
- 70.Dehaene S. 2014. Consciousness and the brain: deciphering how the brain codes our thoughts. London, UK: Penguin. [Google Scholar]
- 71.Crick F, Koch C. 2003. A framework for consciousness. Nature Neurosci. 6, 119–126. ( 10.1038/nn0203-119) [DOI] [PubMed] [Google Scholar]
- 72.Edelman DB, Baars BJ, Seth AK. 2005. Identifying hallmarks of consciousness in non-mammalian species. Conscious. Cogn. 14, 169–187. ( 10.1016/j.concog.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 73.Gold K, Scassellati B. 2007. A Bayesian robot that distinguishes ‘self’ from ‘other’. In Proc. of the 29th annual meeting of the cognitive science society, Nashville, TN, 1--4 August 2007, pp. 1–4. CD distributed by Lawrence Erlbaum Associates, NY, USA. ISBN 978-0-9768318-3-9.
- 74.Reggia JA. 2013. The rise of machine consciousness: studying consciousness with computational models. Neural Netw. 44, 112–131. ( 10.1016/j.neunet.2013.03.011) [DOI] [PubMed] [Google Scholar]
- 75.Oizumi M, Albantakis L, Tononi G. 2014. From the phenomenology to the mechanisms of consciousness: integrated information theory 3.0. PLoS Comput. Biol. 10, e1003588 ( 10.1371/journal.pcbi.1003588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tononi G, Koch C. 2015. Consciousness: here, there and everywhere? Phil. Trans R. Soc. B 370, 20140167 ( 10.1098/rstb.2014.0167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verschure P. 2012. Distributed adaptive control: a theory of the mind, brain, body nexus. Biol. Insp. Cogn. Arch. 1, 55–72. ( 10.1016/j.bica.2012.04.005) [DOI] [Google Scholar]
- 78.Verschure PFMJ. 2016. Synthetic consciousness: the distributed adaptive control perspective. Phil. Trans. R. Soc. B 371, 20150448 ( 10.1098/rstb.2015.0448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.LeCun Y, Bengio Y, Hinton G. 2015. Deep learning. Nature 521, 436–444. ( 10.1038/nature14539) [DOI] [PubMed] [Google Scholar]
- 80.Mnih V, et al. 2015. Human-level control through deep reinforcement learning. Nature 518, 529–533. ( 10.1038/nature14236) [DOI] [PubMed] [Google Scholar]
- 81.Solé RV, Valverde S, Casals MR, Kauffman SA, Farmer D, Eldredge N. 2013. The evolutionary ecology of technological innovations. Complexity 18, 15–27. ( 10.1002/cplx.21436) [DOI] [Google Scholar]
- 82.Nachenberg C. 1997. Computer virus–antivirus coevolution. Comm. ACM 50, 46–51. ( 10.1145/242857.242869) [DOI] [Google Scholar]
- 83.Forrest S, Hofmeyr SA, Somayaji A. 1997. Computer immunology. Comm. ACM 40, 88–96. ( 10.1145/262793.262811) [DOI] [Google Scholar]
- 84.Forrest S, Beauchemin C. 2007. Computer immunology. Immunol. Rev. 216, 176–197. ( 10.1111/j.1600-065X.2007.00499.x) [DOI] [PubMed] [Google Scholar]
- 85.Valverde S. 2016. Major transitions in information technology. Phil. Trans. R. Soc. B 371, 20150450 ( 10.1098/rstb.2015.0450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gualdi S, Yeung CH, Zhang YC. 2011. Tracing the evolution of physics on the backbone of citation networks. Phys. Rev. E 84, 046104 ( 10.1103/PhysRevE.84.046104) [DOI] [PubMed] [Google Scholar]
- 87.Fernández P, Solé RV. 2007. Neutral fitness landscapes in signalling networks. J. R. Soc. Interface 4, 41–47. ( 10.1098/rsif.2006.0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bassett DS, Greenfield DL, Meyer-Lindenberg A, Weinberger DR, Moore SW, Bullmore ET. 2010. Efficient physical embedding of topologically complex information processing networks in brains and computer circuits. PLoS Comput. Biol. 6, e1000748 ( 10.1371/journal.pcbi.1000748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nelson ME, Bower JM. 1990. Brain maps and parallel computers. Trends Neurosci. 13, 403–408. ( 10.1016/0166-2236(90)90119-U) [DOI] [PubMed] [Google Scholar]
- 90.Moses M, Bezerra G, Edwards B, Brown J, Forrest S. 2016. Energy and time determine scaling in biological and computer designs. Phil. Trans. R. Soc. B 371, 20150446 ( 10.1098/rstb.2015.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fellermann H, et al. 2015. Non-equilibrium thermodynamics of self-replicating protocells. (http://arxiv.org/abs/150304683. )
- 92.Wolpert DH, et al. 2014. A framework for optimal high-level descriptions in science and engineering. (http://arxiv.org/abs/1409.7403. )