Abstract
Background
Placement of left ventricular assist devices (LVAD) as a bridge-to-heart transplantation (HTx) has rapidly expanded due to organ donor shortage. However, the timing of LVAD implantation is variable and it remains unclear if earlier implantation improves survival.
Methods
We analyzed 14,187 adult candidates from the United Network of Organ Sharing database. Patients were classified by 3 treatment strategies including patients medically treated alone (MED, n=11,009), patients on LVAD support at listing (Early-LVAD, n=1588) and patients undergoing LVAD placement while awaiting HTx (Delayed-LVAD, n=1590). Likelihood of HTx and event-free survival were assessed in patients subcategorized by clinical strategies and UNOS status at listing.
Results
The device support strategy, despite the timing of placement, was not associated with increased likelihood of HTx compared to MED group. However, both LVAD implantation strategies showed better survival compared to MED group (Early-LVAD: HR 0.811 and 0.633, 95% CI 0.668-0.984 and 0.507-0.789, for 1A and 1B; p=0.034 and p<0.001, Delayed-LVAD: HR 0.553 and 0.696, 95% CI 0.415-0.736 and 0.571-0.847, for 1A and 1B; both p<0.001, respectively). Furthermore, there was no significant difference in survival between these LVAD implantation strategies in patients listed as 1B (p=0.500), although Early-LVAD implantation showed worse survival in patients listed as 1A (HR 1.467, 95% CI 1.076-2.000; p=0.015).
Conclusion
LVAD support strategies offer a safe bridge-to-HTx. Those candidates who receive urgent upfront LVAD implantation for HTx, and improve to 1B status, would achieve competitive survival with those who receive elective LVAD implantation.
Keywords: Heart Failure, LVAD, Heart Transplantation
Introduction
Heart transplantation (HTx) provides considerable survival benefit and improvement of quality-of-life for patients with end-stage heart failure (HF). This therapeutic option is severely limited because of donor shortage (1-3). Therefore, a growing number of HTx candidates require left ventricular assist devices (LVAD) implantation while awaiting HTx (4). However, how the presence or the timing of LVAD placement affects the likelihood of HTx has not been fully elucidated. Whether earlier LVAD implantation improves survival to and following HTx has not been studied.
In the present study, we investigated the incidence of death, de-listing and HTx rates, as well as the likelihood of achieving HTx in patients listed in the United Network of Organ Sharing (UNOS) registry in the current era with and without mechanical support. We also investigated the post-HTx outcome and the cumulative event-free survival from the time of listing in all patients subcategorized according to the clinical course and initial UNOS status at listing.
Methods
Data acquisition
Standard transplant analysis and research files with follow-up data were provided by the UNOS database. We identified 15,979 patients who were 18 years of age or older and listed for HTx in the UNOS database between July 12, 2006 and April 30, 2012. All outcomes on the waiting-list and survival post-HTx were included until October 30, 2012.
Patients listed for re-transplantation or multi-organ transplantations were excluded. Patients who required right ventricular assist device (RVAD), biventricular assist device (BiVAD) or total artificial heart (TAH) placement at listing or during the waiting period were also excluded as support with these devices is less successful than the standard LVAD use (5-8).
The wait-list status at listing and at HTx, mechanical circulatory support (MCS) requirement both at listing and while awaiting HTx and the outcome data including death, delisting and HTx were collected. We analyzed the post-HTx survival data of patients who were successfully transplanted and the cumulative event-free survival from the time of listing.
Patient classification and events while being listed
A total of 14,187 adult HTx candidates were included in this study. The baseline demographics are shown in Figure 1. Patients were first divided into 2 groups according to whether they were medically treated or already supported by LVAD at listing. Next, patients who were medically treated at listing were classified into 2 groups according to whether or not they required LVAD support during the listing period. Finally, patients were classified into 3 groups according to their treatment course: (i) patients medically treated without mechanical support throughout the waitlist-period (MED, n=11,009), (ii) patients who were medically treated at listing and who subsequently underwent LVAD implantation (Delayed-LVAD, n=1590), and (iii) patients on LVAD at listing (Early-LVAD, n=1588). The end-point events during listing periods included death, delisting due to disqualification of transplant eligibility and HTx.
Figure 1.
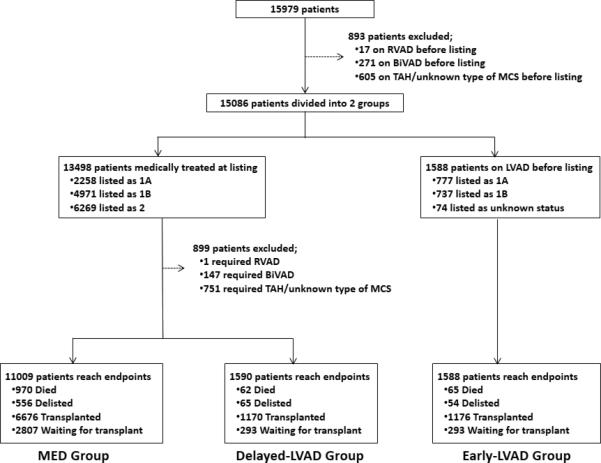
Study design and patient classification.
Clinical characteristics at listing were compared among the three groups (Table 1A). We also compared the clinical characteristics of patients who survived to HTx in each category (Table 1B). The incidence of death, death/delisting and HTx in each group was calculated and we assessed and compared the likelihood of achieving HTx of each group. Subsequently, the post-HTx survival of patients who achieved HTx and the cumulative event-free survival from the time of listing were assessed and compared among the groups. Furthermore, the incidence of end-point events, the likelihood of HTx, the post-HTx survival and the cumulative event-free survival from the time of listing by wait-list status (UNOS 1A and 1B) were compared.
Table 1A.
Patient characteristics at the time of listing for HTx
| All cohort (n=14187) | MED group (n=11009) | Delayed-LVAD group (n=1590) | Early-LVAD group (n=1588) | p-value | |
|---|---|---|---|---|---|
| Age, y | 52.0±12.7 | 52.2±12.8 | 50.7±12.3 | 52.0±12.3 | <0.001 |
| Male sex, n (%) | 10595 (74.7) | 8009 (72.7) | 1297 (81.6) | 1289 (81.2) | <0.001 |
| BMI, Kg/m2 | 26.9±5.9 | 26.6±6.2 | 27.9±5.0 | 27.7±5.0 | <0.001 |
| Race, n (%) | <0.001 | ||||
| White | 9737 (68.6) | 7625 (69.3) | 1031 (64.8) | 1081 (68.1) | |
| Black | 2888 (20.4) | 2132 (19.4) | 405 (25.5) | 351 (22.1) | |
| Hispanic | 1061 (7.5) | 859 (7.8) | 106 (6.7) | 96 (6.0) | |
| Asian | 356 (2.5) | 278 (2.5) | 32 (2.0) | 46 (2.9) | |
| Other | 145 (1.0) | 115 (1.0) | 16 (1.0) | 14 (0.9) | |
| Blood type, n (%) | <0.001 | ||||
| A | 5556 (39.1) | 4471 (40.6) | 500 (31.4)* | 585 (36.8) | |
| B | 1888 (13.3) | 1497 (13.6) | 175 (11.0) | 216 (13.6) | |
| AB | 649 (4.6) | 567 (5.2) | 23 (1.4) | 59 (3.7) | |
| O | 6094 (43.0) | 4474 (40.6) | 892 (56.1) | 728 (45.9) | |
| Etiology, n (%)* | <0.001 | ||||
| Ischemic | 3426 (37.8) | 2445 (36.5) | 430 (36.6) | 551 (46.5) | |
| Non-ischemic | 4411 (48.7) | 3178 (47.5) | 664 (56.5) | 569 (48.1) | |
| Hypertrophic | 191 (2.1) | 170 (2.5) | 16 (1.4) | 5 (0.4) | |
| Congenital | 232 (2.6) | 221 (3.3) | 5 (0.4) | 6 (0.5) | |
| Other | 795 (8.8) | 681 (10.2) | 61 (5.1) | 53 (4.5) | |
| UNOS status at listing, n (%) | <0.001 | ||||
| 1A | 2813 (19.9) | 1711 (15.5) | 325 (20.4)* | 777 (48.9) | |
| 1B | 5308 (37.4) | 3853 (35.0) | 718 (45.2)* | 737 (46.4) | |
| 2 | 5992 (42.2) | 5445 (49.5) | 547 (34.4)* | n/a † | |
| Comorbidities, n (%) * | |||||
| Diabetes mellitus | 4036 (28.4) | 3071 (27.9) | 468 (29.4) | 497 (31.3) | 0.012 |
| Cerebrovascular disease | 235 (4.2) | 202 (4.3) | 15 (3.5) | 18 (4.2) | 0.709 |
| Hypertension | 303 (43.0) | 274 (42.8) | 11 (37.9) | 18 (51.4) | 0.515 |
| Outcome, n (%) | <0.001 | ||||
| Died | 1097 (7.7) | 970 (8.8) | 62 (3.9) | 65 (4.1) | |
| Delisted | 685 (4.8) | 556 (5.1) | 65 (4.1) | 54 (3.4) | |
| Transplanted | 9022 (63.6) | 6676 (60.6) | 1170 (73.6) | 1176 (74.1) | |
| Still waiting/censored | 3383 (23.9) | 2807 (25.5) | 293 (18.4) | 293 (18.4) | |
Data not obtained from all patients, n (%) indicating the numbers/percentages of patients based on the available data.
74 patients in early-LVAD group did not have their listing UNOS status.
HTx indicates heart transplantation; BMI, body mass index; MED, medically treated; LVAD, left ventricular assist device.
Table 1B.
Patient characteristics at the time of HTx
| All recipients (n=9022) | MED group (n=6676) | Delayed-LVAD group (n=1170) | Early-LVAD group (n=1176) | p value | |
|---|---|---|---|---|---|
| Age, y | 52.6±12.6 | 52.6±12.7 | 51.9±12.2 | 52.8±12.4 | 0.149 |
| Male sex, n (%) | 6727 (74.6) | 4789 (71.7) | 974 (83.2) | 964 (81.9) | <0.001 |
| BMI, Kg/m2 | 26.9±6.0 | 26.6±6.2 | 27.9±5.0 | 27.8±5.0 | <0.001 |
| Race, n (%) | 0.007 | ||||
| White | 6177 (68.5) | 4584 (68.7) | 774 (66.2) | 819 (69.6) | |
| Black | 1794 (19.9) | 1275 (19.1) | 283 (24.2) | 236 (20.1) | |
| Hispanic | 708 (7.8) | 553 (8.3) | 79 (6.8) | 76 (6.5) | |
| Asian | 254 (2.8) | 194 (2.9) | 25 (2.1) | 35 (3.0) | |
| Other | 89 (1.0) | 70 (1.1) | 9 (0.8) | 10 (0.8) | |
| Blood type, n (%) | <0.001 | ||||
| A | 3795 (42.1) | 2940 (44.1) | 389 (33.2) | 466 (39.6) | |
| B | 1326 (14.7) | 1031 (15.4) | 131 (11.2) | 164 (13.9) | |
| AB | 522 (5.8) | 455 (6.8) | 18 (1.5) | 49 (4.2) | |
| O | 3379 (37.4) | 2250 (33.7) | 632 (54.0) | 497 (42.4) | |
| Etiology, n (%) | <0.001 | ||||
| Ischemic | 3418 (37.9) | 2441 (36.6) | 429 (36.7) | 548 (46.5) | |
| Non-ischemic | 4389 (48.6) | 3165 (47.4) | 660 (56.4) | 564 (48.0) | |
| Hypertrophic | 191 (2.1) | 170 (2.5) | 16 (1.4) | 5 (0.4) | |
| Congenital | 231 (2.6) | 220 (3.3) | 5 (0.4) | 6 (0.5) | |
| Other | 793 (8.8) | 680 (10.2) | 60 (5.1) | 53 (4.6) | |
| UNOS status at listing, n (%) | <0.001 | ||||
| 1A | 1988 (22.0) | 1161 (17.4) | 234 (20.0) | 593 (50.4) | |
| 1B | 3797 (42.1) | 2748 (41.2) | 525 (44.9) | 524 (44.6) | |
| 2 | 3178 (35.2) | 2767 (41.4) | 411 (35.1) | n/a † | |
| UNOS status at HTx, n (%) | <0.001 | ||||
| 1A | 4661 (51.7) | 2760 (41.3) | 947 (80.9) | 956 (81.2) | |
| 1B | 3468 (38.4) | 3025 (45.3) | 222 (19.0) | 221 (18.8) | |
| Total waiting time to HTx (days) | 143.8±203.0 | 116.8±184.6 | 310.0±257.7 | 131.6±157.8 | <0.001 |
| Comorbidities, n (%)* | |||||
| Diabetes mellitus | 2455 (27.2) | 1746 (26.2) | 344 (29.4) | 365 (31.1) | <0.001 |
| Cerebrovascular accident | 5 (1.2) | 4 (1.0) | 0 (0.0) | 1 (4.0) | <0.001 |
| Infection at HTx | 855 (9.5) | 456 (6.8) | 190 (16.2) | 209 (17.8) | <0.001 |
| Laboratory data | |||||
| Creatinine (mg/dl) | 1.36±0.92 | 1.38±0.98 | 1.28±0.73 | 1.28±0.72 | <0.001 |
| Albumin (g/dl) | 3.68±0.70 | 3.72±0.69 | 3.62±0.69 | 3.50±0.75 | <0.001 |
| Total bilirubin (mg/dl) | 1.19±1.95 | 1.24±2.04 | 1.06±1.43 | 1.02±1.88 | <0.001 |
| HTx-related information | |||||
| Ischemic time (hours) | 3.24±1.05 | 3.22±1.04 | 3.33±1.06 | 3.30±1.07 | <0.001 |
| Donor age, y | 31.6±11.9 | 31.8±12.2 | 30.9±10.8 | 31.0±11.1 | 0.007 |
Data not obtained from all patients, n (%) indicating the numbers/percentages of patients based on the available data.
59 patients in early-LVAD group did not have their listing UNOS status.
HTx indicates heart transplantation; BMI, body mass index; MED, medically treated; LVAD, left ventricular assist device.
The UNOS status 1A is designated for candidates who have the highest priority on the basis of medical urgency. Patients may be listed as UNOS status 1A for 30 days at any time after LVAD implantation or if they are experiencing LVAD-related complications such as infection, thromboembolism or device malfunction. Patients who are supported by LVAD but who do not meet the aforementioned criteria are listed as UNOS status 1B. UNOS status 2 does not apply to patients with LVAD (9).
Statistical analysis
Continuous data are presented as mean ± SD and Student's unpaired t-test and one-way ANOVA were used to compare the variables among the groups. Categorical variables were summarized as frequencies and percentages and were compared using the Pearson χ2 test or the Fisher exact test. A p-value <0.05 was considered statistically significant. The incidence of death, delisting and HTx in each group were calculated and expressed by the number of end-point events per 100 person-month. The competing risk regression models were used to assess the likelihoods of HTx with death, delisting and HTx as the competing events from the time of listing. Variables considered for adjustment were patient background including age, gender, race, blood type and coexistence of diabetes mellitus. The cumulative post-HTx survival was calculated using Kaplan-Meier product-limit estimators and compared using the log-rank test among the groups. The Cox proportional hazard model was used for the analysis of post-HTx survival to adjust patient background which were the same variables used in the comparison analysis of the likelihood of HTx. The cumulative event-free survival from the time of listing was also calculated using Kaplan-Meier product-limit estimators and compared using the competing risk regression models to adjust patient background which were the same variables used in the comparison analysis of the likelihood of successful transplant. We defined the duration from the time of listing to death and delisting due to disqualification of transplant eligibility while listed or the post-HTx death if patients successfully transplanted. And we also defined the duration from the time of listing to the last censoring if patients were still waiting for HTx or underwent HTx and survived as a follow-up period. All statistical analyses were performed using SAS 9.2 software and R package cmprsk (http://cran.r-project.org/web/packages/cmprsk/index.html).
Results
Baseline demographics
There were 15,979 adult candidates listed for de-novo heart-only transplantation in the UNOS database. We excluded 893 patients who were on RVAD, BiVAD, TAH, or on unknown type of MCS at listing. Among the 15,086 patients, 13,498 patients (89.5%) were treated medically only. The remaining 1588 patients (10.5%) were on LVAD support at listing and were classified as the Early-LVAD group. Among patients medically treated at listing, 1590 patients (11.8%) required LVAD implantation during listing (Delayed-LVAD group). 899 patients (6.7%) required RVAD, BiVAD, TAH, and unknown type of MCS implantation during listing and these patients were also excluded from the analysis. The remaining 11,009 patients (81.6%) were medically treated throughout their waiting periods (MED group) (Figure 1).
Clinical characteristics of all patients at listing
Patient characteristics at listing are summarized in Table 1A. Patients were more likely to be male, with larger body mass indexes and blood type O in the Early-LVAD and Delayed-LVAD groups as compared to the MED group. Patients with hypertrophic cardiomyopathy and congenital heart disease were less likely to receive LVAD. Among 11,009 patients in the MED group, 6676 patients (60.6%) underwent HTx while 1170 patients out of 1590 patients (73.6%) in the Delayed-LVAD group and 1176 patients among 1588 patients (74.1%) in the Early-LVAD group received a HTx. Fewer patients died and more were transplanted in LVAD recipients as compared to the MED group. More deaths occurred in the MED group despite the fact that more patients continued to await HTx at the end of the observation period.
Clinical characteristics of patients at the time of HTx
Clinical characteristics of patients undergoing HTx are summarized in Table 1B. Patients supported by LVAD regardless of the timing of implant (both Early-LVAD and Delayed-LVAD groups) showed higher incidence of infection and longer ischemic times as compared to patients in the MED group. Serum albumin levels of patients supported by LVAD were lower than those of the MED group. Among LVAD recipients, albumin levels were even lower in the Early-LVAD compared to the Delayed-LVAD group. Both groups of patients supported by LVAD showed lower serum total bilirubin levels at the time of HTx compared to the MED group.
Incidence of death, delisting and HTx
Table 2 summarizes the incidence of death, death/delisting and achieving HTx in patients listed for HTx according to their treatment course and UNOS waitlist status (1A and 1B). The analysis of the patient clinical course revealed that the Delayed-LVAD group showed the lowest incidence of death and/or delisting; however, also the lowest HTx rates. On the other hand, the Early-LVAD group showed lower incidence of death and/or delisting but higher HTx rates when compared to the MED group.
Table 2.
Incidence of death, delisting and HTx while listed and comparison of the likelihood of HTx among the 3 clinical cohorts.
| Events while listed | Likelihood of HTx | ||||||
|---|---|---|---|---|---|---|---|
| Death | Death/Delisting | HTx | HR (95% CI) ‡ | p-value | HR (95% CI) § | p-value | |
| Clinical Course | |||||||
| MED group (n=11009) | 1.15/100PM | 1.83/100PM | 7.94/100PM | 1.000 | |||
| Delayed-LVAD group (n=1590) | 0.30/100PM | 0.62/100PM | 5.70/100PM | 0.968 (0.921-1.017) | 0.190 | 1.000 | |
| Early-LVAD group (n=1588) | 0.65/100PM | 1.19/100PM | 11.77/100PM | 1.389 (1.307-1.477) | <0.001 | 1.435 (1.339-1.538) | <0.001 |
| Waitlist Status when being listed | |||||||
| Listed as UNOS status 1A | |||||||
| MED group (n=1711) | 5.23/100PM | 8.95/100PM | 31.30/100PM | 1.000 | |||
| Delayed-LVAD group (n=325) | 0.63/100PM | 1.04/100PM | 7.36/100PM | 0.627 (0.563-0.699) | <0.001 | 1.000 | |
| Early-LVAD group (n=777) | 1.15/100PM | 2.07/100PM | 17.00/100PM | 0.980 (0.889-1.081) | 0.690 | 1.563 (1.395-1.751) | <0.001 |
| Listed as UNOS status 1B | |||||||
| MED group (n=3853) | 1.98/100PM | 3.10/100PM | 17.00/100PM | 1.000 | |||
| Delayed-LVAD group (n=718) | 0.30/100PM | 0.73/100PM | 6.22/100PM | 0.651 (0.603-0.702) | <0.001 | 1.000 | |
| Early-LVAD group (n=737) | 0.34/100PM | 0.66/100PM | 8.90/100PM | 0.756 (0.696-0.820) | <0.001 | 1.161 (1.056-1.277) | 0.002 |
Comparison analysis being adjusted by age, gender, race, blood type, and coexisting of diabetes mellitus, MED group as a reference
Comparison analysis being adjusted by age, gender, race, blood type, and coexisting of diabetes mellitus, Delayed-LVAD group as a reference
HTx indicates heart transplantation; MED, medically treated; LVAD, left ventricular assist device; 100MP, 100 person-month; HR hazard ratio; CI, confidential interval.
When we sub-classified patients according to their UNOS status at listing, the incidence of death/delisting were equivalent but HTx rates were higher in the MED group irrespective of the UNOS listing status. As compared to the Early-LVAD group, the Delayed-LVAD group showed lower incidence of death/delisting when they listed as UNOS status 1A.
Likelihood of HTx
The competing risk regression analysis adjusted by patient background was performed to compare the association between the patient clinical course and their likelihood of undergoing HTx (Table 2). Patients in the Early-LVAD group showed the highest likelihood of HTx among the three groups (HR 1.389 95% CI: 1.307-1.477, p<0.001, versus MED; HR 1.435 95% CI 1.339-1.538, p<0.001, versus Delayed-LVAD). Patients in the MED group listed as UNOS status 1A showed a higher likelihood of achieving HTx compared to the Delayed-LVAD group (HR of Delayed-LVAD to MED as a reference: 0.627 95% CI 0.563-0.699, p<0.001). Patients listed as UNOS status 1B showed the highest likelihood of HTx among the three groups (HR of Delayed-LVAD compare to MED as a reference: 0.651 95% CI 0.603-0.702, p<0.001; HR of Early-LVAD 0.756 95% CI 0.696-0.820, p<0.001, respectively). Patients of the Early-LVAD group showed a higher likelihood of HTx compared to the Delayed-LVAD group in the analysis of both the patient overall clinical course and the UNOS waitlist status when being listed (HR 1.435 95% CI 1.339-1.538, p<0.001, for overall clinical course comparison; HR 1.563 95% CI 1.395-1.751, p<0.001, for UNOS status 1A; HR 1.161 95% CI 1.056-1.277, p=0.002, for UNOS status 1B).
Comparison of post-HTx survival
We also calculated and compared the cumulative post-HTx survival among the three groups. The Early-LVAD group showed worse survival following HTx compared to the MED group (HR 1.181, 95% CI 1.006-1.387, p=0.042) Among the patients who were listed at UNOS status 1A, the Early-LVAD group also showed worse survival compared to the MED group (HR 1.305, 95% CI 1.019-1.671, p=0.035) as well as compared to the Delayed-LVAD group (HR 1.617, 95% CI 1.067-2.451, p=0.024). Patients listed at UNOS status 1B showed no significant differences in post-HTx outcome among the three groups.
Event-free survival from the time of listing for HTx
Table 4 shows the cumulative event-free survival from the time of listing for HTx (including the time awaiting and following HTx) and the comparison of event-free survival among the three groups. The Delayed-LVAD group showed the lowest risk of adverse events following listing for HTx which included death, delisting due to worsening status while listed and death after HTx among the three groups. (HR of Delayed-LVAD compared to MED: 0.693, 95% CI: 0.607-0.791, p<0.001; HR of Early-LVAD compared to Delayed-LVAD: 1.278, 95% CI: 1.074-1.520, p=0.006, respectively). Among patients listed as UNOS status 1A, the Delayed-LVAD group also showed the lowest risk of adverse events among the three groups (HR of Delayed-LVAD compared to MED: 0.553, 95% CI: 0.415-0.736, p<0.001; HR of Early-LVAD compared to Delayed-LVAD: 1.467, 95% CI: 1.076-2.000, p=0.015, respectively). Among patients listed as UNOS status 1B, all LVAD-supported patients showed a lower incidence of adverse events compared to the MED group (HR of Early-LVAD: 0.633, 95% CI: 0.507-0.789, p<0.001; HR of Delayed-LVAD: 0.696, 95% CI: 0.571-0.847, p<0.001, respectively). No risk difference was noted between the two groups of LVAD supported patients (Early-LVAD versus Delayed-LVAD, p=0.500).
Table 4.
Comparison of survival from the time of listing among the 3 cohorts.
| Survival from the time of listing | ||||||
|---|---|---|---|---|---|---|
| 3-year survival (%±SE) | 5-year survival (%±SE) | HR (95% CI) ‡ | p-value | HR (95% CI) § | p-value | |
| Clinical Course | ||||||
| MED group (n=11009) | 78.7±0.47 | 72.1±0.71 | 1.000 | |||
| Delayed-LVAD group (n=1590) | 83.1 ± 1.12 | 75.0±2.06 | 0.693 (0.607-0.791) | <0.001 | 1.000 | |
| Early-LVAD group (n=1588) | 81.1 ± 1.34 | 67.7±3.30 | 0.886 (0.775-1.012) | 0.074 | 1.278 (1.074-1.520) | 0.006 |
| UNOS status at the time of listing | ||||||
| Listed as UNOS status 1A | ||||||
| MED group (n=1711) | 74.9±1.23 | 68.5±1.88 | 1.000 | |||
| Delayed-LVAD group (n=325) | 85.7±2.26 | 65.9±9.01 | 0.553 (0.415-0.736) | <0.001 | 1.000 | |
| Early-LVAD group (n=777) | 77.1 ±1.99 | 60.6±5.32 | 0.811 (0.668-0.984) | 0.034 | 1.467 (1.076-2.000) | 0.015 |
| Listed as UNOS status 1B | ||||||
| MED group (n=3853) | 77.5 ±1.24 | 69.6±1.29 | 1.000 | |||
| Delayed-LVAD group (n=718) | 80.3±2.26 | 76.8±2.39 | 0.696 (0.571-0.847) | <0.001 | 1.000 | |
| Early-LVAD group (n=737) | 83.5±2.00 | 74.5 ±4.48 | 0.633 (0.507-0.789) | <0.001 | 0.909 (0.691-1.200) | 0.500 |
Comparison analysis being adjusted by age, gender, race, blood type, and coexisting of diabetes mellitus, MED group as a reference
Comparison analysis being adjusted by age, gender, race, blood type, and coexisting of diabetes mellitus, Delayed-LVAD group as a reference
HTx indicates heart transplantation; MED, medically treated; LVAD, left ventricular assist device; SE, standard error; HR, hazard ratio; CI, confidential interval.
Discussion
Our current study demonstrates that medically-treated patients awaiting HTx have a higher risk of death and delisting but a higher incidence of HTx compared to LVAD supported patients. The likelihood of undergoing HTx was higher in medically treated patients listed both as UNOS status 1A and 1B. Patients already LVAD-supported at listing for HTx who were listed as UNOS status 1A, had a higher likelihood of HTx compared to patients requiring LVAD placement after listing for HTx but demonstrated the lowest post-HTx survival. Finally, patients already LVAD-supported at listing for HTx who were listed as UNOS status 1B also had significantly higher incidence of HTx and demonstrated statistical equivalent cumulative survival from the time of listing including the waiting period and the time following HTx compared to patients required LVAD support after listing for HTx. Therefore, our data suggest that an upfront LVAD implantation strategy is superior to medical treatment alone and equivalent to an elective LVAD implantation strategy in patients who achieved stability as evidenced by downgrading to UNOS status 1B.
LVAD has become a standard therapy for patients with advanced HF both as a bridge-totransplantation (BTT) as well as a destination therapy (4,10-11). In the era of continuous-flow LVADs which have improved patient survival and quality-of-life due to their smaller size, longer durability, increased energy efficiency, and reduction of surgical trauma (4,10,12). LVAD usage has been expanded to less hemodynamically compromised patients including those at INTERMACS level 3-4 (12-15).
Our current analysis utilized the UNOS database in order to investigate whether an early timing of LVAD placement for BTT is superior to a delayed LVAD implantation strategy as it is currently unclear which strategy is associated with better outcomes both before and after HTx. Patients in the medically-treated cohort included a variety of risk profiles and this cohort was not limited to patients with short expected waiting time before HTx due to favorable blood type or body size (16-17), but also patients with an etiology of HF that does not favor LVAD placement such as hypertrophic or congenital cardiomyopathies with bi-ventricular failure (15,18-20). This group had a high likelihood of undergoing HTx during the observation period of this analysis.
The post-HTx survival analysis revealed that patients who were already on LVAD support at listing had worse survival post-HTx compared to patients who were exclusively medically supported in the entire cohort. This was also confirmed in patients initially listed at UNOS status 1A of the medically treated and the Delayed-LVAD groups. LVAD recipients were in an unfavorable condition because of long ischemic times during HTx and a higher prevalence of infections at the time of HTx (21-22). In addition, albumin levels in LVAD recipients at HTx were lower than those in medically-treated patients which might reflect a poor overall status and associate infections. Our group has recently reported that pre-operative hypoalbuminemia is associated with poor prognosis following HTx and LVAD surgery (23-24). These concerning findings related to factors known to affect post-HTx outcomes (25-26) in addition an increased risk of LVAD-associated adverse events during the waiting period question the validity of an early implantation strategy in patients with INTERMACS level 4-6 awaiting HTx.
Our analysis also showed that patients initially listed on medical therapy who later required LVAD implantation had the lowest likelihood of undergoing HTx. While we do not have information on the clinical decision process leading to this strategy, it is assumed that the later LVAD placement is effective for the stabilization of these patients awaiting HTx when clinically deteriorating. Although post-HTx survival of patients undergoing LVAD surgery before listing was poorer than patients who received HTx without requiring LVAD, the survival of patients who required LVAD surgery after listing was not significantly different from that of patients treated medically. In fact, when already in UNOS status 1A, patients requiring LVAD placement after listing showed better post-HTx survival as compared to patients who had already LVAD implanted at listing for HTx. Finally, the comparison of cumulative event-free survival from the time of listing revealed that LVAD placement as a BTT with a delayed timing accomplished the best overall survival rates over the entire observation period that included the waiting period before HTx as well as the post-HTx follow-up. Of note, when patients needed LVAD support before listing for HTx and were able to achieve relatively stable hemodynamics as UNOS status 1B, an upfront LVAD implantation strategy achieved competitive cumulative survival from listing for HTx compared to Delayed-LVAD implantation strategy.
Our analysis has several limitations. This study was based on a retrospective review of the UNOS clinical registry database; therefore, errors in data entry and missing data may be present. By its nature, the UNOS registry is restricted to patients in the United States. Patient demographics recorded in the UNOS registry such as co-morbidities and etiology of HF are based on each institutional report. In addition, pre-HTx information is mainly limited to the data at listing and at HTx and clinical data from the waiting period while being listed for HTx are rarely obtainable. Therefore, there is the possibility that we underestimated the potential risk of delisting in patients on the waitlist who needed urgent LVAD placement before or during listing for HTx. In this analysis, we could also not discriminate patients who were in stable condition on LVAD support with allowance for 30 days of elective 1A status from unstable UNOS 1A patients requiring urgent HTx.
In conclusion, medical therapy alone is associated with an increased clinical event rate during the waiting period but a favorable likelihood of HTx. LVAD support strategies independent of the timing of implantation offer a safe bridge-to-HTx. When patients required upfront LVAD support before listing for HTx, the Early-LVAD implantation strategy achieved a survival comparable with the Delayed-LVAD implantation strategy when these patients could be listed for HTx in relatively stable hemodynamic condition as UNOS status 1B. In contrast, in patients with evidence of hemodynamic instability or other causes to be listed as UNOS status 1A, the Early-LVAD implantation strategy results in decreased survival despite the higher likelihood of HTx compared to a Delayed-LVAD implantation strategy suggesting that deteriorating HF status and overall clinical sickness primarily defines outcomes of these patients. Therefore, regardless of the timing, LVAD implantation for hemodynamic instability seems to provide the best survival benefit for patients with advanced HF awaiting HTx.
Table 3.
Comparison of post-HTx survival among the 3 cohorts.
| Post-HTx survival | ||||||
|---|---|---|---|---|---|---|
| 1-year survival (%±SE) | 2-year survival (%±SE) | HR (95% CI) ‡ | p-value | HR (95% CI) § | p-value | |
| Clinical Course | ||||||
| MED group (n=6676) | 89.9±0.39 | 85.9±0.47 | 1.000 | |||
| Delayed-LVAD group (n=1170) | 88.5±0.98 | 84.5±1.20 | 1.090 (0.926-1.283) | 0.298 | 1.000 | |
| Early-LVAD group (n=1176) | 88.1±1.01 | 84.4±1.24 | 1.181 (1.006-1.387) | 0.042 | 1.083 (0.879-1.335) | 0.453 |
| UNOS status at the time of listing | ||||||
| Listed as UNOS status 1A | ||||||
| MED group (n=1161) | 90.0±0.92 | 85.0±1.18 | 1.000 | |||
| Delayed-LVAD group (n=234) | 92.6±1.78 | 89.2±2.28 | 0.807 (0.542-1.202) | 0.292 | 1.000 | |
| Early-LVAD group (n=593) | 87.7±1.43 | 83.5±1.78 | 1.305 (1.019-1.671) | 0.035 | 1.617 (1.067-2.451) | 0.024 |
| Listed as UNOS status 1B | ||||||
| MED group (n=2748) | 90.5±0.59 | 86.3±0.73 | 1.000 | |||
| Delayed-LVAD group (n=525) | 87.0±1.55 | 82.4±1.90 | 1.235 (0.975-1.564) | 0.081 | 1.000 | |
| Early-LVAD group (n=524) | 89.0±1.47 | 85.7±1.82 | 1.084 (0.837-1.405) | 0.541 | 0.878 (0.639-1.208) | 0.425 |
Comparison analysis being adjusted by age, gender, race, blood type, and coexisting of diabetes mellitus, MED group as a reference
Comparison analysis being adjusted by age, gender, race, blood type, and coexisting of diabetes mellitus, Delayed-LVAD group as a reference
HTx indicates heart transplantation; MED, medically treated; LVAD, left ventricular assist device; SE, standard error; HR, hazard ratio; CI, confidential interval.
Acknowledgements
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of the manuscript and all authors report no potential conflicts.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report—2011. J Heart Lung Transplant. 2011;30:1078–94. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–26. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Fiorelli AI, Stolf NA, Peqo-Fernandes PM, et al. Recommendations for use of marginal donors in heart transplantation: Brazilian Association of Organs Transplantation guideline. Transplant Proc. 2011;43:211–5. doi: 10.1016/j.transproceed.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 5.Wever-Pinzon O, Drakos SG, Kfoury AG, et al. Morbidity and mortality in heart transplant candidates supported with mechanical circulatory support: is reappraisal of the current United network for organ sharing thoracic organ allocation policy justified? Circulation. 2013;127:452–62. doi: 10.1161/CIRCULATIONAHA.112.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrar DJ, Hill JD, Penninqton DG, et al. Preoperative and postoperative comparison of patients with univentricular and biventricular support with the thoratec ventricular assist device as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg. 1997;113:202–9. doi: 10.1016/S0022-5223(97)70416-1. [DOI] [PubMed] [Google Scholar]
- 7.Cleveland JC, Jr, Naftel DC, Reece TB, et al. Survival after biventricular assist device implantation: an analysis of the Interagency Registry for Mechanically Assisted Circulatory Support database. J Heart Lung Transplant. 2011;30:862–9. doi: 10.1016/j.healun.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Gray NA, Jr, Selzman CH. Current status of the total artificial heart. Am Heart J. 2006;152:4–10. doi: 10.1016/j.ahj.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Slaughter MS. UNOS status of heart transplant patients supported with a left ventricular assist device: is it time to reconsider the status criteria? Tex Heart Inst J. 2011;38:549–51. [PMC free article] [PubMed] [Google Scholar]
- 10.Birks EJ, Yacoub MH, Banner NR, Khaqhani A. The role of bridge to transplantation: should LVAD patients be transplanted? Curr Opin Cardiol. 2004;19:148–53. doi: 10.1097/00001573-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–23. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2011;57:1890–8. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 13.Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–7. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Teuteberg JJ, Ewald GA, Adamson RM, et al. Risk assessment for continuous flow left ventricular assist devices: does the destination therapy risk score work? An analysis of over 1,000 patients. J Am Coll Cardiol. 2012;60:44–51. doi: 10.1016/j.jacc.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: heart. Am J Transplant. 2013. 13(Suppl 1):119–148. doi: 10.1111/ajt.12023. [DOI] [PubMed] [Google Scholar]
- 17.Weiss ES, Allen JG, Russell SD, Shah AS, Conte JV. Impact of recipient body mass index on organ allocation and mortality in orthotopic heart transplantation. J Heart Lung Transplant. 2009;28:1150–7. doi: 10.1016/j.healun.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Blume ED, Naftel DC, Bastardi HJ, Duncan BW, Kirklin JK, Webber SA. Pediatric Heart Transplant Study Investigators. Outcomes of children bridged to heart transplantation with ventricular assist devices: a multi-institutional study. Circulation. 2006;113:2313–9. doi: 10.1161/CIRCULATIONAHA.105.577601. [DOI] [PubMed] [Google Scholar]
- 19.Karamlou T, Hirsch J, Welke K, et al. A United Network for Organ Sharing analysis of heart transplantation in adults with congenital heart disease: outcomes and factors associated with mortality and retransplantation. J Thorac Cardiovasc Surg. 2010;140:161–8. doi: 10.1016/j.jtcvs.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Topilsky Y, Pereira NL, Shah DK, et al. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail. 2011;4:266–75. doi: 10.1161/CIRCHEARTFAILURE.110.959288. [DOI] [PubMed] [Google Scholar]
- 21.Yeen W, Polgar A, Guglin M, et al. Outcomes of adult orthotopic heart transplantation with extended allograft ischemic time. Transplant Proc. 2013;45:2399–405. doi: 10.1016/j.transproceed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Schulman AR, Martens TP, Russo MJ, et al. Effect of left ventricular assist device infection on post-transplant outcomes. J Heart Lung Transplant. 2009;28:237–42. doi: 10.1016/j.healun.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Kato TS, Cheema FH, Yang J, et al. Preoperative serum albumin levels predict 1-year postoperative survival of patients undergoing heart transplantation. Circ Heart Fail. 2013;6:785–91. doi: 10.1161/CIRCHEARTFAILURE.111.000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato TS, Kitada S, Yang J, et al. Relation of preoperative serum albumin levels to survival in patients undergoing left ventricular assist device implantation. Am J Cardiol. 2013;112:1484–8. doi: 10.1016/j.amjcard.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss ES, Allen JG, Arnaoutakis GJ, et al. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg. 2011;92:914–21. doi: 10.1016/j.athoracsur.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 26.Arnaoutakis GJ, George TJ, Kilic A, et al. Risk factors for early death in patients bridged to transplant with continuous-flow left ventricular assist devices. Ann Thorac Surg. 2012;93:1549–54. doi: 10.1016/j.athoracsur.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]


