Abstract
Physical and chemical disinfection methods have been proposed with the aim of controlling Legionella water contamination. To date, the most effective procedures for reducing bacterial contamination have not yet been defined. The aim of this study was to assess the long-term effectiveness of various disinfection procedures in order to reduce both culturable and nonculturable (NC) legionellae in different hospital water networks treated with heat, chlorine dioxide, monochloramine, and hydrogen peroxide. The temperature levels and biocide concentrations that proved to give reliable results were analyzed. In order to study the possible effects on the water pipes, we verified the extent of corrosion on experimental coupons after applying each method for 6 months. The percentage of positive points was at its lowest after treatment with monochloramine, followed by chlorine dioxide, hydrogen peroxide, and hyperthermia. Different selections of Legionella spp. were observed, as networks treated with chlorine-based disinfectants were contaminated mainly by Legionella pneumophila serogroup 1, hyperthermia was associated with serogroups 2 to 14, and hydrogen peroxide treatment was associated mainly with non-pneumophila species. NC cells were detected only in heat-treated waters, and also when the temperature was approximately 60°C. The corrosion rates of the coupons were within a satisfactory limit for water networks, but the morphologies differed. We confirm here that chemical disinfection controls Legionella colonization more effectively than hyperthermia does. Monochloramine was the most effective treatment, while hydrogen peroxide may be a promising alternative to chlorine-based disinfectants due to its ability to select for other, less virulent or nonpathogenic species.
INTRODUCTION
The number of cases of Legionnaires' disease has increased steadily over the years, especially in Italy and Europe (1, 2). In 2013 in Italy, most cases were community acquired (83.4%), followed by travel-associated (9.8%) and health care-associated (4.6%) (3) cases. Despite the lower percentage of nosocomial cases, the control of Legionella sp. contamination is essential in health care settings, where patients, in particular those with compromised immune systems, are at high risk of contracting the disease, with a possible fatal outcome (4). For this reason, national and international guidelines recommend using preventive measures to control Legionella water contamination, with particular reference to health care structures (3, 5). A range of physical and chemical disinfection methods have been proposed, but to date, the most effective procedures for controlling contamination have not been defined (6). Moreover, their impacts on pipe deterioration/corrosion have not been documented extensively and are studied mainly in model water distribution systems (7–10).
Molecular investigation tools have been used together with conventional culture for monitoring corrective actions (11–13). Culture is essential for identifying and typing bacterial strains; however, it is time-consuming and can give false-negative results due to the possible presence of cells in a viable but nonculturable (VBNC) state (14). In the VBNC state, pathogens are not generally able to initiate disease but virulence is retained, and infection can follow their resuscitation to the actively metabolizing state (15). Quantitative PCR (qPCR) techniques have proved to have several advantages, including high sensitivity and accuracy and rapid evaluation of germ contamination. The main disadvantage of these techniques is that they cannot distinguish viable and nonviable cells, which is an important factor to take into account in evaluating the effectiveness of corrective actions (16, 17). In order to overcome this problem, new PCR-based strategies, collectively called molecular viability analyses, have been developed (18). Among these, methods based on DNA-intercalating dyes, such as ethidium monoazide (EMA), have been proposed in combination with qPCR (19–21). EMA selectively binds the DNA of cells with compromised membranes, while intact cell membranes represent a barrier for the dye. In the bound state, the DNA cannot be amplified by qPCR, while DNA from cells with an intact membrane can be amplified and quantified (21). The only insurmountable limitation of this method is the inability to detect bacteria inactivated by conditions that do not alter membrane permeability, such as short-wave UV and low-temperature pasteurization (18).
The main aim of the present study was to assess the long-term effectiveness of monochloramine, chlorine dioxide, hydrogen peroxide, and heat in reducing/eliminating both culturable and nonculturable (NC) legionellae in various hospital water networks. For this purpose, traditional culture was used with qPCR alone and in combination with EMA. In our study, the EMA-qPCR method proved to be suitable for measuring bacterial viability because oxidative disinfectants and heat rapidly act against bacterial cells, causing damage to cellular components, including the cytoplasmic membrane (18). In order to verify the possible corrosive action of each disinfection procedure, carbon steel coupons were inserted along the water distribution systems and were periodically examined for weight loss and morphology.
MATERIALS AND METHODS
Hospital setting.
This study was carried out in two hospitals situated in Modena, northern Italy: a private hospital built in the 1980s and a university hospital consisting of a central block, called building 1, and four separate blocks built between the 1970s and the 1990s. The same municipality provides the incoming cold groundwater in both hospitals. Hot water is produced in situ using heat exchangers and reaches the peripheral points through a water recirculation system. Three different water networks (A, B, and C) distribute hot water in parallel in building 1, while the other four buildings of the university hospital have their own hot water networks (D, E, F, and G), as previously described (22). The private hospital has a unique hot water distribution network (H).
Since 2000, sampling plans have been implemented in order to assess Legionella sp. contamination in the water distribution systems. In both hospitals, the samples collected prior to intervention were contaminated mainly by Legionella pneumophila serogroups 2 to 14, at concentrations of >104 CFU liter−1, which led to the implementation of a wide range of control strategies (23). The disinfection strategies still operating in both hospitals are described below.
(i) University hospital, building 1 (nine floors, 40 years old).
A monochloramine device operating since March 2009 is used on water network C; monochloramine is produced in situ from the chemical reaction between a stabilized chlorine-based precursor and an ammonium salt (Sanipur s.r.l., Brescia, Italy). The monochloramine generator is set to maintain a concentration of biocide in the recirculation loop between 2.0 and 4.0 mg liter−1. Residual levels are in line with the guideline value of 3 mg liter−1 and the maximum contaminant level of 4 mg liter−1, established by WHO and the U.S. Environmental Protection Agency (EPA), respectively (24, 25).
Two chlorine dioxide devices (Sanipur s.r.l., Brescia, Italy) operating since January 2005 are used on water network A and, since November 2005, on water network B. Chlorine dioxide is produced in situ by injecting hydrochloric acid and sodium chlorite into the recirculating hot water. The system is set up to ensure concentrations of at least 0.30 mg liter−1 at distal points, as previously reported (22), without exceeding the EPA maximum residual disinfectant level of 0.80 mg liter−1 (25).
(ii) University hospital, building D (four floors, 40 years old).
An experimental hydrogen peroxide device operating since January 2012 (O2 s.r.l., Bergamo, Italy) is used for building D. A 48% hydrogen peroxide solution is injected continuously into the recirculating hot water by a dosing pump in order to ensure concentrations of 15 to 20 mg liter−1 at distal outlets.
(iii) Private hospital H (four floors, 35 years old).
Hyperthermia has been used since April 2012. The hot water is produced at a temperature of at least 60°C and distributed at temperatures constantly at >50°C.
Sample collection.
Over a 3-year period (January 2012 to December 2014), hot water samples (n = 662) were taken from heaters, return loops, and distal outlets (showers and/or taps) of the water networks treated with the disinfection strategies listed above. In both hospitals, the protocols anticipated sampling from at least one remote point every 50 beds, with repeat sampling at the same sites every 3 or 4 months. The network that was experimentally treated with hydrogen peroxide was monitored more frequently, i.e., every week for the first 3 months, every month until the end of the second year, and every 4 months during the last year. Water was collected in sterile plastic bottles without flaming and after flushing for 1 min. Sodium thiosulfate (10 mg liter−1) was added (final concentration, 1 ml liter−1) to neutralize residual free chlorine. At sampling, water temperature (digital thermometer), chlorine dioxide (Microquant DPD method; Merck, Darmstadt, Germany), monochloramine (indophenol method; Hach Lange, Milan, Italy), and hydrogen peroxide (RQflex 2 reflectometer; Merck, Darmstadt, Germany) were measured. The samples were returned to the laboratory immediately after collection and analyzed within 24 h as described elsewhere (26).
Laboratory methods.
Culture and identification of Legionella spp. were carried out according to the ISO 11731:1998 method (27) as previously described (28). The results were expressed as numbers of CFU per liter, and the limit of detection (LOD) of the procedure was 25 CFU liter−1.
DNA was extracted by use of a QIAamp DNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, as reported elsewhere (19). The extracted DNA was stored at −20°C until use.
The minimum number of samples to be analyzed by molecular methods in order to have statistical power was determined by carrying out a power analysis based on the results of a previous pilot study (29). For each treatment, the first 22 negative samples with culture (total of 88 samples) were analyzed by qPCR, with and without the EMA pretreatment. The water samples were treated with EMA (Sigma Chemical Co., St. Louis, MO) at a final concentration of 6 μM (2.53 μg ml−1) prior to DNA extraction, as reported by Mansi et al. (19).
DNA amplification was carried out with a Rotor-Gene Q 2plex instrument (Qiagen, Hilden, Germany) and using a commercial kit (new Legionella quantitative kit; Diatheva, Fano, Italy) validated to be in agreement with the ISO/TS 12869:2012 method (30, 31). The results were expressed as numbers of genome units (GU) per liter. Under the experimental conditions used in this study, the LOD and the limit of quantification of the qPCR method were estimated to be 100 GU liter−1 and 500 GU liter−1, respectively.
Corrosion study.
Rectangular coupons (area, 21.81 cm2; density, 7.87 g cm−3) of carbon steel C1010 foils with frosted surfaces were used to evaluate the type and extent of corrosion according to the standard practices ASTM G1-03:2011 and ASTM G4-01:2014 (32, 33). Nonalloy steel with a carbon content of up to 0.22% is suitable for the conveyance of aqueous liquids, including water for human consumption (34). We selected C1010 steel with a maximum carbon content of 0.13% because it is easily found on the market as coupons proper for our experimental conditions. The coupons were inserted into five separate racks made from polytetrafluoroethylene (Fig. 1). The racks were connected to the return loops of the treated networks (A, B, D, and H) and an untreated network (F). Before the beginning of the study, four coupons were inserted into each rack. After 2 and 4 months, one coupon from each rack was removed and a new coupon was added. After 6 months, all the coupons were removed. In line with this practice, we analyzed six coupons for each rack, i.e., two for each exposure time (2, 4, and 6 months). After collection of the coupons for corrosion analysis, they were immediately dried with dimethyl ketone and placed in vials containing silica gel for transportation to the laboratory. The weight loss method was used in order to determine the corrosion rate. The coupons were scraped with a brass brush to remove surface deposits, washed in an ultrasonic bath for 6 min, and then weighed. The cleaning cycles were suspended as soon as the weight value was stabilized. With the aim of determining the mass loss of the base metal during removal of the corrosion products, an uncorroded control coupon was cleaned using the same procedure performed on the test coupons. The average corrosion rates were calculated by means of the following formula: corrosion rate (in millimeters per year) = (K × W)/(A × T × D), where K is a constant (3.65 × 104), W is the mass loss (in grams), T is the time of exposure (in days), A is the area of the carbon steel coupon (in square centimeters), and D is the density (carbon steel) (in grams per cubic centimeter).
FIG 1.

Example of a rack used to support coupons for the test of corrosion.
An optical microscope (Carl Zeiss, Milan, Italy) equipped with a system for automatic digitization of the images was used in order to characterize the corrosive phenomena.
Statistical analysis.
All statistical analyses were performed with PASW Statistics, version 21.0 (SPSS Inc., Chicago, IL). Logarithmic transformations were used to normalize the bacteriological data, and the results are presented as geometric mean values. The chi-square test, paired t test, and one-way analysis of variance (ANOVA) with the Bonferroni test were applied whenever necessary.
RESULTS
In total, 237 of 662 samples (35.8%) were contaminated by Legionella spp. The disinfection treatments significantly affected both the percentage of positive samples (χ2 = 104.385; P < 0.001) and the bacterial load of positive samples, expressed as the geometric mean (F = 26.007; P < 0.001). Monochloramine showed the lowest percentage of positive results (9/95 samples [9.5%]), followed by chlorine dioxide (60/201 samples [29.8%]), hydrogen peroxide (80/208 samples [38.5%]), and hyperthermia (36/66 samples [54.5%]). Regarding Legionella concentrations, no differences in the geometric mean were observed according to chemical treatments (2.2 × 102 CFU liter−1 for monochloramine, 3.0 × 102 CFU liter−1 for chlorine dioxide, and 1.3 × 102 CFU liter−1 for hydrogen peroxide), while a significantly higher geometric mean (1.7 × 103 CFU liter−1) was observed for the heat-treated positive samples than for the samples treated with all biocides (P < 0.05 by the Bonferroni test).
Table 1 shows the numbers and percentages of positive samples and their geometric means according to the biocides and temperatures examined. No significant difference in the percentage of positives relating to the concentrations of monochloramine was observed, and 3 mg liter−1 was required in order to obtain legionella levels of <102 CFU liter−1. Levels of chlorine dioxide of ≥0.50 mg liter−1 significantly reduced the percentage of positive points, to below 30% (χ2 = 3.930; P < 0.05). Hydrogen peroxide at concentrations between 15 and 19.9 mg liter−1 was associated with a significant reduction in positive points (χ2 = 3.823; P < 0.05), yet levels of ≥20 mg liter−1 were required to obtain fewer than 30% positive distal points. A significant reduction of positive results was observed by increasing the temperature to 55 to 59.9°C (χ2 = 7.796; P < 0.010), but no positive sample was observed when the temperature reached 60°C. For all treatments, the bacterial load did not differ according to the biocide/temperature level, and only a limited number of samples exceeded 1.0 × 104 CFU liter−1 (one each with monochloramine and hyperthermia and two with chlorine dioxide).
TABLE 1.
Numbers and percentages of positive samples and their geometric means determined by culture
| Treatment | No. of positive samples/total no. of samples (%) | χ2 value, P valuea | Geometric mean CFU liter−1 for positive samples (range) | F value, P valuea |
|---|---|---|---|---|
| Monochloramine | 4.131, NS | 1.963, NS | ||
| <2.0 mg liter−1 | 2/8 (25.0) | 2.0 × 103 (1.2 × 102–3.1 × 104) | ||
| 2.0–2.9 mg liter−1 | 4/29 (13.8) | 3.7 × 102 (25–5.7 × 103) | ||
| ≥3.0 mg liter−1 | 3/58 (5.2) | 25 (25) | ||
| Chlorine dioxide | 6.928, <0.05 | 2.058, NS | ||
| <0.30 mg liter−1 | 31/84 (36.9) | 4.0 × 102 (25–2.5 × 104) | ||
| 0.30–0.49 mg liter−1 | 18/54 (33.3) | 3.5 × 102 (25–4.1 × 104) | ||
| ≥0.50 mg liter−1 | 11/63 (17.5) | 1.0 × 102 (25–5.5 × 103) | ||
| Hydrogen peroxide | 10.339, <0.001 | 2.468, NS | ||
| <15.0 mg liter−1 | 46/91 (50.5) | 1.1 × 102 (25–1.4 × 103) | ||
| 15–19.9 mg liter−1 | 14/43 (32.6) | 2.6 × 102 (25–5.0 × 103) | ||
| ≥20.0 mg liter−1 | 20/74 (27.0) | 1.1 × 102 (25–2.3 × 103) | ||
| Hyperthermia | 33.31, <0.001 | 0.481, NS | ||
| 50–54.9°C | 19/21 (90.5) | 2.5 × 103 (25–1.2 × 104) | ||
| 55–59.9°C | 17/27 (63.0) | 1.2 × 103 (25–7.9 × 103) | ||
| ≥60°C | 0/18 (0) |
NS, not significant.
Table 2 shows that waters treated with chlorine-based systems were mainly contaminated with L. pneumophila serogroup 1, hyperthermia was strictly associated with L. pneumophila serogroups 2 to 14, and hydrogen peroxide treatment was mainly associated with non-pneumophila species (54.5% L. jamestowniensis, 36.4% L. anisa, and 9.1% both).
TABLE 2.
Numbers and percentages of species and serogroups of Legionella according to treatment (χ2 = 270.042; P < 0.001)
| Treatment | No. (%) of positive samples |
||
|---|---|---|---|
| L. pneumophila serogroup 1 alone or with others | L. pneumophila serogroups 2–14 | Legionella spp. | |
| Monochloramine | 7 (77.8) | 0 (0.0) | 2 (22.2) |
| Chlorine dioxide | 51 (85.0) | 4 (6.7) | 5 (8.3) |
| Hydrogen peroxide | 21 (26.3) | 5 (6.2) | 54 (67.5) |
| Hyperthermia | 0 (0.0) | 36 (100.0) | 0 (0.0) |
Among the 394 water samples whose cultures were negative, 88 were analyzed by molecular methods (Table 3). Chemical biocides showed similar percentages of positive results by qPCR but no positive sample by EMA-qPCR. Over 95% of the heat-treated samples were positive by qPCR, and 50% were positive by EMA-qPCR. Positive results for EMA-qPCR were also associated with samples at temperatures over 60°C (6/11 [54.5%]).
TABLE 3.
Molecular analysis of culture-negative samples
| Treatment | No. of positive samples/total no. of samples (%)a |
|
|---|---|---|
| qPCR | EMA-qPCR | |
| Monochloramine | 11/22 (50.0) | 0/22 (0.0) |
| Chlorine dioxide | 9/22 (40.9) | 0/22 (0.0) |
| Hydrogen peroxide | 10/22 (45.4) | 0/22 (0.0) |
| Hyperthermia | 21/22 (95.4) | 11/22 (50.0) |
For qPCR, χ2 = 15.246 and P < 0.005; and for EMA-qPCR, χ2 = 27.957 and P < 0.001.
The average losses of thickness of the coupons exposed to treated and untreated waters did not differ significantly according to the type of treatment and time of exposure. The mean corrosion rate was 0.17 ± 0.03 mm/year for the coupons exposed to hydrogen peroxide, 0.15 ± 0.03 mm/year for hyperthermia, 0.14 ± 0.04 mm/year for the untreated network, 0.14 ± 0.03 mm/year for chlorine dioxide, and 0.11 ± 0.05 mm/year for monochloramine.
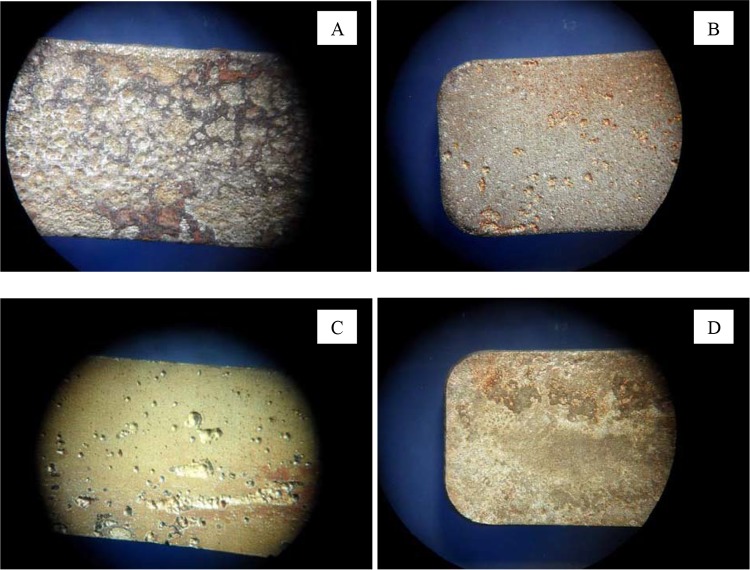
The morphology of corrosion did not change over time but differed according to treatment. As an example, the morphologies of corrosion according to treatment following 6 months of water exposure are shown in Fig. 2. The coupons exposed to chlorine dioxide presented a uniform corrosion with pitting and ulcerations (Fig. 2A), those exposed to monochloramine showed a uniform corrosion as well as rare pitting (Fig. 2B), and those exposed to hydrogen peroxide were characterized by pitting, with pit sizes ranging from a few micrometers to several millimeters (Fig. 2C). Finally, coupons from the heat-treated waters (Fig. 2D) showed a uniform corrosion with some ulcerations similar to those observed on untreated samples (data not shown).
FIG 2.
Digitalized images showing one of the two coupons removed after 6 months of water exposure for each treatment. Magnification, ×3. (A) Coupon treated with chlorine dioxide; (B) coupon treated with monochloramine; (C) coupon treated with hydrogen peroxide; (D) coupon treated with hyperthermia (similar to untreated carbon steel coupons [not shown]).
DISCUSSION
In this study, we followed the trend of contamination by Legionella spp. in hospital hot water networks treated by different disinfection procedures. The effectiveness of these procedures was evaluated using traditional culture, qPCR, and EMA-qPCR in order to detect culturable and NC Legionella cells. Among the studied disinfection strategies, we included monochloramine, an innovative method which proved to be effective for controlling Legionella contamination in a hospital water network (22, 35). We also studied hydrogen peroxide, which has not yet been used extensively to control Legionella in hospital water distribution systems (36, 37). A comparison was carried out between these new procedures and two popular methods, chlorine dioxide and hyperthermia, which have widely been reported to be effective (38–42).
Our study confirms the effectiveness of continuous chemical disinfection, but we emphasize that all systems must be monitored continuously, since none of them eradicates legionellae from water distribution systems (6, 36, 43). On comparing the three disinfectants, monochloramine proved to be the most effective approach, as it gave the best results in reducing the percentage of positive points by culture, followed by chlorine dioxide and hydrogen peroxide. Moreover, for all biocides, approximately 50% of the culture-negative samples analyzed by molecular methods were positive by qPCR but negative by EMA-qPCR. This confirms that qPCR can give false-positive results when the biocides are applied in a contaminated system, as previously reported (17), and that continuous injection of chemicals that are capable of killing the circulating microbes avoids the induction of VBNC forms of legionellae.
In contrast, the network treated with hyperthermia was more contaminated in terms of both percentage of positive sites and bacterial load. The Italian and European guidelines recommend constantly maintaining the water temperature between 55 and 60°C in order to prevent Legionella contamination (44, 45), yet in our study this range proved to be ineffective, as over 60% of the samples remained positive. Interestingly, the presence of NC legionellae was also observed at temperatures of around 60°C, which is considered to be a safe value for preventing Legionella contamination (46). Recent studies demonstrated that VBNC legionellae are again culturable upon resuscitation within amoebae and that infection can be initiated following their resuscitation (14). In this respect, the NC cells generated following the thermal treatment used in our hospital constitute a potential public health hazard. For all these reasons, we do not advise using hyperthermia as the only method for controlling Legionella contamination in hospital water networks.
We stress the importance of finding an adequate level of biocide for controlling Legionella contamination, as our long-term experience suggests that the effectiveness of chlorine-based chemicals changes over time. After 1 year of disinfection applications, we proposed a level of chlorine dioxide of 0.30 to 0.40 mg liter−1 and a level of monochloramine of 2.0 mg liter−1 to lower contamination to below 102 CFU liter−1 (35). In the following 3 years, the levels required to obtain the same reduction were 0.50 to 0.70 mg liter−1 for chlorine dioxide and 2 to 3 mg liter−1 for monochloramine (22). In the present study, chlorine dioxide levels of ≥0.50 mg liter−1 were associated with 103 CFU liter−1, but the percentage of positives was below 30%, a value reported as being an indicator of low risk for disease transmission (47). Similarly, a monochloramine level of ≥3 mg liter−1 was required to maintain Legionella levels below 102 CFU liter−1, in accordance with other studies (48–50), but the percentage of positive sites was less than 30% independently of the biocide levels, thus confirming the satisfactory results obtained with this disinfectant.
Hydrogen peroxide produced satisfactory results in reducing Legionella contamination only when the biocide level was ≥20 mg liter−1, in agreement with other studies (36, 37). For this procedure, the high percentage of positive points, although at low levels, was due to the difficulty in regulating the disinfectant concentration, probably because the building was under renovation and many of the outlets were seldom used. Hydrogen peroxide appears to be a promising alternative for decreasing Legionella colonization; however, further field studies in other health care and community settings are required to confirm its effectiveness.
The chlorine-based biocides caused a shift from L. pneumophila serogroups 2 to 14 to L. pneumophila serogroup 1, while hydrogen peroxide favored a switch from L. pneumophila to other species, mainly L. jamestowniensis, which has not yet been associated with human disease (51, 52). Contrastingly, no shift was observed with hyperthermia, which is in line with its ineffectiveness at reducing Legionella colonization. The continuous injection of chlorine-based biocides evidently selects the most resistant Legionella spp., in our experience L. pneumophila serogroup 1, which is also the most virulent (1). In order to support this hypothesis, other authors reported the persistence of serogroup 1 in hospital water systems despite the adoption of chlorine-based disinfection strategies (53, 54). Duda et al. (50) reported a shift from L. pneumophila serogroup 1 to L. bozemanae, both of which are associated with human pathologies, following 24 months of monochloramine applications.
It is well known that disinfection can speed up corrosion and cause plumbing leaks, even though contradictory results are reported in the literature concerning the impact of disinfection on corrosion (7–10). To complete the study, we studied the appearance of a favorable environment for corrosion within the water networks according to the disinfection methods applied. The loss of thickness of the carbon steel foils which were used to evaluate the extent of corrosion over a 6-month period did not exceed the average value of 0.50 mm/year that is considered satisfactory for water networks (55), and no significant differences were observed between treated and untreated networks. On the other hand, differences were observed regarding the morphology of corrosion. Hydrogen peroxide and chlorine dioxide caused pitting, which is a type of corrosion that can create holes in tubes (9). Monochloramine and hyperthermia appeared to be less aggressive, since monochloramine caused a uniform corrosion with rare formation of pitting and hyperthermia showed a morphology of corrosion similar to that observed on the untreated coupons. The results encouraged us to continue the corrosion study; therefore, we are now analyzing the long-term corrosive effects of these four disinfection procedures on commonly used plumbing materials, such as copper, stainless steel, galvanized steel, and polyvinyl chloride, the last two of which are also used in our hospitals.
REFERENCES
- 1.Rota MC, Caporali MG, Bella A, Ricci ML, Napoli C. 2013. Legionnaires' disease in Italy: results of the epidemiological surveillance from 2000 to 2011. Euro Surveill 18:20497. [DOI] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control (ECDC). 2014. Legionnaires' disease in Europe 2012. ECDC, Stockholm, Sweden. [Google Scholar]
- 3.Istituto Superiore di Sanità (ISS). 2014. Rapporto annuale sulla legionellosi in Italia nel 2013. Not Ist Super Sanita 27:3–9. http://www.legionellaonline.it/not_ISS_ottobre_2014.pdf. [Google Scholar]
- 4.Ferranti G, Marchesi I, Favale M, Borella P, Bargellini A. 2014. Etiology, source and prevention of waterborne healthcare-associated infections: a review. J Med Microbiol 63:1247–1259. doi: 10.1099/jmm.0.075713-0. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). 2007. Legionella and the prevention of legionellosis. WHO, Geneva, Switzerland. [Google Scholar]
- 6.Lin YE, Stout JE, Yu VL. 2011. Controlling Legionella in hospital drinking water: an evidence-based review of disinfection methods. Infect Control Hosp Epidemiol 32:166–173. doi: 10.1086/657934. [DOI] [PubMed] [Google Scholar]
- 7.Eisnor JD, Gagnon GA. 2004. Impact of secondary disinfection on corrosion in a model water distribution system. J Water Supply Res Technol 53:441–452. [Google Scholar]
- 8.Chord F, Fascia P, Mallaval F, Cornillon J, Roesch L, Pozzetto B, Grattard F, Berthelot P. 2011. Chlorine dioxide for Legionella spp. disinfection: a danger for cross-linked polyethylene pipes? J Hosp Infect 78:242–243. doi: 10.1016/j.jhin.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Castillo Montes J, Hamdani F, Creus J, Touzain S, Correc O. 2014. Impact of chlorinated disinfection on copper corrosion in hot water systems. Appl Surf Sci 314:686–696. doi: 10.1016/j.apsusc.2014.07.069. [DOI] [Google Scholar]
- 10.Masters S, Wang H, Pruden A, Edwards MA. 2015. Redox gradients in distribution systems influence water quality, corrosion, and microbial ecology. Water Res 68:140–149. doi: 10.1016/j.watres.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 11.Ballard AL, Fry NK, Chan L, Surman SB, Lee JV, Harrison TG, Towner KJ. 2000. Detection of Legionella pneumophila using a real-time PCR hybridization assay. J Clin Microbiol 38:4215–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wellinghausen N, Frost C, Marre R. 2001. Detection of legionellae in hospital water samples by quantitative real-time LightCycler PCR. Appl Environ Microbiol 67:3985–3993. doi: 10.1128/AEM.67.9.3985-3993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joly P, Falconnet PA, André J, Weill N, Reyrolle M, Vandenesch F, Maurin M, Etienne J, Jarraud S. 2006. Quantitative real-time Legionella PCR for environmental water samples: data interpretation. Appl Environ Microbiol 72:2801–2808. doi: 10.1128/AEM.72.4.2801-2808.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epalle T, Girardot F, Allegra S, Maurice-Blanc C, Garraud O, Riffard S. 2015. Viable but not culturable forms of Legionella pneumophila generated after heat shock treatment are infectious for macrophage-like and alveolar epithelial cells after resuscitation on Acanthamoeba polyphaga. Microb Ecol 69:215–224. doi: 10.1007/s00248-014-0470-x. [DOI] [PubMed] [Google Scholar]
- 15.Oliver JD. 2010. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev 34:415–425. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen NT, Chang CW. 2010. Rapid quantification of viable legionellae in water and biofilm using ethidium monoazide coupled with real-time quantitative PCR. J Appl Microbiol 109:623–634. doi: 10.1111/j.1365-2672.2010.04678.x. [DOI] [PubMed] [Google Scholar]
- 17.Delgado-Viscogliosi P, Solignac L, Delattre JM. 2009. Viability PCR, a culture-independent method for rapid and selective quantification of viable Legionella pneumophila cells in environmental water samples. Appl Environ Microbiol 75:3502–3512. doi: 10.1128/AEM.02878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cangelosi GA, Meschke JS. 2014. Dead or alive: molecular assessment of microbial viability. Appl Environ Microbiol 80:5884–5891. doi: 10.1128/AEM.01763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansi A, Amori I, Marchesi I, Marcelloni AM, Proietto AR, Ferranti G, Magini V, Valeriani F, Borella P. 2014. Legionella spp. survival after different disinfection procedures: comparison between conventional culture, qPCR and EMA-qPCR. Microchem J 112:65–69. doi: 10.1016/j.microc.2013.09.017. [DOI] [Google Scholar]
- 20.Qin T, Tian Z, Ren H, Hu G, Zhou H, Lu J, Luo C, Liu Z, Shao Z. 2012. Application of EMA-qPCR as a complementary tool for the detection and monitoring of Legionella in different water systems. World J Microbiol Biotechnol 28:1881–1890. doi: 10.1007/s11274-011-0986-x. [DOI] [PubMed] [Google Scholar]
- 21.Fittipaldi M, Codony F, Adrados B, Camper AK, Morató J. 2011. Viable real-time PCR in environmental samples: can all data be interpreted directly? Microb Ecol 61:7–12. doi: 10.1007/s00248-010-9719-1. [DOI] [PubMed] [Google Scholar]
- 22.Marchesi I, Ferranti G, Bargellini A, Marchegiano P, Predieri G, Stout JE, Borella P. 2013. Monochloramine and chlorine dioxide for controlling Legionella pneumophila contamination: biocide levels and disinfection by-product formation in hospital water networks. J Water Health 11:738–747. doi: 10.2166/wh.2013.079. [DOI] [PubMed] [Google Scholar]
- 23.Marchesi I, Marchegiano P, Bargellini A, Cencetti S, Frezza G, Miselli M, Borella P. 2011. Effectiveness of different methods to control Legionella in the water supply: ten-year experience in an Italian university hospital. J Hosp Infect 77:47–51. doi: 10.1016/j.jhin.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO). 2011. Guidelines for drinking-water quality, 4th ed WHO, Geneva, Switzerland. [Google Scholar]
- 25.US Environmental Protection Agency. 2012. Drinking water standards and health advisories. EPA 822-S-12–001. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- 26.Borella P, Montagna MT, Stampi S, Stancanelli G, Romano-Spica V, Triassi M, Marchesi I, Bargellini A, Tatò D, Napoli C, Zanetti F, Leoni E, Moro M, Scaltriti S, Ribera D'Alcalà G, Santarpia R, Boccia S. 2005. Legionella contamination in hot water of Italian hotels. Appl Environ Microbiol 71:5805–5813. doi: 10.1128/AEM.71.10.5805-5813.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ISO. 1998. ISO 11731:1998. Water quality—detection and enumeration of Legionella. ISO, Geneva, Switzerland. [Google Scholar]
- 28.Bargellini A, Marchesi I, Leoni E, Mansi A, Cristino S, Marcelloni AM, Borella P. 2010. Inter-laboratory validation of a rapid assay for the detection and quantification of Legionella spp. in water samples. Lett Appl Microbiol 51:421–427. doi: 10.1111/j.1472-765X.2010.02910.x. [DOI] [PubMed] [Google Scholar]
- 29.Mansi A, Amori I, Marchesi I, Proietto AR, Marcelloni AM, Giugliano R, Ferranti G, Bargellini A, Borella P. 2013. Abstr 8th Int Conf Legionella, Melbourne, Australia, p 80. [Google Scholar]
- 30.ISO. 2012. ISO/TS 12869:2012. Water quality—detection and quantification of Legionella spp. and/or Legionella pneumophila by concentration and genic amplification by quantitative polymerase chain reaction (qPCR). ISO, Geneva, Switzerland. [Google Scholar]
- 31.Omiccioli E, Schiavano GF, Ceppetelli V, Amagliani G, Magnani M, Brandi G. 2015. Validation according to ISO/TS 12869:2012 of a molecular method for the isolation and quantification of Legionella spp. in water. Mol Cell Probes 29:86–91. doi: 10.1016/j.mcp.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 32.ASTM. 2011. ASTM G1-03:2011. Standard practice for preparing, cleaning, and evaluating corrosion test specimens. Book of standards volume 03.02. ASTM, West Conshohocken, PA. [Google Scholar]
- 33.ASTM. 2014. ASTM G4-01:2014. Standard guide for conducting corrosion tests in field applications. Book of standards volume 03.02. ASTM, West Conshohocken, PA. [Google Scholar]
- 34.Anonymous. 2006. UNI EN 10224:2006. Non-alloy steel tubes and fittings for the conveyance of aqueous liquids including water for human consumption—technical delivery conditions. Ente Nazionale Italiano di Unificazione, Rome, Italy. [Google Scholar]
- 35.Marchesi I, Cencetti S, Marchegiano P, Frezza G, Borella P, Bargellini A. 2012. Control of Legionella contamination in a hospital water distribution system by monochloramine. Am J Infect Control 40:279–281. doi: 10.1016/j.ajic.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Shuval H, Yarom R, Shenman R. 2009. An innovative method for the control of Legionella infections in the hospital hot water systems with a stabilized hydrogen peroxide-silver formulation. Int J Infect Control 5:1. doi: 10.3396/ijic.V5i1.006.09. [DOI] [Google Scholar]
- 37.Cristino S, Legnani PP, Leoni E. 2012. Plan for the control of Legionella infections in long-term care facilities: role of environmental monitoring. Int J Hyg Environ Health 215:279–285. doi: 10.1016/j.ijheh.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Darelid J, Löfgren S, Malmvall BE. 2002. Control of nosocomial Legionnaires' disease by keeping the circulating hot water temperature above 55 degrees C: experience from a 10-year surveillance programme in a district general hospital. J Hosp Infect 50:213–219. doi: 10.1053/jhin.2002.1185. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan A, Bova G, Ross T, Mackie K, Paquette N, Merz W, Perl TM. 2003. A 17-month evaluation of a chlorine dioxide water treatment system to control Legionella species in a hospital water supply. Infect Control Hosp Epidemiol 24:575–579. doi: 10.1086/502254. [DOI] [PubMed] [Google Scholar]
- 40.Sidari FP III, Stout JE, VanBriesen JM, Bowman AM, Grubb D, Neuner A, Wagener MM, Yu VL. 2004. Keeping Legionella out of water systems. J Am Water Works Assoc 96:111–119. [Google Scholar]
- 41.Blanc DS, Carrara P, Zanetti G, Francioli P. 2005. Water disinfection with ozone, copper and silver ions, and temperature increase to control Legionella: seven years of experience in a university teaching hospital. J Hosp Infect 60:69–72. doi: 10.1016/j.jhin.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Tesauro M, Bianchi A, Consonni M, Pregliasco F, Galli MG. 2010. Environmental surveillance of Legionella pneumophila in two Italian hospitals. Ann Ist Super Sanita 46:274–278. doi: 10.4415/ANN_10_03_08. [DOI] [PubMed] [Google Scholar]
- 43.Casini B, Buzzigoli A, Cristina ML, Spagnolo AM, Del Giudice P, Brusaferro S, Poscia A, Moscato U, Valentini P, Baggiani A, Privitera G. 2014. Long-term effects of hospital water network disinfection on Legionella and other waterborne bacteria in an Italian university hospital. Infect Control Hosp Epidemiol 35:293–299. doi: 10.1086/675280. [DOI] [PubMed] [Google Scholar]
- 44.Istituto Superiore di Sanità (ISS). 5 May 2000. Linee guida italiane per la prevenzione e il controllo della legionellosi. Gazzetta ufficiale della Repubblica Italiana serie generale, no.103. [Google Scholar]
- 45.European Working Group for Legionella Infections (EWGLI). 2011. Technical guidelines for the investigation, control and prevention of travel associated Legionnaires' disease, version 1.1. EWGLI, London, United Kingdom. [Google Scholar]
- 46.Kim BR, Anderson JE, Mueller SA, Gaines WA, Kendall AM. 2002. Efficacy of various disinfectants against Legionella in water systems. Water Res 36:4433–4444. doi: 10.1016/S0043-1354(02)00188-4. [DOI] [PubMed] [Google Scholar]
- 47.Allegheny County Health Department (ACHD). 1997. Approaches to the prevention and control of Legionella infection in Allegheny County health care facilities. ACHD, Pittsburgh, PA. [Google Scholar]
- 48.Chien SH, Chowdhury I, Hsieh MK, Li H, Dzombak DA, Vidic RD. 2012. Control of biological growth in recirculating cooling systems using treated secondary effluent as makeup water with monochloramine. Water Res 46:6508–6518. doi: 10.1016/j.watres.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 49.Kandiah S, Yassin MH, Stout J. 2013. Monochloramine use for prevention of Legionella in hospital water systems. Infect Disord Drug Targets 13:184–190. doi: 10.2174/1871526511313030006. [DOI] [PubMed] [Google Scholar]
- 50.Duda S, Kandiah S, Stout JE, Baron JL, Yassin M, Fabrizio M, Ferrelli J, Hariri R, Wagener MM, Goepfert J, Bond J, Hannigan J, Rogers D. 2014. Evaluation of a new monochloramine generation system for controlling Legionella in building hot water systems. Infect Control Hosp Epidemiol 35:1356–1363. doi: 10.1086/678418. [DOI] [PubMed] [Google Scholar]
- 51.Brenner DJ, Steigerwalt AG, Gorman GW, Wilkinson HW, Bibb WF, Hackel M, Tyndall RL, Campbell J, Feeley JC, Thacker WL, Skaliy P, Martin WT, Brake BJ, Fields BS, McEachern HV, Corcoran LK. 1985. Ten new species of Legionella. Int J Syst Evol Microbiol 35:50–59. [Google Scholar]
- 52.Casati S, Conza L, Bruin J, Gaia V. 2010. Compost facilities as a reservoir of Legionella pneumophila and other Legionella species. Clin Microbiol Infect 16:945–947. doi: 10.1111/j.1469-0691.2009.03009.x. [DOI] [PubMed] [Google Scholar]
- 53.Scaturro M, Dell'eva I, Helfer F, Ricci ML. 2007. Persistence of the same strain of Legionella pneumophila in the water system of an Italian hospital for 15 years. Infect Control Hosp Epidemiol 28:1089–1092. doi: 10.1086/519869. [DOI] [PubMed] [Google Scholar]
- 54.Casini B, Valentini P, Baggiani A, Torracca F, Frateschi S, Nelli LC, Privitera G. 2008. Molecular epidemiology of Legionella pneumophila serogroup 1 isolates following long-term chlorine dioxide treatment in a university hospital water system. J Hosp Infect 69:141–147. doi: 10.1016/j.jhin.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Campbell FC. 2012. Fatigue and fracture: understanding the basics. ASM International, Materials Park, OH. [Google Scholar]