Abstract
Therapeutic monoclonal antibodies (mAbs) have made a tremendous impact in treating patients with various diseases. MAbs are designed to target specifically a cell and illicit a response from the immune system to destroy the cell. As originator mAb drug patents are coming to an end, generic pharmaceutical companies are poised to replicate and produce so-called biosimilar drugs. MAbs are significantly more complicated than small drugs to analyze and produce. The mAb proteoform and glycoform must be as similar to the original drug as possible to be a viable replacement. The mAb proteoform is well characterized but can be altered through various undesirable reactions such as deamidation. The mAb glycoform is harder to replicate as the glycan formation is a complicated template-less one; it is proving difficult for the originator companies to produce a homogenous population of mAbs from batch to batch. Severe side-effects have occurred in patients taking mAbs with immunogenic glycans, highlighting the importance of quality control mechanisms.
The complex nature of mAbs requires sensitive and robust tools amenable to the high-throughput analysis required by a manufacturing setting. Miniaturized analytical platforms for complex biosimilar analysis are still in their infancy but have shown great promise for sample preparation. Capillary electrophoresis-laser induced fluorescence remains a powerful and fast technique for routine glycan analysis. Mass spectrometry is the method of choice for the analysis of mAb proteoforms and is emerging as a powerful tool for glycoform analysis.
Keywords: IgG, Microscale immobilized enzyme reactors, CHIPS, IgG, Biosimilars, LC-MS
Introduction
Glycosylation is one of the most common post-translational modifications (PTMs) found in living organisms as evidenced by their ubiquitous presence on cell surfaces and in the surrounding extracellular matrix [1, 2]. Extracellular glycosylation serves several vital functions including but not limited to cell adhesion, cell-extracellular matrix adhesion, signal transduction, immune responses, embryogenesis, and various adult physiological processes [3–12]. Glycosylation has a pronounced effect on protein folding, stability, and localization [13].
The importance of protein glycosylation is especially prominent in current disease models. The changes in individual proteoglycans are thought to mediate various pathophysiological events related to cancer tumor progression and have been suggested as biomarkers for prostate cancer (prostate-specific antigen), colon cancer (carbohydrate antigen CA-19-9), liver cancer (α-fetoprotein), and germ cell tumors (and β-human chorionic gonadotropin) [14–19]. Global changes in glycan and glycoproteins are correlated with: inflammatory conditions, age, pregnancy, following vaccination, infection, hereditary disorders, immune deficiencies, cardiovascular disease, and neurodegenerative disorders [20–23]. An increase in glycan fucosylation of haptoglobin has been observed in pancreatic and hepatocellular cancer [24, 25].
Immunoglobulin Gs (IgGs) are a high percentage of the antibodies present in humans [26]. IgGs are large (~150 kDa) glycoproteins comprised of two light and two heavy chains. The quaternary structure results in three distinct moieties: two identical antigen-binding fragment (Fab) regions composed of the light and heavy chains and one effector/crystallizable region (Fc) consisting of only the heavy chains. The Fab region binds foreign antigens while the Fc region alerts the various responses from the immune system by binding to Fc receptor types (FcγRI, FcγRII, FcγRIII, FcRn) or the complement fixation receptor c1q. Antibody activity and effectiveness depend on N-linked glycosylation at Fc asparagine 297 (N297); the Fab regions have also been reported to have glycosylation, but it does not seem to be as critical for antibody activity. Protein engineering has taken advantage of the immune response to modify monoclonal antibodies (mAb) for drug therapy. Most mAb therapeutics are IgG1 based. There are over 40 mAbs approved for use in the United States and Europe and an equal number in various stages of clinical trials [27, 28]. In addition to proteins, carbohydrates are also starting to be targeted for mAb therapy; the anticarbohydrate antibody Unituxin (dinutuximab) targeting GD2 ganglioside was approved for use in treating pediatric patients with high-risk neuroblastoma [28].
The utility of therapeutic mAbs lies in their hallmark ability to specifically bind to a target molecule, reducing side-effects and required dosage amounts. Some mAbs are engineered to bind to unique antigens present on various tumor cells and subsequently recruit the innate immune system to destroy the cancer cells via antibody-dependent cellular cytotoxicity (ADCC) or complement-mediated cytotoxicity (CMC) [29–32]. Other mAbs are designed to inhibit a specific transmembrane receptor; bevacizumab binds to and inhibits the vascular endothelial growth factor (VEGF) which results in angiogenesis inhibition and a decrease in nutrients and oxygen reaching the tumor [33, 34]. Antibody-drug conjugates (ADCs) are a hybrid of mAb and a small drug molecule; ADCs combine the specificity of an mAb with the potency of a highly toxic small molecule [35]. After ADCs bind to the outside of the target cell, they are endocytosed; the linker between the small molecule drug and the mAb breaks, typically pH or protease controlled, releasing the drug into the cell [36]. In addition to single mAb therapeutics, combinations of mAbs, antibody cocktails, can result in improved pharmacological profiles compared to a single mAb [37, 38]. Glycosylation has an immense impact on the efficacy and effectiveness of mAb therapeutics. All antibodies have at least one N-linked glycan at asparagine 297 in the CH2 domain of the crystallizable fragment (Fc) [39, 40]; some glycans are also present in the fragment antigen-binding (Fab) portion of some mAbs. The N-linked glycans found in the Fc domain are core fucosylated, biantennary oligosaccharides with up to two galactose residues whereas the Fab domain glycans are reported to be core fucosylated, biantennary oligosaccharides with one or two N-glycolylneuraminic acid (NeuGc) residues, have zero or one α-linked galactose, and zero or one β-linked GalNac [41]. Differences in mAb pharmacodynamics and pharmacokinetics can be attributed to glycosylation. The importance of glycosylation is further illustrated by the loss of binding activity and effector functions upon mAb deglycosylation [42, 43].
Molecular biology and cloning techniques, i.e. protein engineering, have allowed for better control over the production of mAb glycosylation that has improved the clinical efficacy of mAb therapeutics. It has been observed that the removal of fucose from the biantennary structure allows for an increase in binding to FCγRIIIa and subsequently an increase in cytotoxicity [44]. Dramatic clinical results were observed with chronic lymphocytic leukemia patients after treatment with an improved anti-CD20 antibody; a bisecting N-acetyl-D-glucosamine (GlcNAc) was added to the mAb [30, 31]. It has been suggested that mAb modifications could also aid in the treatment of HIV patients [45].
The patent protection on some therapeutic mAb are scheduled to expire soon [28], thus prompting the generic pharmaceutical market to start production of so-called biosimilar drugs, a biological compound highly similar in primary sequence to a reference drug with no clinically meaningful differences in safety, purity, and potency [46]. It has become readily apparent that replicating mAb drugs will be a very different and arduous process. Homogenous batches of small molecule drugs are routinely manufactured in large quantities through various well-controlled chemical reactions. This is in contrast to mAb manufacturing that utilizes biological processes. The mAb itself is disclosed in patents, but the manufacturing process remains undisclosed.
The numerous decisions made in manufacturing biosimilars have a profound effect on the final product, including the quaternary structure, proteoforms, glycoforms, aggregation, and ultimately the mAb activity. The proteoforms can be affected by either incorrect sequences in the cDNA used for mAb expression or in deamidation of asparagine/glutamine to eventually aspartic/glutamic; deamidated therapeutic mAb have been shown to have reduced [47–50]. The choice of cell line has a profound effect on the mAb glycoform with different cell lines yielding different glycoforms. Cetuximab, an mAb targeting EGFR approved for use with squamous-cell carcinoma of the head and neck and colorectal cancer, produced in murine SP2/0 cell line resulted in an anaphylaxis response in patients [51]. It was originally produced in Chinese Hamster Ovary (CHO) cells for clinical trials and scaled up for production in SP2/0 cells. The cause of the illicit immune response was the presence of galactose-α-1,3-galactose glyco-type; a foreign glycan in humans and diagnostic for SP2/0 cell production [51].
Some of the challenges associated with biosimilar production can be attributed to the heterogeneity associated with mAbs. Glycans attached at N297 of the Fc are of the complex biantennary type with a core pentasaccharide with variable addition of fucose, galactose, bisecting N-acetylglucosamine and sialic acid to a lesser extent. Conversely, glycans derived from the Fab portion of IgGs are reported to be extremely heterogeneous with extensive galactosylation, substantial sialyation, complex biantennary, hybrid oligosaccharides, and N-glycolylneuraminic acid residues. The combination of Fc and Fab glycoforms has been estimated at 128 unique glycoforms. Maintaining glycoform fidelity at both sites has proved a daunting challenge in mAb manufacturing, much less biosimilar manufacturing. Analysis of four batches of Trastuzumab, an approved mAb targeting human epidermal growth factor receptor 2 (HER2) in breast cancer, revealed the batches had the same types of glycoforms but in different relative abundances. Analysis of an undisclosed IgG1 mAb from ImClone also revealed a range of isoforms [52].
In addition to the micro-heterogeneity of the mAb glycoform, the amino acid sequence can also be modified. As mentioned above, deamidation is a common issue in the biological drug manufacturing community. Other inadvertent protein modifications report include but are not limited to disulfide bridge formation between cysteines, N-terminal glutamine cyclization, C-terminal lysine clipping, oxidation, deamidation, non-enzymatic glycation, sequence truncation or point mutations resulting in single amino acid substitutions [53–56].
The extensive proteoforms and glycoforms of mAbs require a multitude of analytical techniques for complete compositional analysis and verification. Ayoub et al. [57] characterized Cetuximab employing several mass spectrometry-based (MS) strategies. The intact mAb was analyzed with MS1 while the amino acid sequence and PTMs, especially the glycoform, was verified with middle-up, middle-down, and bottom-up proteomic approaches. This approach yielded a comprehensive glycosylation profile for the Fab and Fc domains as well as an error in the amino acid sequence of Cetuximab. Gahoual et al. [58] also characterized Cetuximab but utilized capillary zone electrophoresis coupled to a mass spectrometer with a sheathless interface (CESI-MS). A bottom-up strategy allowed for 100% amino acid sequence coverage in addition to a glycoform of 16 glycans. Henninot et al. [59] characterized an undisclosed mAb from cell culture media with liquid chromatography-mass spectrometry time of flight (LC-MS-TOF). A middle-down approach utilizing IdeS yielded large fragments that allowed for proteoform analysis in one experimental run; EndoS was employed for fucosylation yield analysis. The glycan information yield was equivalent to the gold standard technique, high-performance capillary electrophoresis-laser induced fluorescence (HPCE-LIF) but consumed significantly less media, 500 µL vs 200 mL.
The analytical techniques and examples highlighted in this review can be divided into several sections. The first section discusses advances in mAb sample preparation. We have chosen to focus on mAb for the examples cited in this review. Currently, there is no technique to analyze completely an intact mAb for sequence and glycoform elucidation in one experiment; this is an active area of research in the top-down mass spectrometry community. The mAb must be broken down into smaller pieces before primary sequence and glycoform identification. Typically, N-glycans are released with peptide-N-glycosidase F (PNGase F), an endoglycosidase, followed by digestion of the protein. The following sections highlight research that has allowed for comprehensive mAb proteoform identification and glycan determination. The combination of rapid, and efficient sample preparation coupled to sensitive detectors yields a powerful approach for accurate mAb analysis.
Importance of Miniaturization in general
In the ultrasensitive measurements of biological and pharmaceutical samples, an extreme caution must be exercised in manipulating the minute quantities of such biomolecules. Biological samples at the low microgram scale, while becoming measurable with the modern instrumental techniques, can easily be adsorbed on the surface of glassware before such measurements. Sample loss during ultrafiltration, dialysis, lyophilization, etc., can easily become a bottleneck of the entire analysis. Another problem with working at such a reduced scale is contamination (dust, solvent, reagent impurities, etc.). It is thus crucial to minimize the number of handling and transfer steps during the analysis. Miniaturized forms of separation, regarding reduced column diameters, solvent flow-rates, and the overall surface area that a pharmaceutical sample may encounter during analysis are becoming crucial in high-sensitivity work.
Microscale immobilized enzyme reactors
Microscale immobilized enzyme reactors (IMERs) are steadily gaining acceptance as a rapid means of sample preparation for peptide and glycan analysis. IMERs increase enzymatic efficiency by decreasing the substrate to enzyme ratio and by reducing the diffusion space between the enzyme and substrate. Enzymes are covalently bound to an immobilized monolith inside a capillary; the sample is passed through the capillary exposing the protein of interest to the enzyme. Some labs have reported dramatic improvements in sample preparation time with protease digestion and deglycosylation steps.
Korecka et al. [60] utilized IMERs for the preparation and study of IgG fragments; chymotrypsin, trypsin, and papain were utilized to determine the presence of a heterogeneous population of IgG glycoproteins. Krenkova et al. [61]demonstrated an extremely efficient IMER with two proteases, trypsin, and endoproteinase Lys-C, coupled either online to ESI-TOF MS or offline to MALDI-TOF MS. An IMER digest of IgG for 6 minutes at room temperature produced comparable and in some cases, better peptide MS data as a soluble enzyme digest at 37°C for 24 hours. Krenkova et al. [62], also demonstrated efficient deglycosylation of IgGs with immobilized PNGase F coupled to MALDI-TOF-MS/MS; a 5.5-minute reactor deglycosylation was equivalent to a 24-hour solution based deglycosylation. Weng et al. [63] produced a PNGase F based IMER in conjunction with dimethyl labeling to identify 43 up regulated and 30 down regulated N-glycosylation sites in hepatocarcinoma ascites syngeneic cell lines; they also quantitated 11 N-glycoproteins related to tumorigenesis and tumor metastasis.
Microfluidic Devices/chips
Several microfluidic devices have been employed for the rapid analysis of glycans in academic labs. Zhuang et al. [64] were able to separate N-glycan positional isomers derived from standards ribonuclease B, and linkage isomers from asialofetuin; their method further separated N-glycans derived from a cancer patient’s serum. Mellors et al. [65] employed a more robust analytical system utilizing a hybrid capillary/microfluidic system for comprehensive online capillary electrophoresis liquid chromatography-electrospray ionization mass spectrometry. This allowed the identification of two different N-linked glycosylation sites from an mAb amid a complex mixture of tryptic peptides. Macchi et al. [66] was able to quantitate mAb derived glycans utilizing a chip-based nanoflow reversed-phase liquid chromatography coupled with the a time of flight mass spectrometer (nRPLC-Chip-TOFMS) approach.
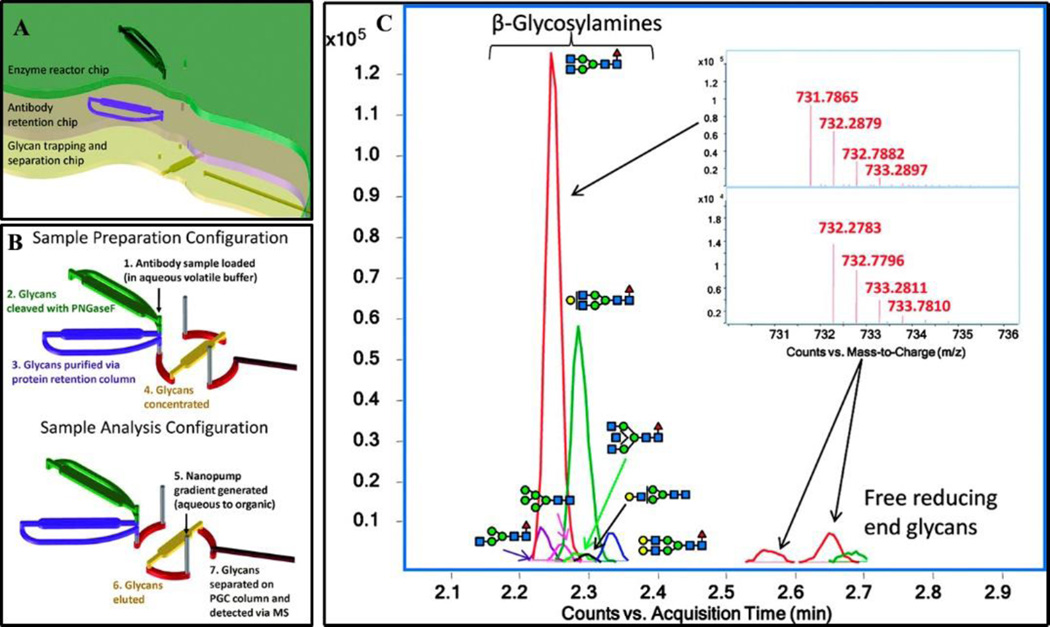
Two commercially available chip kits are enabling the most widespread use of microfluidic devices. Both chips utilize an immobilized enzyme reactor for rapid deglycosylation, followed by capillary electrophoresis for glycan separation and subsequent detection by either LIF or MS. The Caliper LabChip GXII Microchip-CE platform has already been utilized by Amgen as a high-throughput assay to generate rapidly glycans derived from undisclosed mAb produced in cell cultures followed by LIF detection [67]. Agilent’s mAB-glyco kit (Figure 1) has also been utilized for mAb sample preparation before MS-analysis [68–70].
Figure 1. Illustration of a commercially available microfluidic device, Agilent’s Mab-glyco chip.
(A). Schematic showing the location and orientation of the deglycosylation enzyme reactor, antibody retention region and the glycan trap to concentrate. (2). Diagram illustrating the sample loading and handling scheme. (B). Samples are initially sent to the enzyme reactor for deglycosylation with PNGase F. The deglycosylated proteins are trapped in the protein retention column while the glycans pass through to the glycan trap where they are concentrated. A rotor shift allows for exclusive elution of the glycans from the glycan trap; the proteins remain on the retention column. The eluted glycans pass through an analytical column followed by ESI-MS analysis (C). Figure reproduced with permission [68].
mAb Characterization
After sample preparation and separation, a detector is required for a more detailed qualitative and quantitative analysis. The current approaches to glycoprotein analysis require separation of the glycan from the protein. Current methods are further limited by identification of only N-glycans; O-glycan identification remains a difficult and arduous task. MAb proteoform analysis is dominated by various mass spectrometry-based approaches while the current gold standard in routine glycan detection is high-performance capillary electrophoresis-laser induced fluorescence. Before analysis, glycans are labeled with a fluorophore, commonly commonly 9-Aminopyrene-1,4,6-trisulfonic acid (APTS). HPCE-LIF provides short analysis times, efficient separations and high sensitivity making it ideally suited for routine analysis of well-characterized glycoproteins.
Mass Spectrometry for mAb proteoform identification
Mass spectrometry (MS) is becoming a more widely adopted approach to glycoprotein and mAb analysis because it can characterize both the protein and glycan of glycoproteins through multiple experimental approaches. An MS1 experiment, either denatured or natively folded (native MS), allows for intact mAb analysis allowing for initial qualitative and quantitative assessment of sample heterogeneity, a particularly useful technique in the emerging field of bispecific mAb and antibody cocktails/mixtures.
Native MS employs aqueous buffers and nano-electrospray to maintain a protein’s tertiary and quaternary structure (Figures 2A and 2B). This results in a smaller charge-state envelope than a denatured protein; this allows for easier data interpretation and an increase in sensitivity because the ions are condensed into fewer charge states, improving the signal to noise ratio [71]. Native MS allows for rapid measurements (on the order of minutes for a single analysis), very sensitive (picomoles of mAbs) and minimal sample preparation (often times a buffer exchange column is used right before MS injection) [72]. Several disadvantages handicap the native MS approach for mAb analysis. Native MS cannot differentiate small mass modifications (+1 Da for deamidation) and cannot determine the location of PTMs other than C-terminal lysine clipping, N-terminal glutamine cyclization, and glycosylation [72]. Work is being done to improve the mass resolution at the higher m/z observed in native MS as well as to improve fragmentation techniques for protein sequence and glycan identification.
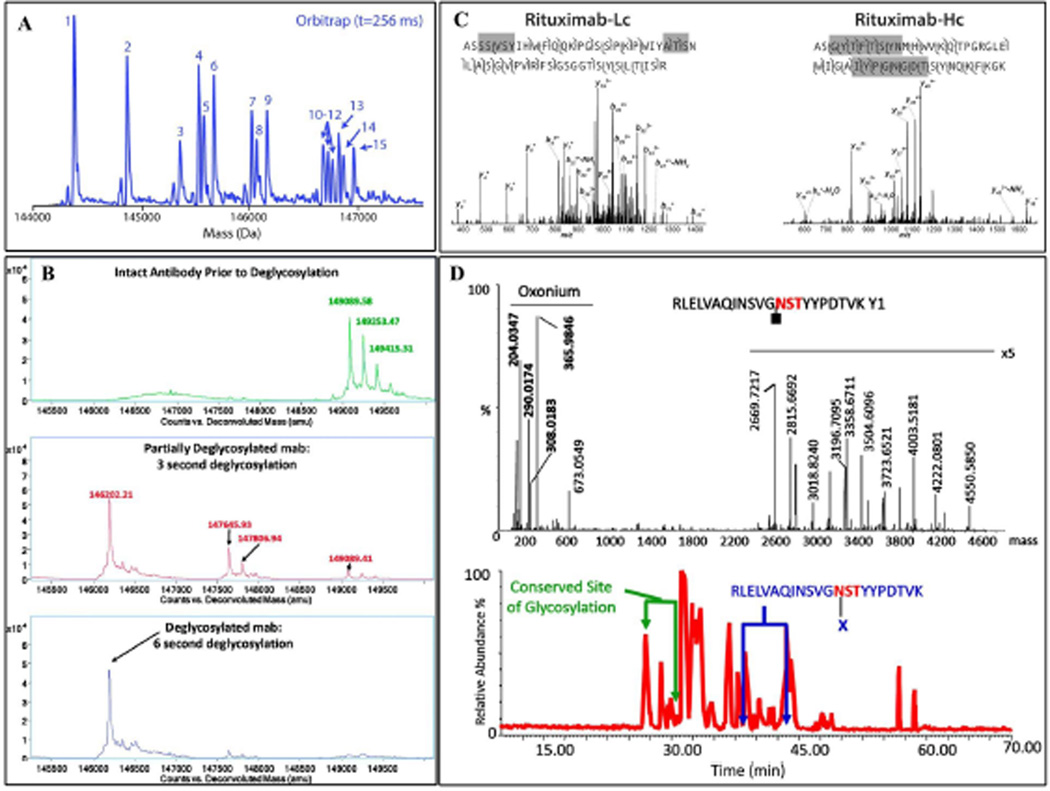
Figure 2. The different MS-based proteomics approaches to mAb proteoform identification.
(A) The intact mAb analysis yields rapid insight into the mAb population homogeneity and can be used to assess the deglycosylation efficiency of an enzyme reactor (B). Large peptide fragments result from a middle-out or ‘extended bottom-up’ approach. The large fragments can be used for determining the various components of the mAb, or they can be fragmented in the instrument for primary sequence elucidation. (C). Product ions from a fragmented glycopeptide illustrate a bottom-up proteomic approach which utilizes small tryptic peptides to verify sequence identity and in this example, glycan attachment site. Figure reproduced with permission [104] [68] [84] [86].
Ion mobility mass spectrometry (IM-MS) provides shape to charge information along with mass to charge; this technique is well situated to identify differences in the shape of an isobaric mAb population. Bagal et al. [73] utilized IM-MS to resolve differences in disulfide structural isomers of IgG2 mAbs; these mAbs have the same m/z but differ in shape because of differences in the disulfide bonds formed. IM-MS is also being utilized to assess the structural flexibility of mAbs [74] and other large protein complexes [75, 76].
MS1 experiments yield relevant information about the intact mAb but do not yield more detailed information about the protein of interest. To gain information about a protein’s sequence or glycan information, tandem mass spectrometry (MS2) experiments are performed. Several MS2 experiments are currently used with differing results. Top-down MS, fragmentation of the intact protein in the MS with electron-transfer dissociation (ETD), electron-capture dissociation (ECD) or ultraviolet photodissociation (UVPD), is a very promising technique that has yielded tremendous information and insight into the histone biology field; numerous histone proteoforms have been identified utilizing this approach [77, 78]. The current top-down fragmentation techniques have resulted in poor sequence coverage for mAbs; approximately 33–34% of mAb was identified with either ECD- Fourier transform ion cyclotron resonance (FTICR) MS [79] and ETD-orbitrap Fourier transform mass spectrometry (FTMS) [80]. A top-down approach coupled with UVPD fragmentation has resulted in 100% sequence coverage of model monomer proteins [81] and 67% sequence coverage of two protein complexes, β-lactoglobulin and hexameric insulin [82].
Middle down mass spectrometry utilizes restrictive proteases, such as papain or IdeS for mAb, to generate large peptides for fragmentation. Fornelli et al. [83] utilized IdeS to generate large peptides and was able to observe 70% sequence coverage when several LC runs were averaged. Srzentic et al. [84] employed an ‘extended bottom-up proteomics’ approach using Sap9 for mAb analysis; this resulted in 100% sequence coverage of the light chain and 99–100% coverage of the heavy chain in a single collision induced dissociation (CID) LC-MS/MS run (Figure 2C).
Bottom-up mass spectrometry employs enzymatic digestion, usually with trypsin, to generate numerous peptides. Xie et al. [85] utilized this approach and was able to differentiate a difference of 2 amino acids of a biosimilar mAb as well as the difference in relative abundance of biosimilar derived glycans and originator drugs. Mechref et al. [86] combined several methodologies including capillary electrophoresis (CE) and matrix assisted laser desorption ionization mass spectrometry (MALDI-MS) to comprehensively characterize the glycans derived from murine immunoglobulin Gκ; tryptically generated glycopeptides were essential to identifying the difference in glycan microheterogeneity and abundance between the conserved and variable domains (Figure 2D).
Glycan analysis
Characterization of mAb also entails the analysis of the glycan moieties associated with the protein. Several analytical approaches have been adopted or developed to facilitate the characterization of the glycan moieties of mAb, including capillary electrophoresis (CE), capillary electrophoresis mass spectrometry (CE-MS), liquid chromatography ultraviolet (LC-UV), liquid chromatography fluorescence (LC-FL), and liquid chromatography mass spectrometry (LC-MS).
Capillary Electrophoresis and CE-MS
Capillary electrophoresis (CE) is a well-proven method for rapidly separating biological molecules; separation times are much faster than LC. The reduced sample and reagent requirements allow for a cost-reduction over a traditional LC separation approach. The small dimensions of CE are especially conducive to coupling with nanoflow electrospray ionization mass spectrometry (nESI-MS) as there is less solvent to evaporate before introduction into the instrument. Some laboratories have utilized CE for mAb analysis. Hunt and Nashabeh [87] developed a robust CE-LIF based assay to replace sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for routine mAb quality control experiments. The mAb is kept intact and labeled with 5-carboxytetramethylrhodamine succinimidyl ester, a neutral fluorophore, for low concentration detection. The method offered the sensitivity of a silver-stained SDS-PAGE gel as well as the advantages of a high-throughput assay: increased reproducibility, increased precision, and short analysis times. The same group [88] also developed an assay to assess better the N-glycans derived from the mAb, rituximab. It was known that the N-glycans affect the mAb’s efficacy, but there was not a robust assay in use for routine glycan analysis. Their method derivatizes glycans with APTS followed by CE-LIF analysis. This approach is still used as a quick assessment for well-characterized glycans. Gahoual et al. [89] utilized sheathless capillary electrophoresis and tandem mass spectrometry (CESI-MS/MS) with a bottom-up proteomic approach for comprehensive mAb proteoform and glycoform analysis. The same group further showed their approach was well situated to discern differences between the biosimilar and original drug mAbs [58]. An automated offline approach was employed for the comprehensive analysis of an mAb; a novel capillary electrophoresis ultraviolet detection separation method was interfaced with matrix-assisted laser desorption ionization mass spectrometry (CE-UV/MALDI-MS) [90]. A bottom-up proteomic approach was employed and resulted in 100% sequence coverage of the light chain and 92% of the heavy chain along with four major glycosylated peptides and their structural characterization.
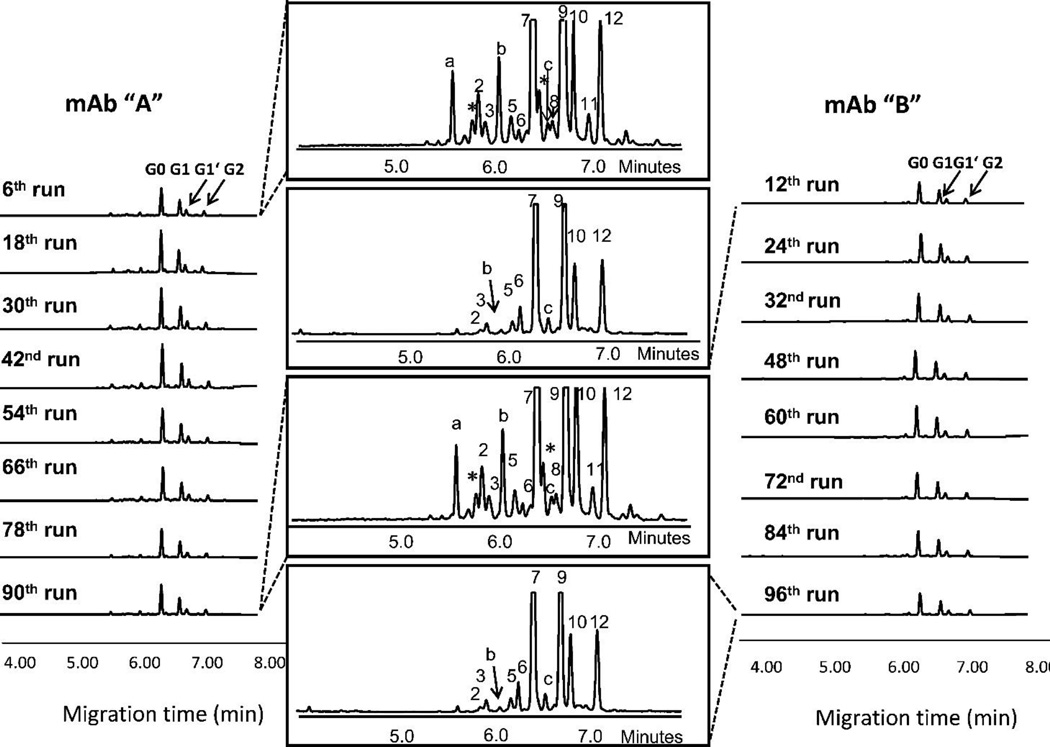
Routine glycan analysis has typically been performed by HPCE-LIF because of the short analysis times, efficient separations, and relatively inexpensive equipment compared to MS. For these reasons, HPCE-LIF has been used as a high-throughput routine analysis to monitor mAb glycoforms. Glycans are labeled with a fluorophore, usually APTS, passed through a CE separation, excited with a laser and subsequent fluorescence detection. Ruhaak et al. [91] optimized a gel-based CE method for mAb derived N-glycan profiling in a high-throughput format. This method was able to characterize glycans derived from a pregnant woman’s serum; serum was collected during each trimester as well as 6 weeks, 3 months and 6 months post-partum. Szabo et al. [92] has utilized this approach to separate fucosylated and non-fucosylated glycans from several high mannose oligosaccharides derived from mAb (Figure 3). Szabo et al. [93] also utilized a CE-based method to detect the presence of the immunogenic α-1,3-Gal in mAb derived from mouse cell lines.
Figure 3. Representative Data for Capillary Electrophoresis.
A 96-well plate based method was developed for the rapid analysis of N-glycans derived from two different mAbs. Figure reproduced with permission [92].
MS
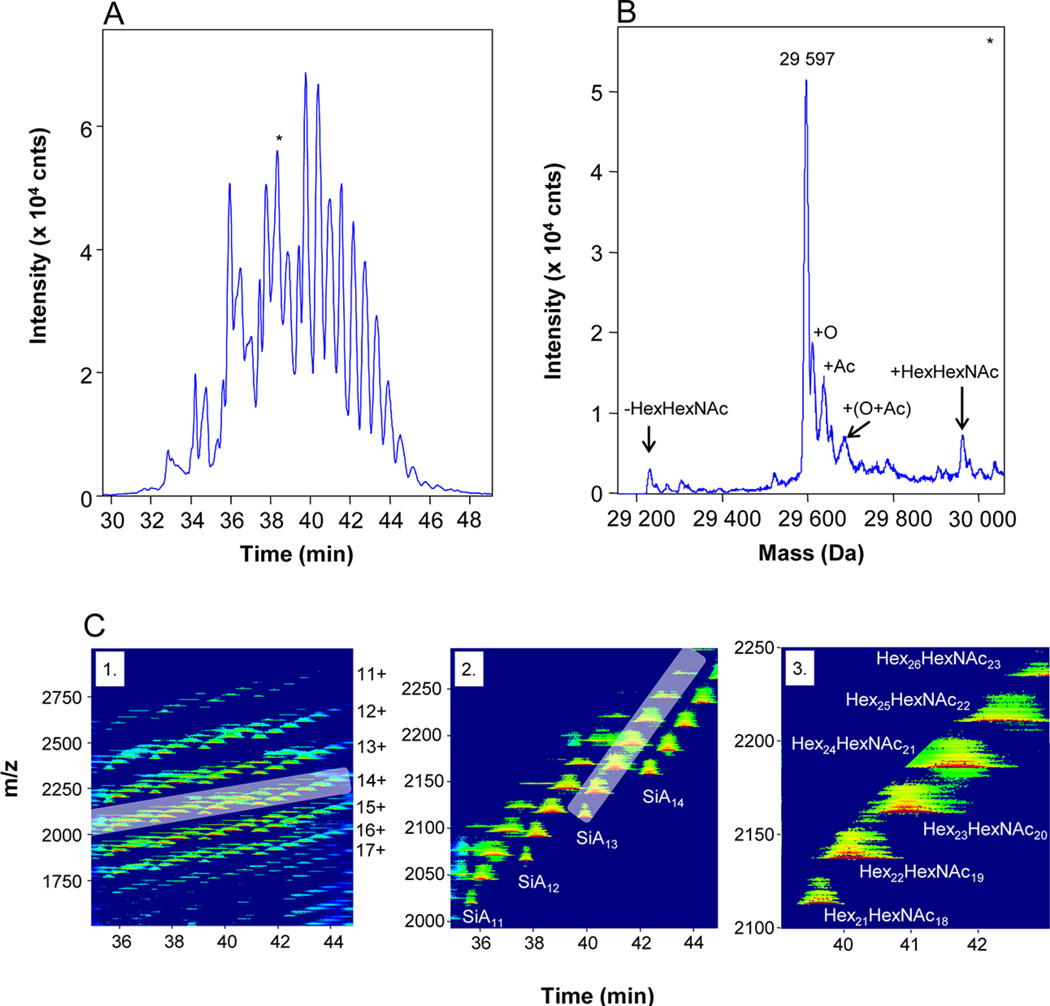
Some different MS only-based approaches have been utilized for glycan characterization including but not limited to electrospray ionization-ion mobility-quadrupole-time of flight mass spectrometry (ESI-IM-Q-TOF MS) [94], matrix assisted laser desorption ionization-quadrupole- time of flight-mass spectrometry (MALDI-Q-TOF MS) and tandem mass spectrometry MS/MS [95], MALDI-TOF and LCQ®-Ion Trap MS [52]. Haselberg et al. [96] utilized CE coupled to TOF-MS via sheathless electrospray ionization to characterize the proteoform and glycoform of biological pharmaceuticals (Figure 4). The sheathless ESI source increased the ionization efficiency for intact proteins while simultaneously decreasing ionization suppression. This allowed for the identification of multiple glycoforms on interferon-β (18 glycans) and recombinant human erythropoietin (74 glycans) in addition to multiple PTMs on both proteins.
Figure 4. Sheathless capillary electrophoresis for extensive glycoform analysis of recombinant human erythropoietin (rhEPO).
(A) TIC of eluted glycans and (B) deconvoluted MS of 38 minute LC window. (C) Contour plot of glycans illustrating the intensity of each m/z value over the LC run. The authors highlighted the 14+ glycoforms in (2) and zoomed in (3) to show they could distinguish a subset, the 14+ SiA13 sialoforms. This sheathless CE-MS allowed the authors to identify 74 glycoforms derived from rhEPO. Figure reproduced with permission [96].
Combined Analytical Techniques to attain comprehensive characterizations of mAb
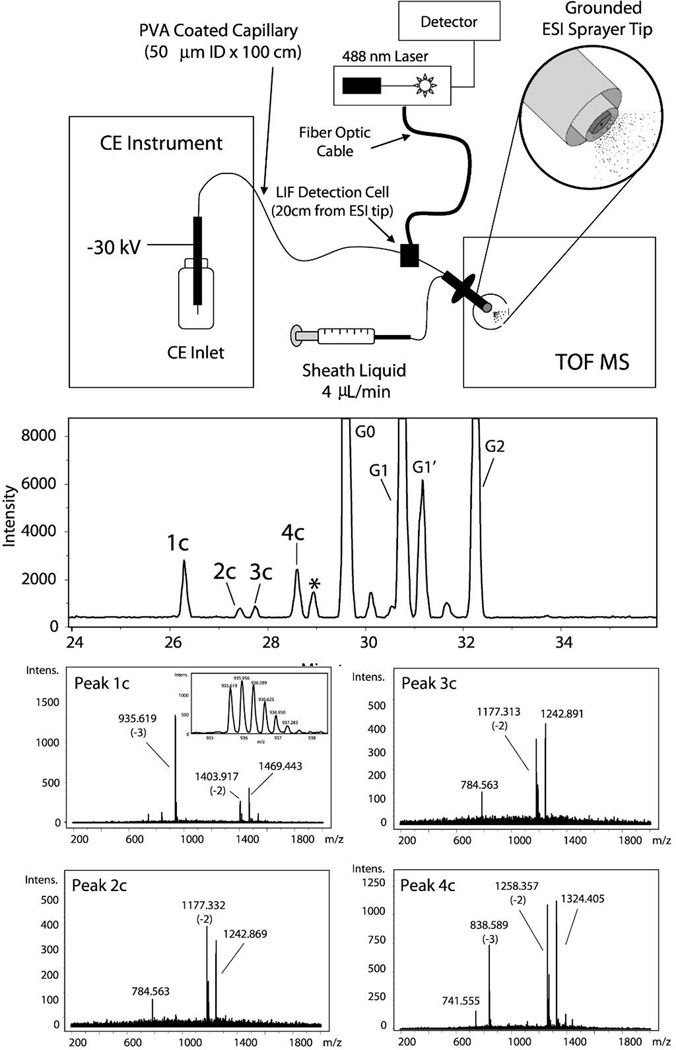
A combination of analytical techniques has resulted in a number of sensitive and high-throughput assays that can identify routine glycans and characterize novel or low abundant glycans. Gennaro et al. [97] developed a high-throughput glycan assay by combining the rapid separation of CE with the immediate information from LIF detection to a sensitive MS. This allowed for routine assays as well as more in-depth characterization should an anomaly appear on the LIF trace (Figure 5).
Figure 5. Schematic of a hybrid capillary electrophoresis-laser induced fluorescence (CE-LIF) method coupled to TOF-MS detection.
The CE-LIF method allows for rapid separation and analysis of the glycans for routine quality control experiments while the TOF-MS allows for more detailed analysis should an anomaly appear in the CE-LIF experiment. (A) The instrumentation configuration shows the CE-LIF detection first followed by TOF-MS. (B). Representative data from a CE-LIF experiment. In this example, the 1c–4c peaks were not accounted for by the standards. (C) MS of the 1c–4c peaks allows for more detailed analysis of the peaks resulting in their identification as sialylated species. This approach combines the advantages of CE-LIF and MS resulting in a robust assay amenable to high-throughput environments. Figure reproduced with permission [97].
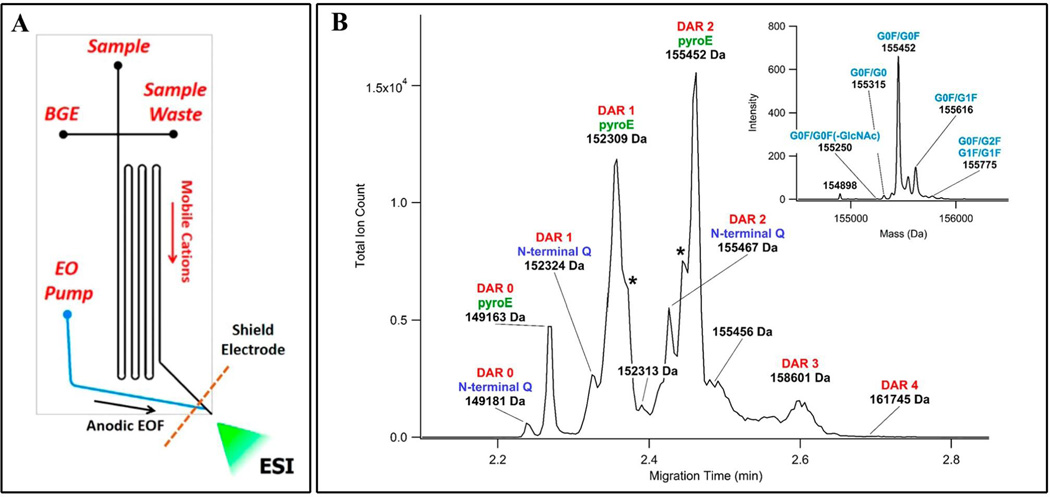
Maeda et al. [98] combined CE-LIF and ESI-MS to develop a high-throughput assay aimed at distinguishing immunogenic carbohydrates from human N-glycans in six commercial therapeutic mAbs. Hamm et al. [99] combined CE-LIF with off-line MS to characterize APTS-labeled-glycans derived from mAb produced in murine myeloma (NS0) cells. This approach allowed for routine analysis of well-characterized glycans with the more in-depth characterization of minor glycans utilizing MS analysis. Reusch et al. [100] also took a combined method approach but introduced a DNA analyzer for fluorescence detection. APTS-labeled glycans were initially identified with hydrophilic interaction liquid chromatography-ultra performance liquid chromatography-tandem mass spectrometry (HILIC-UPLC-MS/MS) analysis and subsequent capillary gel electrophoresis with laser-induced fluorescence (CGE-LIF) with a DNA analyzer. Mahan et al. [101] also coupled CE with a DNA analyzer to develop an inexpensive, routine, method for the high-throughput analysis of glycans derived from mAb. Wiegandt et al. [102] combined a high-throughput MS based assay to identify 37 N-glycan compositions derived from the therapeutic originator mAb Cetuximab; the ten most abundant glycans were unambiguously characterized with proton nuclear magnetic resonance (1H NMR). Redman et al. [103] integrated CE with MS in a microfluidic platform to characterize intact ADCs and their drug-to-antibody ratio (Figure 6).
Figure 6. Intact ADC analysis with CE-MS in a microfluidic platform.
A novel microfluidic CE-MS device (A) was used to rapidly analyze the products of the drug conjugation reaction (B). [103]
Concluding Remarks
The sensitive and robust tools amenable to the high-throughput analysis required by a manufacturing setting are essential for the characterization of mAbs and biosimilars. Although miniaturized analytical platforms for complex biosimilar analysis are still in the early stages of development, they have demonstrated great promise for sample preparation. Capillary electrophoresis-laser induced fluorescence remains a powerful and fast technique for the routine analysis of glycans derived from mAbs and biosimilars. Mass spectrometry is the method of choice for defining the proteoforms of mAbs and biosimilars. MS is emerging as a powerful tool for glycoform analysis.
Table 1.
Glycan identified by LC-MS/MS. Symbols Square: N-acetylhexosamine; triangle: fucose; diamond: N-glycolylneuraminic acid; dark grey circle: mannose; light grey circle: galactose. Reproduced from [102] with permission.
| Glycan compositiona | Identified by | Quant. | Glycan compositiona | Identified by | Quant. | ||
|---|---|---|---|---|---|---|---|
| MS [Da] | Acc. [ppm] |
MS [%] | MS [Da] | Acc. [ppm] |
MS [%] | ||
| 1624.604* | 3.7 | 31.4 | 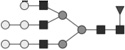 |
2475.888* | 1.9 | 0.4 | |
| 1462.552* | 3.7 | 19.6 | 2239.802 | 1.5 | 0.4 | ||
| 2110.762* | 2.7 | 13.9 | 1931.692 | 1.9 | 0.3 | ||
| 2255.800* | 2.3 | 7.7 | 2313.838 | 0.6 | 0.3 | ||
| 1786.656* | 2.7 | 7.5 | 2620.931* | 1.5 | 0.3 | ||
| 1234.436 | 2.2 | 2.9 | 2637.945* | 1.6 | 0.3 | ||
| 1259.465 | 1.0 | 2.0 | 1761.621 | 1.6 | 0.3 | ||
| 1745.626* | 1.7 | 1.9 | 2296.8198 | 0.3 | 0.2 | ||
| 2094.763* | 1.3 | 1.5 | 2458.868 | 0.6 | 0.1 | ||
| 2093.746* | 2.2 | 1.5 | 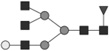 |
1827.678* | 0.2 | 0.3 | |
| 1421.519 | 1.1 | 0.4 | 1890.758 | 2.2 | 0.2 | ||
| 1583.574* | 1.3 | 1.0 | 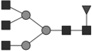 |
1665.625 | 0.6 | 0.2 | |
| 1478.542* | 1.4 | 1.0 | 1437.514 | 1.8 | 0.2 | ||
| 2151.782 | 1.3 | 0.8 | 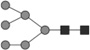 |
1396.486 | 0.7 | 0.2 | |
| 2400.832* | 1.9 | 0.6 | 2077.751 | 4.5 | 0.1 | ||
| 1989.734* | 0.8 | 0.6 | 2052.718 | 1.9 | 0.1 | ||
| 1907.680* | 1.5 | 0.6 | 2928.016* | 1.9 | 0.1 | ||
| 2782.984* | 1.4 | 0.5 | 1316.486* | 2.3 | <0.1 | ||
| 1599.566* | 1.4 | 0.5 | |||||
Table 2.
The 10 most abundant glycans identified with 1H-NMR. Symbols as in Table 1. Reproduced from [102] with permission.
| Glycan structureb | Identified by | Quantification | Compound Number |
|||
|---|---|---|---|---|---|---|
| MS [Da] | Accuracy [ppm] | NMR (L,C,S)c | MS [%]d | NMR [%/nmol]e | ||
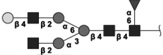 |
1624.604 | 3.9 | L,C,S | 20.4 | 13.9/5.2 | 1 |
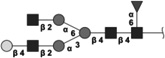 |
1624.604 | 3.9 | L,C,S | 11.4 | 14.6/5.5 | 2 |
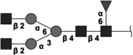 |
1462.552 | 4.9 | L,C,S | 19.6 | 22.9/8.6 | 3 |
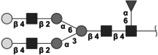 |
1786.656 | 3.1 | L,S | 7.5 | 3.0/1.1 | 4 |
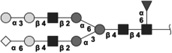 |
2255.800 | 2.8 | L,C,S | 7.7 | 5.8/2.2 | 5 |
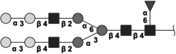 |
2110.762 | 2.8 | L,C,S | 13.9 | 15.9/5.9 | 6 |
| 1234.436 | 1.9 | L,C,S | 2.9 | 7.9/3.0 | 7 | |
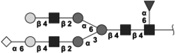 |
2093.746 | 2.5 | L,S | 1.4 | 4.8/1.8 | 8 |
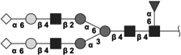 |
2400.832 | 0.4 | L,S | 0.6 | 10.0/3.7 | 9 |
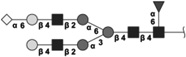 |
2093.746 | 2.5 | L,S | 0.1 | 1.2/0.4 | 10 |
Acknowledgments
This work was supported by grants from National Institutes of Health (1R01GM112490-01) and Cancer Prevention and Research Institute of Texas (RP130624).
References
- 1.Tian Y, Zhang H. Glycoproteomics and clinical applications. Proteomics. Clinical applications. 2010;4:124–132. doi: 10.1002/prca.200900161. [DOI] [PubMed] [Google Scholar]
- 2.Tian Y, Zhang H. Characterization of disease-associated N-linked glycoproteins. Proteomics. 2013;13:504–511. doi: 10.1002/pmic.201200333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 4.Brückner K, et al. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406:411–415. doi: 10.1038/35019075. [DOI] [PubMed] [Google Scholar]
- 5.Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annual review of biochemistry. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 6.Helenius A. Intracellular Functions of N-Linked Glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 7.Isaji T, et al. Introduction of bisecting GlcNAc into integrin alpha5beta1 reduces ligand binding and down-regulates cell adhesion and cell migration. The Journal of biological chemistry. 2004;279:19747–19754. doi: 10.1074/jbc.M311627200. [DOI] [PubMed] [Google Scholar]
- 8.Rudd P, Woods R. The effects of variable glycosylation on the functional activities of ribonuclease, plasminogen and tissue plasminogen activator. … et Biophysica Acta (BBA …. 1995 doi: 10.1016/0167-4838(94)00230-e. [DOI] [PubMed] [Google Scholar]
- 9.Rudd P, Wormald M, Stanfield R. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. Journal of molecular …. 1999 doi: 10.1006/jmbi.1999.3104. [DOI] [PubMed] [Google Scholar]
- 10.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–845. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Zheng M, Fang H, Hakomori S. Functional role of N-glycosylation in alpha 5 beta 1 integrin receptor. De-N-glycosylation induces dissociation or altered association of alpha 5 and beta 1 subunits and concomitant loss of fibronectin binding activity. The Journal of biological chemistry. 1994;269:12325–12331. [PubMed] [Google Scholar]
- 13.Dwek R. Glycobiology: toward understanding the function of sugars. Chemical Reviews. 1996 doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 14.Catalona WJ, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. The New England journal of medicine. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 15.Dwek MV, Brooks SA. Harnessing changes in cellular glycosylation in new cancer treatment strategies. Current cancer drug targets. 2004;4:425–442. doi: 10.2174/1568009043332899. [DOI] [PubMed] [Google Scholar]
- 16.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nature reviews. Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 17.Gebauer G, Müller-Ruchholtz W. Tumor marker concentrations in normal and malignant tissues of colorectal cancer patients and their prognostic relevance. Anticancer research. 1996;17:2939–2942. [PubMed] [Google Scholar]
- 18.Seregni E, et al. Serum and cerebrospinal fluid human chorionic gonadotropin (hCG) and alpha-fetoprotein (AFP) in intracranial germ cell tumors. The International journal of biological markers. 2001;17:112–118. doi: 10.1177/172460080201700206. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World journal of gastroenterology : WJG. 2006;12:1175–1181. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis J, Granovsky M, Warren C. Protein glycosylation in development and disease. Bioessays. 1999 doi: 10.1002/(SICI)1521-1878(199905)21:5<412::AID-BIES8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Drake PM, et al. Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clinical chemistry. 2010;56:223–236. doi: 10.1373/clinchem.2009.136333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowe J, Marth J. A genetic approach to mammalian glycan function. Annual review of biochemistry. 2003 doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 23.Rademacher TW, et al. The role of IgG glycoforms in the pathogenesis of rheumatoid arthritis. Springer seminars in immunopathology. 1988;10:231–249. doi: 10.1007/BF01857227. [DOI] [PubMed] [Google Scholar]
- 24.Lin Z, et al. Mass Spectrometric Assay for Analysis of Haptoglobin Fucosylation in Pancreatic Cancer. Journal of Proteome Research. 2011;10:2602–2611. doi: 10.1021/pr200102h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pompach P, et al. Site-specific glycoforms of haptoglobin in liver cirrhosis and hepatocellular carcinoma. Molecular & cellular proteomics : MCP. 2013;12:1281–1293. doi: 10.1074/mcp.M112.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nature reviews. Drug discovery. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton GS. Antibody-drug conjugates for cancer therapy: The technological and regulatory challenges of developing drug-biologic hybrids. Biologicals : journal of the International Association of Biological Standardization. 2015;43:318–332. doi: 10.1016/j.biologicals.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Reichert JM. Antibodies to watch in 2015. mAbs. 2015;7:1–8. doi: 10.4161/19420862.2015.988944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nature reviews. Immunology. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 30.Davies J, et al. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnology and bioengineering. 2001;74:288–294. [PubMed] [Google Scholar]
- 31.Goede V, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. The New England journal of medicine. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 32.Shibata-Koyama M, et al. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcgammaRIIIb and MHC class II expression on the phagocytotic neutrophils. Experimental hematology. 2009;37:309–321. doi: 10.1016/j.exphem.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara N, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature reviews. Drug discovery. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 34.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nature Reviews Cancer. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nature Reviews Cancer. 2015;15:361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen B-Q, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nature biotechnology. 2012;30:184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- 37.Logtenberg T. Antibody cocktails: next-generation biopharmaceuticals with improved potency. Trends in Biotechnology. 2007;25:390–394. doi: 10.1016/j.tibtech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Robak T. The emerging therapeutic role of antibody mixtures. Expert opinion on biological therapy. 2013;13:953–958. doi: 10.1517/14712598.2013.799133. [DOI] [PubMed] [Google Scholar]
- 39.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Archives of Biochemistry and Biophysics. 2012;526:159–166. doi: 10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcgammaR: current models. Immunology letters. 2002;82:57–65. doi: 10.1016/s0165-2478(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 41.Huang L, et al. Impact of variable domain glycosylation on antibody clearance: an LC/MS characterization. Analytical biochemistry. 2006;349:197–207. doi: 10.1016/j.ab.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Krapp S, et al. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. Journal of molecular biology. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 43.Matsumiya S, et al. Structural Comparison of Fucosylated and Nonfucosylated Fc Fragments of Human Immunoglobulin G1. Journal of Molecular Biology. 2007;368:767–779. doi: 10.1016/j.jmb.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara C, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proceedings of the National Academy of Sciences. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung AW, et al. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS. 2014;28:2523–2530. doi: 10.1097/QAD.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epstein MS, Ehrenpreis ED, Kulkarni PM. Biosimilars: the need, the challenge, the future: the FDA perspective. The American journal of gastroenterology. 2014;109:1856–1859. doi: 10.1038/ajg.2014.151. [DOI] [PubMed] [Google Scholar]
- 47.Harris RJ, et al. Identification of multiple sources of charge heterogeneity in a recombinant antibody. Journal of chromatography. B, Biomedical sciences and applications. 2001;752:233–245. doi: 10.1016/s0378-4347(00)00548-x. [DOI] [PubMed] [Google Scholar]
- 48.O'Connor PB, et al. Differentiation of aspartic and isoaspartic acids using electron transfer dissociation. Journal of the American Society for Mass Spectrometry. 2006;17:15–19. doi: 10.1016/j.jasms.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Yan B, et al. Succinimide formation at Asn 55 in the complementarity determining region of a recombinant monoclonal antibody IgG1 heavy chain. Journal of Pharmaceutical Sciences. 2009;98:3509–3521. doi: 10.1002/jps.21655. [DOI] [PubMed] [Google Scholar]
- 50.Yang H, Zubarev RA. Mass spectrometric analysis of asparagine deamidation and aspartate isomerization in polypeptides. Electrophoresis. 2010;31:1764–1772. doi: 10.1002/elps.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung CH, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. The New England journal of medicine. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundaram S, et al. An innovative approach for the characterization of the isoforms of a monoclonal antibody product. mAbs. 2011;3:505–512. doi: 10.4161/mabs.3.6.18090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brady LJ, Martinez T, Balland A. Characterization of Nonenzymatic Glycation on a Monoclonal Antibody. Analytical Chemistry. 2007;79:9403–9413. doi: 10.1021/ac7017469. [DOI] [PubMed] [Google Scholar]
- 54.Leymarie N, et al. Interlaboratory study on differential analysis of protein glycosylation by mass spectrometry: the ABRF glycoprotein research multi-institutional study 2012. Mol. Cell Proteomics. 2013;12:2935–2951. doi: 10.1074/mcp.M113.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. mAbs. 2012;4:17–23. doi: 10.4161/mabs.4.1.18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pace AL, et al. Asparagine deamidation dependence on buffer type, pH, and temperature. Journal of Pharmaceutical Sciences. 2013;102:1712–1723. doi: 10.1002/jps.23529. [DOI] [PubMed] [Google Scholar]
- 57.Ayoub D, et al. Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. mAbs. 2013;5:699–710. doi: 10.4161/mabs.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gahoual R, et al. Monoclonal antibodies biosimilarity assessment using transient isotachophoresis capillary zone electrophoresis-tandem mass spectrometry. mAbs. 2014;6:1464–1473. doi: 10.4161/mabs.36305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henninot A, et al. Characterization of monoclonal antibodies by a fast and easy LC- MS ToF analysis on culture supernatant. Analytical biochemistry. 2015 doi: 10.1016/j.ab.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Korecká L, et al. Utilization of newly developed immobilized enzyme reactors for preparation and study of immunoglobulin G fragments. Journal of Chromatography B. 2004;808:15–24. doi: 10.1016/j.jchromb.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 61.Krenkova J, Lacher NA, Svec F. Highly efficient enzyme reactors containing trypsin and endoproteinase LysC immobilized on porous polymer monolith coupled to MS suitable for analysis of antibodies. Analytical chemistry. 2009;81:2004–2012. doi: 10.1021/ac8026564. [DOI] [PubMed] [Google Scholar]
- 62.Krenkova J, Lacher NA, Svec F. Multidimensional system enabling deglycosylation of proteins using a capillary reactor with peptide-N-glycosidase F immobilized on a porous polymer monolith and hydrophilic interaction liquid chromatography-mass spectrometry of glycans. Journal of chromatography. A. 2009;1216:3252–3259. doi: 10.1016/j.chroma.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 63.Weng Y, et al. An integrated sample pretreatment platform for quantitative N-glycoproteome analysis with combination of on-line glycopeptide enrichment, deglycosylation and dimethyl labeling. Analytica Chimica Acta. 2014;833:1–8. doi: 10.1016/j.aca.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 64.Zhuang Z, et al. Electrophoretic analysis of N-glycans on microfluidic devices. Analytical chemistry. 2007;79:7170–7175. doi: 10.1021/ac071261v. [DOI] [PubMed] [Google Scholar]
- 65.Mellors JS, et al. Hybrid capillary/microfluidic system for comprehensive online liquid chromatography-capillary electrophoresis-electrospray ionization-mass spectrometry. Analytical chemistry. 2013;85:4100–4106. doi: 10.1021/ac400205a. [DOI] [PubMed] [Google Scholar]
- 66.Macchi FD, et al. Absolute Quantitation of Intact Recombinant Antibody Product Variants Using Mass Spectrometry. Analytical chemistry. 2015 doi: 10.1021/acs.analchem.5b02627. [DOI] [PubMed] [Google Scholar]
- 67.Primack J, Flynn GC, Pan H. A high-throughput microchip-based glycan screening assay for antibody cell culture samples. Electrophoresis. 2011;32:1129–1132. doi: 10.1002/elps.201000619. [DOI] [PubMed] [Google Scholar]
- 68.Bynum MA, et al. Characterization of IgG N-glycans employing a microfluidic chip that integrates glycan cleavage, sample purification, LC separation, and MS detection. Analytical chemistry. 2009;81:8818–8825. doi: 10.1021/ac901326u. [DOI] [PubMed] [Google Scholar]
- 69.Schiel JE, Rogstad SM, Boyne MT. Comparison of Traditional 2-AB Fluorescence LC-MS/MS and Automated LC-MS for the Comparative Glycan Analysis of Monoclonal Antibodies. Journal of Pharmaceutical Sciences. 2015;104:2464–2472. doi: 10.1002/jps.24522. [DOI] [PubMed] [Google Scholar]
- 70.Wang B, et al. Structural comparison of two anti-CD20 monoclonal antibody drug products using middle-down mass spectrometry. The Analyst. 2013;138:3058–3065. doi: 10.1039/c3an36524g. [DOI] [PubMed] [Google Scholar]
- 71.Beck A, et al. Cutting-edge mass spectrometry characterization of originator, biosimilar and biobetter antibodies. Journal of mass spectrometry : JMS. 2015;50:285–297. doi: 10.1002/jms.3554. [DOI] [PubMed] [Google Scholar]
- 72.Rosati S, et al. Detailed mass analysis of structural heterogeneity in monoclonal antibodies using native mass spectrometry. Nature protocols. 2014;9:967–976. doi: 10.1038/nprot.2014.057. [DOI] [PubMed] [Google Scholar]
- 73.Bagal D, et al. Resolving disulfide structural isoforms of IgG2 monoclonal antibodies by ion mobility mass spectrometry. Analytical chemistry. 2010;82:6751–6755. doi: 10.1021/ac1013139. [DOI] [PubMed] [Google Scholar]
- 74.Pacholarz KJ, et al. Dynamics of Intact Immunoglobulin G Explored by Drift-Tube Ion-Mobility Mass Spectrometry and Molecular Modeling. Angewandte Chemie International Edition. 2014;53:7765–7769. doi: 10.1002/anie.201402863. [DOI] [PubMed] [Google Scholar]
- 75.Hall Z, et al. The role of salt bridges, charge density, and subunit flexibility in determining disassembly routes of protein complexes. Structure (London, England: 1993) 2013;21:1325–1337. doi: 10.1016/j.str.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laganowsky A, et al. Mass spectrometry of intact membrane protein complexes. Nature protocols. 2013;8:639–651. doi: 10.1038/nprot.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia BA, et al. Pervasive combinatorial modification of histone H3 in human cells. Nature methods. 2007;4:487–489. doi: 10.1038/nmeth1052. [DOI] [PubMed] [Google Scholar]
- 78.Pesavento JJ, et al. Combinatorial modification of human histone H4 quantitated by two-dimensional liquid chromatography coupled with top down mass spectrometry. The Journal of biological chemistry. 2008;283:14927–14937. doi: 10.1074/jbc.M709796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao Y, et al. Top-down structural analysis of an intact monoclonal antibody by electron capture dissociation-Fourier transform ion cyclotron resonance-mass spectrometry. Analytical chemistry. 2013;85:4239–4246. doi: 10.1021/ac303525n. [DOI] [PubMed] [Google Scholar]
- 80.Fornelli L, et al. Analysis of intact monoclonal antibody IgG1 by electron transfer dissociation Orbitrap FTMS. Molecular & cellular proteomics : MCP. 2012;11:1758–1767. doi: 10.1074/mcp.M112.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw JB, et al. Complete protein characterization using top-down mass spectrometry and ultraviolet photodissociation. Journal of the American Chemical Society. 2013;135:12646–12651. doi: 10.1021/ja4029654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Brien JP, et al. Characterization of native protein complexes using ultraviolet photodissociation mass spectrometry. Journal of the American Chemical Society. 2014;136:12920–12928. doi: 10.1021/ja505217w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fornelli L, et al. Middle-down analysis of monoclonal antibodies with electron transfer dissociation orbitrap fourier transform mass spectrometry. Analytical chemistry. 2014;86:3005–3012. doi: 10.1021/ac4036857. [DOI] [PubMed] [Google Scholar]
- 84.Srzentić K, et al. Advantages of extended bottom-up proteomics using Sap9 for analysis of monoclonal antibodies. Analytical chemistry. 2014;86:9945–9953. doi: 10.1021/ac502766n. [DOI] [PubMed] [Google Scholar]
- 85.Xie H, et al. Rapid comparison of a candidate biosimilar to an innovator monoclonal antibody with advanced liquid chromatography and mass spectrometry technologies. mAbs. 2010;2:379–394. doi: 10.4161/mabs.2.4.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mechref Y, Muzikar J, Novotny MV. Comprehensive assessment of N-glycans derived from a murine monoclonal antibody: a case for multimethodological approach. Electrophoresis. 2005;26(10):2034–2046. doi: 10.1002/elps.200410345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunt G, Nashabeh W. Capillary electrophoresis sodium dodecyl sulfate nongel sieving analysis of a therapeutic recombinant monoclonal antibody: a biotechnology perspective. Analytical chemistry. 1999;71:2390–2397. doi: 10.1021/ac981209m. [DOI] [PubMed] [Google Scholar]
- 88.Ma S, Nashabeh W. Carbohydrate analysis of a chimeric recombinant monoclonal antibody by capillary electrophoresis with laser-induced fluorescence detection. Analytical chemistry. 1999;71:5185–5192. doi: 10.1021/ac990376z. [DOI] [PubMed] [Google Scholar]
- 89.Gahoual R, et al. Rapid and multi-level characterization of trastuzumab using sheathless capillary electrophoresis-tandem mass spectrometry. mAbs. 2013;5:479–490. doi: 10.4161/mabs.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biacchi M, et al. Analysis of monoclonal antibody by a novel CE-UV/MALDI-MS interface. Electrophoresis. 2014;35:2986–2995. doi: 10.1002/elps.201400276. [DOI] [PubMed] [Google Scholar]
- 91.Ruhaak LR, et al. Optimized workflow for preparation of APTS-labeled N-glycans allowing high-throughput analysis of human plasma glycomes using 48-channel multiplexed CGE-LIF. Journal of proteome research. 2010;9:6655–6664. doi: 10.1021/pr100802f. [DOI] [PubMed] [Google Scholar]
- 92.Szabo Z, et al. Rapid high-resolution characterization of functionally important monoclonal antibody N-glycans by capillary electrophoresis. Analytical chemistry. 2011;83:5329–5336. doi: 10.1021/ac2007587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szabo Z, et al. Ultrasensitive capillary electrophoretic analysis of potentially immunogenic carbohydrate residues in biologics: galactose-α-1,3-galactose containing oligosaccharides. Molecular pharmaceutics. 2012;9:1612–1619. doi: 10.1021/mp200612n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Damen CWN, et al. Electrospray ionization quadrupole ion-mobility time-of-flight mass spectrometry as a tool to distinguish the lot-to-lot heterogeneity in N-glycosylation profile of the therapeutic monoclonal antibody trastuzumab. Journal of the American Society for Mass Spectrometry. 2009;20:2021–2033. doi: 10.1016/j.jasms.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 95.Qian J, et al. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzy. Analytical biochemistry. 2007;364:8–18. doi: 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 96.Haselberg R, de Jong GJ, Somsen GW. Low-flow sheathless capillary electrophoresis-mass spectrometry for sensitive glycoform profiling of intact pharmaceutical proteins. Analytical chemistry. 2013;85:2289–2296. doi: 10.1021/ac303158f. [DOI] [PubMed] [Google Scholar]
- 97.Gennaro LA, Salas-Solano O. On-line CE-LIF-MS technology for the direct characterization of N-linked glycans from therapeutic antibodies. Analytical chemistry. 2008;80:3838–3845. doi: 10.1021/ac800152h. [DOI] [PubMed] [Google Scholar]
- 98.Maeda E, et al. Analysis of nonhuman N-glycans as the minor constituents in recombinant monoclonal antibody pharmaceuticals. Analytical chemistry. 2012;84:2373–2379. doi: 10.1021/ac300234a. [DOI] [PubMed] [Google Scholar]
- 99.Hamm M, Wang Y, Rustandi RR. Characterization of N-Linked Glycosylation in a Monoclonal Antibody Produced in NS0 Cells Using Capillary Electrophoresis with Laser-Induced Fluorescence Detection. Pharmaceuticals (Basel, Switzerland) 2013;6:393–406. doi: 10.3390/ph6030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reusch D, et al. High-throughput glycosylation analysis of therapeutic immunoglobulin G by capillary gel electrophoresis using a DNA analyzer. mAbs. 2014;6:185–196. doi: 10.4161/mabs.26712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahan AE, et al. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. Journal of immunological methods. 2015;417:34–44. doi: 10.1016/j.jim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiegandt A, Meyer B. Unambiguous Characterization of N -Glycans of Monoclonal Antibody Cetuximab by Integration of LC-MS/MS and 1 H NMR Spectroscopy. Analytical Chemistry. 2014;86:4807–4814. doi: 10.1021/ac404043g. [DOI] [PubMed] [Google Scholar]
- 103.Redman EA, et al. Characterization of Intact Antibody Drug Conjugate Variants Using Microfluidic Capillary Electrophoresis-Mass Spectrometry. Anal Chem. 2016;88(4):2220–2226. doi: 10.1021/acs.analchem.5b03866. [DOI] [PubMed] [Google Scholar]
- 104.Thompson NJ, et al. Complex mixtures of antibodies generated from a single production qualitatively and quantitatively evaluated by native Orbitrap mass spectrometry. MAbs. 2014;6(1):197–203. doi: 10.4161/mabs.27126. [DOI] [PMC free article] [PubMed] [Google Scholar]