ABSTRACT
Human norovirus (HuNoV) genogroup II genotype 4 (GII.4) strains account for about 80% of the gastroenteritis outbreaks in the United States. Contaminated food is a major transmission vehicle for this virus. In humans, pigs, and oysters, histo-blood group antigens (HBGAs) act as attachment factors for HuNoVs. In lettuce, although the virus-like particles (VLPs) of a GII.4 HuNoV were found to bind to cell wall carbohydrates, the exact binding site has not been investigated. Here, we show the presence of HBGA-like carbohydrates in the cell wall of lettuce. The digestion of lettuce leaves with cell wall-degrading enzymes exposed more binding sites and significantly increased the level of binding of GII.4 HuNoV VLPs. Competition assays showed that both the HBGA monoclonal antibody, recognizing the H type, and plant lectins, recognizing α-l-fucose in the H type, effectively inhibited VLP binding to lettuce tissues. Lettuce cell wall components were isolated and their NoV VLP binding characteristics were tested by enzyme-linked immunosorbent assays. The binding was inhibited by pretreatment of the lettuce cell wall materials with α-1,2-fucosidase. Collectively, our results indicate that H-type HBGA-like carbohydrates exist in lettuce tissues and that GII.4 HuNoV VLPs can bind the exposed fucose moiety, possibly in the hemicellulose component of the cell wall.
IMPORTANCE Salad crops and fruits are increasingly recognized as vehicles for human norovirus (HuNoV) transmission. A recent study showed that HuNoVs specifically bind to the carbohydrates of the lettuce cell wall. Histo-blood group antigens (HBGAs) are carbohydrates and are known as the attachment factors for HuNoV infection in humans. In this study, we show the presence of HBGA-like carbohydrates in lettuce, to which HuNoVs specifically bind. These results suggest that specifically bound HuNoVs cannot be removed by simple washing, which may allow viral transmission to consumers. Our findings provide new information needed for developing potential inhibitors to block binding and prevent contamination.
INTRODUCTION
Foodborne illnesses constitute a recurring public health burden, with an estimated 9.4 million illnesses and 1,351 deaths annually in the United States (1). Currently, most illnesses (58%) are caused by human noroviruses (HuNoVs), and others are caused by human bacterial pathogens, such as nontyphoidal Salmonella spp. (11%) and Clostridium perfringens (10%) (1). Noroviruses (NoVs) are nonenveloped single-stranded RNA viruses belonging to the Norovirus genus of the family Caliciviridae. NoVs have been classified into five genogroups (GI–GV), and GI, GII, and GIV viruses cause gastroenteritis in humans (2). Each NoV genogroup is further subdivided into several genotypes based on genetic similarities. As a single genogroup, GII currently accounts for about 86% of HuNoV cases (3, 4). HuNoVs have a very low infectious dose, and the 50% infective dose (ID50) was estimated as about 18 viruses and 2,800 genomic equivalents (GEs) (5, 6). Moreover, NoVs are extremely stable in the environment and show resistance to freezing, high temperatures (60°C), disinfection with chlorine, acidic conditions, and alcohol treatment (7, 8). Therefore, the low infective dose, coupled with this stability, contributes to the high transmission rate of HuNoVs (4).
Many studies that have dealt with foodborne illnesses have focused on the transmission of human pathogens from foods of animal origin; however, fruits and vegetables are increasingly being recognized as the source of many foodborne disease outbreaks (9). Fruits and salad crops, such as raspberries, strawberries, and lettuce, are recognized as vehicles for HuNoV transmission (10–12). Fruit and vegetable produce can be contaminated at any stage of the production chain from the preharvest to the postharvest stages. Sources of pollution may include polluted irrigation water, soil amendments, sick farm workers, and food handlers with poor hygienic practices (13, 14). Although HuNoVs are the leading cause of acute viral gastroenteritis worldwide, controlling virus transmission and contamination of produce is challenging, in part due to the lack of a tissue culture model for propagating HuNoVs and testing the effectiveness of treatments. It was reported recently that HuNoVs can infect B cells in vitro with the help of enteric bacteria expressing histo-blood group antigens (HBGAs) (15). However, HuNoVs still cannot grow efficiently in B-cell cultures. Therefore, recombinant virus-like particles (VLPs) of HuNoVs are often used as surrogates for investigating HuNoV attachment to food surfaces (16). HuNoV VLPs are not only morphologically similar to the native virions but they also harbor the same viral antigenic epitopes and binding sites as native virions (4).
Several studies suggested that electrostatic forces contribute to the attachment of HuNoV surrogates (such as feline calicivirus and porcine sapovirus) to lettuce (8, 17). In addition, certain plant-associated factors, such as the stomata, minor veins, and injuries on the leaf surface, enhance either virus attachment or entrapment (18, 19). Moreover, carbohydrate moieties in the lettuce cell wall were reported to play an important role in the specific binding of HuNoV VLPs to lettuce (19). The plant cell wall is a complex matrix of polysaccharides that supports and strengthens plant cells and confers resistance against biotic and abiotic stress (20). It is a dynamic and highly specialized complex formed by a heterogeneous mixture of celluloses, pectins, hemicelluloses, and proteins and phenolic compounds. Celluloses and hemicelluloses, embedded in the amorphous pectin polymers, provide rigidity to the cell wall, whereas pectins provide fluidity through the gelatinous polysaccharide matrix (21). Although HBGAs were identified as ligands in human, pig, and oyster tissues for HuNoV binding (22–24), neither HBGAs nor their analogues have been reportedly found in lettuce tissues.
Therefore, the objective of this study was to identify the carbohydrates in the cell wall responsible for HuNoV VLP binding and to investigate which cell wall component harbors these carbohydrates. Understanding the mechanism of virus attachment to food can provide insights into suitable prevention strategies.
MATERIALS AND METHODS
Production and purification of NoV VLPs and NoV antibodies.
The recombinant baculovirus (rBac) carrying the VP1 gene (ORF2) of the Hu/NoV/GII.4/HS194/2009/US strain (GenBank accession number GU325839) was produced using the BaculoDirect baculovirus expression system (Invitrogen, Carlsbad, CA). The Gateway entry clone containing the capsid protein (VP1) of HS194 (pENTR/SD/D-TOPO-HS194) was generated and used with BaculoDirect linear DNA to perform an LR (attL [entry clone] × attR [destination vector]) recombination reaction to generate recombinant baculovirus DNA carrying the VP1 gene of HS194 (25). Sf9 cells were cultured at a density of 3 × 107 cells/flask (162 cm2) and inoculated with rBac GII.4 at a multiplicity of infection (MOI) of 10, after which the medium was harvested at 10 days postinoculation. VLPs were semipurified through sucrose cushions and then purified by CsCl gradient ultracentrifugation in 4.5 ml of TNC buffer (10 mM Tris-HCl, 140 mM NaCl, and 10 mM CaCl2 [pH 7.4]) containing a protease inhibitor cocktail of 10 μM leupeptin, 100 μM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), 1 μM pepstatin A, and 1 μM E-64 (Roche, Indianapolis, IN). The band around 1.34 g/cm3 was collected and resuspended with TNC buffer followed by ultracentrifugation to remove CsCl. The collected VLPs were then suspended in TNC buffer with the protease inhibitor cocktail. The VLP concentrations were quantified by the Bradford method (Bio-Rad, Hercules, CA). The morphology of the VLPs was examined by electron microscopy, and the antigenic reactivity of the VLPs was confirmed by Western blotting. Hyperimmune antiserum against HuNoV VLPs was generated previously in our lab using guinea pigs (19). Total IgG antibodies from the guinea pig hyperimmune serum against NoV/GII.4/HS194 were further purified using an Immunopure IgG purification kit (Thermo/Pierce, Rockford, IL) according to manufacturer instructions. The production and purification of NoV/GII.12/HS206 VLPs were performed as described previously (26). NoV/GII.4/1997 and NoV/GII.4/2004 VLPs were obtained from the lab of Ralph Baric at the University of North Carolina at Chapel Hill.
Evaluation of the effect of different enzymatic digestions on binding between NoV VLPs and plant leaves assessed by using immunofluorescence microscopy.
All plants used here (romaine lettuce, red leaf lettuce, basil, and celery) were purchased from a local grocery store. The leaves and veins were cut into small pieces (0.5 × 0.5 cm2), and about 30 pieces of samples were dehydrated in 30 ml of a gradient ethanol series (70% [vol/vol], 90%, 95%, 100%, and 100%) for 6 h. Following dehydration, the plant pieces were incubated in Pro-par clearant solution (Anatech, Battle Creek, MI) for 3 h at room temperature (RT) and then in paraffin for 2 h at 63°C. Five-micrometer sections were cut using a microtome and collected on glass slides (Thermo Fisher Scientific, Pittsburgh, PA). Slides were dried at 37°C for 2 h, deparaffinized in xylene for 15 min, and rehydrated in 300 ml of a gradient ethanol series (100%, 90%, 70%, 50% [vol/vol]) at RT for 15 min per ethanol step. Different slides were incubated with one of four cell wall-digesting enzymes, including cellulase R-10 and macerozyme R-10 (PhytoTechnology Laboratories, Lenexa, KS), cellulase from Trichoderma viride (Sigma-Aldrich, St. Louis, MO), and pectinase from Aspergillus niger (Sigma-Aldrich). The enzymes were added at a final concentration of 1 μg/μl per slide and incubated at 37°C for 1 h. For NaIO4 oxidation, after cellulase R-10 digestion, slides were incubated with 200 μl of 50 mM NaIO4 (Sigma-Aldrich) at RT for 30 min in the dark. For fucosidase digestion, each slide was digested with 20 U of α-1,2-fucosidase (NEB, Ipswich, MA) at 37°C for 1 h. Following three washes with 300 ml of phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T), blocking was performed at RT with PBS supplemented with 1% bovine serum albumin (BSA). Subsequently, the slides were incubated with 200 μl of NoV VLPs (final concentration, 10 μg/ml) at RT for 1 h. The HuNoV VLPs on lettuce were detected by immunofluorescence assay (IFA) as follows. For the detection of GII.4 HS194 and GII.12 HS206 VLPs, 200 μl of anti-HS194 or anti-HS206 VLP guinea pig serum (1:1,800) and 200 μl of the Alexa Fluor 488-conjugated goat anti-guinea pig IgG (1:700; Invitrogen, Eugene, OR) were used as the primary and secondary antibodies, respectively; for NoV/GII.4/1997 and NoV/GII.4/2004 VLPs, 200 μl of rabbit anti-NoV VLP antiserum (1:1,000) and Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:700; Invitrogen, Eugene, OR) were used. Two controls were used, including slides treated as described above but without the VLPs (to check the specificity of the primary antibody) and slides without the primary antibody (to check the specificity of the secondary antibody). Controls with no secondary antibody were also used to assess the autofluorescence of the tissue, and the results revealed no background fluorescence (data not shown). All antibody incubation steps were performed at RT for 1 h. The slides were washed three times by immersing them in jars containing 300 ml of PBS-T after each incubation step.
Detection of HBGA-like carbohydrates in digested lettuce by monoclonal antibodies to human HBGAs.
To unmask the HBGA binding sites, paraffin-embedded lettuce slides were digested by 200 μl of 1 μg/μl cellulase R-10 at 37°C for 1 h, followed by washing with 300 ml of PBS buffer and blocking with 200 μl of PBS containing 1% BSA for 1 h. For porcine intestine paraffin slides, 200 μl of 20 μg/ml proteinase K (Roche, Indianapolis, IN) was applied for 10 min. After washing, the slides were blocked with 200 μl of PBS containing 1% BSA for 1 h (23). After blocking, slides were incubated with 200 μl of various HBGA monoclonal antibodies (anti-A type I and II [BG1, Covance, Emeryville, CA], anti-B [BG2, Immucor, Norcross, GA], and anti-H type 1 [BG3, Covance]) diluted in PBS (1:50). Alexa Fluor 488-conjugated goat anti-mouse IgG serum (Invitrogen) was used as the secondary antibody. All antibody incubation steps were performed at RT for 1 h. The slides were washed three times by immersing them in jars containing 300 ml of PBS-T after each incubation step.
Inhibition effect of monoclonal antibodies to HBGAs on HuNoV VLP binding to lettuce.
For fresh leaf samples, the abaxial wax layer was peeled away using tweezers. Both leaves and paraffin slides were digested with 1 μg/μl cellulase R-10 at 37°C for 1 h, followed by blocking with 200 μl of PBS containing 1% BSA. Two hundred microliters of HBGA monoclonal antibodies diluted in PBS (1:50) was added and incubated for 1 h. Subsequently, 200 μl of 10 μg/ml NoV VLPs (GII.4, HS194) was added and incubated for 1 h. Alexa Fluor 488-conjugated goat anti-guinea pig IgG (Invitrogen, Carlsbad, CA) diluted in PBS (1:700) was used as the secondary antibody. The VLPs were detected by IFA as described above.
Inhibition effect of plant lectins on NoV VLP binding to lettuce.
Paraffin-embedded lettuce was digested with 200 μl of 1 μg/μl cellulase R-10 at 37°C for 1 h before the blocking step. Plant lectins, including BS-I (Bandeiraea simplicifolia), LcH (Lens culinaris), and UEA-I (Ulex europaeus), were diluted in TBS buffer (50 mM Tris-HCl and 150 mM NaCl, supplemented with 2.5 mM MnCl2 and CaCl2) to a final concentration of 100 μg/ml. Two hundred microliters of each lectin was incubated on different digested slides for 1 h at RT. After washing with 300 ml of PBS-T, the slides were incubated with 200 μl of 10 μg/ml NoV VLPs for 1 h at RT. The VLPs were detected by IFA as described above.
Extraction of lettuce hemicellulose.
Extraction of lettuce cell wall materials (CWMs) was performed using the method described by Hoffman et al. (44). Mature romaine lettuce was purchased from a local grocery store, and about 200 g of lettuce was washed thoroughly with sterile water (21). Then, the leaves were cut to separate the midrib, also known as the principal vein, from the green leaf lamina. Each of the two tissues was first ground to a fine powder in liquid nitrogen, homogenized in 80% ethanol using a Polytron homogenizer (Cole-Parmer Instruments, Vernon Hill, IL), and centrifuged for 20 min at 2,500 × g. The resulting pellet was subjected to a series of ethanol washes (80% twice and 100% once) and then stirred in 60 ml of methanol-chloroform (1:1 [vol/vol]) for 2 h at 4°C. The pellet was collected by filtration using Whatman glass microfiber filters (Fisher Scientific, Pittsburgh, PA) and washed with 100 ml of methanol-chloroform, followed by 100 ml of ice-cold acetone. The pellet was dried in a chemical hood overnight. The resulting CWMs were stored at 4°C in sealed bottles under dark conditions.
For hemicellulose (xylan) extraction from CWM, we used a previously described method (27). Xylan delignification and solubilization were done simultaneously in warm 3.25 M NaOH (60°C) at an alkali charge of 13% (wt/vol) and a liquid-to-biomass ratio of 3:1 for 2 h. The liquid portion containing the xylan was collected, and the residue was washed with 20 ml of 60°C water. The pH of xylan extract was adjusted with acetic acid to pH 5.0. The xylan polymers were precipitated in 2 volumes of 95% ethanol overnight at 4°C before centrifugation at 7,500 × g for 10 min. The xylan pellets were washed with 30 ml of ethanol before freezing at −20°C.
Detection of NoV VLP binding to cell wall fractions by ELISA.
The enzyme-linked immunosorbent assay (ELISA) was performed according to the method of Esseili et al. (19). Briefly, the xylan fraction of CWMs was resuspended in PBS (pH 7.4) at 0.2 mg/ml to coat 96-well MaxiSorp plates (Thermo Fisher Scientific, Rochester, NY). For cellulase R-10 digestion, samples were digested with 1 μg/μl cellulase R-10 at RT for 1 h. For fucosidase digestion, samples were digested with 20 U of α-1,2-fucosidase (NEB) at 37°C for 1 h after cellulase R-10 digestion. The plates were coated with CWMs at 4°C for 18 h, washed three times with PBS-T, and blocked with 1% BSA in PBS for 1 h at 37°C. Then, NoV VLPs (50 μl of 10 μg/ml) were added and incubated for 1 h at RT. The optimal dilutions of the purified primary antibody (guinea pig anti-HS194 VLP serum) and the secondary antibody (goat anti-guinea pig IgG–horseradish peroxidase [HRP] conjugated, KPL, Gaithersburg, MD) were 1:2,000 and 1:4,000, respectively. Plates were washed with PBS-T four times following each incubation step. Plates were incubated with the peroxidase substrate tetramethylbenzidine (KPL) for 8 min at RT in the dark, and the reaction was stopped by adding 0.3 M sulfuric acid. The absorbance was read at 450 nm. Controls consisted of wells coated with CWMs (with neither VLPs nor the primary antibodies added) and PBS-coated wells with VLPs added.
Statistical analyses.
GraphPad Prism was used for statistical analyses. One-way analysis of variance (ANOVA) was used to determine significant differences in VLP binding to different cell wall fractions. Student's t test was used to determine significant differences in VLP binding after fucosidase digestion. Each treatment in the ELISA was run in triplicate wells, and the entire ELISA was repeated at least three times. The reported results are representative of those from one ELISA, but the same trend was shown among different ELISA runs. Differences in means are considered significant when the P value is <0.05, and data are represented as means and standard deviations (SDs).
RESULTS
Binding of HuNoV VLPs to lettuce tissues was enhanced after cell wall digestion.
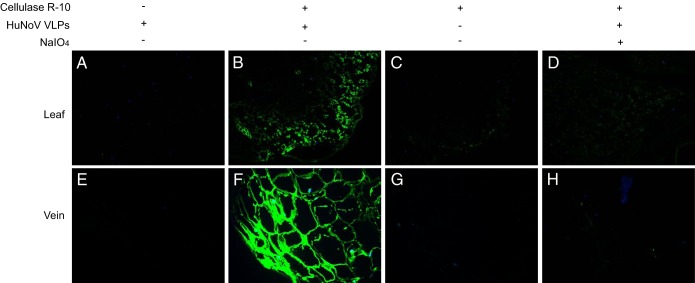
Our previous results showed that minor veins inside the lettuce leaf and leaf cut edges had the highest levels of Hu/NoV/GII.4/HS194 VLP binding (19). We hypothesized that the binding sites of the lettuce cell wall are located under the leaf surface and are not exposed to bind to the HuNoV VLPs but that lesions on the leaves may expose them. To test the hypothesis, lettuce paraffin slides were incubated with cell wall digestion enzymes, and then HuNoV VLP binding was tested by immunofluorescence assay. The results show that lettuce sections from both green lamina and veins had strong binding signal after cellulase R-10 digestion. The binding was inhibited by treatment with 50 mM NaIO4 (Fig. 1). Several other commercial enzymes, such as cellulase, macerozyme R-10, and pectinase, were also tested, and all of them increased HuNoV VLP binding (see Fig. S1 in the supplemental material). HuNoV VLP binding specificity by lettuce CWMs was also determined by testing the VLPs of several other Hu/NoV/GII.4 strains. As in the case of NoV GII.4/HS194, both GII.4/1997 and GII.4/2004 bound to the lettuce after cellulase R-10 digestion (see Fig. S2 in the supplemental material). As a control, the Hu/NoV/GII.12/HS206 VLPs that did not show HBGA binding in vitro (26) did not bind to lettuce (see Fig. S2D). We also investigated the binding between Hu/NoV/GII.4/HS194 VLPs and several other vegetables, red leaf lettuce, celery, and basil. The results show that HuNoV VLPs bound to both digested red leaf lettuce and celery veins (see Fig. S3A and B in the supplemental material) but not the digested basil leaves (see Fig. S3C).
FIG 1.
Binding of HuNoV VLPs to lettuce tissues. Immunofluorescence microscopy was performed on lettuce paraffin slides. NoV/GII.4/HS194 VLPs attached to lettuce green leaf lamina (A–D) and vein (E–H). (A and E) The control sample was not subjected to cellulase R-10 digestion before incubation with NoV VLPs. (B and F) Samples were digested with cellulase R-10 and then incubated with NoV VLPs. (C and G) Samples were digested with cellulase R-10 but not incubated with NoV VLPs. (D and H) Samples were digested with cellulase R-10 and then exposed to NaIO4, followed by incubation with NoV VLPs. The binding signal (green) was detected by primary antiserum against NoV/GII.4/HS194 VLPs and the Alex Fluor 488-conjugated goat anti-guinea pig IgG antibody. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). All pictures were taken using the same exposure time.
HBGA-like carbohydrates can be detected in digested lettuce tissues.
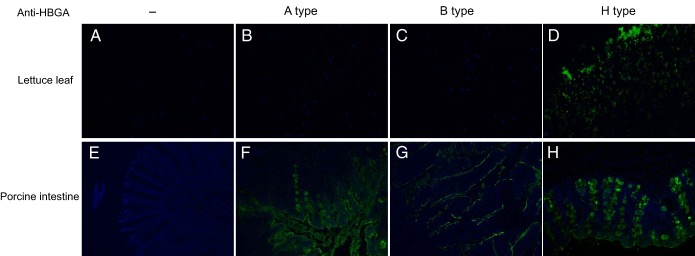
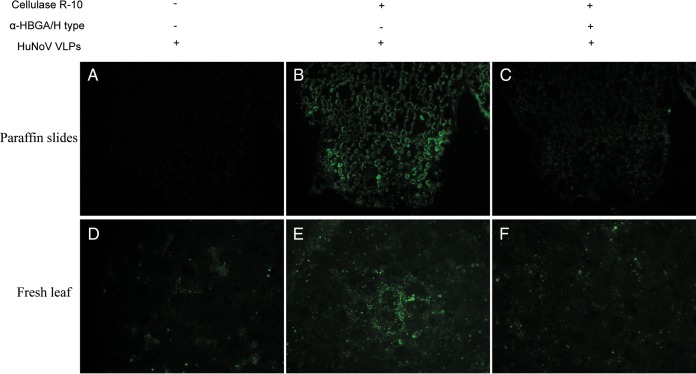
HBGA-like carbohydrates on lettuce leaves were detected using HBGA monoclonal antibodies after enzymatic digestion. The H-type antigen was detected on lettuce, but the A- and B-type antigens were not (Fig. 2). Competition assays with HBGA monoclonal antibodies were performed to confirm the specific binding between HuNoV VLPs and H-type antigens in lettuce. The results show that pretreatment of lettuce with H-type monoclonal antibodies inhibited the interaction between HuNoV VLPs and lettuce (Fig. 3C). Similar competition results were also obtained by using fresh lettuce leaves whose surface cuticle was peeled off with tweezers (Fig. 3F).
FIG 2.
Detection of HBGA-like carbohydrates on lettuce leaves with anti-HBGA monoclonal antibodies. Paraffin slides of lettuce were digested with 1 μg/μl cellulase R-10 at room temperature (RT) for 1 h before addition of PBS (no anti-HBGA), anti-A-type HBGA monoclonal antibody (A type), anti-B-type HBGA monoclonal antibody (B type), or anti-H-type HBGA monoclonal antibody (H type). Porcine intestine paraffin slides were used as positive controls for A-type and H-type antigens and were digested with proteinase K at RT for 10 min before incubation with antibodies. The binding signal (green) was detected by Alex Fluor 488-conjugated goat anti-mouse IgG antibody. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). All pictures were taken using the same exposure time.
FIG 3.
Inhibition of HuNoV binding to lettuce tissues by anti-H-type HBGA monoclonal antibodies. Competition assays were performed by adding anti-H-type HBGA monoclonal antibodies prior to HuNoV VLP incubation. Paraffin slides of lettuce and fresh lettuce leaves whose cuticle was peeled away were incubated with anti-H-type HBGA monoclonal antibody (1:50). (A and D) The control sample was not subjected to cellulase R-10 digestion before incubation with NoV VLPs. (B and E) The sample was digested with cellulase R-10 and then incubated with NoV VLPs. (C and F) The sample was digested with cellulase R-10 and then exposed to anti-H-type HBGA antibody, followed by incubation with HuNoV VLPs. The binding signal (green) was detected by primary antiserum against NoV/GII.4/HS194 VLPs and the Alex Fluor 488-conjugated goat anti-guinea pig IgG antibody. All pictures were taken using the same exposure time.
HuNoV VLPs recognize α-1,2-fucose of HBGA-like carbohydrates in lettuce tissue.
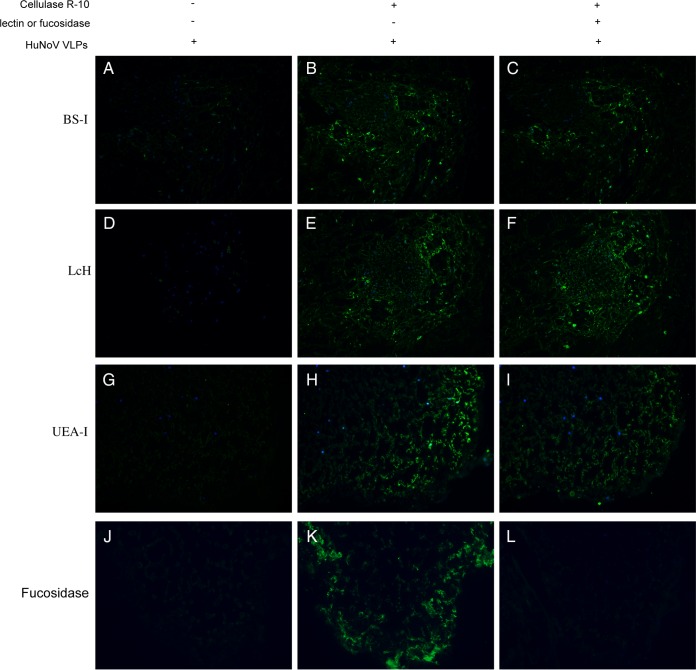
The central binding pocket of the GII.4/NoV/VA387 strain fits well with an α-fucose, and VA387 VLPs used α-1,2-fucose as the major binding saccharide (H epitope) (28). Lectins, plant-derived carbohydrate-binding proteins, are highly specific for sugar moieties and can be used to test binding specificity by blocking NoV VLP binding sites (19). Competition assays with lectins were performed to investigate sugar moieties for NoV VLP recognition. The results show that treatment of digested lettuce slides with lectin UEA-I (Ulex europaeus agglutinin), recognizing α-1,2-fucose, partially inhibited NoV/GII.4 VLP binding to lettuce (Fig. 4I). However, two lectins (BS-I and LcH) against other sugar moieties (α-d-galactose, α-d-mannose, and α-d-glucose) showed no clear inhibition effects (Fig. 4C and F). Moreover, incubation with α-1,2-fucosidase after cell wall digestion completely inhibited NoV/GII.4 VLP binding (Fig. 4L). These results suggest that recognition by NoV VLPs is likely via α-1,2-fucose moieties in HBGA-like carbohydrates in the lettuce leaves.
FIG 4.
Inhibition of HuNoV binding to lettuce tissues by plant lectins and fucosidase. Competition assays were performed by adding carbohydrate-specific lectins before NoV VLP incubation. Lettuce paraffin slides were incubated with different plant lectins (BS-I/anti-B-type HBGA, recognize d-galactose; LcH/no HBGA specificity, recognize d-mannose and d-glucose; UEA-I/anti-H-type HBGA, recognize l-fucose). (A, D, G, and J) Control slides incubated with NoV VLPs but not subjected to cellulase R-10 digestion. (B, E, H, and K) Slides digested with cellulase R-10 and then incubated with HuNoV VLPs. (C, F, I, and L) Cellulase R-10-digested slides exposed to plant lectins or α-1,2-fucosidase, followed by incubation with HuNoV VLPs. The binding signal (green) was detected by primary antiserum against NoV/GII.4/HS194 VLPs and the Alex Fluor 488-conjugated goat anti-guinea pig IgG antibody. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). All pictures were taken using the same exposure time.
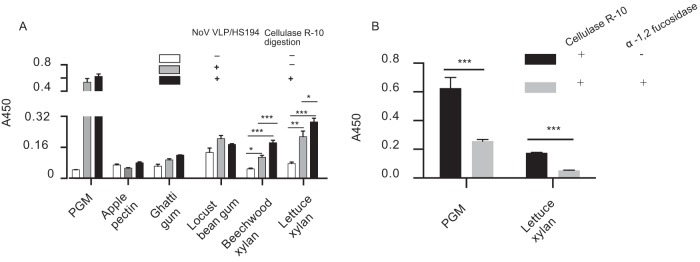
HBGA-like carbohydrates are present mainly in lettuce hemicellulose fraction.
To identify the cell wall component that contains the HBGA-like carbohydrates to which NoV/GII.4 VLPs bind, the binding between several purchased cell wall polysaccharides and NoV/GII.4 VLPs was tested by ELISA. Apple pectin, ghatti gum, and locust bean gum did not show VLP binding, even after cellulase R-10 digestion, whereas lettuce xylan and beechwood xylan showed VLP binding (Fig. 5A). Moreover, the binding of beechwood xylan increased after digestion with cellulase R-10 (Fig. 5A), suggesting that xylan, a group of hemicelluloses in the plant cell wall, might account for NoV VLP binding. Indeed, the extracted lettuce xylan bound to HuNoV VLPs, and its digestion with cellulase R-10 further enhanced the signal (Fig. 5A). Next, lettuce xylan was digested with α-1,2-fucosidase, and this treatment also significantly inhibited NoV/GII.4 VLP binding (Fig. 5B). These results suggest that HBGA-like carbohydrates in the lettuce hemicellulose component were responsible for NoV/GII.4 VLP binding.
FIG 5.
Binding of HuNoV VLPs to α-1,2-fucose of lettuce hemicellulose. (A) HuNoV VLP binding to commercial plant polysaccharides. Commercial plant polysaccharides, including lettuce xylan, were suspended in PBS (pH 7.4) at 0.2 μg/ml and used to coat ELISA plates. For cellulase R-10 digestion, 1 μg/μl enzyme was added to the ELISA plates and incubated at 37°C for 30 min. Porcine gastric mucin (PGM) was used as a positive control (50 ng/well). Error bars indicate standard deviations, and significance was determined by one-way ANOVA. (B) HuNoV VLPs bound to fucose of extracted lettuce hemicellulose. For fucose digestion, 20 U of α-1,2-fucosidase was added to the wells and incubated at 37°C for 1 h. Error bars indicate standard deviations, and significance was determined by one-way Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Each sample was assayed in triplicate.
DISCUSSION
Human HBGAs are complex carbohydrates distributed mainly on red blood cells and mucosal epithelia of the genitourinary, respiratory, and digestive tracts (29), and they are also present as free oligosaccharides in the secretions (including biologic fluids, such as saliva, intestinal content, and milk) of secretor-positive individuals (28). HuNoV/GI.1 and GII.4 VLPs bound to pig duodenal and buccal tissues expressing either A or H antigens (23), and HuNoV/GI.1 VLPs also bound to A antigens in oyster gastrointestinal cells (24, 30). A recent study also showed the existence of an HBGA-like substance in extracellular polymeric substances of the enteric bacterium strain Enterobacter sp. SENG-6 (31). All of these results suggest the important role of HBGAs in specific HuNoV binding. HBGAs are synthesized from disaccharide precursors (types I and II) through sequential additions of sugar residues with specific linkages catalyzed by three different glycosyltransferases (28). Lewis (Le), O secretor/nonsecretor (Se−), and AB phenotypes are the products of FUT3 (α-1,3- or α-1,4-fucosyltransferase), FUT2 (α-1,2-fucosyltransferase), and two glycosyltransferases (A and B), respectively (28). Hu/NoV/GII.4/VA387 interacts with all major ABO antigens, while α-1,2-fucose (H epitope) is the major binding saccharide (32). Interestingly, plant carbohydrates also share oligosaccharide branches similar to those of human Lea antigen (33). Moreover, two nonredundant α-1,2-fucosyltransferases, which catalyzed the formation of terminal α-1,2-fucose residues, were identified in Arabidopsis thaliana (34). The results of all these studies raise the possibility of HBGA-like carbohydrates being present in lettuce tissues, to which HuNoVs potentially bind. Our data show that H-type HBGA-like carbohydrates on lettuce leaves can be detected by monoclonal antibodies to the human HBGAs and that preincubation of these antibodies with lettuce leaves reduces NoV VLP binding. In addition, fucose in HBGA-like carbohydrates might also play a main role in HuNoV/GII.4 attachment, because lectin UEA-I against fucose and α-1,2-fucosidase can significantly inhibit the binding.
Fruit and vegetables, such as raspberries and romaine lettuce, are being recognized as sources of HuNoV transmission (10, 19). Besides romaine lettuce, the binding between HuNoV VLPs and several other green leaf vegetables, such as red leaf lettuce (Lactuca sativa L.), celery (Apium graveolens), and basil (Ocimum basilicum), was also investigated. HuNoV/GII.4 VLPs bound to both lettuce and celery veins after digestion with cellulase R-10. These results suggest that HuNoV bound not only specifically to lettuce but also to other plants. Moreover, HuNoV/GII.4 VLPs could not bind to basil leaves, even after cellulase R-10 digestion. Whether basil leaves contain H-type HBGA carbohydrates or bind to other genogroups of HuNoVs needs to be further investigated.
Hemicelluloses are heterogeneous cell wall polysaccharides that are characterized as having β-(1-4)-linked backbones of glucose, xylose, or mannose. Xylan, a group of hemicelluloses with β-(1-4)-linked xylose residues, is the dominant noncellulosic polysaccharide in the secondary wall of dicots (35). Plant fucosyltransferases can transfer fucose to xyloglucan in cell walls as part of the hemicelluloses with α-Fuc-(1-2)-β-Gal linkage (36). Our results confirm the binding between lettuce hemicellulose of the cell wall and NoV VLPs. Pretreatment with cell wall digestion enzymes enhanced the binding, while α-1,2-fucosidase inhibited it. These results and the immunofluorescence microscopy data suggest that H-type HBGA-like carbohydrates present in lettuce tissue and α-1,2-fucose residues are involved in HuNoV VLP binding.
The epidermis of plant leaves is usually covered with a protective cuticle, which consists of lipid and hydrocarbon polymers impregnated with wax that is synthesized by the epidermal cells. This extracellular hydrophobic layer prevents water loss and provides protection against both external environmental stresses (physical damages, etc.) and biotic stress (virus and bacterium penetration, etc.) (20). We reported previously that the cut edges and injured surfaces of lettuce leaves had the highest level of HuNoV VLP binding signal (19). This was probably due to the damaged cuticle of those areas on the leaf surface. Therefore, to detect the HBGA-like carbohydrates on fresh lettuce leaves, we first peeled away the cuticle layer on the leaf surfaces to expose the cell wall. Bacteria and fungi on plant surfaces secrete enzymes to depolymerize the main structural polysaccharide components of the plant cell wall, and these enzymes can be used to decipher the carbohydrate structure (37–39). We found significantly increased HuNoV VLP binding to lettuce after digestion with several commercial cell wall-digesting enzymes from fungi. Cellulase R-10, a multienzymatic system from the fungus Trichoderma viride, contains cellulase, pectinase, and hemicellulase activity. Combined with macerozyme R-10, it was used in the preparation of plant protoplasts (40). In this study, the purified cellulase from the fungus Trichoderma viride exposed HuNoV VLP binding sites on lettuce, and such binding was inhibited by HBGA antibodies. These data suggest that lettuce HBGA-like carbohydrates can be exposed after cellulase digestion. Several ready-to-eat fresh vegetables, including lettuce, can be contaminated by the mold Aspergillus flavus, resulting in a decay process, such as slime formation and loss of firmness (41). These decay processes are accompanied by cell wall degradation, which may also increase the chance of NoV binding and enhance virus persistence and transmission. Moreover, GII.4 HuNoV can infect human B cells with the help of either HBGA-expressing bacteria or synthesized free HBGA (15). The human gastrointestinal tract harbors extremely dense microbial communities, and these regions are also the sites of active microbial breakdown of dietary plant polysaccharides (42). Commensal microbes determined if enteric murine norovirus infections were persistent, while antibiotic treatment prevented persistent murine norovirus infections (43). Therefore, polysaccharide metabolites in the human gut, such as HBGA-like carbohydrates from bacteria and plant cell walls, may play a role in norovirus infection. Antibiotics may prevent certain viral infections in the intestine by inhibiting the growth of gut bacteria, which can express HBGA and digest the plant cell wall.
Noroviruses have been classified into five genogroups (GI–GV) based on the similarity of complete capsid (VP1) sequences (2). However, GII.4 NoVs currently account for most of the HuNoV outbreaks (4). Besides GII.4/HS194 VLPs, two other GII.4 VLPs of 1997 and 2004 strains showed positive binding signals, whereas GII.12/HS206 did not. Our results confirm the specific binding between lettuce cell wall and the Hu/NoV/GII.4 strains.
In conclusion, we have demonstrated the presence of HBGA-like carbohydrates in romaine lettuce leaves and the fact that fucose was responsible for NoV VLP recognition. Also, HBGA-like carbohydrates were distributed mainly in the hemicellulose fraction of the lettuce cell wall polysaccharides. Since current food-processing technologies rely on simple washing to remove pathogens adsorbed to the surface of leaves, specifically bound viruses require more robust technologies to remove or inactivate them. Knowledge about the mechanisms of NoV binding to the surface of ready-to-eat vegetables is of paramount importance for developing better technologies to control NoV transmission through vegetables.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ralph Baric of the University of North Carolina for providing NoV/GII.4/1997 and NoV/GII.4/2004 VLPs and the corresponding hyperimmune serum samples. We thank Susan Sommer-Wagner for her editing of the manuscript.
Funding Statement
Salaries and research support were provided by state and federal funds provided to the Ohio Agricultural Research and Development Center (OARDC), The Ohio State University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04096-15.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. 2014. Advances in norovirus biology. Cell Host Microbe 15:668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall AJ, Wikswo ME, Pringle K, Gould LH, Parashar UD. 2014. Vital signs: foodborne norovirus outbreaks—United States, 2009–2012. Morb Mortal Wkly Rep 63:491–495. [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. 2008. Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev 225:190–211. doi: 10.1111/j.1600-065X.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 5.Teunis PF, Moe CL, Liu P, Miller ES, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. 2008. Norwalk virus: how infectious is it? J Med Virol 80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 6.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Ramani S, Hill H, Ferreira J, Graham DY. 2014. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webby RJ, Carville KS, Kirk MD, Greening G, Ratcliff RM, Crerar SK, Dempsey K, Sarna M, Stafford R, Patel M, Hall G. 2007. Internationally distributed frozen oyster meat causing multiple outbreaks of norovirus infection in Australia. Clin Infect Dis 44:1026–1031. doi: 10.1086/512807. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Zhang Z, Saif LJ. 2012. Stability of and attachment to lettuce by a culturable porcine sapovirus surrogate for human caliciviruses. Appl Environ Microbiol 78:3932–3940. doi: 10.1128/AEM.06600-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, Frankel G. 2010. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol 12:2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 10.Le Guyader FS, Mittelholzer C, Haugarreau L, Hedlund KO, Alsterlund R, Pommepuy M, Svensson L. 2004. Detection of noroviruses in raspberries associated with a gastroenteritis outbreak. Int J Food Microbiol 97:179–186. doi: 10.1016/j.ijfoodmicro.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Mäde D, Trübner K, Neubert E, Höhne M, Johne R. 2013. Detection and typing of norovirus from frozen strawberries involved in a large-scale gastroenteritis outbreak in Germany. Food Environ Virol 5:162–168. doi: 10.1007/s12560-013-9118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ethelberg S, Lisby M, Bottiger B, Schultz AC, Villif A, Jensen T, Olsen KE, Scheutz F, Kjelso C, Muller L. 2010. Outbreaks of gastroenteritis linked to lettuce, Denmark, January 2010. Euro Surveill 15:pii=19484 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19484. [PubMed] [Google Scholar]
- 13.da Silva AK, Le Saux JC, Parnaudeau S, Pommepuy M, Elimelech M, Le Guyader FS. 2007. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl Environ Microbiol 73:7891–7897. doi: 10.1128/AEM.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Jin Y, Sims T, Kniel KE. 2010. Manure- and biosolids-resident murine norovirus 1 attachment to and internalization by romaine lettuce. Appl Environ Microbiol 76:578–583. doi: 10.1128/AEM.02088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinjé J, Tibbetts SA, Wallet SM, Karst SM. 2014. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiCaprio E, Purgianto A, Ma Y, Hughes J, Dai X, Li J. 2015. Attachment and localization of human norovirus and animal caliciviruses in fresh produce. Int J Food Microbiol 211:101–108. doi: 10.1016/j.ijfoodmicro.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Vega E, Garland J, Pillai SD. 2008. Electrostatic forces control nonspecific virus attachment to lettuce. J Food Prot 71:522–529. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi KM, Mandrell RE, Tian P. 2010. Binding of virus-like particles of Norwalk virus to romaine lettuce veins. Appl Environ Microbiol 76:7997–8003. doi: 10.1128/AEM.01566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esseili MA, Wang Q, Saif LJ. 2012. Binding of human GII.4 norovirus virus-like particles to carbohydrates of romaine lettuce leaf cell wall materials. Appl Environ Microbiol 78:786–794. doi: 10.1128/AEM.07081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeats TH, Rose JK. 2013. The formation and function of plant cuticles. Plant Physiol 163:5–20. doi: 10.1104/pp.113.222737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerouxel O, Cavalier DM, Liepman AH, Keegstra K. 2006. Biosynthesis of plant cell wall polysaccharides—a complex process. Curr Opin Plant Biol 9:621–630. doi: 10.1016/j.pbi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Hutson AM, Atmar RL, Graham DY, Estes MK. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis 185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 23.Cheetham S, Souza M, McGregor R, Meulia T, Wang Q, Saif LJ. 2007. Binding patterns of human norovirus-like particles to buccal and intestinal tissues of gnotobiotic pigs in relation to A/H histo-blood group antigen expression. J Virol 81:3535–3544. doi: 10.1128/JVI.01306-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian P, Bates AH, Jensen HM, Mandrell RE. 2006. Norovirus binds to blood group A-like antigens in oyster gastrointestinal cells. Lett Appl Microbiol 43:645–651. doi: 10.1111/j.1472-765X.2006.02010.x. [DOI] [PubMed] [Google Scholar]
- 25.Jung K, Wang Q, Kim Y, Scheuer K, Zhang Z, Shen Q, Chang KO, Saif LJ. 2012. The effects of simvastatin or interferon-α on infectivity of human norovirus using a gnotobiotic pig model for the study of antivirals. PLoS One 7:e41619. doi: 10.1371/journal.pone.0041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takanashi S, Wang Q, Chen N, Shen Q, Jung K, Zhang Z, Yokoyama M, Lindesmith LC, Baric RS, Saif LJ. 2011. Characterization of emerging GII.g/GII.12 noroviruses from a gastroenteritis outbreak in the United States in 2010. J Clin Microbiol 49:3234–3244. doi: 10.1128/JCM.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chimphango AF, van Zyl WH, Görgens JF. 2012. Isolation, characterization and enzymatic modification of water soluble xylans from Eucalyptus grandis wood and sugarcane bagasse. J Chem Technol Biotechnol 87:1419–1429. [Google Scholar]
- 28.Tan M, Jiang X. 2014. Histo-blood group antigens: a common niche for norovirus and rotavirus. Expert Rev Mol Med 16:e5. doi: 10.1017/erm.2014.2. [DOI] [PubMed] [Google Scholar]
- 29.Ravn V, Dabelsteen E. 2000. Tissue distribution of histo-blood group antigens. APMIS 108:1–28. doi: 10.1034/j.1600-0463.2000.d01-1.x. [DOI] [PubMed] [Google Scholar]
- 30.Le Guyader FS, Loisy F, Atmar RL. 2006. Norwalk virus–specific binding to oyster digestive tissues. Emerg Infect Dis 12:931–936. doi: 10.3201/eid1206.051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura T, Sano D, Suenaga A, Yoshimura T, Fuzawa M, Nakagomi T, Nakagomi O, Okabe S. 2013. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J Virol 87:9441–9451. doi: 10.1128/JVI.01060-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan M, Jiang X. 2011. Norovirus-host interaction: multi-selections by human histo-blood group antigens. Trends Microbiol 19:382–388. doi: 10.1016/j.tim.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitchette-Lainé AC, Gomord V, Cabanes M, Michalski JC, Saint Macary M, Foucher B, Cavelier B, Hawes C, Lerouge P, Faye L. 1997. N-Glycans harboring the Lewis a epitope are expressed at the surface of plant cells. Plant J 12:1411–1417. doi: 10.1046/j.1365-313x.1997.12061411.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Williams M, Bernard S, Driouich A, Showalter AM, Faik A. 2010. Functional identification of two nonredundant Arabidopsis alpha(1,2)fucosyltransferases specific to arabinogalactan proteins. J Biol Chem 285:13638–13645. doi: 10.1074/jbc.M110.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheller HV, Ulvskov P. 2010. Hemicelluloses. Annu Rev Plant Biol 61:263–289. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 36.Perrin RM, DeRocher AE, Bar-Peled M, Zeng W, Norambuena L, Orellana A, Raikhel NV, Keegstra K. 1999. Xyloglucan fucosyltransferase, an enzyme involved in plant cell wall biosynthesis. Science 284:1976–1979. doi: 10.1126/science.284.5422.1976. [DOI] [PubMed] [Google Scholar]
- 37.Kubicek CP, Starr TL, Glass NL. 2014. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol 52:427–451. doi: 10.1146/annurev-phyto-102313-045831. [DOI] [PubMed] [Google Scholar]
- 38.De Vries R, Visser J. 2001. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev 65:497–522. doi: 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esseili MA, Chin A, Saif L, Miller SA, Qu F, Lewis Ivey ML, Wang Q. 2015. Postharvest survival of porcine sapovirus, a human norovirus surrogate, on phytopathogen-infected leafy greens. J Food Prot 78:1472–1480. doi: 10.4315/0362-028X.JFP-14-518. [DOI] [PubMed] [Google Scholar]
- 40.Wu FH, Shen SC, Lee LY, Lee SH, Chan MT, Lin CS. 2009. Tape-Arabidopsis Sandwich—a simpler Arabidopsis protoplast isolation method. Plant Methods 5:16. doi: 10.1186/1746-4811-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corato U. 2012. Fungal population dynamics in ready-to-eat salads during a shelf-life in Italy. J Agric Sci Technol A 2:569–576. [Google Scholar]
- 42.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 43.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. 2015. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science 347:266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman M, Jia Z, Peña MJ, Cash M, Harper A, Blackburn AR II, Darvill A, York WS. 2005. Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydr Res 340:1826–1840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.