ABSTRACT
During fermentation, increased ethanol concentration is a major stress for yeast cells. Vacuolar H+-ATPase (V-ATPase), which plays an important role in the maintenance of intracellular pH homeostasis through vacuolar acidification, has been shown to be required for tolerance to straight-chain alcohols, including ethanol. Since ethanol is known to increase membrane permeability to protons, which then promotes intracellular acidification, it is possible that the V-ATPase is required for recovery from alcohol-induced intracellular acidification. In this study, we show that the effects of straight-chain alcohols on membrane permeabilization and acidification of the cytosol and vacuole are strongly dependent on their lipophilicity. These findings suggest that the membrane-permeabilizing effect of straight-chain alcohols induces cytosolic and vacuolar acidification in a lipophilicity-dependent manner. Surprisingly, after ethanol challenge, the cytosolic pH in Δvma2 and Δvma3 mutants lacking V-ATPase activity was similar to that of the wild-type strain. It is therefore unlikely that the ethanol-sensitive phenotype of vma mutants resulted from severe cytosolic acidification. Interestingly, the vma mutants exposed to ethanol exhibited a delay in cell wall remodeling and a significant increase in intracellular reactive oxygen species (ROS). These findings suggest a role for V-ATPase in the regulation of the cell wall stress response and the prevention of endogenous oxidative stress in response to ethanol.
IMPORTANCE The yeast Saccharomyces cerevisiae has been widely used in the alcoholic fermentation industry. Among the environmental stresses that yeast cells encounter during the process of alcoholic fermentation, ethanol is a major stress factor that inhibits yeast growth and viability, eventually leading to fermentation arrest. This study provides evidence for the molecular mechanisms of ethanol tolerance, which is a desirable characteristic for yeast strains used in alcoholic fermentation. The results revealed that straight-chain alcohols induced cytosolic and vacuolar acidification through their membrane-permeabilizing effects. Contrary to expectations, a role for V-ATPase in the regulation of the cell wall stress response and the prevention of endogenous oxidative stress, but not in the maintenance of intracellular pH, seems to be important for protecting yeast cells against ethanol stress. These findings will expand our understanding of the mechanisms of ethanol tolerance and provide promising clues for the development of ethanol-tolerant yeast strains.
INTRODUCTION
The budding yeast Saccharomyces cerevisiae has been widely used in industrial fermentation, such as in the production of alcoholic beverages and ethanol fuel. During fermentation, yeast cells encounter several environmental stresses, such as increased alcohol concentration, high osmolarity, and temperature fluctuations. Among these, ethanol is a major stress factor that affects the vitality and viability of yeast cells, thereby leading to an arrest of the fermentation process (1). High ethanol concentrations are known to influence a number of metabolic processes, including the inhibition of glucose and amino acid transport, denaturation of the key glycolytic enzymes pyruvate kinase and hexokinase, and increased membrane permeability (1).
Cellular membranes, especially the plasma membrane, have been suggested to be the main target of ethanol stress in yeast cells (2). Ethanol and other short-chain alcohols are believed to disturb the cellular membrane through the association of their aliphatic chains with the hydrophobic interior of membranes, thereby affecting membrane permeability and stability (3). The loss of membrane integrity decreases the cell's ability to maintain a concentration gradient across the plasma membrane, which is important for coupled transport of several metabolites, such as amino acids and ions (4, 5). Since the budding yeast normally lives in a slightly acidic environment with a high proton concentration, the increase in membrane permeability caused by alcohol may lead to an increased passive influx of protons across the membrane, thereby inducing intracellular acidification (6). Compounds with high lipophilicity, usually expressed in terms of the log octanol-water partition coefficient (log Pow), are known to possess a high affinity for the cellular membrane (7). In addition, the toxicities of alcohols have been shown to be correlated with their lipophilicity (3, 8–10). For instance, alcohols with high lipophilicity, such as 1-octanol (log Pow = 3.07), were more toxic to yeast cells than those with low lipophilicity, such as ethanol (log Pow = −0.30) (8). Taken together, these results indicate it is possible that alcohols with high lipophilicity trigger a high degree of membrane permeabilization, leading to greater proton influx and a significant decrease in intracellular pH. In agreement with this idea, Leão and Van Uden (6) reported that upon exposure to alkanols, i.e., ethanol, isopropanol, propanol, and butanol, the extracellular pH increased in a concentration- and lipophilicity-dependent manner, possibly as a consequence of the increased proton influx. However, since the alterations in intracellular pH in response to alcohols with different lipophilicities have not been determined, the correlation between the lipophilicity of alcohols and their effects on membrane permeability and intracellular pH remains unclear.
A number of genes associated with vacuolar H+-ATPase (V-ATPase), including genes encoding structural components of V-ATPase and genes involved in the assembly of V-ATPase, have been shown to be required for tolerance not only to ethanol but also to other alcohols (9, 10). The yeast V-ATPase is a multisubunit proton pump composed of a peripheral V1 ATP-hydrolytic domain and an integral V0 proton-translocating domain. The V-ATPase plays a vital role in the maintenance of intracellular pH homeostasis through proton translocation across the vacuolar membrane driven by ATP hydrolysis, resulting in vacuolar acidification (11). In addition to the V-ATPase, the plasma membrane H+-ATPases, which are composed of the essential Pma1p and its homolog, the nonessential Pma2p, are also known to function in pumping protons out of the cell to control the intracellular pH balance (12, 13). The activities of both V-ATPase and plasma membrane H+-ATPase Pma1p are induced under acidic pH conditions, possibly in response to cytosolic acidification (14, 15). The vma mutants lacking V-ATPase activity exhibit a more-alkaline vacuolar pH than the wild-type strain (16) and are unable to grow at pH values of >7 and <4 (17, 18). Moreover, a loss of V-ATPase activity triggers ubiquitination and endocytosis of Pma1p, possibly to balance overall pH homeostasis (16, 19). These phenotypes of the vma mutants suggest that the V-ATPase and the plasma membrane H+-ATPases together play an interdependent role in the regulation of intracellular pH homeostasis. In addition to the expected phenotypes of the vma mutants, which are a direct consequence of impaired intracellular pH regulation, these mutants also exhibited other pleiotropic phenotypes, including sensitivity to a variety of oxidants, such as H2O2 (20), sensitivity to transition metals, such as copper and zinc (21, 22), poor growth under conditions of both high and low concentrations of calcium and iron (18, 23, 24), poor growth on nonfermentable carbon sources (25), and defective vacuolar morphology and vacuolar protein sorting (26, 27). Some of these phenotypes can be easily explained by an increased accumulation of reactive oxygen species (ROS) or defective vacuolar function, important for storage and sequestration of several metabolites and toxins in these mutants (20, 25). Based on these previous observations, it is possible that the V-ATPase, possibly in collaboration with the plasma membrane H+-ATPase, is required for recovery from cytosolic acidification or other harmful effects caused by alcohols. However, the roles of these proton pumps during alcohol stress remain unclear.
In this report, we show a clear correlation between the lipophilicity of straight-chain alcohols and their effects on membrane permeability and intracellular pH alteration, suggesting that the alcohol-induced cytosolic and vacuolar acidification may result from increased membrane permeability. However, the ethanol-sensitive phenotype of vma mutants lacking V-ATPase activity is likely due to elevated intracellular ROS levels and a defect in the response to cell wall damage caused by ethanol rather than the increased cytosolic acidification.
MATERIALS AND METHODS
Strains and culture conditions.
The yeast strains used in this study were the BY4742 wild-type strain (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and the congenic Δvma2::kanMX, Δvma3::kanMX, and Δpma2::kanMX strains. All strains were obtained from Open Biosystems (Huntsville, AL). YPD (1% yeast extract, 2% peptone, 2% dextrose) and SD (2% dextrose and 0.67% yeast nitrogen base without amino acids) media supplemented with appropriate nutrients were prepared as described previously (28) and adjusted to pH 6.0 with the optional addition of 200 mg/liter G418 (Geneticin; Sigma-Aldrich).
Growth assay.
A serial-dilution spot test was used to examine alcohol sensitivity. Exponential-phase cells were diluted to an optical density at 600 nm (OD600) of 1.0 (approximately 107 cells/ml) and then serially diluted 10-fold. Aliquots of 3 μl were spotted onto freshly prepared YPD agar plates containing 18% methanol, 10% ethanol, 7% 1-propanol, 3% 1-butanol, and 100 μg/ml calcofluor white and incubated at 30°C for 3 days. For growth under anaerobic conditions, the inoculated plates were incubated at 30°C for 3 to 4 days in an AnaeroPack rectangular jar (Mitsubishi Gas Chemical Co., Tokyo, Japan) containing an AnaeroPack working as an oxygen absorber-CO2 generator (Mitsubishi Gas Chemical Co.). To test amphotericin B (AmB) sensitivity, log-phase cultures were inoculated into YPD medium containing 0.5 or 1 μM AmB to an OD600 of 0.1 and incubated with shaking at 30°C. Cell growth was monitored at 2-h intervals for 24 h by measuring the OD600.
Sytox green uptake.
Yeast membrane permeabilization was monitored by measuring Sytox green uptake, as described previously (29). Briefly, log-phase cells were harvested and resuspended in 50 mM morpholineethanesulfonic acid (MES)-NaOH buffer (pH 5.5) containing 1 μM Sytox green (Invitrogen) to an OD600 of 1. The Sytox green fluorescence was measured with a SpectraMax M3 microplate reader (Molecular Devices) at an excitation wavelength of 488 nm and an emission wavelength of 544 nm. The relative fluorescence units were expressed as a percentage of the fluorescence values of the wild-type strain grown in YPD medium.
Measurements of cytosolic and vacuolar pH.
Cytosolic pH was measured using pHluorin (30), a pH-sensitive green fluorescent protein (GFP) expressed from plasmid pYES-PACT1-pHluorin under the control of the ACT1 promoter (a generous gift from Gertien J. Smits, University of Amsterdam, the Netherlands), according to a method described previously (16), with some modifications. Briefly, yeast cells were transformed with this plasmid, and transformants were selected in SD medium lacking uracil (SD–Ura) and confirmed by fluorescence microscopy (30). Yeast cells grown to log phase in the specified medium were harvested, washed, and resuspended at 1 g of wet cell mass/ml in glucose-free medium. Fluorescence intensities at excitation wavelengths of 390 and 470 nm were measured at a constant emission wavelength of 512 nm by the SpectraMax M3 microplate reader (Molecular Devices). Calibration of fluorescence with pH was performed for each strain and included buffers titrated to different pH values within a range of 4.5 to 8.0, as described previously (16).
Vacuolar pH was measured according to a previously described method (31). Yeast cells grown to log phase in the specified medium were harvested, washed, resuspended in the same culture medium containing 50 μM 2′,7′-bis-(2-carboxyethyl)-5-(and -6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM; Sigma), and incubated at 30°C for 30 min. Fluorescence intensities at excitation wavelengths of 450 and 490 nm were measured at a constant emission wavelength of 535 nm by the SpectraMax M3 microplate reader (Molecular Devices). The vacuolar pH was estimated from the calibration curve for each strain, which was constructed by incubating the yeast strain in buffers titrated to different pH values within a range of 4.0 to 8.0, as described previously (31).
Zymolyase susceptibility test.
Susceptibility to Zymolyase was carried out according to the method of Ovalle et al. (32), with some modifications. Log-phase cells were diluted to an OD600 of 0.5 in TE buffer (10 mM Tris-HCl and 1 mM EDTA [pH 7.5]) containing 100 μg/ml (1 U) Zymolyase 20T (Zymo Research). Changes in the OD600 was monitored at 15-min intervals for 2 h.
Measurement of intracellular ROS levels.
The oxidant-sensitive probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma) was used to determine the level of intracellular ROS as described previously (33). Briefly, exponential-phase cells were treated with 10 μM DCFH-DA in culture medium for 1 h. The cells were then harvested, washed twice in ice-cold phosphate-buffered saline, resuspended in phosphate-buffered saline, and disrupted with glass beads. After being centrifuged at 13,000 × g for 10 min at 4°C, the supernatant was collected and used for the measurement of fluorescence intensity at an excitation wavelength of 504 nm and an emission wavelength of 524 nm by the SpectraMax M3 microplate reader (Molecular Devices). The fluorescence intensity value was normalized to the protein level in the supernatant.
RESULTS
Effects of straight-chain alcohols on yeast cell growth and membrane permeability are correlated with their lipophilicity.
To determine the effects of straight-chain alcohols with different lipophilicities expressed in terms of log Pow values on yeast cells, we examined the growth of the wild-type strain (BY4742) on YPD agar plates containing methanol (log Pow, −0.74), ethanol (log Pow, −0.3), 1-propanol (log Pow, 0.25), or 1-butanol (log Pow, 0.84), which have similar structures and chemical properties but different aliphatic chain lengths, by using a spot susceptibility test. The wild-type strain was able to grow in the presence of methanol, ethanol, 1-propanol, and 1-butanol at concentrations of up to 20%, 15%, 7.5%, and 5%, respectively (see Fig. S1 in the supplemental material), showing a clear correlation between yeast cell growth and lipophilicity (log Pow value) of straight-chain alcohols.
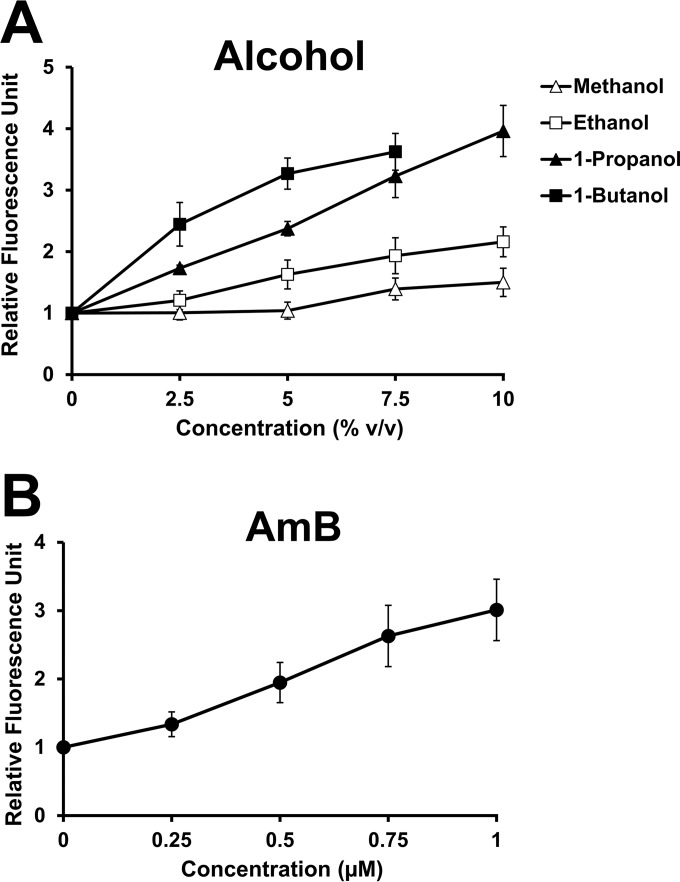
Since it has been suggested that an effect of ethanol on enhancing membrane permeability is probably due to an insertion of its hydrophobic portion into the hydrophobic interior of the biological membrane (34), it is possible that highly lipophilic alcohols with high affinity to the cellular membrane may have high membrane-permeabilizing effects. To explore the relationship between the lipophilicity of straight-chain alcohols and their effects on membrane permeability, we determined the membrane permeability of the wild-type strain exposed to increasing concentrations of methanol, ethanol, 1-propanol, and 1-butanol by using the Sytox green uptake assay. Sytox green is a high-affinity nucleic acid-staining fluorescent dye that can penetrate the plasma membrane only when the membrane integrity is compromised (29). Our results revealed that the fluorescence intensities of cells treated with straight-chain alcohols increased with increasing concentrations of (in decreasing order) 1-butanol, 1-propanol, ethanol, and methanol, indicating a strong dependence of fluorescence intensity on the lipophilicity and concentration of a straight-chain alcohol (Fig. 1A). To determine whether increased fluorescence intensities of the cells treated with alcohols correlate with a known instance of increased membrane permeability, we performed this assay in the wild-type strain challenged with amphotericin B (AmB), a polyene antifungal drug that, together with ergosterol, forms an ion-permeable self-assembling transmembrane pore (35), and we found that the fluorescence intensities increased with increasing concentrations of AmB (Fig. 1B). Taken together, these results suggest that the membrane-permeabilizing effects of straight-chain alcohols are correlated with their lipophilicity.
FIG 1.
Sytox green uptake by yeast cells exposed to straight-chain alcohols and amphotericin B (AmB). The exponentially growing wild-type cells (BY 4742) were incubated in YPD medium containing the indicated concentrations of methanol (△), ethanol (□), 1-propanol (▲), or 1-butanol (■) (A) or of AmB (●) (B) at 30°C for 1 h. The cells with an OD600 of 1 loaded with Sytox green were immediately used for fluorescence measurement at an excitation wavelength of 544 nm and an emission wavelength of 488 nm. The fluorescence intensities of the treated cells were normalized to that of the untreated control and plotted as relative fluorescence units (RFU). All experiments were carried out in triplicate. The error bars indicate the standard deviations (SD). v/v, vol/vol.
Degrees of cytosolic and vacuolar acidification induced by straight-chain alcohols are dependent on their lipophilicity.
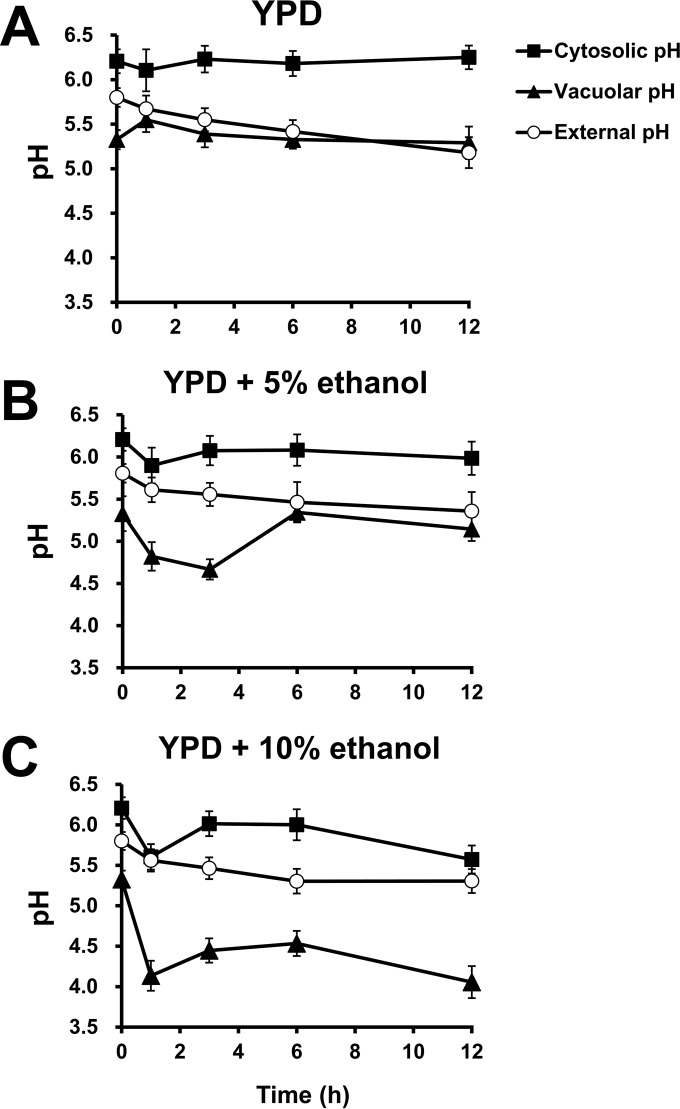
It has been shown that straight-chain alcohols induce passive fluxes of ions, including protons, across the plasma membrane due to increased membrane permeability (6), which may disturb the intracellular pH. To investigate the dynamic changes in intracellular and extracellular pHs in response to ethanol, time courses of the changes in cytosolic, vacuolar, and external pHs of the wild-type strain were monitored. The cytosolic and vacuolar pH values of the wild-type cells precultivated to exponential phase in YPD medium for 12 h were 6.2 and 5.3, respectively, while the external pH of the preculture before a shift to fresh medium was 5.2. We first investigated the pH fluctuations under normal conditions by measuring the cytosolic, vacuolar, and external pH values of wild-type cells grown in YPD medium adjusted to pH 5.8 (Fig. 2A). After a shift to fresh YPD medium, the cytosolic pH of wild-type cells was maintained at approximately 6.1 to 6.2 throughout an incubation period of 12 h, whereas the vacuolar pH of this strain increased to 5.6 within 1 h after a shift and dropped to approximately 5.3 after incubation for >3 h (Fig. 2A). On the other hand, the external pH gradually dropped to 5.2 during a 12-h cultivation (Fig. 2A).
FIG 2.
Dynamic changes in cytosolic, vacuolar, and external pHs of yeast cells during ethanol exposure. Cytosolic, vacuolar, and external pH values of the wild-type cells (BY4742) treated with 0% (A), 5% (B), or 10% ethanol (C) were measured. For the cytosolic pH (■), log-phase cells transformed with pYES-PACT1-pHluorin were harvested at the indicated times and used for the measurement of fluorescence intensity. For the vacuolar pH (▲), the exponentially growing cells without the plasmid were harvested at the indicated times and loaded with BCECF-AM prior to the measurement of fluorescence intensity. The extracellular pH (○) at the indicated times was also monitored. All experiments were carried out in triplicate. The error bars indicate the SD.
To investigate the effect of ethanol on pH fluctuations, the cytosolic, vacuolar, and external pH values of wild-type cells challenged with 5% and 10% ethanol were determined (Fig. 2B and C). The cytosolic pH of the wild-type strain challenged with 5% ethanol dropped to 5.9 within 1 h and was maintained at 6.0 to 6.1 after exposure for >3 h, whereas during exposure to 10% ethanol, it strikingly dropped to 5.6 within the first hour, increased to 6.0 during 3 to 6 h of incubation, and declined again to 5.6 after a 12-h exposure (Fig. 2B and C). After the wild-type strain was challenged with 5% ethanol, the vacuolar pH decreased to 4.7 within 3 h and increased to 5.1 to 5.3 after exposure for >6 h (Fig. 2B). When wild-type cells were treated with 10% ethanol, the vacuolar pH remarkably dropped to 4.1 within 1 h, was maintained at 4.5 during 3 to 6 h of incubation, and fell to 4.1 after a 12-h exposure (Fig. 2C). With regard to the external pH, when cells were treated with 5% or 10% ethanol, it gradually decreased to 5.4 or 5.3, respectively, during a 12-h exposure (Fig. 2B and C). These results suggest a dose-dependent effect of ethanol on cytosolic acidification, which seems to be recovered by vacuolar acidification and proton extrusion from cells.
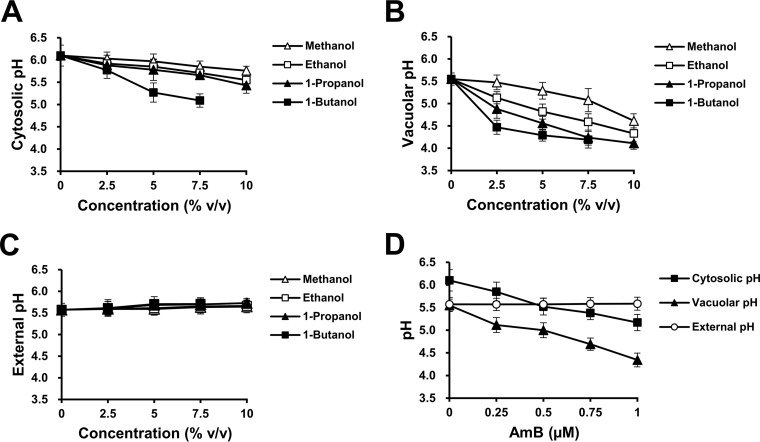
To determine the effect of straight-chain alcohols with different lipophilicity values on intracellular pH disturbance, we measured the cytosolic, vacuolar, and external pH values of the wild-type strain after 1 h of exposure to various concentrations (0 to 10%) of methanol, ethanol, 1-propanol, and 1-butanol. Our results revealed that the acidification of the cytosol and vacuole was enhanced in a concentration- and lipophilicity-dependent manner (Fig. 3A and B). Meanwhile, during short-term exposure, the external pH of wild-type cells grown in the presence or absence of straight-chain alcohols was not significantly altered (Fig. 3C). To determine whether the effect of straight-chain alcohols on cytosolic and vacuolar acidification correlates with increased membrane permeability, the cytosolic, vacuolar, and external pH values of the wild-type strain challenged with 0 to 1 μM AmB for 1 h were measured. Similar to the treatments with straight-chain alcohols, the cytosolic and vacuolar pHs decreased with increasing concentrations of AmB while the external pH was not affected (Fig. 3D). Taken together, these results suggest that the increased membrane permeability caused by straight-chain alcohols induces cytosolic and vacuolar acidification in a concentration- and lipophilicity-dependent manner.
FIG 3.
Changes in cytosolic, vacuolar, and external pH in response to straight-chain alcohols and AmB in the wild-type strain. Cytosolic, vacuolar, and external pH values of the wild-type cells (BY4742) incubated in YPD medium containing 0 to 10% methanol (△), ethanol (□), 1-propanol (▲), or 1-butanol (■) (A to C) or 0 to 1 μM AmB (D) at 30°C for 1 h were measured. The log-phase wild-type cells transformed with pYES-PACT1-pHluorin were used for the measurement of cytosolic pH (A), whereas log-phase wild-type cells without the plasmid were used for the measurement of vacuolar pH (B) and external pH (C). All experiments were carried out in triplicate. The error bars indicate the SD.
Cytosolic acidification does not account for the ethanol-sensitive phenotype of the vma mutants lacking V-ATPase activity.
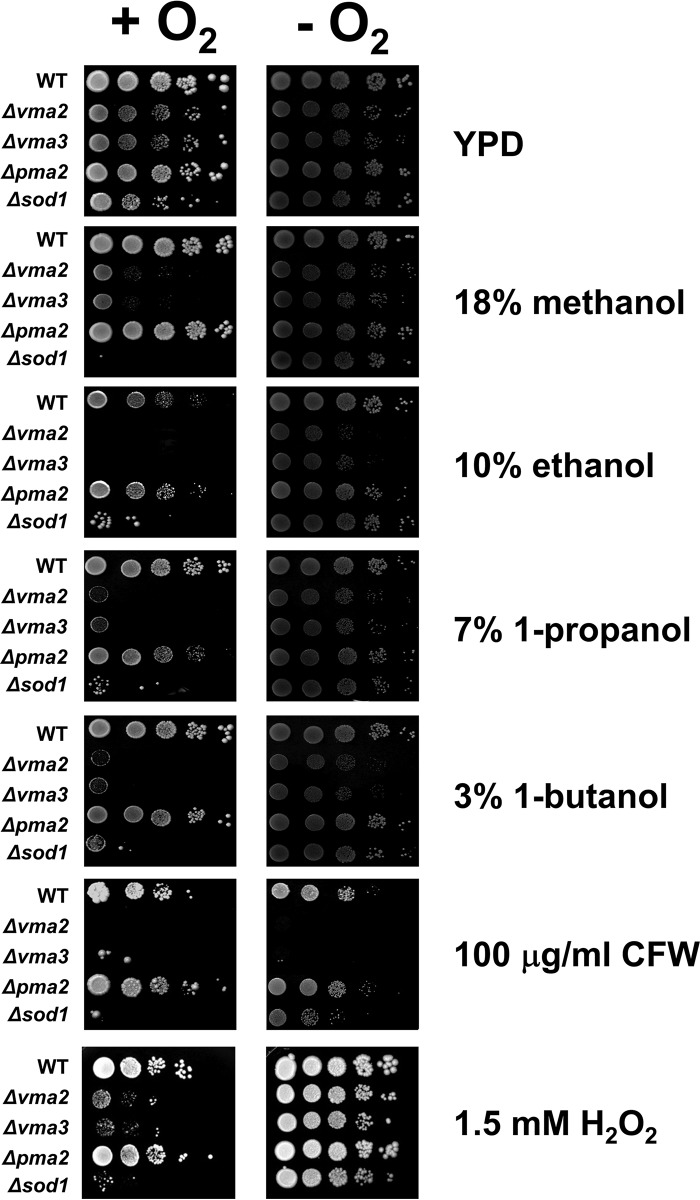
The V-ATPase and plasma membrane H+-ATPase functioning in pumping protons into the vacuole and out of cells, respectively, are known to play a pivotal role in the maintenance of cytosolic pH homeostasis (16). Previously, a number of mutants lacking V-ATPase activity have been shown to be sensitive to alcohols, including ethanol (9, 10). It is therefore possible that the V-ATPase and/or the plasma membrane H+-ATPase is required for recovery from cytosolic acidification as a consequence of increased membrane permeability induced by alcohols. To confirm the importance of V-ATPase for alcohol tolerance, the Δvma2 and Δvma3 mutants, lacking the V0 and V1 subunits of the V-ATPase, respectively, were used. To investigate the role of plasma membrane H+-ATPase, we used the Δpma2 mutant, lacking the minor plasma membrane H+-ATPase, because PMA1, which encodes the major plasma membrane H+-ATPase, is an essential gene, precluding the construction of such a deletion mutant. We examined the growth of these mutants in the presence and absence of various straight-chain alcohols and found that, compared to the wild-type strain, the Δvma2 and Δvma3 mutants, but not the Δpma2 mutant, were more sensitive to 18% methanol, 10% ethanol, 7% 1-propanol, and 3% 1-butanol (Fig. 4). In addition, only the vma mutants were also more sensitive to AmB than the wild-type strain (see Fig. S2 in the supplemental material).
FIG 4.
V-ATPase is important for tolerance to not only straight-chain alcohols but also cell wall and oxidative stresses. Wild-type, Δvma2, Δvma3, Δpma2, and Δsod1 cells grown to log phase in YPD broth were serially diluted 10-fold from an initial OD600 of 0.1. Aliquots (3 μl) were spotted on the YPD agar plate containing 18% methanol, 10% ethanol, 7% 1-propanol, 3% 1-butanol, 100 μg/ml calcofluor white (CFW), or 1.5 mM H2O2 and incubated at 30°C under aerobic (+O2) and anaerobic (−O2) conditions for 3 days.
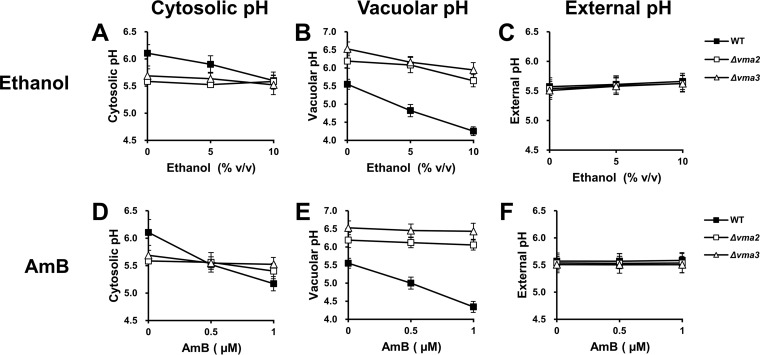
To investigate whether the ethanol-sensitive phenotype of the vma mutants is a consequence of severe cytosolic acidification due to the lack of an ability to pump excess protons from the cytosol into the vacuole, we monitored the cytosolic, vacuolar, and external pH values of the Δvma2 and Δvma3 mutants after challenge with ethanol or AmB for 1 h. As expected, under all conditions, the vacuolar pH of the vma mutants was more alkaline than that of the wild-type strain (Fig. 5B and E). For the external pH, no significant differences were observed between the wild-type and mutant strains (Fig. 5C and F). In contrast to the wild-type strain, the cytosolic pH of vma mutants was not significantly changed in response to ethanol or AmB (Fig. 5A and D). Furthermore, although the cytosolic pH of vma mutants was lower than that of the wild-type strain when they were grown in YPD medium, it was similar to that of the wild-type strain under conditions of 10% ethanol or 0.5 μM AmB and even more alkaline than the wild-type strain when treated with 1 μM AmB (Fig. 5A and D). However, it was still possible that the effect of alcohol on cytosolic acidification in vma mutants might occur after long-term exposure; therefore, we measured the cytosolic pH of these mutants treated with ethanol or AmB for 12 h. As with short-term exposure, the cytosolic pH of vma mutants was not altered by long-term exposure to ethanol or AmB and was at levels similar to those of the wild-type strain when high concentrations were used (see Fig. S3A and D in the supplemental material). Based on our observations, it is unlikely that the sensitivity of vma mutants to ethanol results from severe cytosolic acidification.
FIG 5.
Effects of straight-chain alcohols and AmB on cytosolic, vacuolar, and external pH in the mutants lacking V-ATPase. Cytosolic, vacuolar, and external pH values of the wild-type (BY 4742) (■), Δvma2 (□), and Δvma3 (△) cells incubated in YPD medium containing 0, 5, or 10% ethanol (A to C) or 0, 0.5 or 1 μM AmB (D to F) at 30°C for 1 h were measured. Log-phase cells transformed with pYES-PACT1-pHluorin were used for the measurement of cytosolic pH (A and D), whereas log-phase cells without the plasmid were used for the measurement of vacuolar pH (B and E) and external pH (C and F). All experiments were carried out in triplicate. The error bars indicate the SD.
Loss of V-ATPase activity leads to delayed cell wall remodeling in response to ethanol stress.
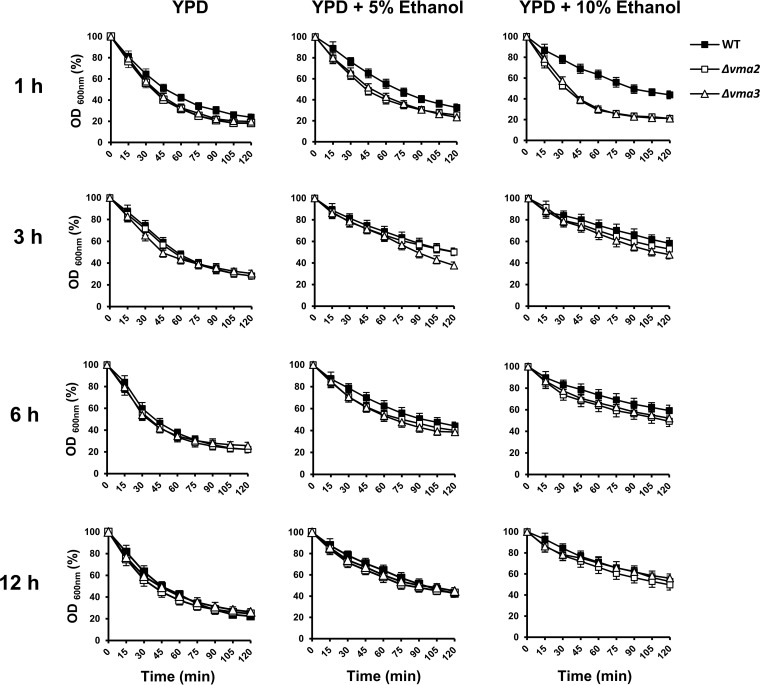
It has been reported that several cell wall-related genes are important for ethanol tolerance and that cell wall composition is remodeled in response to ethanol stress (9, 10, 36, 37). To test whether the ethanol-sensitive phenotype of the vma mutants is a consequence of a defect in cell wall remodeling, we first examined the aerobic growth of the Δvma2 and Δvma3 mutants in the presence of the cell wall-perturbing agent calcofluor white. The Δvma2 and Δvma3 mutants were highly sensitive to 100 μg/ml calcofluor white (Fig. 4), suggesting impaired cell wall stress responses in these mutants. To investigate remodeling of the cell wall in response to ethanol stress, the wild-type, Δvma2, and Δvma3 cells challenged with 5% and 10% ethanol for up to 12 h were examined for sensitivity to Zymolyase, a cell wall-degrading enzyme whose major activities are β-1,3-glucanase and β-1,3-glucan laminaripentaohydrolase. Zymolyase resistance (as determined by measuring the optical density of the cell suspensions) of wild-type cells increased with increasing ethanol concentration and exposure time to ethanol (Fig. 6), suggesting the concentration- and exposure time-dependent regulation of cell wall remodeling in response to ethanol. On the other hand, the vma mutants were more sensitive to Zymolyase than the wild-type strain after short-term exposure to ethanol for 1 h but displayed increased resistance to Zymolyase to wild-type levels after exposure to ethanol for ≥3 h (Fig. 6). Since the vma mutants exhibited a delay, but not a complete loss, of cell wall remodeling in response to cell wall stress caused by ethanol, it seems that the defect in the cell wall stress response may not be a major cause of ethanol sensitivity resulting from a loss of V-ATPase activity.
FIG 6.
Role of V-ATPase in the early response to ethanol-induced cell wall stress. The exponentially growing wild-type (BY4742) (■), Δvma2 (□), and Δvma3 (△) cells were incubated in YPD medium lacking ethanol or in YPD medium containing 5% or 10% ethanol at 30°C for 1 and 3 h. Cells were harvested and adjusted to an OD600 of 0.5 in TE buffer containing 100 μg/ml (1 U) Zymolyase 20T. Susceptibility to Zymolyase was monitored by measuring the OD600 at the indicated times and expressed as a percentage of OD600 relative to that at 0 min. All experiments were carried out in triplicate. The error bars indicate the SD.
V-ATPase is important for protecting yeast cells against ethanol-induced endogenous oxidative stress.
Previously, it was demonstrated that the loss of V-ATPase function leads to chronic oxidative stress and that the level of intracellular reactive oxygen species (ROS) of yeast cells increases during exposure to ethanol (20, 38). To address whether the sensitivity of vma mutants to ethanol stress results from an elevated endogenous oxidative stress induced by ethanol, we examined the growth of the wild-type, Δvma2, Δvma3, and Δsod1 strains (the last lacking cytoplasmic Cu/Zn-superoxide dismutase, required for the elimination of cytosolic ROS) on YPD agar plates supplemented with straight-chain alcohols, calcofluor white, or H2O2 under aerobic and anaerobic conditions. When grown aerobically, the Δsod1 mutant was sensitive to not only H2O2 but also all straight-chain alcohols examined and calcofluor white, and the growth defect of this mutant was restored by cultivation under anaerobic conditions (Fig. 4). These findings suggest that in addition to ethanol, the other straight-chain alcohols and calcofluor white are capable of inducing endogenous oxidative stress, whereas in the vma mutants, only alcohol sensitivity but not calcofluor white sensitivity was suppressed under anaerobic conditions (Fig. 4). These results suggest that in the vma mutants lacking V-ATPase activity, the alcohol sensitivity may result from elevated endogenous oxidative stress induced by straight-chain alcohols, whereas the calcofluor white-sensitive phenotype is caused by oxidative-stress-independent pathways.
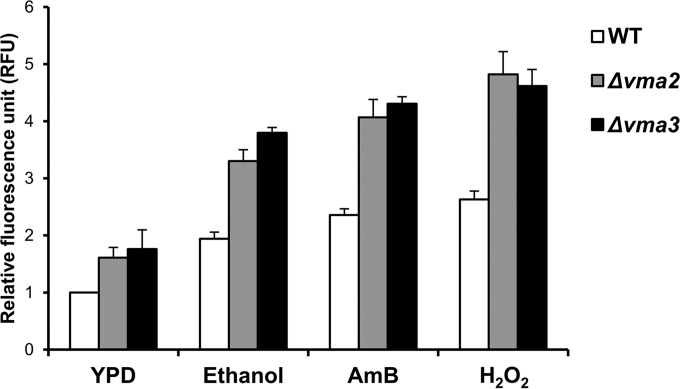
To further investigate the role of V-ATPase in the protection of yeast cells against ethanol-induced oxidative stress, we measured intracellular ROS levels in the wild-type, Δvma2, and Δvma3 strains after exposure to ethanol, AmB, or H2O2 for 1 h. Our results showed that ROS production was significantly enhanced in response to not only H2O2 and ethanol but also AmB (Fig. 7), suggesting that increased membrane permeability may induce endogenous oxidative stress. Furthermore, the vma mutants exhibited higher intracellular ROS levels than the wild-type strain under both stress and nonstress conditions (Fig. 7), raising the possibility that the loss of V-ATPase function may result in a defect in the regulation of intracellular redox homeostasis, which is potentially disturbed by the membrane-permeabilizing effect of ethanol.
FIG 7.
Loss of V-ATPase activity promotes ROS accumulation. Exponentially growing wild-type (BY4742) (white bars), Δvma2 (gray bars), and Δvma3 (black bars) cells loaded with DCFH-DA were incubated in YPD medium containing 10% ethanol, 1 μM AmB, or 2 mM H2O2 at 30°C for 1 h. The fluorescence intensity of each sample was normalized to that of the untreated wild-type cells and expressed as relative fluorescence units (RFU). All experiments were carried out in triplicate. The error bars indicate the SD.
DISCUSSION
In this study, a clear correlation between the lipophilicity of straight-chain alcohols and their effects on growth inhibition and membrane permeabilization was demonstrated. The straight-chain alcohols, such as ethanol, have been proposed to disturb the integrity of the cellular membrane through partitioning into a lipid bilayer with their aliphatic chains associated with the fatty acyl chains of phospholipids (39). It is therefore likely that the straight-chain alcohols with long aliphatic chains will be deeply inserted into the hydrophobic interior of the membrane lipid bilayer, thereby causing a high degree of membrane permeabilization. Since in our experiments the pH of fresh YPD medium (pH = 5.8) was more acidic than the cytosolic pH of the precultivated wild-type cells (pH = 6.2), it is plausible that acidification of the cytosol and vacuole after alcohol challenge was caused by the passive influx of protons into alcohol-permeabilized cells. In agreement with the effect of straight-chain alcohols on membrane permeabilization, the degrees of cytosolic and vacuolar acidification were strictly dependent on both the concentration and lipophilicity of straight-chain alcohols. Our findings are consistent with the previous work of Leão and Van Uden (6), who showed that upon exposure to alkanols, i.e., ethanol, isopropanol, propanol, and butanol, the extracellular pH increased in a concentration- and lipophilicity-dependent manner, possibly due to increased proton influx.
Since the V-ATPase is involved in the maintenance of intracellular pH homeostasis through its role in pumping excessive protons into the vacuole (11), it has been thought that almost all phenotypes of the vma mutants lacking V-ATPase activity are caused by a highly acidified cytosol, which would inhibit cell function. Contrary to this common notion, our results revealed that the alcohol-sensitive phenotype of the vma mutants was not caused by severe cytosolic acidification but rather by elevated levels of endogenous oxidative stress. The generation of ROS by the mitochondrial electron transport chain is frequently the major cause of endogenous oxidative stress in eukaryotic cells (40). However, this does not account for ROS accumulation in the Δvma2 mutant, because a loss of mitochondrial DNA did not improve the growth of this mutant or its resistance to the oxidative stress-inducing agent H2O2 (20). The mechanism of oxidative stress induction in the mutant lacking V-ATPase activity is still unclear. Recently, Diab and Kane (41) showed that the Δvma2 mutant accumulated a higher level of intracellular iron than the wild-type strain, in spite of the upregulation of the iron regulon, which is induced by the activated Aft1p and Aft2p transcription factors in response to iron deprivation (42). Excessive iron has a high potential to induce oxidative stress through its promotion of a Fenton reaction, which converts H2O2 and O2 into toxic radicals, i.e., hydroxyl radicals and superoxide anions, respectively (43). It is therefore possible that the increased intracellular iron levels may be one of the causes of oxidative stress in the mutant lacking V-ATPase activity. Although the vma mutants did not display acute severe cytosolic acidification in response to straight-chain alcohols, the possibility that the increased oxidative stress in these mutants was a consequence of chronic cytosolic acidification could not be excluded. It is well known that the structures of cellular macromolecules, such as protein and DNA, are disturbed under acidic pH conditions, leading to dysfunctional organelles and molecules, especially those required for important cellular functions, including the oxidative defense system (44). Hence, the chronic oxidative stress in the mutant lacking V-ATPase activity may be induced by the enhancement of cellular damage and/or reduction of cellular antioxidant activity resulting from cytosolic acidification. If this is the case, the vma mutants associated with cellular dysfunction may be unable to promptly respond to alcohol-induced oxidative stress, leading to high-level accumulation of intracellular ROS and eventually growth inhibition.
It is known that in response to cell wall stress, the cell surface sensors, i.e., Wsc1-3p, Mid2p, and Mtl1p, transmit stress signals via Rho1p GTPase to the cell wall integrity signaling pathway, resulting in the increased expression of several cell wall biosynthesis genes, such as FKS2 and CRH1, which encode the catalytic subunits of 1,3-β-glucan synthase and chitin transglycosylase, respectively. The structural components and networks of the yeast cell wall are thereby remodeled in order to maintain cell wall integrity and function (45). Endocytosis and recycling of cell wall sensors from endosomes back to the plasma membrane have been shown to play an important role in regulation of the cell wall integrity pathway (46–48). Similar to the mislocalization of plasma membrane H+-ATPase Pma1p in the vma mutants (16), the deletion of VMA genes has recently been reported to result in mislocalization of Wsc1p from the plasma membrane to the vacuole (49), suggesting a role for V-ATPase in the maintenance of yeast cell wall integrity through the endocytic recycling of Wsc1p. It is therefore possible that the defect in cell wall remodeling upon ethanol exposure in the vma mutants may be a consequence of the mislocalization of cell wall stress sensor(s), at least Wsc1p, which potentially leads to a reduced sensing efficiency for ethanol-induced cell wall stress. Since our results revealed that the vma mutants displayed only a delay, and not a complete loss, of cell wall remodeling in response to ethanol, it is likely that the cell wall stress sensors remaining at the cell surface (if any) retain the ability to sense cell wall stress, although longer detection times seem to be required.
In conclusion, although straight-chain alcohols induced cytosolic and vacuolar acidification through their effects on increasing membrane permeability, the ethanol sensitivity of mutants lacking V-ATPase activity, which is generally involved in the maintenance of intracellular pH homeostasis, seemed to result from increased endogenous oxidative stress and a defect in cell wall remodeling.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maleeya Kruatrachue, Metha Meetam (Mahidol University), and Saranya Phunpruch (King Mongkut's Institute of Technology Ladkrabang) for helpful suggestions, Nisarut Udom for help with the preliminary experiments, and Ferdinand Carlo Warg for editing the manuscript.
This work was supported by a grant from the faculty of science, Mahidol University. S.C. and T.D. were supported by scholarships from the Center of Excellence on Environmental Health and Toxicology, Science & Technology Postgraduate Education and Research Development Office (PERDO), Ministry of Education. T.B. and T.T. were supported by scholarships from the Faculty of Science, Mahidol University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00376-16.
REFERENCES
- 1.Stanley D, Bandara A, Fraser S, Chambers PJ, Stanley GA. 2010. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J Appl Microbiol 109:13–24. doi: 10.1111/j.1365-2672.2009.04657.x. [DOI] [PubMed] [Google Scholar]
- 2.Alexandre H, Rousseaux I, Charpentier C. 1994. Ethanol adaptation mechanisms in Saccharomyces cerevisiae. Biotechnol Appl Biochem 20:173–183. [PubMed] [Google Scholar]
- 3.Weber FJ, de Bont JA. 1996. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim Biophys Acta 1286:225–245. doi: 10.1016/S0304-4157(96)00010-X. [DOI] [PubMed] [Google Scholar]
- 4.Ohsumi Y, Anraku Y. 1981. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem 256:2079–2082. [PubMed] [Google Scholar]
- 5.Forster C, Kane PM. 2000. Cytosolic Ca2+ homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J Biol Chem 275:38245–38253. doi: 10.1074/jbc.M006650200. [DOI] [PubMed] [Google Scholar]
- 6.Leão C, Van Uden N. 1984. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 774:43–48. doi: 10.1016/0005-2736(84)90272-4. [DOI] [PubMed] [Google Scholar]
- 7.Salter GJ, Kell DB. 1995. Solvent selection for whole cell biotransformations in organic media. Crit Rev Biotechnol 15:139–177. doi: 10.3109/07388559509147404. [DOI] [PubMed] [Google Scholar]
- 8.Fujita K, Matsuyama A, Kobayashi Y, Iwahashi H. 2004. Comprehensive gene expression analysis of the response to straight-chain alcohols in Saccharomyces cerevisiae using cDNA microarray. J Appl Microbiol 97:57–67. doi: 10.1111/j.1365-2672.2004.02290.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujita K, Matsuyama A, Kobayashi Y, Iwahashi H. 2006. The genome-wide screening of yeast deletion mutants to identify the genes required for tolerance to ethanol and other alcohols. FEMS Yeast Res 6:744–750. doi: 10.1111/j.1567-1364.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 10.Auesukaree C, Damnernsawad A, Kruatrachue M, Pokethitiyook P, Boonchird C, Kaneko Y, Harashima S. 2009. Genome-wide identification of genes involved in tolerance to various environmental stresses in Saccharomyces cerevisiae. J Appl Genet 50:301–310. doi: 10.1007/BF03195688. [DOI] [PubMed] [Google Scholar]
- 11.Kane PM. 2006. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev 70:177–191. doi: 10.1128/MMBR.70.1.177-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira T, Mason AB, Slayman CW. 2001. The yeast Pma1 proton pump: a model for understanding the biogenesis of plasma membrane proteins. J Biol Chem 276:29613–29616. doi: 10.1074/jbc.R100022200. [DOI] [PubMed] [Google Scholar]
- 13.Supply P, Wach A, Thinés-Sempoux D, Goffeau A. 1993. Proliferation of intracellular structures upon overexpression of the PMA2 ATPase in Saccharomyces cerevisiae. J Biol Chem 268:19744–19752. [PubMed] [Google Scholar]
- 14.Eraso P, Gancedo C. 1987. Activation of yeast plasma membrane ATPase by acid pH during growth. FEBS Lett 224:187–192. doi: 10.1016/0014-5793(87)80445-3. [DOI] [PubMed] [Google Scholar]
- 15.Padilla-López S, Pearce DA. 2006. Saccharomyces cerevisiae lacking Btn1p modulate vacuolar ATPase activity to regulate pH imbalance in the vacuole. J Biol Chem 281:10273–10280. doi: 10.1074/jbc.M510625200. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Muñoz GA, Kane P. 2008. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J Biol Chem 283:20309–20319. doi: 10.1074/jbc.M710470200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson H, Nelson N. 1990. Disruption of genes encoding subunits of yeast vacuolar H+-ATPase causes conditional lethality. Proc Natl Acad Sci U S A 87:3503–3507. doi: 10.1073/pnas.87.9.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambade M, Alba M, Smardon AM, West RW, Kane PM. 2005. A genomic screen for yeast vacuolar membrane ATPase mutants. Genetics 170:1539–1551. doi: 10.1534/genetics.105.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smardon AM, Kane PM. 2014. Loss of vacuolar H+-ATPase activity in organelles signals ubiquitination and endocytosis of the yeast plasma membrane proton pump Pma1p. J Biol Chem 289:32316–32326. doi: 10.1074/jbc.M114.574442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milgrom E, Diab H, Middleton F, Kane PM. 2007. Loss of vacuolar proton-translocating ATPase activity in yeast results in chronic oxidative stress. J Biol Chem 282:7125–7136. doi: 10.1074/jbc.M608293200. [DOI] [PubMed] [Google Scholar]
- 21.Eide DJ, Bridgham JT, Zhao Z, Mattoon JR. 1993. The vacuolar H+-ATPase of Saccharomyces cerevisiae is required for efficient copper detoxification, mitochondrial function, and iron metabolism. Mol Gen Genet 241:447–456. [DOI] [PubMed] [Google Scholar]
- 22.Eide DJ, Clark S, Nair TM, Gehl M, Gribskov M, Guerinot ML, Harper JF. 2005. Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biol 6:R77. doi: 10.1186/gb-2005-6-9-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis-Kaplan SR, Ward DM, Shiflett SL, Kaplan J. 2004. Genome-wide analysis of iron-dependent growth reveals a novel yeast gene required for vacuolar acidification. J Biol Chem 279:4322–4329. doi: 10.1074/jbc.M310680200. [DOI] [PubMed] [Google Scholar]
- 24.Yadav J, Muend S, Zhang Y, Rao R. 2007. A phenomics approach in yeast links proton and calcium pump function in the Golgi. Mol Biol Cell 18:1480–1489. doi: 10.1091/mbc.E06-11-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Supek F, Supekova L, Nelson N. 1994. Features of vacuolar H+-ATPase revealed by yeast suppressor mutants. J Biol Chem 269:26479–26485. [PubMed] [Google Scholar]
- 26.Bonangelino CJ, Chavez EM, Bonifacino JS. 2002. Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol Biol Cell 13:2486–2501. doi: 10.1091/mbc.02-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeley ES, Kato M, Margolis N, Wickner W, Eitzen G. 2002. Genomic analysis of homotypic vacuole fusion. Mol Biol Cell 13:782–794. doi: 10.1091/mbc.01-10-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke D, Dawson D, Stearns T. 2000. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 29.Zakrzewska A, Boorsma A, Delneri D, Brul S, Oliver SG, Klis FM. 2007. Cellular processes and pathways that protect Saccharomyces cerevisiae cells against the plasma membrane-perturbing compound chitosan. Eukaryot Cell 6:600–608. doi: 10.1128/EC.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orij R, Postmus J, Ter Beek A, Brul S, Smits GJ. 2009. In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology 155:268–278. doi: 10.1099/mic.0.022038-0. [DOI] [PubMed] [Google Scholar]
- 31.Ali R, Brett CL, Mukherjee S, Rao R. 2004. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J Biol Chem 279:4498–4506. doi: 10.1074/jbc.M307446200. [DOI] [PubMed] [Google Scholar]
- 32.Ovalle R, Lim ST, Holder B, Jue CK, Moore CW, Lipke PN. 1998. A spheroplast rate assay for determination of cell wall integrity in yeast. Yeast 14:1159–1166. doi:. [DOI] [PubMed] [Google Scholar]
- 33.Wu CY, Bird AJ, Winge DR, Eide DJ. 2007. Regulation of the yeast TSA1 peroxiredoxin by ZAP1 is an adaptive response to the oxidative stress of zinc deficiency. J Biol Chem 282:2184–2195. doi: 10.1074/jbc.M606639200. [DOI] [PubMed] [Google Scholar]
- 34.Sikkema J, de Bont JA, Poolman B. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumori N, Yamaji N, Matsuoka S, Oishi T, Murata M. 2002. Amphotericin B covalent dimers forming sterol-dependent ion-permeable membrane channels. J Am Chem Soc 124:4180–4181. doi: 10.1021/ja012026b. [DOI] [PubMed] [Google Scholar]
- 36.van Voorst F, Houghton-Larsen J, Jønson L, Kielland-Brandt MC, Brandt A. 2006. Genome-wide identification of genes required for growth of Saccharomyces cerevisiae under ethanol stress. Yeast 23:351–359. doi: 10.1002/yea.1359. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira MC, Raposo LR, Mira NP, Lourenço AB, Sá-Correia I. 2009. Genome-wide identification of Saccharomyces cerevisiae genes required for maximal tolerance to ethanol. Appl Environ Microbiol 75:5761–5772. doi: 10.1128/AEM.00845-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du X, Takagi H. 2007. N-Acetyltransferase Mpr1 confers ethanol tolerance on Saccharomyces cerevisiae by reducing reactive oxygen species. Appl Microbiol Biotechnol 75:1343–1351. doi: 10.1007/s00253-007-0940-x. [DOI] [PubMed] [Google Scholar]
- 39.Westerman PW, Pope JM, Phonphok N, Doane JW, Dubro DW. 1988. The interaction of n-alkanols with lipid bilayer membranes: a 2H-NMR study. Biochim Biophys Acta 939:64–78. doi: 10.1016/0005-2736(88)90048-X. [DOI] [PubMed] [Google Scholar]
- 40.Dröse S, Brandt U. 2012. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol 748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 41.Diab HI, Kane PM. 2013. Loss of vacuolar H+-ATPase (V-ATPase) activity in yeast generates an iron deprivation signal that is moderated by induction of the peroxiredoxin TSA2. J Biol Chem 288:11366–11377. doi: 10.1074/jbc.M112.419259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philpott CC, Protchenko O. 2008. Response to iron deprivation in Saccharomyces cerevisiae. Eukaryot Cell 7:20–27. doi: 10.1128/EC.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halliwell B, Gutteridge JM. 1984. Role of iron in oxygen radical reactions. Methods Enzymol 105:47–56. doi: 10.1016/S0076-6879(84)05007-2. [DOI] [PubMed] [Google Scholar]
- 44.Booth IR, Statford N. 2003. Acidulants and low pH, p 25–48. In Russell NJ, Gould GW (ed), Food preservatives, 2nd ed Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 45.Levin DE. 2011. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piao HL, Machado IM, Payne GS. 2007. NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol Biol Cell 18:57–65. doi: 10.1091/mbc.E06-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilk S, Wittland J, Thywissen A, Schmitz HP, Heinisch JJ. 2010. A block of endocytosis of the yeast cell wall integrity sensors Wsc1 and Wsc2 results in reduced fitness in vivo. Mol Genet Genomics 284:217–229. doi: 10.1007/s00438-010-0563-2. [DOI] [PubMed] [Google Scholar]
- 48.Chapa-y-Lazo B, Allwood EG, Smaczynska-de Rooij Jr, Snape ML, Ayscough KR. 2014. Yeast endocytic adaptor AP-2 binds the stress sensor Mid2 and functions in polarized cell responses. Traffic 15:546–557. doi: 10.1111/tra.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ueno K, Saito M, Nagashima M, Kojima A, Nishinoaki S, Toshima JY, Toshima J. 2014. V-ATPase-dependent luminal acidification is required for endocytic recycling of a yeast cell wall stress sensor, Wsc1p. Biochem Biophys Res Commun 443:549–555. doi: 10.1016/j.bbrc.2013.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.