Abstract
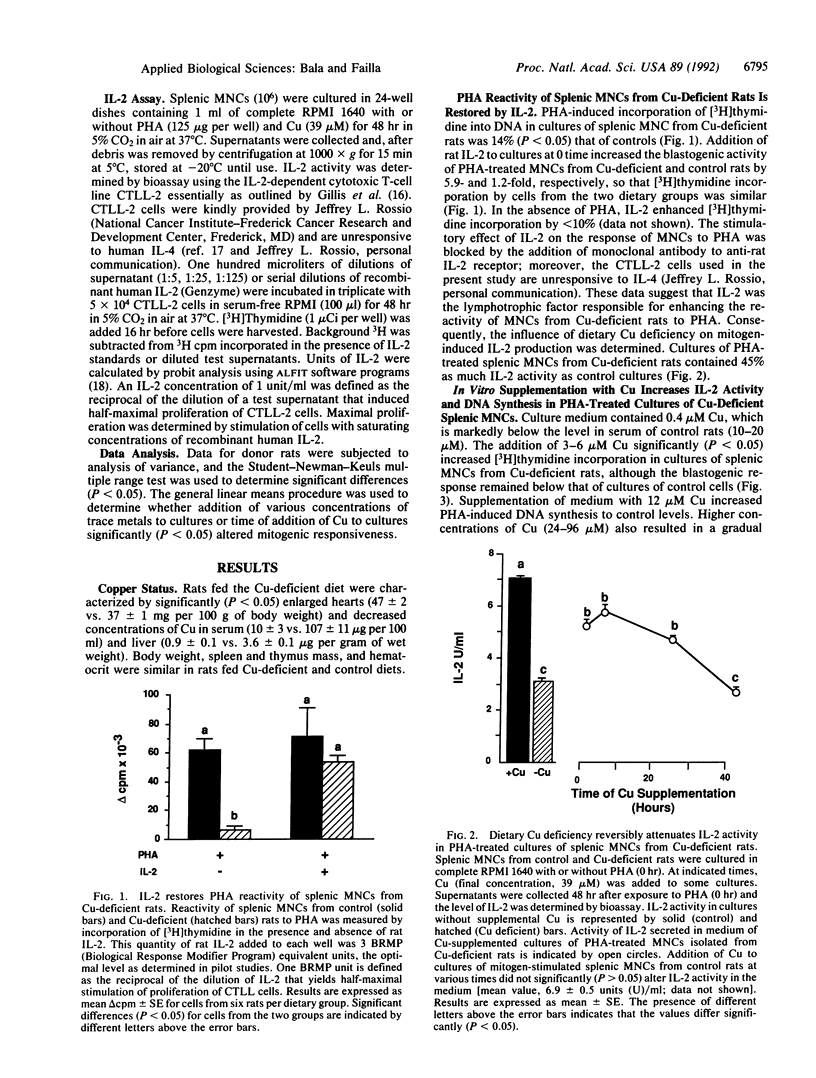
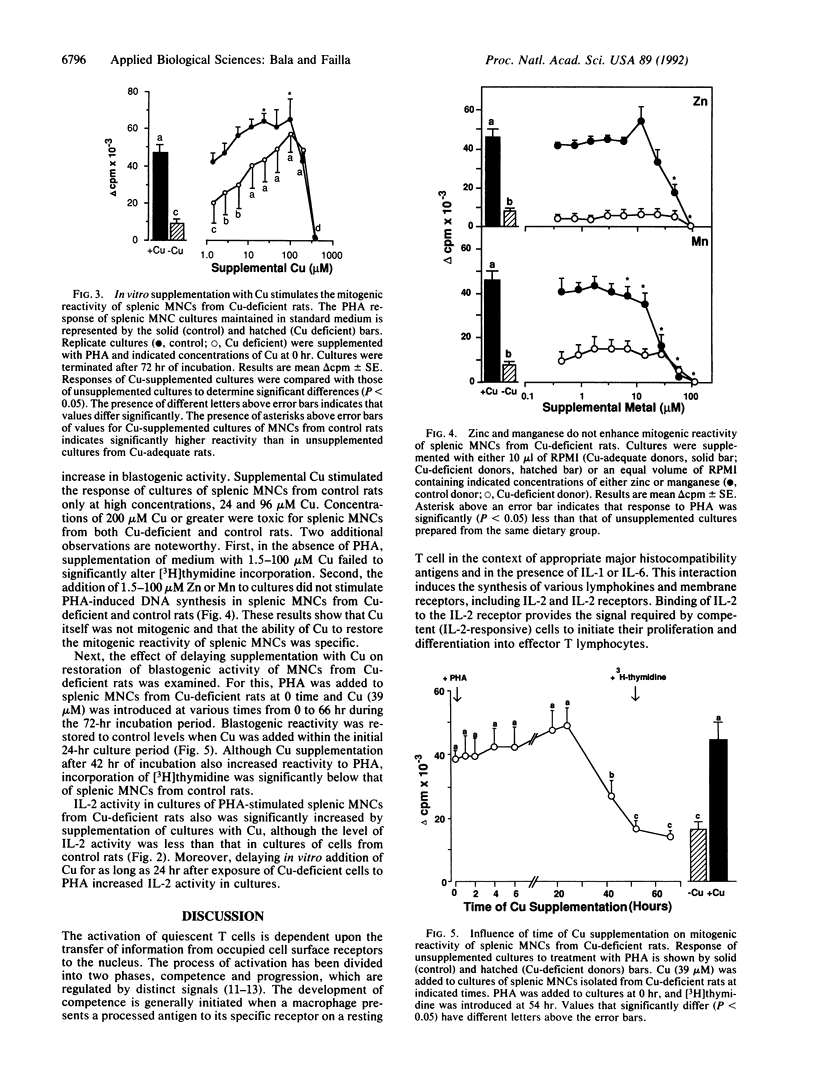
The essentiality of adequate copper (Cu) nutriture for normal T-cell function in laboratory and domestic animals is well established. However, specific biochemical roles of Cu in the maturation and activation of T cells have not been defined. Previous work showed that when cultures of splenic mononuclear cells (MNCs) from Cu-deficient rats were exposed to T-cell mitogens, DNA synthesis was markedly reduced despite normal up-regulation of interleukin 2 (IL-2) receptors, transferrin receptors, and class II major histocompatibility complex molecules. In the present study, IL-2 activity in PHA-treated cultures of MNCs from Cu-deficient rats was 40-50% that of controls as determined by bioassay. Addition of rat IL-2 to phytohemagglutinin-treated cultures of MNCs from Cu-deficient rats increased blastogenic activity to control levels, demonstrating that Cu deficiency does not inhibit transition of quiescent cells to the competence phase of the activation process. Moreover, supplementation of MNC cultures from Cu-deficient rats with physiological levels of Cu enhanced IL-2 activity and DNA synthesis in response to phytohemagglutinin. These data indicate that IL-2 activity in cultures of activated splenic T lymphocytes from Cu-deficient rats is insufficient for optimal blastogenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bala S., Failla M. L., Lunney J. K. Alterations in splenic lymphoid cell subsets and activation antigens in copper-deficient rats. J Nutr. 1991 May;121(5):745–753. doi: 10.1093/jn/121.5.745. [DOI] [PubMed] [Google Scholar]
- Carpentieri U., Myers J., Daeschner C. W., 3rd, Haggard M. E. Effects of iron, copper, zinc, calcium, and magnesium on human lymphocytes in culture. Biol Trace Elem Res. 1988 Jul;16(2):165–176. doi: 10.1007/BF02797101. [DOI] [PubMed] [Google Scholar]
- Dowd P. S., Kelleher J., Guillou P. J. T-lymphocyte subsets and interleukin-2 production in zinc-deficient rats. Br J Nutr. 1986 Jan;55(1):59–69. doi: 10.1079/bjn19860010. [DOI] [PubMed] [Google Scholar]
- Failla M. L., Babu U., Seidel K. E. Use of immunoresponsiveness to demonstrate that the dietary requirement for copper in young rats is greater with dietary fructose than dietary starch. J Nutr. 1988 Apr;118(4):487–496. doi: 10.1093/jn/118.4.487. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R. Soluble cytokine receptors: their role in immunoregulation. FASEB J. 1991 Aug;5(11):2567–2574. doi: 10.1096/fasebj.5.11.1868981. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Jones D. G., Suttle N. F. Some effects of copper deficiency on leucocyte function in sheep and cattle. Res Vet Sci. 1981 Sep;31(2):151–156. [PubMed] [Google Scholar]
- Kramer T. R., Johnson W. T., Briske-Anderson M. Influence of iron and the sex of rats on hematological, biochemical and immunological changes during copper deficiency. J Nutr. 1988 Feb;118(2):214–221. doi: 10.1093/jn/118.2.214. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E. Immunosuppression by D-penicillamine in vitro. Inhibition of human T lymphocyte proliferation by copper- or ceruloplasmin-dependent generation of hydrogen peroxide and protection by monocytes. J Clin Invest. 1984 Jan;73(1):53–65. doi: 10.1172/JCI111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasewycz O. A., Prohaska J. R. The immune response in copper deficiency. Ann N Y Acad Sci. 1990;587:147–159. doi: 10.1111/j.1749-6632.1990.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Zhang N., Schmandt R., Fung M., Greene W., Mellors A., Hogg D. Transmembrane signalling by interleukin 2. Biochem Soc Trans. 1991 Apr;19(2):277–287. doi: 10.1042/bst0190277. [DOI] [PubMed] [Google Scholar]
- Modiano J. F., Kolp R., Lamb R. J., Nowell P. C. Protein kinase C regulates both production and secretion of interleukin 2. J Biol Chem. 1991 Jun 5;266(16):10552–10561. [PubMed] [Google Scholar]
- Moulder K., Steward M. W. Experimental zinc deficiency: effects on cellular responses and the affinity of humoral antibody. Clin Exp Immunol. 1989 Aug;77(2):269–274. [PMC free article] [PubMed] [Google Scholar]
- Prohaska J. R., Lukasewycz O. A. Copper deficiency suppresses the immune response of mice. Science. 1981 Jul 31;213(4507):559–561. doi: 10.1126/science.7244654. [DOI] [PubMed] [Google Scholar]
- Scuderi P. Differential effects of copper and zinc on human peripheral blood monocyte cytokine secretion. Cell Immunol. 1990 Apr 1;126(2):391–405. doi: 10.1016/0008-8749(90)90330-t. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Favata M. F., Oroszlan S. Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol. 1983 Oct;131(4):1808–1815. [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Suttle N. F., Jones D. G. Recent developments in trace element metabolism and function: trace elements, disease resistance and immune responsiveness in ruminants. J Nutr. 1989 Jul;119(7):1055–1061. doi: 10.1093/jn/119.7.1055. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Cerottini J. C. Antigen presentation. FASEB J. 1989 Nov;3(13):2496–2502. doi: 10.1096/fasebj.3.13.2572499. [DOI] [PubMed] [Google Scholar]



