Abstract
BACKGROUND
While cardiac allograft vasculopathy (CAV) is typically characterized by diffuse coronary intimal thickening with pathological vessel remodeling, plaque instability may also play an important role in CAV. Previous studies of native coronary atherosclerosis have demonstrated associations between attenuated-signal plaque (ASP), plaque instability, and adverse clinical events.
OBJECTIVES
This study’s aim was to characterize the association between ASP and long-term mortality post-heart transplantation.
METHODS
In 105 heart transplant recipients, serial (baseline and 1-year post-transplant) intravascular ultrasound (IVUS) was performed in the first 50 mm of the left anterior descending artery. ASP score was calculated by grading the measured angle of attenuation from grades 0 to 4 (specifically, 0°, 1° to 90°, 91° to 180°, 181° to 270°, and >270°) at 1 mm intervals. The primary endpoint was all-cause death or retransplantation.
RESULTS
At 1-year post-transplant, 10.5% of patients demonstrated ASP progression (newly developed or increased ASP). Patients with ASP progression had a higher incidence of acute cellular rejection during the first year (63.6% vs. 22.3%; p = 0.006) and tendency for greater intimal growth (percent intimal volume: 9.2% ± 9.3% vs. 4.4% ± 5.3%; p = 0.07) than those without. Over a median follow-up of 4.6 years, there was a significantly lower event-free survival rate in patients with ASP progression at 1-year post-transplant compared to those without. In contrast, maximum intimal thickness did not predict long-term mortality.
CONCLUSIONS
ASP progression appears to reflect chronic inflammation related to acute cellular rejection and is an independent predictor of long-term mortality after heart transplantation. Serial assessments of plaque instability may enhance identification of high-risk patients who may benefit from closer follow-up and targeted medical therapies.
Keywords: acute cellular rejection, intima, intravascular ultrasound, plaque instability, remodeling
Cardiac allograft vasculopathy (CAV), a leading cause of long-term mortality after heart transplantation (1), is the predominant form of chronic rejection (2). Both immunologic and nonimmunologic factors drive this process, resulting in localized inflammation with persistent vascular injury and endothelial dysfunction (3). Unlike angiography, intravascular ultrasound (IVUS) can directly visualize the wall structure, offering a sensitive method to evaluate CAV (4). While CAV is typically characterized by coronary intimal thickening with pathological remodeling, several virtual histology IVUS studies of transplant recipients reported that increasing amounts of necrotic core and dense calcium (inflammatory plaques) are related to early recurrent rejection and subsequent CAV progression (5–7).
In coronary atherosclerosis, attenuated-signal plaque (ASP) represents increased necrotic core and fibrofatty tissue or microcalcification (8). Recent studies also showed that ASP is associated with poor prognosis in patients with coronary artery disease (9–11). Hence, we hypothesized that ASP, as a surrogate marker of inflammatory plaque (plaque instability), may also serve as an important measure of CAV. This study aimed to investigate the natural evolution of ASP early after transplantation and to characterize its association with CAV progression and long-term outcomes.
METHODS
Between January 2002 and January 2013, stable heart transplant recipients with preserved renal function (baseline serum creatinine ≤2.0 mg/dl) who survived at least 1 year post-transplantation and underwent pre-scheduled serial IVUS at baseline (within 6 weeks) and 1 year were eligible for enrollment in this retrospective study. This population included patients from 2 prospective trials performed to evaluate the role of cytomegalovirus or an angiotensin-converting enzyme inhibitor in CAV development (ClinicalTrials.gov: NCT01078363). All recipients received standard immunosuppressive therapy, including induction therapy with daclizumab or rabbit antithymocyte globulin (rATG), corticosteroids, an antiproliferative agent (sirolimus or mycophenolate mofetil), and a calcineurin inhibitor (cyclosporine or tacrolimus). Patients were monitored for acute cellular rejection using right ventricular endomyocardial biopsies at scheduled intervals post-transplant: weekly during the first month, biweekly until the third month, monthly until the sixth month, and then at 9 and 12 months. Significant acute cellular rejection was defined as the International Society for Heart and Lung Transplantation 2004 revised grade of ≥2R (7,12). Biopsy-proven antibody-mediated rejection (AMR) of grade ≥pAMR2, development of de novo donor-specific antibodies (DSA), and severe rejection with hemodynamic compromise (defined as a decrease in relative left ventricular ejection fraction [LVEF] >25%) during the first year were also evaluated. Lipid profile and LVEF by echocardiography were evaluated at 1-year post-transplant. Patients were followed beyond the first year, and the primary endpoint was all-cause death or re-transplantation. Informed consent was obtained from all patients, and the protocol was Institutional Review Board-approved.
INTRAVASCULAR ULTRASOUND
IVUS was performed using a 40-MHz mechanical system (Galaxy with Atlantis SR Pro or OptiCross with iLab; Boston Scientific Corporation, Marlborough, Massachusetts) in a standard manner. Images were analyzed with a validated system (echoPlaque; Indec Systems, Santa Clara, California) at a core laboratory blinded to clinical and angiographic information. Cross-sectional areas were traced at 1 mm intervals throughout the first 50 mm of the left anterior descending artery (LAD) with automated interpolated measurements of the remaining frames (4,7,12). Volumes calculated using Simpson’s method were standardized as volume index (volume/analyzed length, mm3/mm). ASP was evaluated on each cross-section by 2 experienced observers and discrepancy in presence of ASP was adjudicated by consensus (intraobserver and interobserver kappa coefficients were 0.996 and 0.994, respectively). ASP was identified by absence of ultrasound signal behind plaque that was either hypoechoic or isoechoic to the reference adventitia, but contained no bright calcium. The arc of attenuation was measured in degrees with a protractor centered on the lumen. ASP score was calculated by grading the measured angle of attenuation as 0 to 4 at 1 mm intervals: 0 = no attenuation, 1 = 1 to 90°; 2 = 91 to 180°, 3 = 181 to 270°, and 4 = 271 to 360°; total ASP score was standardized by the segment length (13). ASP progression was defined as newly developed or increased ASP during the first year, calculated as Δ standardized ASP score >0. The arc of calcium was also measured and graded similarly to the ASP score. According to literature, paradoxical remodeling (increased intima with negative remodeling or decreased intima with positive remodeling) at the proximal LAD, significant increase in maximal intimal thickness (MIT) (ΔMIT ≥0.5 mm), and donor-transmitted atherosclerosis (MIT ≥0.5 mm at baseline) were also evaluated (4,14–16).
STATISTICAL ANALYSIS
Statistical calculations were performed with JMP® 10 (SAS Institute Inc., Cary, North Carolina). Data are expressed as frequencies and percentages for categorical variables, and as mean ± SD or median with interquartile range for continuous variables. Categorical comparisons were performed using a chi-square test or Fisher exact test. Continuous values were compared by using the unpaired or paired Student t test, Wilcoxon rank sum test, Mann-Whitney U test, or 1-way analysis of variance, as appropriate. A 2-way repeated measures analysis of variance was used to test for group and time effects and their interactions. Associations between continuous variables were investigated using linear regression analysis. Survival analysis was performed by applying the Kaplan-Meier method and the log-rank test. Hazard ratios (HR) and 95% confidence intervals (CI) were analyzed with Cox proportional hazards regression models to identify factors associated with the study’s primary endpoint. Variables with p value ≤ 0.05 on the univariate analysis were entered into the multivariate model. A p value < 0.05 was considered statistically significant.
RESULTS
The inclusion and exclusion criteria were met in 118 heart transplant recipients. Among these, 13 were excluded because of missing IVUS images (n = 3) or poor-quality images due to nonuniform rotational distortion (n = 4), air bubbles (n = 1), partial scanning (n = 4), or inconsistent pullback (n = 1). As a result, 105 patients were enrolled in this analysis and followed for up to 12 years (median: 4.6 years). During this period, 19 patients died (12 cardiac deaths, 1 from acute cellular rejection, 1 from cancer, 2 from infection, and 3 of unknown causes) and 1 patient underwent retransplantation.
Baseline clinical characteristics and IVUS indexes in patients who met the primary endpoint versus those who did not are summarized in Tables 1 and 2. During the first year, 26.7% experienced >1 episode(s) of significant acute cellular rejection, and its incidence was significantly higher in patients with subsequent mortality or retransplantation (Table 1).
TABLE 1.
Clinical Characteristics
| All-cause Death or Retransplantation
|
||||
|---|---|---|---|---|
| Variables | All (n = 105) | Present (n = 20) | Absent (n = 85) | p Value |
| Recipient profile | ||||
|
| ||||
| Age, yrs | 52 (42–60) | 45 (20–56) | 53 (43–61) | 0.015 |
| Male | 68.6 | 60.0 | 70.6 | 0.37 |
| Body mass index, kg/m2 | 25.2 (22.6–29.0) | 26.4 (22.0–30.5) | 25.0 (22.6–27.7) | 0.24 |
| Cytomegalovirus IgG positive | 67.6 | 65.0 | 68.2 | 0.78 |
| Diabetes mellitus | 14.3 | 20.0 | 12.9 | 0.43 |
| Hypertension | 36.2 | 10.0 | 42.4 | 0.003 |
| Hyperlipidemia | 27.6 | 15.0 | 30.6 | 0.14 |
| Ischemic cardiomyopathy | 23.8 | 20.0 | 24.7 | 0.65 |
| Statins at 1 year | 93.3 | 95.0 | 92.9 | 0.73 |
| Antidiabetic medications* at 1 year | 30.5 | 35.0 | 29.4 | 0.63 |
| Insulin at 1 year | 21.9 | 30.0 | 20.0 | 0.34 |
| Total cholesterol at 1 year, mg/dl | 169 (149–190) | 163 (152–222) | 171 (145–190) | 0.63 |
| Triglycerides at 1 year, mg/dl | 114 (85–177) | 152 (111–187) | 105 (81–167) | 0.06 |
| LVEF at 1 year, % | 61.0 (57.0–65.0) | 60.0 (54.4–66.1) | 61.0 (58.0–64.5) | 0.58 |
| Acute cellular rejection ≥2R† | 26.7 | 55.0 | 20.0 | 0.002 |
| pAMR ≥2† | 1.0 | 0 | 1.2 | 0.51 |
| De novo DSA | 9.5 | 10.0 | 9.4 | 0.94 |
| Severe rejection‡ | 4.8 | 5.0 | 4.7 | 0.96 |
| Immunosuppressive regimen§ | ||||
| rATG | 37.1 | 5.0 | 44.7 | 0.0002 |
| Tacrolimus | 50.5 | 10.0 | 60.0 | <0.0001 |
| Sirolimus | 13.3 | 35.0 | 8.2 | 0.004 |
|
| ||||
| Donor profile | ||||
|
| ||||
| Age, yrs | 25 (20–40) | 29 (21–44) | 24 (19–40) | 0.18 |
| Male | 73.3 | 60.0 | 76.5 | 0.15 |
| Donor-recipient sex mismatch | 21.9 | 10.0 | 24.7 | 0.13 |
| Cold ischemic time, min | 221 ± 49 | 221 ± 31 | 222 ± 53 | 0.96 |
Values are median (IQR), %, or mean ± SD.
Oral antidiabetic drugs and/or insulin.
1 or more episodes of grade ≥2R or 2 during the first year post-transplant.
Severe rejection with hemodynamic compromise, defined as a decrease in relative LVEF >25%.
Primarily used during the first year post-transplant.
ASP = attenuated-signal plaque; DSA = donor-specific antibodies (during the first year); IgG = immunoglobulin G; IQR = interquartile range; LVEF = left ventricular ejection fraction; pAMR = pathologic antibody-mediated rejection; rATG = rabbit antithymocyte globulin.
TABLE 2.
Baseline IVUS Indexes
| All-cause Death or Retransplantation
|
||||
|---|---|---|---|---|
| IVUS indexes | All (n = 105) | Present (n = 20) | Absent (n = 85) | p Value |
| Vessel volume, mm3/mm | 15.0 ± 3.6 | 14.8 ± 3.5 | 15.0 ± 3.6 | 0.81 |
| Lumen volume, mm3/mm | 12.3 ± 3.0 | 12.6 ± 3.2 | 12.2 ± 2.9 | 0.58 |
| Intimal volume, mm3/mm | 2.4 (1.8–3.2) | 1.9 (1.4–3.4) | 2.4 (2.0–3.2) | 0.06 |
| Percent intimal volume, % | 16.6 (12.9–20.3) | 12.9 (9.9–18.1) | 16.7 (14.1–20.8) | 0.03 |
| Maximum intimal thickness, mm | 0.64 (0.42–1.09) | 0.68 (0.42–1.24) | 0.64 (0.42–1.05) | 0.73 |
| Donor atherosclerosis* | 61.0 | 55.0 | 62.4 | 0.55 |
| ASP | 15.2 | 5.0 | 17.7 | 0.12 |
| Calcium | 19.0 | 20.0 | 18.8 | 0.90 |
Values are mean ± SD, median (IQR), or %.
Donor atherosclerosis = maximum intimal thickness at baseline ≥0.5 mm.
IVUS = intravascular ultrasound; other abbreviations as in Figure 1.
IVUS CHANGES
Overall, both vessel (15.0 ± 3.6 to 13.9 ± 3.6 mm3/mm; p < 0.0001) and lumen volumes (12.3 ± 3.0 to 10.7 ± 2.9 mm3/mm; p < 0.0001) decreased significantly, whereas intimal volume (2.7 ± 1.4 to 3.2 ± 1.7 mm3/mm; p < 0.0001) and MIT (0.79 ± 0.48 to 0.94 ± 0.57 mm; p < 0.0001) increased from baseline to 1 year. At 1 year, patients with all-cause death or retransplantation showed a greater loss of vessel (p < 0.05 for interaction) and lumen volumes (p < 0.01 for interaction) compared to those without (Figure 1). These patients also demonstrated a greater increase in percent intimal volume (8.0 ± 7.0 vs. 4.2 ± 5.5%; p = 0.008), whereas increases in other intimal parameters (intimal volume or MIT) did not differ significantly between the 2 groups (Figure 1). The proportion of patients with a significant MIT increase was higher in patients with all-cause death or retransplantation (20.0% vs. 9.4%, respectively; p = 0.21), although the difference was not statistically significance.
FIGURE 1. Patients with Versus Without All-cause death or Retransplantation.
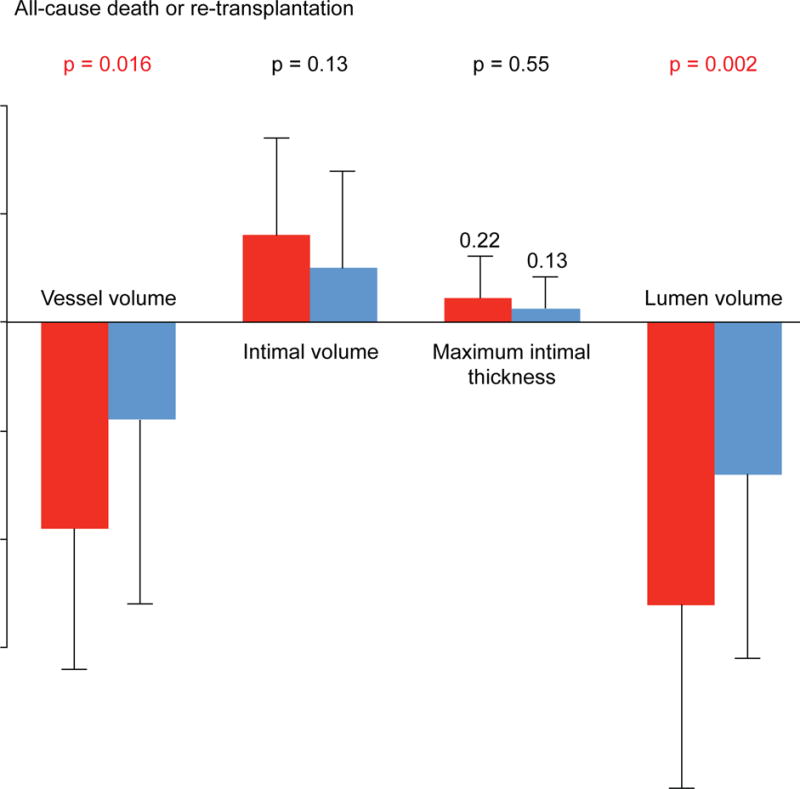
At 1 year, patients with all-cause death or retransplantation demonstrated greater decreases in vessel and lumen volumes compared to those without; there was no significant difference in changes in intimal volume or maximal intimal thickness.
Patients who experienced significant acute cellular rejection during the first year had greater increases in intimal volume, MIT, and percent intimal volume (7.3% ± 7.2% vs. 4.1% ± 5.2%; p = 0.02) compared to those without rejection, with significant group-by-time interaction effects (Figure 2). The change in vessel or lumen volume did not differ significantly between the 2 groups. On the other hand, patients with and without AMR (grade ≥2) or severe rejection with hemodynamic compromise during the first year showed similar changes in all IVUS indexes at 1 year. Patients who developed de novo DSA during the first year tended to have greater intimal increase (volume: 0.9 ± 1.2 vs. 0.5 ± 0.8 mm3/mm; percent volume: 7.7% ± 6.9% vs. 4.6% ± 5.8%; p = 0.13 for both) at 1 year compared to those without, whereas changes in other IVUS indexes were not significantly different between the groups.
FIGURE 2. Patients with Versus Without Acute Cellular Rejection.
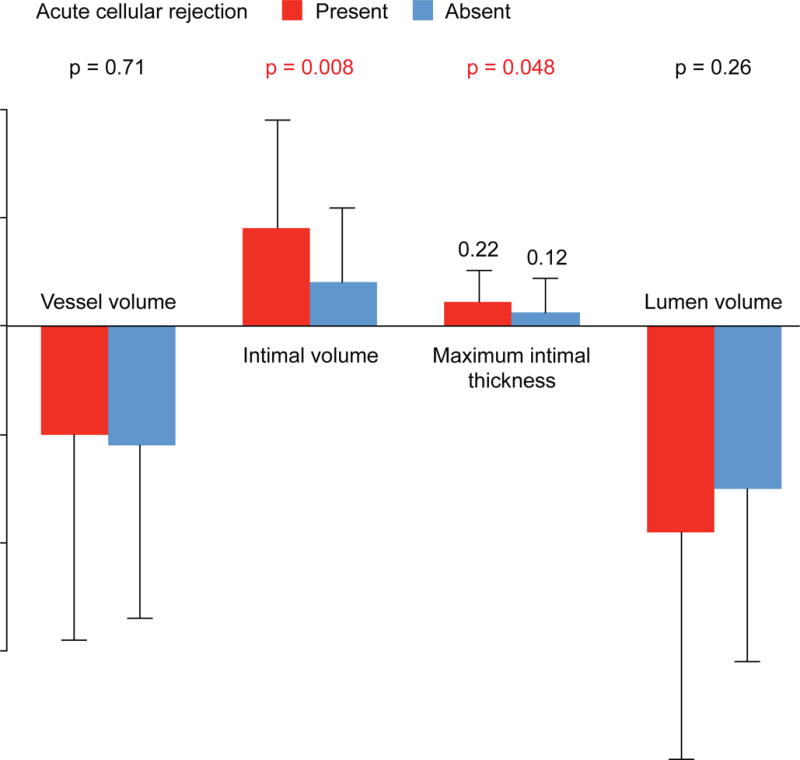
Patients with acute cellular rejection during the first year demonstrated greater increases in intimal volume and maximal intimal thickness compared to those without. Changes in vessel and lumen volumes did not differ significantly between the 2 groups.
ASP PROGRESSION AND IVUS CHANGES
At baseline, ASP was detected in 16 patients (15.2%): 5 patients had an increase in ASP from baseline to 1 year, while 11 demonstrated regression (9 patients) or no change (2 patients). Among patients with no ASP at baseline, 6 patients had newly developed ASP at 1 year. As a result, a total of 11 patients (10.5%) had ASP progression (increased or newly developed ASP) at 1 year (Figure 3). Patients with ASP progression at 1 year showed a significantly higher incidence of subsequent mortality or retransplantation compared to those without (Figure 4); in particular, all patients with newly developed ASP subsequently died after the follow-up IVUS (median: 5.9 years post-transplant).
FIGURE 3. ASP Post-transplant.
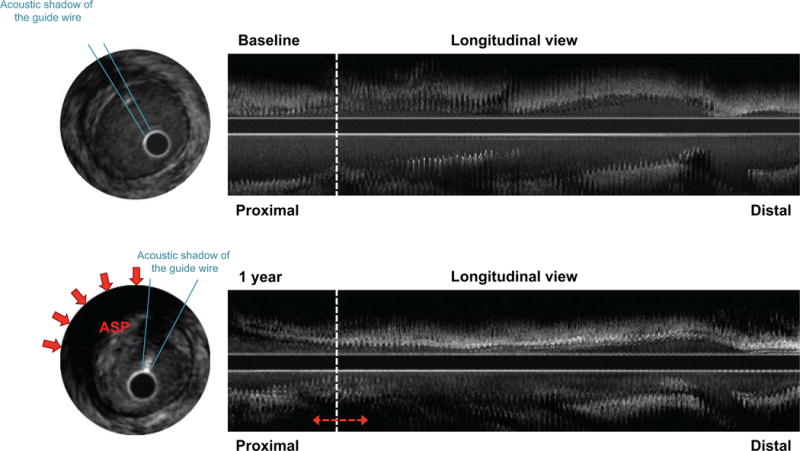
At 1-year post-transplant, attenuated-signal plaque (ASP) progression (red arrows) was seen at the proximal segment of the left anterior descending artery, accompanied by prominent intimal proliferation.
FIGURE 4. ASP Progression: All-cause Death or Retransplantation.
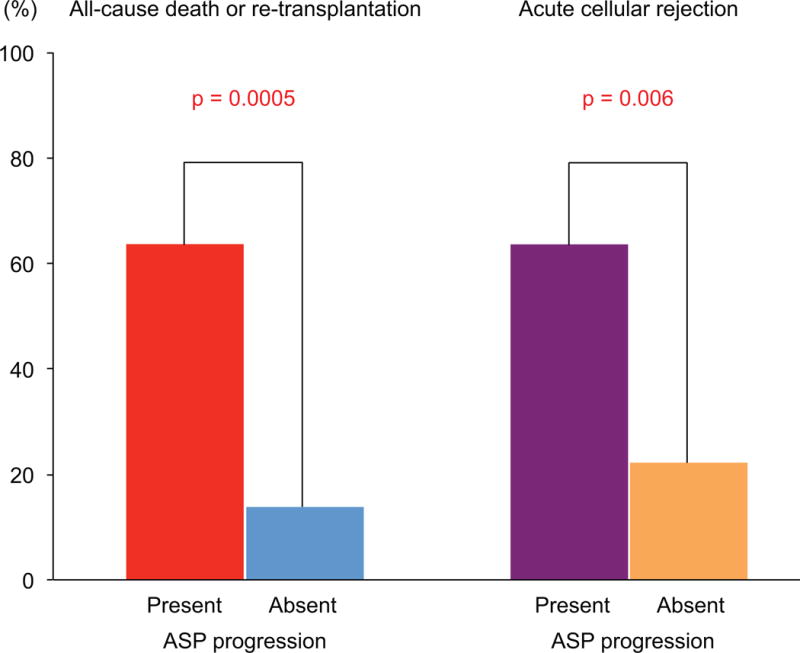
Patients with ASP progression at 1 year had a significantly higher incidence of subsequent mortality or retransplantation. Abbreviations as in Figure 3.
Patients with and without ASP progression showed similar profiles at heart transplantation, except for higher body mass index, lower prevalence of hypertension, older donor age, and higher prevalence of donor-transmitted atherosclerosis in patients with ASP progression (Table 3), who also experienced more frequent significant acute cellular rejection during the first year (Figure 4). Regarding medical treatment, patients with ASP progression had lower rates of rATG and tacrolimus use, a higher rate of sirolimus use, and a tendency toward more use of antidiabetic drugs (especially insulin) at 1 year (Table 3). While severe rejection with hemodynamic compromise tended to be more frequent in patients with ASP progression compared to those without, occurrence of AMR (grade 2 or higher) or development of de novo DSA during the first year was not significantly different between the 2 groups. Among several clinical differences, the occurrence of acute cellular rejection during the first year was strongly associated with 1-year ASP progression (odds ratio: 11.99; 95% CI: 1.83 to 145.35; p = 0.008) while immunosuppressive drugs were not (p = 0.47 for rATG; p = 0.18 for tacrolimus; p = 0.43 for sirolimus). Donor-transmitted atherosclerosis was also significantly associated with ASP progression (p = 0.03).
TABLE 3.
Patients with and Without ASP Progression
| ASP Progression
|
|||
|---|---|---|---|
| Variables | Present (n = 11) | Absent (n = 94) | p Value |
| Recipient profile | |||
|
| |||
| Age, yrs | 49 (40–55) | 52 (42–61) | 0.25 |
| Male | 72.7 | 68.1 | 0.75 |
| Body mass index, kg/m2 | 29.7 (26.0–31.8) | 25.0 (22.5–27.8) | 0.02 |
| Cytomegalovirus IgG positive | 63.6 | 68.1 | 0.77 |
| Diabetes mellitus | 18.2 | 13.8 | 0.70 |
| Hypertension | 9.1 | 39.4 | 0.03 |
| Hyperlipidemia | 27.3 | 27.7 | 0.98 |
| Ischemic cardiomyopathy | 27.3 | 23.4 | 0.78 |
| Statins at 1 year | 90.9 | 93.6 | 0.74 |
| Antidiabetic medications* at 1 year | 54.6 | 27.7 | 0.08 |
| Insulin at 1 year | 45.5 | 19.2 | 0.06 |
| Total cholesterol at 1 year, mg/dl | 172 (153–206) | 169 (147–190) | 0.80 |
| Triglycerides at 1 year, mg/dl | 151 (107–172) | 109 (81–182) | 0.25 |
| LVEF at 1 year, % | 61.0 (56.2–66.1) | 61.0 (57.0–64.5) | 0.99 |
| Acute cellular rejection ≥2R† | 63.6 | 22.3 | 0.006 |
| pAMR ≥2† | 0 | 1.1 | 0.64 |
| De novo DSA | 18.2 | 8.5 | 0.35 |
| Severe rejection‡ | 18.2 | 3.2 | 0.07 |
| Immunosuppressive regimen§ | |||
| rATG | 9.1 | 40.4 | 0.03 |
| Tacrolimus | 18.2 | 54.3 | 0.02 |
| Sirolimus | 36.4 | 10.6 | 0.04 |
|
| |||
| Donor profile | |||
|
| |||
| Age, yrs | 40 (25–50) | 24 (19–40) | 0.03 |
| Male | 81.8 | 72.3 | 0.49 |
| Donor-recipient sex mismatch | 9.1 | 23.4 | 0.24 |
| Cold ischemic time, min | 203±43 | 224±50 | 0.18 |
|
| |||
| Baseline IVUS indexes | |||
|
| |||
| Vessel volume, mm3/mm | 16.4±2.3 | 14.8±3.7 | 0.16 |
| Lumen volume, mm3/mm | 12.8±3.1 | 12.2±3.0 | 0.56 |
| Intimal volume, mm3/mm | 3.5 (3.1–3.8) | 2.3 (1.8–3.0) | 0.003 |
| Percent intimal volume, % | 22.2 (16.8–26.8) | 16.3 (12.6–19.8) | 0.017 |
| Maximum intimal thickness, mm | 1.41 (1.18–1.86) | 0.58 (0.40–0.92) | <0.0001 |
| Donor atherosclerosis | 100 | 56.4 | 0.0006 |
Values are median (IQR), %, or mean ± SD.
Oral antidiabetic drugs and/or insulin.
1 or more episodes of grade ≥2R or 2 during the first year post-transplant.
Severe rejection with hemodynamic compromise, defined as a decrease in relative LVEF >25%.
Primarily used during the first year post-transplant.
Patients with ASP progression at 1 year tended to have more intimal increase (volume: 1.1 ± 1.4 vs. 0.4 ± 0.8 mm3/mm; p = 0.13; percent intimal volume: 9.2% ± 9.3% vs. 4.4% ± 5.3%; p = 0.07) and lumen decrease (−2.4 ± 2.1 vs. −1.5 ± 1.7 mm3/mm; p = 0.11) at 12 months compared to those without. In contrast, change in vessel volume at 1 year did not differ significantly between the 2 groups (−1.3 ± 1.5 vs. −1.1 ± 1.7 mm3/mm; p = 0.68).
Patients with ASP at baseline showed greater intimal increase (volume: 1.0 ± 1.2 vs. 0.4 ± 0.8 mm3/mm; p = 0.02; percent volume: 9.4% ± 7.4% vs. 4.1% ± 5.3%; p = 0.007) and lumen decrease (−2.5 ± 1.6 vs. −1.4 ± 1.7 mm3/mm, p = 0.02) compared to those without. In contrast, change in vessel volume did not differ significantly between patients with and without ASP at baseline (−1.5 ± 1.2 vs. −1.0 ± 1.7 mm3/mm; p = 0.32).
FACTORS ASSOCIATED WITH ASP REGRESSION AND OUTCOMES
Among patients with ASP at baseline, patients who presented ASP regression (or no change) at 1 year had a significantly lower frequency of significant acute cellular rejection during the first year compared to those without (11.1% vs. 71.4%; p = 0.01). These patients also showed a lower rate of insulin use at 1 year (9.1% vs. 60.0%; p = 0.03) and a tendency toward a higher rate of tacrolimus use (63.6% vs. 20.0%; p = 0.097). Other clinical profiles were similar between the 2 groups.
Kaplan-Meier analysis over a median follow-up of 4.6 years demonstrated a significantly lower event-free survival rate in patients with ASP progression at 1-year post-transplant compared to those without (Central Illustration). On univariate analysis, acute cellular rejection during the first year was associated with all-cause death or retransplantation whereas younger recipient age, lower prevalence of hypertension at heart transplantation, total cholesterol values at 1 year, and immunosuppressive drugs were not (Table 4). In terms of IVUS indexes, greater decreases in vessel and lumen volumes, greater increase in percent intimal volume, ASP progression, and paradoxical vessel remodeling of the proximal LAD at 1 year were all associated with the primary endpoint of all-cause death or retransplantation on univariate analysis (Table 4). Conversely, donor-transmitted atherosclerosis (MIT ≥0.5 mm at baseline or baseline intimal volume), changes in MIT and intimal volume at 1 year, and calcium progression did not predict the primary endpoint. In multivariate analysis, ASP progression and paradoxical vessel remodeling of the proximal LAD at 1 year were independent predictors of subsequent all-cause death or retransplantation (Table 4). This association was preserved in a sensitivity analysis restricted to cardiac death and retransplantation (n = 13) (Table 5). Acute cellular rejection during the first year was also associated with all-cause death or retransplantation but not with cardiac death or retransplantation (Tables 4 and 5).
TABLE 4.
Factors Associated with All-cause Death or Retransplantation
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Recipient age, per 1 year | 0.98 | 0.96–1.00 | 0.10 | |||
| Diabetes at baseline | 2.03 | 0.58–5.63 | 0.24 | |||
| Hypertension at baseline | 0.37 | 0.06–1.31 | 0.14 | |||
| Hyperlipidemia at baseline | 0.45 | 0.10–1.35 | 0.16 | |||
| Total cholesterol at 1 year, per 1 mg/dl | 1.01 | 0.998–1.022 | 0.10 | |||
| Triglycerides at 1 year, per 1 mg/dl | 1.00 | 0.997–1.005 | 0.44 | |||
| LVEF at 1 year, per −1% | 1.02 | 0.97–1.07 | 0.43 | |||
| Acute cellular rejection ≥grade 2R during the first year | 4.25 | 1.74–10.64 | 0.002 | 2.80 | 1.04–7.51 | 0.04 |
| rATG | 0.53 | 0.03–3.22 | 0.53 | |||
| Tacrolimus | 0.29 | 0.05–1.07 | 0.07 | |||
| Sirolimus | 1.70 | 0.63–4.22 | 0.28 | |||
| Female donor | 1.90 | 0.74–4.62 | 0.18 | |||
| Donor-recipient sex mismatch | 0.40 | 0.06–1.39 | 0.17 | |||
| Baseline intimal volume, per 1 mm3/mm | 0.92 | 0.64–1.22 | 0.57 | |||
| Baseline percent intimal volume, per 1% | 0.98 | 0.92–1.04 | 0.54 | |||
| Donor atherosclerosis (MIT at baseline ≥0.5 mm) | 0.80 | 0.33–1.98 | 0.62 | |||
| ASP at baseline | 0.28 | 0.02–1.38 | 0.14 | |||
| Increase in MIT ≥0.5 mm during the first year | 1.62 | 0.46–4.45 | 0.41 | |||
| Increase in intimal volume, per 1 mm3/mm | 1.24 | 0.72–2.06 | 0.42 | |||
| Increase in percent intimal volume, per 1% | 1.08 | 1.00–1.16 | 0.05 | 1.08 | 0.90–1.29 | 0.40 |
| Decrease in vessel volume, per 1 mm3/mm | 1.42 | 1.07–1.89 | 0.01 | 2.81 | 0.93–8.64 | 0.07 |
| Decrease in lumen volume, per 1 mm3/mm | 1.44 | 1.10–1.90 | 0.007 | 0.40 | 0.10–1.54 | 0.18 |
| ASP progression | 4.64 | 1.72–11.64 | 0.004 | 3.41 | 1.03–10.78 | 0.04 |
| Calcium progression | 0.79 | 0.18–2.36 | 0.70 | |||
| Paradoxical vessel remodeling of the proximal LAD segment | 10.63 | 3.06–66.96 | <0.0001 | 6.95 | 1.79–45.98 | 0.003 |
TABLE 5.
Factors Associated with Cardiac Death or Retransplantation
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Recipient age, per 1 year | 0.97 | 0.945–0.999 | 0.04 | 0.98 | 0.94–1.01 | 0.16 |
| Diabetes at baseline | 1.40 | 0.22–5.27 | 0.67 | |||
| Hypertension at baseline | 0.30 | 0.02–1.52 | 0.17 | |||
| Hyperlipidemia at baseline | 0.20 | 0.01–1.02 | 0.05 | 0.16 | 0.01–1.00 | 0.05 |
| Total cholesterol at 1 year, per 1 mg/dl | 1.01 | 0.99–1.02 | 0.27 | |||
| Triglycerides at 1 year, per 1 mg/dl | 1.00 | 0.99–1.00 | 0.997 | |||
| LVEF at 1 year, per −1% | 1.01 | 0.94–1.07 | 0.79 | |||
| Acute cellular rejection ≥grade 2R during the first year | 2.92 | 0.93–8.86 | 0.06 | |||
| rATG | 1.72 | 0.08–14.78 | 0.66 | |||
| Tacrolimus | 0.58 | 0.09–2.29 | 0.47 | |||
| Sirolimus | 2.63 | 0.84–7.99 | 0.09 | |||
| Female donor | 1.87 | 0.57–5.61 | 0.29 | |||
| Donor-recipient sex mismatch | 0.63 | 0.10–2.34 | 0.53 | |||
| Baseline intimal volume, per 1 mm3/mm | 1.00 | 0.67–1.36 | 0.99 | |||
| Baseline percent intimal volume, per 1% | 1.01 | 0.94–1.08 | 0.68 | |||
| Donor atherosclerosis (MIT at baseline ≥0.5 mm) | 1.03 | 0.34–3.41 | 0.96 | |||
| ASP at baseline | 0.43 | 0.02–2.19 | 0.36 | |||
| Increase in MIT ≥0.5 mm during the first year | 1.12 | 0.17–4.22 | 0.88 | |||
| Increase in intimal volume, per 1 mm3/mm | 1.10 | 0.54–2.07 | 0.79 | |||
| Increase in percent intimal volume, per 1% | 1.07 | 0.98–1.18 | 0.14 | |||
| Decrease in vessel volume, per 1 mm3/mm | 1.33 | 0.94–1.89 | 0.11 | |||
| Decrease in lumen volume, per 1 mm3/mm | 1.33 | 0.95–1.89 | 0.10 | |||
| ASP progression | 4.83 | 1.45–14.66 | 0.01 | 6.58 | 1.70–26.33 | 0.008 |
| Calcium progression | 0.79 | 0.12–2.94 | 0.75 | |||
| Paradoxical vessel remodeling of the proximal LAD segment | 13.84 | 2.72–252.29 | 0.0004 | 10.35 | 1.98–190.65 | 0.003 |
DISCUSSION
The main findings of this study are: 1) 10.5% experienced ASP progression during the first year post-transplant; 2) ASP progression at 1 year was an independent predictor of long-term mortality or retransplantation; and 3) ASP progression at 1 year was also associated with the occurrence of significant acute cellular rejection during the first year post-transplant. On the other hand, early coronary intimal thickening or arterial remodeling alone did not predict long-term mortality or retransplantation in multivariate analyses. To the best of our knowledge, this was the first study to suggest the potential clinical utility of serial IVUS assessment of coronary plaque instability in CAV to predict long-term outcomes after heart transplantation.
ASP PROGRESSION IN CAV
Our findings suggested that importantly, ASP can be used to identify high-risk patients after heart transplantation. In native coronary atherosclerosis, ASP is predominantly caused by a combination of factors related to plaque instability (inflammatory plaque), such as large plaque burden, microcalcification, plaque rupture, and presence of thin-capped fibroatheroma characterized by lipid-rich plaque with macrophage infiltration and a large necrotic core (8,17). As in native atherosclerosis (18,19), these components of plaque instability can be encountered in CAV lesions, too, (7,20,21) and some of them (particularly increased lipid-rich, necrotic core and dense calcium) are associated with adverse clinical events in heart transplant recipients, such as recurrent acute cellular rejection, CAV progression, and need for revascularization (7,22). A histopathological study also demonstrated that in contrast to fibro-cellular plaques associated with different inflammatory cell subtypes in CAV versus native atherosclerosis (fewer macrophages and more lymphocytes in CAV), fibro-lipid plaques had the same inflammatory patterns between the 2 pathologies (23). That study also showed that intraplaque hemorrhage – another risk factor for plaque progression and instability in native atherosclerosis – was seen more frequently in CAV lesions than in native atherosclerosis and were colocalized with lipids and necrotic cores in these lesions. Furthermore, several autopsy studies have revealed evidence of lipid accumulation, including lipid-rich inflammatory cells and extracellular lipid deposition, in the early stages of CAV, indicating that early lipid accumulation can play a major role in the process of CAV (21). Thus, although CAV is pathologically quite different from native coronary atherosclerosis, it is reasonable to hypothesize that ASP in CAV might also reflect an aspect of plaque instability, as in the case of native atherosclerosis, and could be used to evaluate CAV severity after heart transplantation.
Our findings also suggest a close link between ASP progression at 1 year and chronic inflammation related to acute cellular rejection. Vascular inflammation plays a pivotal role in the initiation and progression of native coronary atherosclerosis (24). In heart transplantation, CAV is the predominant form of chronic rejection and both immunologic and nonimmunologic factors are involved in this process that ultimately causes localized sustained inflammation, persistent vascular injury, and endothelial dysfunction (2,3). Although CAV may develop at any stage after transplantation, events that occur during the first year, most likely arising from immunologically mediated injury to the vascular endothelium, appear to play an important role in CAV pathogenesis (7, 25). Previous studies have demonstrated an association of acute cellular rejection in the early post-transplant period with inflammatory plaque (increased necrotic core and dense calcium contents) and CAV progression late after heart transplantation (3, 7,12). Immunohistochemical studies also suggested that typical CAV lesions display a similar pattern of inflammatory changes when compared to the inflamed myocardium during acute cellular rejection (23,26). The current study used serial IVUS in the early post-transplant period and showed that early acute cellular rejection can also affect plaque destabilization (ASP progression) during the first year, supporting the major contribution of inflammatory pathways to the etiology of CAV.
Interestingly, compared to the episode(s) of acute cellular rejection during the first year, ASP progression at 1 year appears to have a greater impact on subsequent mortality. As described earlier, development of CAV is a multifactorial and complex process characterized by repetitive vascular injury and a localized sustained inflammatory response (2,3). Therefore, serial IVUS assessment of plaque instability (inflammatory plaque) may more accurately represent the cumulative effects of these inflammatory processes on CAV, compared to the stand-alone assessment of 1 mechanism (acute cellular rejection), indicating the incremental predictive value of inflammatory plaque for long-term prognosis. Particularly, the predictive value of ASP progression in clinical prognosis appeared to be more apparent in patients with newly developed ASP.
CORONARY INTIMAL THICKENING VERSUS PLAQUE INSTABILITY
In contrast to previous studies where a significant MIT increase during the first year was predictive of long-term mortality (14,15), the present study failed to show a significant association between coronary intimal thickening at 1 year and subsequent mortality or retransplantation. In the current era, most patients are treated with tacrolimus, rATG, and statins; multiple studies have shown a protective effect of these treatments on intimal proliferation (2,27,28). Therefore, in the era of modern medical treatments utilizing better immunosuppression and aggressive statin use, early intimal thickening alone may not be sufficiently sensitive to predict long-term mortality (4).
Another possible explanation for this discordance may derive from increasing donor age over the past decades, since increased prevalence of donor-transmitted atherosclerosis may affect the prognostic value of early intimal thickening. In fact, in the present study, significant MIT increase at 1 year was not associated with subsequent mortality in patients with donor-transmitted atherosclerosis (HR: 1.34; 95% CI: 0.29 to 4.69; p = 0.67), whereas this parameter tended to predict mortality in patients without donor-transmitted atherosclerosis (HR: 19.25; 95% CI: 0.76 to 486.31; p = 0.07). Similar results were also observed in other intimal parameters (volume and percent volume), possibly suggesting that early intimal thickening may remain a predictor for long-term mortality if patients show no significant donor-transmitted atherosclerosis at baseline. In contrast, the present study confirmed that paradoxical vessel remodeling of the proximal LAD at 1 year is a more consistent determinant of subsequent mortality or retransplantation, supporting the utility of the combined assessment of arterial remodeling with coronary intimal thickening, rather than intimal thickening alone (4).
Despite no significant difference in early intimal thickening or arterial remodeling found in patients with versus without ASP progression, ASP progression at 1 year was independently associated with long-term mortality or retransplantation. One reason why: plaque instability was associated with subsequent CAV progression; an IVUS study showed that inflammatory plaque is associated with subsequent coronary intimal growth and positive vessel remodeling (7). Indeed, patients with ASP at baseline demonstrated significantly greater intimal growth compared to those without ASP in the present study. Additionally, plaque destabilization may involve CAV progression in a manner distinct from the current concept of diffuse concentric intimal thickening and/or pathological arterial remodeling. According to previous studies of native coronary atherosclerosis, vulnerable plaque does not always accompany severe coronary stenosis, and plaque rupture (or erosion) can occur even in patients with mild-to-moderate disease (29,30). Recent transplant studies also demonstrated the presence of silent plaque rupture and nonocclusive mural thrombi (suggestive of healed plaque rupture and/or erosion) late after transplantation (12,20,31). The present study extended these investigations, indicating that the thrombosis theory might also apply to CAV and that plaque instability could cause cardiac events regardless of significant coronary intimal thickening and/or negative vessel remodeling, suggesting plaque instability as an important independent risk factor for CAV progression.
POSSIBLE EFFECTS OF MEDICAL TREATMENTS
Mechanisms of ASP regression and medical treatments related to this phenomenon are of great interest. In this study, ASP regression was associated with less frequent acute cellular rejection during the first year, a lower rate of insulin use at 1 year, and a higher rate of tacrolimus use. Additionally, donor-transmitted atherosclerosis was independently associated with ASP progression, suggesting that both immunologic and nonimmunologic factors play important roles in ASP changes. However, retrospective analyses are susceptible to confounding factors; therefore, further studies are needed, particularly to determine the effects of specific medical therapies on ASP changes.
Compared to other immunosuppressants, sirolimus was used more frequently in patients with ASP progression at 1 year and subsequent mortality in the categorical analysis. Potential selection bias should be noted when interpreting these results, as sirolimus is generally used for patients with severe or recurrent rejection, significant CAV progression, and/or renal insufficiency. Also, in the present study, sirolimus was used more frequently in combination with cyclosporine (92.9%) than with tacrolimus (7.1%); the latter showed trends toward ASP regression and improved long-term mortality. Thus, the selection bias of sirolimus, rather than sirolimus itself, might have contributed to the univariate categorical analysis results. Indeed, sirolimus use was not associated with ASP progression or subsequent mortality in multivariate analysis or Cox proportional hazard analysis. Furthermore, patients with sirolimus had a smaller increase in intima (volume: 0.1 ± 0.7 vs. 0.6 ± 0.9 mm3/mm; p = 0.01; percent volume: 2.2 ± 7.5% vs. 5.4 ± 5.6%; p = 0.006) at 1 year compared to those without, which might even suggest a possible protective effect on CAV progression.
STUDY LIMITATIONS
First, we hypothesized that ASP reflects one aspect of plaque instability; however, unlike native coronary atherosclerosis, ASP in CAV has not been validated with histopathology and its exact pathology needs to be elucidated in further studies. Second, we analyzed CAV only in the LAD, which has been recognized as an important determinant of patient prognosis. Although previous studies have demonstrated good correlation between LAD imaging and incidence of CAV (32), it remains unknown whether 3-vessel analyses could offer additional prognostic value. Third, the present study did not routinely measure inflammatory biomarkers; therefore, their possible associations with ASP progression remain unknown. Fourth, as this was a retrospective, single-center study, our findings need to be confirmed in prospective studies with pre-defined endpoints. Finally, the multivariable model in this study is still over-fitted by conventional standards and requires replication in a larger sample.
CONCLUSIONS
The present study demonstrated that ASP progression at 1-year post-transplant appeared to represent immune-mediated chronic inflammation related to acute cellular rejection, and was an independent predictor of long-term mortality or retransplantation. Therefore, serial IVUS assessment of plaque instability in CAV early after transplantation may add incremental prognostic value to the standard assessment of intimal thickening and pathological arterial remodeling, enhancing the identification of high-risk patients who may benefit from closer follow-up and targeted medical therapies.
CENTRAL ILLUSTRATION ASP Post-Transplantation.
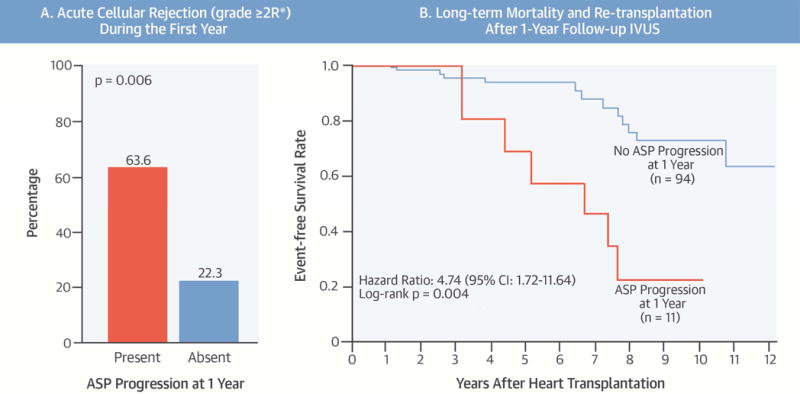
This retrospective study aimed to characterize the association between attenuated-signal plaque (ASP) and long-term mortality post-heart transplantation. (A) During the first year post-implant, patients with ASP progression experienced significantly greater acute cellular rejection. (B) Patients with ASP progression at 1 year also demonstrated a significantly lower long-term event-free survival rate.
Perspectives.
COMPETENCY IN PATIENT CARE
IVUS assessment of plaque instability (ASP progression) early after heart transplantation identifies high-risk plaques.
TRANSLATIONAL OUTLOOK
Further studies are needed to define the mechanisms of plaque instability after transplantation and evaluate the efficacy of specific medical therapies in patients with allograft vasculopathy.
Acknowledgments
Funds: This study was funded in part by grants R01 HL093475-01A1 (WFF), and 1 PO1-AI50153 (HAV) from the National Institutes of Health, (National Heart Lung and Blood Institute), Bethesda, MD.
ABBREVIATIONS AND ACRONYMS
- ASP
attenuated-signal plaque
- CAV
cardiac allograft vasculopathy
- IVUS
intravascular ultrasound
- LAD
left anterior descending artery
- MIT
maximal intimal thickness
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Nothing to disclose
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report–2012. J Heart Lung Transplant. 2012;31:1052–64. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131–41. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 3.Vassalli G, Gallino A, Weis M, et al. Alloimmunity and nonimmunologic risk factors in cardiac allograft vasculopathy. Eur Heart J. 2003;24:1180–8. doi: 10.1016/s0195-668x(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 4.Okada K, Kitahara H, Yang H-M, et al. Paradoxical Vessel Remodeling of the Proximal Segment of the Left Anterior Descending Artery Predicts Long-Term Mortality After Heart Transplantation. J Am Coll Cardiol Fail. 2015;3:942–52. doi: 10.1016/j.jchf.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 5.König A, Kilian E, Sohn H-Y, et al. Assessment and characterization of time-related differences in plaque composition by intravascular ultrasound-derived radiofrequency analysis in heart transplant recipients. J Heart Lung Transplant. 2008;27:302–9. doi: 10.1016/j.healun.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez JM, de L T, de Prada JAV, Burgos V, et al. Virtual histology intravascular ultrasound assessment of cardiac allograft vasculopathy from 1 to 20 years after heart transplantation. J Heart Lung Transplant. 2009;28:156–62. doi: 10.1016/j.healun.2008.11.915. [DOI] [PubMed] [Google Scholar]
- 7.Raichlin E, Bae J-H, Kushwaha SS, et al. Inflammatory burden of cardiac allograft coronary atherosclerotic plaque is associated with early recurrent cellular rejection and predicts a higher risk of vasculopathy progression. J Am Coll Cardiol. 2009;53:1279–86. doi: 10.1016/j.jacc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 8.Pu J, Mintz GS, Biro S, et al. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2,294 human coronary artery segments. J Am Coll Cardiol. 2014;63:2220–33. doi: 10.1016/j.jacc.2014.02.576. [DOI] [PubMed] [Google Scholar]
- 9.Lee SY, Mintz GS, Kim S-Y, et al. Attenuated plaque detected by intravascular ultrasound: clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. J Am Coll Cardiol Interv. 2009;2:65–72. doi: 10.1016/j.jcin.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Kimura S, Kakuta T, Yonetsu T, et al. Clinical significance of echo signal attenuation on intravascular ultrasound in patients with coronary artery disease. Circ Cardiovasc Interv. 2009;2:444–54. doi: 10.1161/CIRCINTERVENTIONS.108.821124. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Mintz GS, Xu K, et al. The relationship between attenuated plaque identified by intravascular ultrasound and no-reflow after stenting in acute myocardial infarction: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol Interv. 2011;4:495–502. doi: 10.1016/j.jcin.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Dong L, Maehara A, Nazif TM, et al. Optical coherence tomographic evaluation of transplant coronary artery vasculopathy with correlation to cellular rejection. Circ Cardiovasc Interv. 2014;7:199–206. doi: 10.1161/CIRCINTERVENTIONS.113.000949. [DOI] [PubMed] [Google Scholar]
- 13.Xu K, Mintz GS, Kubo T, et al. Long-term follow-up of attenuated plaques in patients with acute myocardial infarction: an intravascular ultrasound substudy of the HORIZONS-AMI trial. Circ Cardiovasc Interv. 2012;5:185–92. doi: 10.1161/CIRCINTERVENTIONS.111.964684. [DOI] [PubMed] [Google Scholar]
- 14.Tuzcu EM, Kapadia SR, Sachar R, et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J Am Coll Cardiol. 2005;45:1538–42. doi: 10.1016/j.jacc.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 15.Kobashigawa JA, Tobis JM, Starling RC, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532–7. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Tanaka K, Anzai H, et al. Influence of pre-existing donor atherosclerosis on the development of cardiac allograft vasculopathy and outcomes in heart transplant recipients. J Am Coll Cardiol. 2006;47:2470–6. doi: 10.1016/j.jacc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 17.Lee T, Kakuta T, Yonetsu T, et al. Assessment of echo-attenuated plaque by optical coherence tomography and its impact on post-procedural creatine kinase-myocardial band elevation in elective stent implantation. J Am Coll Cardiol Interv. 2011;4:483–91. doi: 10.1016/j.jcin.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 19.Akyildiz AC, Speelman L, van Brummelen H, et al. Effects of intima stiffness and plaque morphology on peak cap stress. Biomed Eng Online. 2011;10:25. doi: 10.1186/1475-925X-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassar A, Matsuo Y, Herrmann J, et al. Coronary atherosclerosis with vulnerable plaque and complicated lesions in transplant recipients: new insight into cardiac allograft vasculopathy by optical coherence tomography. Eur Heart J. 2013;34:2610–7. doi: 10.1093/eurheartj/eht236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng B, Maehara A, Mintz GS, et al. Increased coronary lipid accumulation in heart transplant recipients with prior high-grade cellular rejection: novel insights from near-infrared spectroscopy. Int J Cardiovasc Img. 2016;32:225–34. doi: 10.1007/s10554-015-0777-9. [DOI] [PubMed] [Google Scholar]
- 22.Sarno G, Lerman A, Bae J-H, et al. Multicenter assessment of coronary allograft vasculopathy by intravascular ultrasound-derived analysis of plaque composition. Nat Clin Pract Cardiovasc Med. 2009;6:61–9. doi: 10.1038/ncpcardio1410. [DOI] [PubMed] [Google Scholar]
- 23.Angelini A, Castellani C, Fedrigo M, et al. Coronary cardiac allograft vasculopathy versus native atherosclerosis: difficulties in classification. Virchows Arch. 2014;464:627–35. doi: 10.1007/s00428-014-1586-6. [DOI] [PubMed] [Google Scholar]
- 24.Choi H, Cho DH, Shin HH, Park JB. Association of high sensitivity C-reactive protein with coronary heart disease prediction, but not with carotid atherosclerosis, in patients with hypertension. Circ J. 2004;68:297–303. doi: 10.1253/circj.68.297. [DOI] [PubMed] [Google Scholar]
- 25.Kobashigawa JA. First-year intravascular ultrasound results as a surrogate marker for outcomes after heart transplantation. J Heart Lung Transplant. 2003;22:711–4. doi: 10.1016/s1053-2498(03)00210-9. [DOI] [PubMed] [Google Scholar]
- 26.van Loosdregt J, van Oosterhout MFM, Bruggink AH, et al. The chemokine and chemokine receptor profile of infiltrating cells in the wall of arteries with cardiac allograft vasculopathy is indicative of a memory T-helper 1 response. Circulation. 2006;114:1599–607. doi: 10.1161/CIRCULATIONAHA.105.597526. [DOI] [PubMed] [Google Scholar]
- 27.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 28.Azarbal B, Cheng R, Vanichsarn C, et al. Induction Therapy With Antithymocyte Globulin in Patients Undergoing Cardiac Transplantation Is Associated With Decreased Coronary Plaque Progression as Assessed by Intravascular Ultrasound. Circ Heart Fail. 2016;9:e002252. doi: 10.1161/CIRCHEARTFAILURE.115.002252. [DOI] [PubMed] [Google Scholar]
- 29.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–66. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 30.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–61. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo Y, Cassar A, Li J, et al. Repeated episodes of thrombosis as a potential mechanism of plaque progression in cardiac allograft vasculopathy. Eur Heart J. 2013;34:2905–15. doi: 10.1093/eurheartj/eht209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–58. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]


