Abstract
Dexamethasone treatment of newborn rats inhibited cardiomyocyte proliferation and stimulated premature terminal differentiation of cardiomyocytes in the developing heart. Yet mechanisms remain undetermined. The present study tested the hypothesis that the direct effect of glucocorticoid receptor-mediated epigenetic repression of cyclin D2 gene in the cardiomyocyte plays a key role in the dexamethasone-mediated effects in the developing heart. Cardiomyocytes were isolated from 2-day-old rats. Cells were stained with a cardiomyocyte marker α-actinin and a proliferation marker Ki67. Cyclin D2 expression was evaluated by Western blot and quantitative real-time polymerase chain reaction. Promoter methylation of CcnD2 was determined by methylated DNA immunoprecipitation (MeDIP). Overexpression of Cyclin D2 was conducted by transfection of FlexiCcnD2 (+CcnD2) construct. Treatment of cardiomyocytes isolated from newborn rats with dexamethasone for 48 hours significantly inhibited cardiomyocyte proliferation with increased binucleation and decreased cyclin D2 protein abundance. These effects were blocked with Ru486 (mifepristone). In addition, the dexamethasone treatment significantly increased cyclin D2 gene promoter methylation in newborn rat cardiomyocytes. 5-Aza-2'-deoxycytidine inhibited dexamethasone-mediated promoter methylation, recovered dexamethasone-induced cyclin D2 gene repression, and blocked the dexamethasone-elicited effects on cardiomyocyte proliferation and binucleation. In addition, the overexpression of cyclin D2 restored the dexamethasone-mediated inhibition of proliferation and increase in binucleation in newborn rat cardiomyocytes. The results demonstrate that dexamethasone acting on glucocorticoid receptors has a direct effect and inhibits proliferation and stimulates premature terminal differentiation of cardiomyocytes in the developing heart via epigenetic repression of cyclin D2 gene.
Introduction
Dexamethasone is used to treat preterm infants and mothers at risk of preterm birth to reduce the incidence and severity of respiratory distress syndrome (Liggins and Howie, 1972; NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes, 1995). Yet synthetic glucocorticoid exposure may be harmful to other tissues and organs (Ortiz et al., 2003; Shoener et al., 2006; Kamphuis et al., 2007; Bal et al., 2008; Davis et al., 2011; Kelly et al., 2012). Recently, we demonstrated that treatment of newborn rats with dexamethasone during a critical window of the heart development inhibited cardiomyocyte proliferation, stimulated premature cardiomyocyte binucleation, and reduced the total cardiomyocyte number in the heart (Gay et al., 2015). These findings provided new insights in the regulation of cardiomyocyte maturation by glucocorticoids, yet the underlying mechanisms remain largely elusive.
During the heart development cardiomyocyte growth occurs in two phases, hyperplasia and hypertrophy (Li et al., 1996; Poolman and Brooks, 1998). Early cardiac growth is by hyperplasia, in which cardiomyocytes proliferate and endow the heart with adequate amount of myocytes. In rodents during late gestation and within the first 2 weeks of life, cardiomyocyte proliferative growth is progressively replaced by hypertrophic growth as myocytes exit the cell cycle and lose the ability to divide, resulting in binucleated cells (Clubb and Bishop, 1984; Li et al., 1996). As binucleation is occurring, the expression of genes for mitosis, cytokinesis, and cell cycle reentry declines, resulting in loss of the proliferative capacity (Brooks et al., 1997, 1998; Kang and Koh, 1997). The critical widow during the heart development when myocyte proliferation is still possible is therefore an especially influential time on the cardiomyocyte developmental trajectory.
Although much is still unknown about the mechanisms underlying the transition of cardiomyocytes from proliferative to terminally differentiated binucleation, many studies have been focused on molecules involved in cell cycle regulation and cytokinesis, as well as epigenetic modifications (Engel et al., 2006; Kou et al., 2010; Liu et al., 2010; Di Stefano et al., 2011). Cyclin D2 is a cell cycle promoter that plays an important role in the regulation of cardiomyocyte proliferation and terminal differentiation (McGill and Brooks, 1995; Brooks et al., 1997; Poolman and Brooks, 1998; Nagai et al., 2001; Paradis et al., 2014). Glucocorticoids are known to influence the cell cycle and proliferation in a variety of cell types including the heart (de Vries et al., 2006; Sundberg et al., 2006; Bird et al., 2007). Of importance, cyclin D proteins are established targets of glucocorticoids (Fernandes et al., 1999; Sundberg et al., 2006; Gay et al., 2015). In rodent hearts, we demonstrated that hypoxia and dexamethasone treatments significantly decreased cyclin D2 protein abundance (Tong et al., 2013; Gay et al., 2015; Paradis et al., 2015), suggesting a role of cyclin D2 in dexamethasone-induced inhibition of cardiomyocyte proliferation in the developing heart. In the present study, we sought to test the hypothesis that dexamethasone has a direct effect on newborn rat cardiomyocytes in repressing the cyclin D2 gene via increasing promoter methylation, and the downregulation of cyclin D2 expression plays a causal role in dexamethasone-mediated transition of cardiomyocyte proliferation to terminal differentiation in the developing heart.
Methods and Materials
Experimental Animals.
All procedures and protocols in the present study were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Time-dated pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI). Postnatal day 2 pups were anesthetized using isoflurane and hearts were removed for isolation of cardiomyocytes. The adequacy of anesthesia was monitored by foot withdrawal reflex.
Cardiomyocyte Isolation and Culture.
Cardiomyocytes were isolated from hearts by enzymatic digestion (0.1% trypsin and 0.5 mg/ml type II collagenase), as previously described (Xiao et al., 2000). Cells were cultured in Hyclone media 199 (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Gemini Bio-Products, Sacramento, CA) and 1% antibiotics (10,000 IU/ml penicillin, 10,000 μg/ml streptomycin) at 37°C in 95% air/5% CO2. Myocytes were plated on 6-well plates, some wells contained poly-l-lysine (Sigma, St. Louis, MO)-coated coverslips. The seeding density was about 0.67 × 106 cells per well. 5-Bromo-2′-deoxyuridine was used to enrich the population of cardiomyocytes by limiting the proliferation of nonmyocytes (Xiao et al., 2000). After 24 hours of attachment, the media was changed and replaced with Hyclone media 199 containing 5-bromo-2′-deoxyuridine (0.1 mM, Sigma) and allowed to grow for 24 hours.
Cell Treatments.
Cardiomyocytes were treated for 48 hours with dexamethasone (100 nM; Sigma) or media alone in the presence or absence of glucocorticoid receptor inhibitor Ru486 (1 μM; Sigma). In addition, a methylation inhibitor, 5-aza-2'-deoxycytidine (5-AZA, 10 μM; Sigma) was used to determine the effect of DNA methylation in dexamethasone-mediated effects. Thus, there were six treatment groups: 1) control, 2) dexamethasone, 3) Ru486, 4) Ru486+dexamethasone, 5) 5-AZA, and 6) 5-AZA+dexamethasone.
Immunocytochemistry.
After treatments, cells were washed twice with phosphate-buffered saline and fixed with paraformaldehyde (3.7%) followed by permeabilization with Triton X-100 (0.5%). Cells were then incubated with 1% bovine serum albumin for 1 hour, followed by incubation with primary antibodies for 1 hour at room temperature, mouse anti-α actinin (1:200; Sigma), and rabbit anti-Ki-67 (1:100 Abcam, Cambridge, MA). Next, cells were incubated for 1 hour at room temperature with secondary anti-rabbit Alexa Fluor 647 conjugated antibody (1:400; Life Technologies, Carlsbad, CA) and anti-mouse Alexa Flour 488 conjugated antibody (1:400; Life Technologies). The nuclei were stained with Hoechst. A Zeiss Axio imager (Carl Zeiss AG, Germany) was used for all immunofluorescent imaging and the analysis was performed using Image J software (https://imagej.nih.gov/ij/).
Western Immunoblotting.
After treatments, cardiomyocytes were washed with phosphate-buffered saline, and RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA) was added to each well on ice. Cells were scraped and total protein was determined using BCA protein assay (Thermo Fisher Scientific). Equal amounts of protein were loaded onto a 10% polyacrylamide gel with 0.1% sodium dodecyl sulfate (SDS). Samples were transferred to polyvinylidene difluoride membrane and were blocked with milk for 1 hour at room temperature. Membranes were incubated with anti-cyclin D2 (1:1000, Abcam) overnight at 4°C followed by washing. Secondary antibody was applied for 1 hour at room temperature. Proteins were visualized with Western blot chemiluminescence reagent and hyperfilm. β-Actin was used as the housekeeping protein to assure equal loading.
Real-time Polymerase Chain Reaction.
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) extraction protocol. SuperScript III first strand synthesis system for reverse transcription-polymerase chain reaction (PCR; Invitrogen) was used for reverse transcription of cDNA. The abundance of cyclin D2 mRNA was determined by quantitative real-time PCR with IQ5 (BioRad, Irvine, CA) using 2× SYBR green PCR mix (Biotool, Houston, Texas). The primer sequences for cyclin D2 mRNA quantification were forward: 5′-cctcacgacttcattgagca and reverse: 5′-ggtagcacacagagcgatga. Each PCR was of 25 µl volume and contained 500 nM each primer, 1× SYBR green master mix and nuclease free water (Qiagen, Valencia, CA). The real time-PCR protocol was 95°C for 10 minutes, followed by 40 cycles of 95°C for 10 seconds, anneal at 56°C for 15 seconds, extension 72°C for 15 seconds. All samples were analyzed in triplicate and threshold cycle number for each sample (Ct) was averaged. Real-time quantitation was done by 2−△△Ct method, as described by Livak and Schmittgen (2001).
Methylated DNA Immunoprecipitation Assay.
Rat cyclin D2 gene (CcnD2) is located on rat chromosome 4 (http://www.ncbi.nlm.nih.gov/gene/64033). To assess if dexamethasone induced methylation of this promoter, a 920-bp proximal promoter region of CcnD2 was evaluated by MeDIP assay using the MeDIP kit (Active Motif). Briefly, genomic DNA was purified from rat cardiomyocytes using Flexi Gene DNA Kit (Qiagen). Twenty micrograms genomic DNA from each sample were fragmented to 200–600 bp range and confirmed by 2% agarose gel electrophoresis. Eight hundred nanograms of each fragmented DNA was next denatured at 95°C and subjected to immunoprecipitation. Briefly, methylated DNA fragments were pulled down and purified by 5-methylcytosine (5-mC) antibody and protein G magnetic beads (Active Motif). The immunoprecipitated DNA fragments were phenol-chloroform-isoamyl alcohol extracted and ethanol precipitated along with 20 µl glycogen as inert carrier. The precipitate was washed, dried, and reconstituted in 50 µl of 10 mM Tris-HCl buffer, pH 7.5. Two microliters of the 5-mC antibody-enriched DNA fragments were next subjected to quantitative real-time PCR with IQ5 (BioRad) using four pairs of primers targeting on the CcnD2 promoter: zone 1 (most proximal), forward: 5′-gcgaagcagttcagagggaagg, reverse: 5′-cagcacagcagctccatagc; zone 2, forward: 5′-tatccggggcccctagcatg, reverse: 5′-ccttccctctgaactgcttcgc; zone 3, forward: 5′-cttattcttccttcaggggtc, reverse: 5′-aaccctcaaaacccacgg; zone 4 (most distal), forward: 5′-ttccgcacgagggtcatatt, reverse: 5′-aagggaaggctgatttgagaa. Parallel to the enrichment of 5-mC antibody-pulled DNA fragments, a second aliquot of each fragmented genomic DNA sample (80 ng) were first heat denatured for 10 minutes, processed the same way as the immunoprecipitated DNA without 5-mC antibody/protein G to generate the input DNA and was in parallel subjected to quantitative real-time PCR using the same PCR primers. In each 5-mC antibody-enriched DNA sample, the abundance of methylated DNA was first calculated as fold of corresponding input DNA and expressed as % of control samples.
Overexpression of Cyclin D2.
Total RNA was isolated from the rat heart by Trizol reagent (Invitrogen) and cDNA was synthesized using SuperScript III first strand synthesis system for reverse transcription-PCR (Invitrogen). By using 100 ng of cDNA as template, the protein-coding domain of rat cyclin D2 (Genbank accession number: NM_022267) was PCR amplified as an 898-bp amplicon, with the help of following PCR primers: forward: 5′-gagacccgcgatcgccatggagctgctgtgctgtgagg, reverse: 5′-gagacccgtttaaactcacaggtcaacatcccgcacg. To facilitate cloning, the forward and reverse PCR primers, respectively, contained SgfI (isoschizomer: AsiSI was used) and PmeI cloning sites (bold). The 898-bp CcnD2 amplicon was next cloned in pF4A CMV Flexi vector (Promega, Madison, WI) between SgfI (5′) and Pme I (3′) orientation and sequence was confirmed. This CcnD2 overexpressing clone was named FlexiCcnD2. To generate corresponding negative control (for transfection experiments), the pF4A CMV Flexi vector was double digested with above two restriction enzymes to remove the cytotoxic Barnase sequence, blunt-ended, 5′-phosphorylated using the Quick blunting kit (New England Biolab, Ipswich, MA), and blunt end ligated with T4 DNA ligase enzyme. These constructs, namely, FlexiCcnD2 and Flexi empty construct were transfected into H9C2 cells using the X-treme GENE HP DNA Transfection reagent (Roche, Indianapolis, IN) for 24 hours followed by recovery for another 24 hours in fresh medium without transfection reagent. Whole cell lysates in RIPA buffer containing protease inhibitors were next made and protein concentration was determined. Fifty micrograms total proteins was electrophoresed by SDS-PAGE, followed by immunoblotting with rat cyclin D2 antibody (Abcam). In subsequent experiments, the same CcnD2 expression constructs were used to successfully overexpress CcnD2 in isolated neonatal rat cardiomyocytes.
5-mC DNA Enzyme-Linked Immunosorbent Assay.
Global CpG methylation in cardiomyocytes was determined with measuring 5-methylcytosine (5-mC) using a 5-mC DNA enzyme-linked immunosorbent assay (ELISA) kit (Zymo Research, Irvine, CA). The kit features a unique anti-5-mC monoclonal antibody that is both sensitive and specific for 5-mC. The protocol for measurement of 5-mC level is described in the manufacturer’s instruction. Briefly, 100 ng of genomic DNA from cardiomyocytes and standard controls provided by the kit were denatured and used to coat the plate wells with 5-mC coating buffer. After incubation at 37°C for 1 hour, the wells were washed with 5-mC ELISA buffer, and then an antibody mix consisting of anti-5-mC and a secondary antibody was added to each well. The plate was covered with foil and incubated at 37°C for 1 hour. After washing out the antibody mix from the wells with 5-mC ELISA buffer, a horseradish peroxidase developer was added to each well and incubated at room temperature for 1 hour. The absorbance at 405 nm was measured using an ELISA plate reader. The percent 5-mC was calculated using the second-order regression equation of the standard curve that was constructed with negative control and positive controls in the same experiment.
Statistical Analysis.
Data are expressed as means ± S.E.M. and analyzed using GraphPad Prism (GraphPad Software, San Diego, CA). Experimental number (n) represents animals from different dams. Statistical significance (P < 0.05) was determined by analysis of variance followed by Neuman-Keuls post hoc testing or Student's t test, where appropriate.
Results
Dexamethasone Inhibited Proliferation and Increased Binucleation in Newborn Rat Cardiomyocytes.
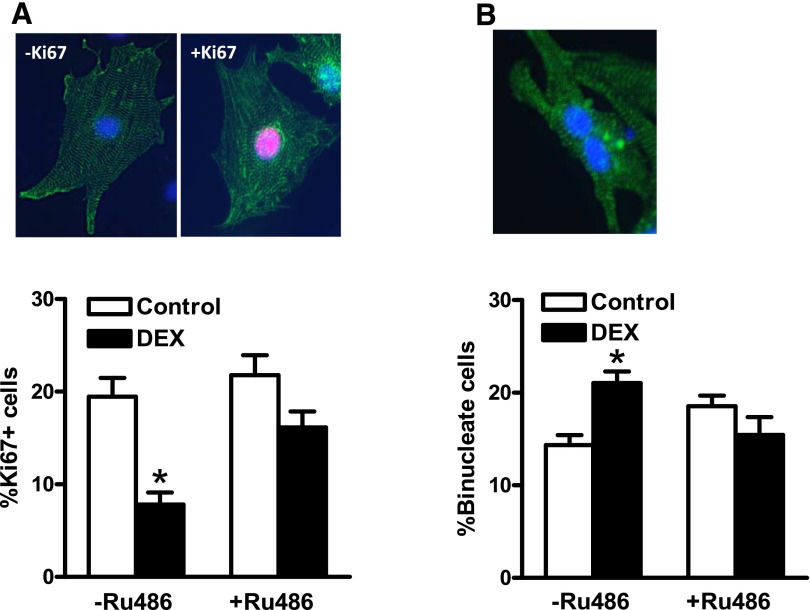
The direct effect of dexamethasone on cardiomyocyte proliferation and binucleation was determined by immunocytochemistry staining with a cardiomyocyte marker α-actinin and Ki67, a marker of proliferation. As shown in Fig. 1A, dexamethasone treatment significantly decreased Ki67 positive cardiomyocytes, which was blocked by Ru486, a glucocorticoid receptor antagonist. We further evaluated the effect of dexamethasone treatment on cardiomyocyte binucleation and terminal differentiation. As shown in Fig. 1B, dexamethasone induced an approximate 50% increase in binucleated cardiomyocytes, and Ru486 reversed the dexamethasone-mediated effect. These findings suggest a direct effect of dexamethasone in increasing the transition of cardiomyocyte proliferation to terminal differentiation in a glucocorticoid receptor-dependent manner.
Fig. 1.
Dexamethasone decreased proliferation and increased binucleation in newborn cardiomyocytes. Cardiomyocytes were isolated from 2-day-old rats and treated with dexamethasone (DEX) in the absence or presence of Ru486 for 48 hours. Cells were then fixed and stained with Ki67 and α-actinin. The nuclei were stained with Hoechst. (A) Ki67 positive cardiomyocytes. (B) Binucleate cardiomyocytes. Data are mean ± S.E.M., n = 7–9. *P < 0.05, DEX versus Control.
Dexamethasone Downregulated Cyclin D2 Expression in Newborn Rat Cardiomyocytes.
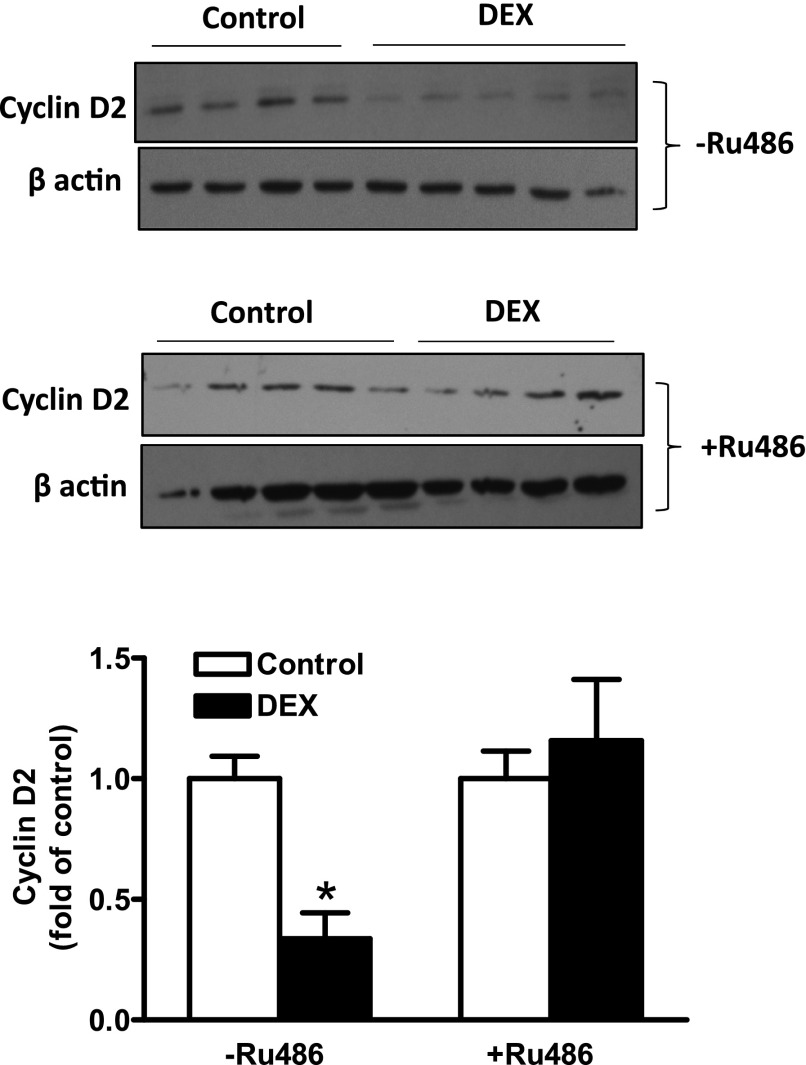
Cyclin D2 is a protein that is implicated in cardiomyocyte proliferation G1 phase progression. We therefore evaluated whether dexamethasone downregulated cyclin D2 expression in newborn rat cardiomyocytes. Consistent with the decrease in proliferating cardiomyocytes, dexamethasone treatment induced a 65% decline in cyclin D2 protein abundance in cardiomyocytes (Fig. 2). To confirm the involvement of the glucocorticoid receptor, cardiomyocytes were treated with dexamethasone in the presence of Ru486. As shown in Fig. 2, Ru486 blocked the effect of dexamethasone and restored cyclin D2 protein expression, further implicating the glucocorticoid receptor in the dexamethasone-mediated effects.
Fig. 2.
Dexamethasone downregulated cyclin D2 protein abundance in newborn cardiomyocytes. Cardiomyocytes were isolated from 2-day-old rats and treated with dexamethasone (DEX) in the absence or presence of Ru486 for 48 hours. Cyclin D2 protein abundance was determined by Western blot. Data are mean ± S.E.M., n = 4 or 5, from different experiments. *P < 0.05, DEX versus Control.
Dexamethasone Increased Promoter Methylation in the CcnD2 Gene in Newborn Rat Cardiomyocytes.
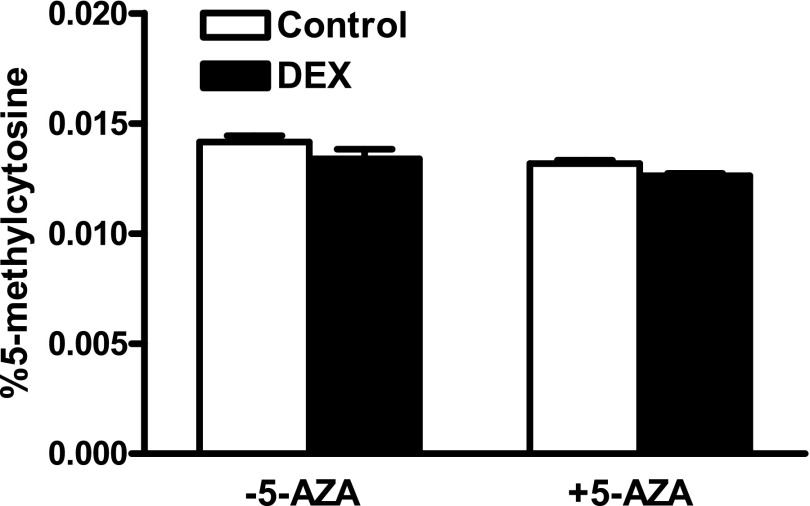
Dexamethasone treatment had no significant effect on global DNA methylation in cardiomyocytes (Fig. 3). We further evaluated the effect of dexamethasone on promoter methylation of the CcnD2 gene. The proximal CcnD2 gene promoter was divided into four regions denoted as zones based on the proximity to the transcription start site. The dexamethasone treatment significantly increased methylation in the two zones most proximal to the transcription start site, zones 1 and 2, which was blocked by 5-AZA (Fig. 4).
Fig. 3.
Effect of dexamethasone on global methylation in newborn cardiomyocytes. Cardiomyocytes were isolated from 2-day-old rats and treated with dexamethasone (DEX) in the absence or presence of 5-aza-2'-deoxycytidine (5-AZA) for 48 hours. Genomic DNA was extracted and 5-methylcytosine was determined using a 5-mC ELISA kit. Data are mean ± S.E.M., n = 4.
Fig. 4.
Dexamethasone increased cyclin D2 promoter methylation in newborn cardiomyocytes. Cardiomyocytes were isolated from 2-day-old rats and treated with dexamethasone (DEX) in the absence or presence of 5-aza-2'-deoxycytidine (5-AZA) for 48 hours. Genomic DNA was extracted and 5-methylcytosine in CcnD2 proximal promoter was determined by MeDIP assays. Data are mean ± S.E.M., n = 4. *P < 0.05, DEX versus control.
5-AZA Reversed Dexamethasone-Induced Downregulation of Cyclin D2 Expression, Decrease in Proliferation, and Increase in Binucleation in Newborn Rat Cardiomyocytes.
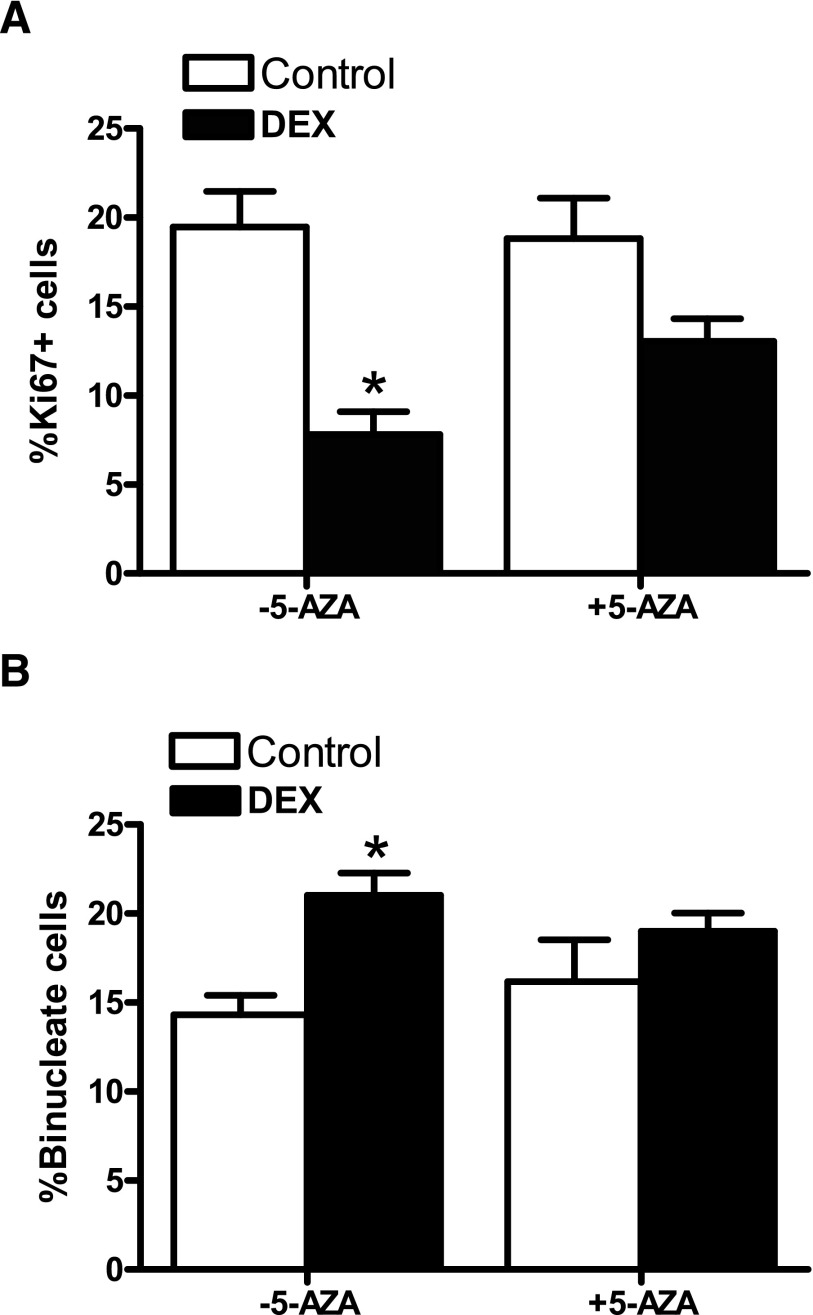
To establish whether methylation plays a causal role in the dexamethasone-induced reduction of cyclin D2 expression in cardiomyocytes, we evaluated the effect of 5-AZA treatment on cyclin D2 protein and mRNA abundance. As shown in Fig. 5, 5-AZA blocked the dexamethasone-mediated effects and restored cyclin D2 protein (Fig. 5A) and mRNA (Fig. 5B) abundance. The functional significance of restoration of cyclin D2 by 5-AZA was determined by examining cardiomyocyte proliferation and binucleation. As shown in Fig. 6, 5-AZA alone had no significant effect on cardiomyocyte proliferation and binucleation but blocked dexamethasone-induced decrease in proliferation and increase in binucleation in newborn rat cardiomyocytes.
Fig. 5.
5-AZA blocked dexamethasone-induced downregulation of cyclin D2 protein and mRNA abundance in newborn cardiomyocytes. Cardiomyocytes were isolated from 2-day-old rats and treated with dexamethasone (DEX) in the absence or presence of 5-aza-2'-deoxycytidine (5-AZA) for 48 hours. Cyclin D2 protein and mRNA abundance was determined by Western blot and quantitative real-time PCR, respectively. Data are mean ± S.E.M., n = 4 or 5, from different experiments. *P < 0.05, DEX versus Control.
Fig. 6.
5-AZA inhibited dexamethasone-induced terminal differentiation in newborn cardiomyocytes. Cardiomyocytes were isolated from 2-day-old rats and treated with dexamethasone (DEX) in the absence or presence of 5-aza-2'-deoxycytidine (5-AZA) for 48 hours. Cells were then fixed and stained with Ki67 and α-actinin. The nuclei were stained with Hoechst. (A) Ki67 positive cardiomyocytes. (B) Binucleate cardiomyocytes. Data are mean ± S.E.M., n = 6–9. * P < 0.05, DEX versus Control.
Overexpression of Cyclin D2 Rescued Dexamethasone-Mediated Decrease in Proliferation and Increase in Binucleation in Newborn Rat Cardiomyocytes.
H9C2 cells were transfected for 24 hours with either Flexi-empty (-CcnD2, control) or Flexi-CcnD2 (+CcnD2, S and M) constructs. As shown in Fig. 7, robust expression of cyclin D2 protein was demonstrated in H9C2 cells transfected with FlexiCcnD2 (+CcnD2, S and M). To further establish a cause and effect relation between dexamethasone-induced reduction of cyclin D2 expression and the transition of cardiomyocytes from proliferation to terminal differentiation of binucleation, we overexpressed the cyclin D2 gene in newborn rat cardiomyocytes. As shown in Fig. 8, dexamethasone significantly decreased cyclin D2 protein in cardiomyocytes transfected with an empty vector Flexi-empty (−CCnD2). Cardiomyocytes transfected with Flexi-CcnD2 expression vector (+CCnD2) expressed about threefold more cyclin D2 protein, which was not affected by dexamethasone treatment (Fig. 8). As shown in Fig. 9, similar to the findings above, in Flexi-empty (−CCnD2)-transfected cardiomyocytes, dexamethasone significantly decreased proliferation (Fig. 9A) and increased binucleation (Fig. 9B). Of importance, in Flexi-CcnD2 expression vector (+CcnD2)-transfected cardiomyocytes, dexamethasone had no significant effect on cardiomyocyte proliferation and binucleation (Fig. 9), suggesting that overexpression of cyclin D2 protein in cardiomyocytes is sufficient to rescue dexamethasone-mediated effects on proliferation and binucleation, and thus demonstrating a causal role of reduced cyclin D2 in dexamethasone-induced transition of proliferation to terminal differentiation of binucleation in newborn rat cardiomyocytes.
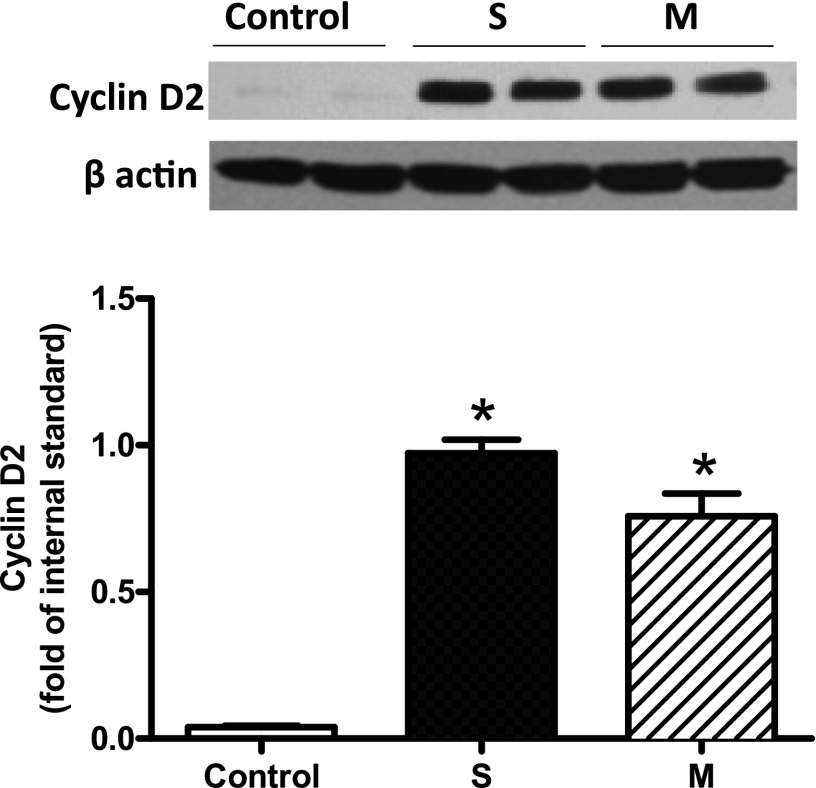
Fig. 7.
Overexpression of cyclin D2 in H9C2 cells. H9C2 cells were transfected with either Flexi-empty (−CcnD2) or Flexi-CcnD2 (+CcnD2) constructs for 24 hours, followed by recovery for another 24 hours in fresh medium without transfection reagent. Transfection was performed in duplicate with one clone of Flexi-empty (Control) and two different clones of Flexi-CcnD2 (S and M). Whole cell lysates in RIPA buffer containing protease inhibitors were next made and protein concentration determined. Fifty micrograms total protein from each sample was electrophoresed by SDS-PAGE, followed by immunoblotting with cyclin D2 monoclonal antibody. Data are mean ± S.E.M., n = 4, from different experiments. *P < 0.05, S or M versus Control.
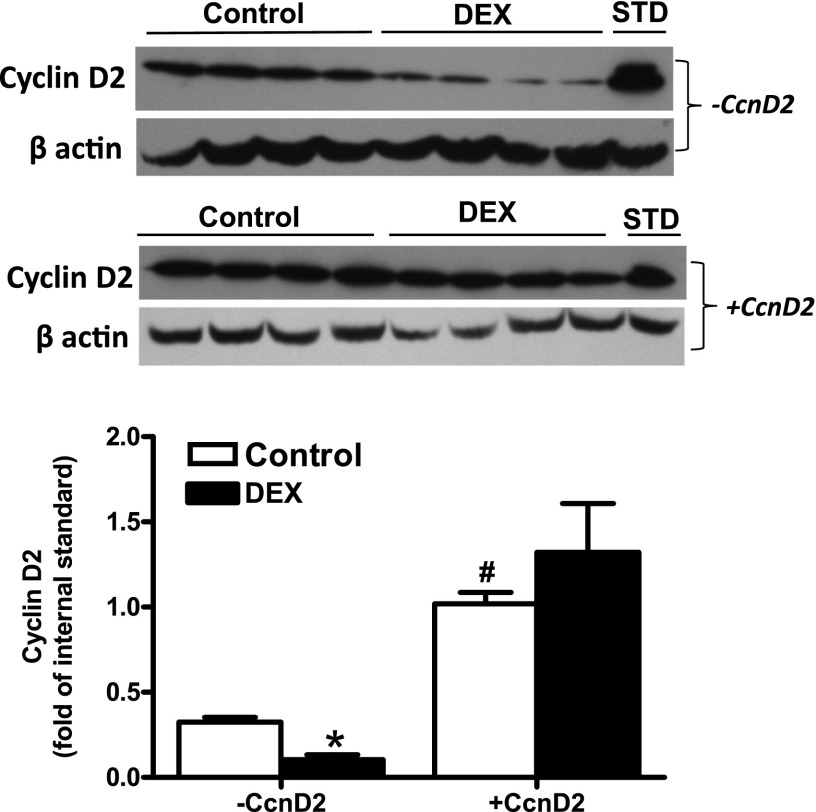
Fig. 8.
Overexpression of cyclin D2 in newborn cardiomyocytes. Cardiomyocytes were isolated from 2-day-old rats and transfected with FlexiCcnD2 (+CcnD2) or Flexi empty construct (−CcnD4). Myocytes were then treated with dexamethasone (DEX) for 48 hours. Cyclin D2 protein abundance was determined by Western blot. STD, internal standard. Data are mean ± S.E.M., n = 4, from different experiments. *P < 0.05, DEX versus Control; #P < 0.05, +CcnD2 versus −CcnD2.
Fig. 9.
Overexpression of cyclin D2 abrogated dexamethasone-induced terminal differentiation in newborn cardiomyocytes. Cardiomyocytes were isolated from 2-day-old rats and transfected with FlexiCcnD2 (+CcnD2) or Flexi empty construct (−CcnD4). Myocytes were then treated with dexamethasone (DEX) for 48 hours. Cells were fixed and stained with Ki67 and α-actinin. The nuclei were stained with Hoechst. (A) Ki67 positive cardiomyocytes. (B) Binucleate cardiomyocytes. Data are mean ± S.E.M., n = 5 or 6. *P < 0.05, DEX versus Control.
Discussion
Our previous study demonstrated that in vivo treatment of newborn rats with clinically relevant doses of dexamethasone significantly inhibited proliferation and increased binucleation in the hearts of day 4 and day 7 pups and decreased cardiomyocyte number in the hearts of day 14 pups (Gay et al., 2015). In the present study, we provided novel evidence of a direct effect of dexamethasone in inhibiting cardiomyocyte proliferation and stimulating myocyte binucleation in a glucocorticoid receptor-dependent manner. Of importance, we demonstrated a cause and effect relation between dexamethasone-mediated epigenetic repression of cyclin D2 gene and dexamethasone-induced transition of proliferation to terminal differentiation of binucleation in newborn rat cardiomyocytes.
Cardiomyocytes terminal differentiation is characterized by the loss of proliferative potential, cell cycle exit, and a transition to binucleate phenotype (Li et al., 1996; Soonpaa et al., 1996). It is evident that cardiomyocyte terminal differentiation is closely associated with the cell cycle, because the vast majority of adult cardiomyocytes are arrested in the G0/G1 phase (Capasso et al., 1992; Walsh et al., 2010). Previous studies by Poolman and Brooks (1998) observed a marked increase in G0/G1 phase cardiomyocytes, because the switch from hyperplasic to hypertrophic growth occurs between neonatal days 2 and 5 in rodents. Analysis of G1 phase cell cycle regulators identified a marked decrease in cyclin D2, from the fetal phase to barely detectable in adult cardiomyocytes (Brooks et al., 1997), further supporting a role of cell cycle regulation in the terminal differentiation. The cell cycle is influenced by glucocorticoids that have been shown to inhibit proliferation and result in cellular arrest in the G0 or G1 phase (Goya et al., 1993; Rogatsky et al., 1997; Zou et al., 2015). Yet in fetal sheep, cortisol infusion did not influence the rate of proliferation or the expression of mitogen-activated protein kinase signaling proteins (Lumbers et al., 2005). This likely illustrates that the effects of glucocorticoid are dependent on timing and duration of exposure. It has been shown that glucocorticoids play a role in regulating gene expression of G1 activators such as c-Myc and cyclin D1 (Goya et al., 1993), as well as the expression of cell cycle inhibitors including p21 (Bird et al., 2007) and p53 (Li et al., 2012). It is therefore possible that glucocorticoid exposure inhibits cardiomyocyte proliferation and accelerates cardiomyocyte maturation by regulating the expression of cell cycle genes. In the present study, we demonstrated that the direct effects of dexamethasone are mediated by the glucocorticoid receptor. The concentration of dexamethasone (100 nM, ∼0.04 µg/ml) used in the present study is clinically relevant, because tapered doses of dexamethasone (0.5, 0.3, 0.1 mg/kg) used in preterm infants estimate blood concentrations around 0.1–5 µg/ml. Although terminal differentiation is normal during cardiomyocyte development, dexamethasone appears to accelerate the process by reducing proliferation and increasing binucleation of cardiomyocytes prematurely. Additionally, cyclin D2 that is instrumental in cellular progression through the G1 phase of the cell cycle was downregulated as a result of dexamethasone treatment. Similarly, previous studies showed that stresses such as hypoxia and anoxia also decrease cyclin D2 protein expression and inhibit proliferation of cardiomyocytes (Tong et al., 2013; Paradis et al., 2015). During hypoxia and other physiologic stresses, glucocorticoids are increased and are believed to be important physiologic mediators during stress response (Raff et al., 2003). In terms of gene regulation there seems to be a consistent mechanism initiated independent of glucocorticoid source, whether endogenous or exogenous. Interestingly, in our previous study, neonatal dexamethasone exposure was not associated with changes in the cell cycle inhibitor p27 (Gay et al., 2015), yet hypoxia and anoxia treatment resulted in a significant increase in p27 (Tong et al., 2013; Paradis et al., 2015). This highlights a difference between hypoxic/anoxic stress (which stimulates endogenous glucocorticoid release) and exogenous glucocorticoid exposure, potentially indicating alternate pathways.
Substantial evidence indicates the importance of epigenetic modifications including DNA methylation in glucocorticoid signaling (Crudo et al., 2013; Sharma et al., 2013; Petropoulos et al., 2014). It has been shown that glucocorticoids repress gene expression by interacting with DNA (cytosine-5)-methyltransferase 3β and methyl CpG binding protein 2 and increasing promoter methylation (Sharma et al., 2013). Furthermore glucocorticoid exposure is associated with both methylation and demethylation of promoter and enhancer regions, illustrating a potentially complex regulatory pathway (Crudo et al., 2013; Thomassin et al., 2001). Crudo et al. (2012) demonstrated that the endogenous glucocorticoid surge alters the expression of methylation specific genes such as DNA (cytosine-5)-methyltransferase 3β and methyl-CpG-binding domain protein 2 and is instrumental in defining DNA methylation patterns in an organ-specific manner (Crudo et al., 2012). The effects of glucocorticoids on methylation patterns are long lasting and are sustained into adulthood and even in subsequent generations (Crudo et al., 2012, 2013). In the present study, we found that treatment with dexamethasone was not associated with changes in global DNA methylation. Yet dexamethasone induced hypermethylation of the cyclin D2 gene promoter, which was consistent with the decrease in cyclin D2 mRNA and protein expression. Interestingly, inhibition of methylation by 5-AZA blocked dexamethasone-mediated cyclin D2 promoter hypermethylation and restored the expression of cyclin D2 protein and mRNA in cardiomyocytes. Of importance, 5-AZA also blocked the dexamethasone-induced effects in inhibiting cardiomyocyte proliferation and increasing myocyte binucleation. These findings illustrate an important role of DNA methylation in cardiomyocyte terminal differentiation and further implicate methylation as a vital factor in the induction of dexamethasone-mediated effects. Other studies also support the importance of methylation in perpetuating cardiomyocyte terminal differentiation (Kou et al., 2010; Gilsbach et al., 2014). For instance, Kou et al. (2010) found that inhibition of DNA methylation in cardiomyocytes using 5-azacytidine resulted in increased DNA synthesis and delayed terminal differentiation during the development of postnatal days 7 and 10 rats. This is consistent with the present findings that dexamethasone treatment induced terminal differentiation prematurely and was associated with hypermethylation of the CcnD2 gene promoter. Inhibition of methylation restored the developmental trajectory in myocytes treated with dexamethasone, suggesting that hypermethylation is a contributing factor in terminal differentiation. It is apparent that methylation is also important in cell cycle regulation, and studies in HeLa cells showed dynamic DNA methylation patterns in a single cell cycle with differential methylation occurring between the G1 and S phases (Brown et al., 2007). Methylation of specific G1 proteins such as P16 resulted in transcriptional loss of protein expression (Matsuda et al., 1999) and the cyclin D2 promoter was also a target of aberrant hypermethylation (Matsubayashi et al., 2003), indicating that methylation is of importance in cell cycle control and transition between the various stages.
In cardiomyocytes, cyclin D2 has been identified as an essential protein related to cardiomyocyte proliferative potential (McGill and Brooks, 1995; Brooks et al., 1997; Poolman and Brooks, 1998; Nagai et al., 2001; Paradis et al., 2014). Considering that most adult cardiomyocytes are locked in the G1/G0 phase of the cell cycle (Capasso et al., 1992; Walsh et al., 2010), it is likely that decreased expression of the G1 cell cycle promoter cyclin D2 contributes to the permanent cell cycle exit characteristic of terminal differentiation. Pasumarthi et al. (2005) demonstrated that reestablishing cyclin D2 protein expression levels resulted in DNA synthesis in adult mitotically inactive cardiomyocytes. Studies in transgenic mice expressing cyclin D2 in the heart demonstrated a robust increase in cardiomyocyte DNA synthesis in the infarct border zone 7 days after myocardial injury, which was sustained up to 150 days after the injury (Pasumarthi et al., 2005). This was associated with an increase in cardiomyocyte number (Pasumarthi et al., 2005). Additionally, the regenerated myocardium of cyclin D2 expressing animals was associated with functional improvements of the heart (Hassink et al., 2008). Consistent with these findings, the present study demonstrated that overexpression of cyclin D2 rescued the effects of dexamethasone on cardiomyocyte terminal differentiation, providing evidence of cause and effect relation between dexamethasone-induced downregulation of cyclin D2 and the acceleration of terminal differentiation in cardiomyocytes.
In summary, the present study revealed a novel mechanism by which dexamethasone stimulates premature terminal differentiation in newborn cardiomyocytes. We demonstrated a causal role of downregulating cyclin D2 in the direct effect of dexamethasone in inhibiting cardiomyocyte proliferation and increasing myocyte binucleation. Although the present study established a key role of promoter methylation in dexamethasone-mediated epigenetic repression of cyclin D2 gene in cardiomyocytes, other mechanisms, e.g., histone modifications, in the regulation of cyclin D2 gene expression may not be precluded. Given the clinical importance of perinatal dexamethasone treatment in preterm infants and mothers at risk of preterm birth and the role of endogenous glucocorticoids in mediating physiologic stresses such as hypoxia, the present study provides a critical insight into the mechanisms of glucocorticoid-mediated harmful effects that may negatively impact in cardiomyocyte endowment in the heart and cardiac function later in life. It is not clear at present whether this effect is peculiar to dexamethasone or rather pertains to other glucocorticoids, e.g., hydrocortisone and methylprednisolone, that have been used in clinics to treat respiratory distress in premature newborns. This remains to be determined in the future studies.
Acknowledgments
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure and the Loma Linda University School of Medicine.
Abbreviations
- 5-AZA
5-aza-2'-deoxycytidine
- CcnD2
cyclin D2 gene
- ELISA
enzyme-linked immunosorbent assay
- 5-mC
5-methylcytosine
- MeDIP
methylated DNA immunoprecipitation
- PCR
polymerase chain reaction
Authorship Contributions
Participated in research design: Zhang.
Conducted experiments: Gay, Dasgupta, Li, and Kanna.
Performed data analysis: Gay and Zhang.
Wrote or contributed to the writing of the manuscript: Gay and Zhang.
Footnotes
This study was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL118861].
References
- Bal MP, de Vries WB, van Oosterhout MF, Baan J, van der Wall EE, van Bel F, Steendijk P. (2008) Long-term cardiovascular effects of neonatal dexamethasone treatment: hemodynamic follow-up by left ventricular pressure-volume loops in rats. J Appl Physiol (1985) 104:446–450. [DOI] [PubMed] [Google Scholar]
- Bird AD, Tan KH, Olsson PF, Zieba M, Flecknoe SJ, Liddicoat DR, Mollard R, Hooper SB, Cole TJ. (2007) Identification of glucocorticoid-regulated genes that control cell proliferation during murine respiratory development. J Physiol 585:187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G, Poolman RA, Li JM. (1998) Arresting developments in the cardiac myocyte cell cycle: role of cyclin-dependent kinase inhibitors. Cardiovasc Res 39:301–311. [DOI] [PubMed] [Google Scholar]
- Brooks G, Poolman RA, McGill CJ, Li JM. (1997) Expression and activities of cyclins and cyclin-dependent kinases in developing rat ventricular myocytes. J Mol Cell Cardiol 29:2261–2271. [DOI] [PubMed] [Google Scholar]
- Brown SE, Fraga MF, Weaver IC, Berdasco M, Szyf M. (2007) Variations in DNA methylation patterns during the cell cycle of HeLa cells. Epigenetics 2:54–65. [DOI] [PubMed] [Google Scholar]
- Capasso JM, Bruno S, Cheng W, Li P, Rodgers R, Darzynkiewicz Z, Anversa P. (1992) Ventricular loading is coupled with DNA synthesis in adult cardiac myocytes after acute and chronic myocardial infarction in rats. Circ Res 71:1379–1389. [DOI] [PubMed] [Google Scholar]
- Clubb FJ, Jr, Bishop SP. (1984) Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Lab Invest 50:571–577. [PubMed] [Google Scholar]
- Crudo A, Petropoulos S, Moisiadis VG, Iqbal M, Kostaki A, Machnes Z, Szyf M, Matthews SG. (2012) Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: multigenerational effects. Endocrinology 153:3269–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crudo A, Suderman M, Moisiadis VG, Petropoulos S, Kostaki A, Hallett M, Szyf M, Matthews SG. (2013) Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology 154:1168–1180. [DOI] [PubMed] [Google Scholar]
- Davis EP, Waffarn F, Sandman CA. (2011) Prenatal treatment with glucocorticoids sensitizes the hpa axis response to stress among full-term infants. Dev Psychobiol 53:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries WB, Bal MP, Homoet-van der Kraak P, Kamphuis PJ, van der Leij FR, Baan J, Steendijk P, de Weger RA, van Bel F, van Oosterhout MF. (2006) Suppression of physiological cardiomyocyte proliferation in the rat pup after neonatal glucocorticosteroid treatment. Basic Res Cardiol 101:36–42. [DOI] [PubMed] [Google Scholar]
- Di Stefano V, Giacca M, Capogrossi MC, Crescenzi M, Martelli F. (2011) Knockdown of cyclin-dependent kinase inhibitors induces cardiomyocyte re-entry in the cell cycle. J Biol Chem 286:8644–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, Keating MT. (2006) Anillin localization defect in cardiomyocyte binucleation. J Mol Cell Cardiol 41:601–612. [DOI] [PubMed] [Google Scholar]
- Fernandes D, Guida E, Koutsoubos V, Harris T, Vadiveloo P, Wilson JW, Stewart AG. (1999) Glucocorticoids inhibit proliferation, cyclin D1 expression, and retinoblastoma protein phosphorylation, but not activity of the extracellular-regulated kinases in human cultured airway smooth muscle. Am J Respir Cell Mol Biol 21:77–88. [DOI] [PubMed] [Google Scholar]
- Gay MS, Li Y, Xiong F, Lin T, Zhang L. (2015) Dexamethasone treatment of newborn rats decreases cardiomyocyte endowment in the developing heart through epigenetic modifications. PLoS One 10:e0125033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsbach R, Preissl S, Grüning BA, Schnick T, Burger L, Benes V, Würch A, Bönisch U, Günther S, Backofen R, et al. (2014) Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat Commun 5:5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya L, Maiyar AC, Ge Y, Firestone GL. (1993) Glucocorticoids induce a G1/G0 cell cycle arrest of Con8 rat mammary tumor cells that is synchronously reversed by steroid withdrawal or addition of transforming growth factor-alpha. Mol Endocrinol 7:1121–1132. [DOI] [PubMed] [Google Scholar]
- Hassink RJ, Pasumarthi KB, Nakajima H, Rubart M, Soonpaa MH, de la Rivière AB, Doevendans PA, Field LJ. (2008) Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res 78:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis PJ, de Vries WB, Bakker JM, Kavelaars A, van Dijk JE, Schipper ME, van Oosterhout MF, Croiset G, Heijnen CJ, van Bel F, et al. (2007) Reduced life expectancy in rats after neonatal dexamethasone treatment. Pediatr Res 61:72–76. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Koh GY. (1997) Differential and dramatic changes of cyclin-dependent kinase activities in cardiomyocytes during the neonatal period. J Mol Cell Cardiol 29:1767–1777. [DOI] [PubMed] [Google Scholar]
- Kelly BA, Lewandowski AJ, Worton SA, Davis EF, Lazdam M, Francis J, Neubauer S, Lucas A, Singhal A, Leeson P. (2012) Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics 129:e1282–e1290. [DOI] [PubMed] [Google Scholar]
- Kou CY, Lau SL, Au KW, Leung PY, Chim SS, Fung KP, Waye MM, Tsui SK. (2010) Epigenetic regulation of neonatal cardiomyocytes differentiation. Biochem Biophys Res Commun 400:278–283. [DOI] [PubMed] [Google Scholar]
- Li F, Wang X, Capasso JM, Gerdes AM. (1996) Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol 28:1737–1746. [DOI] [PubMed] [Google Scholar]
- Li H, Qian W, Weng X, Wu Z, Li H, Zhuang Q, Feng B, Bian Y. (2012) Glucocorticoid receptor and sequential P53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3-E1 cells. PLoS One 7:e37030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins GC, Howie RN. (1972) A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50:515–525. [PubMed] [Google Scholar]
- Liu Z, Yue S, Chen X, Kubin T, Braun T. (2010) Regulation of cardiomyocyte polyploidy and multinucleation by CyclinG1. Circ Res 106:1498–1506. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lumbers ER, Boyce AC, Joulianos G, Kumarasamy V, Barner E, Segar JL, Burrell JH. (2005) Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am J Physiol Regul Integr Comp Physiol 288:R567–R574. [DOI] [PubMed] [Google Scholar]
- Matsubayashi H, Sato N, Fukushima N, Yeo CJ, Walter KM, Brune K, Sahin F, Hruban RH, Goggins M. (2003) Methylation of cyclin D2 is observed frequently in pancreatic cancer but is also an age-related phenomenon in gastrointestinal tissues. Clin Cancer Res 9:1446–1452. [PubMed] [Google Scholar]
- Matsuda Y, Ichida T, Matsuzawa J, Sugimura K, Asakura H. (1999) p16(INK4) is inactivated by extensive CpG methylation in human hepatocellular carcinoma. Gastroenterology 116:394–400. [DOI] [PubMed] [Google Scholar]
- McGill CJ, Brooks G. (1995) Cell cycle control mechanisms and their role in cardiac growth. Cardiovasc Res 30:557–569. [PubMed] [Google Scholar]
- Nagai T, Takano H, Komuro I. (2001) The cell cycle can be a new target for the treatment of cardiac hypertrophy? J Mol Cell Cardiol 33:1769–1771. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes (1995) Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 273:413–418. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. (2003) Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis AN, Gay MS, Wilson CG, Zhang L. (2015) Newborn hypoxia/anoxia inhibits cardiomyocyte proliferation and decreases cardiomyocyte endowment in the developing heart: role of endothelin-1. PLoS One 10:e0116600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis AN, Gay MS, Zhang L. (2014) Binucleation of cardiomyocytes: the transition from a proliferative to a terminally differentiated state. Drug Discov Today 19:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. (2005) Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res 96:110–118. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Matthews SG, Szyf M. (2014) Adult glucocorticoid exposure leads to transcriptional and DNA methylation changes in nuclear steroid receptors in the hippocampus and kidney of mouse male offspring. Biol Reprod 90:43. [DOI] [PubMed] [Google Scholar]
- Poolman RA, Brooks G. (1998) Expressions and activities of cell cycle regulatory molecules during the transition from myocyte hyperplasia to hypertrophy. J Mol Cell Cardiol 30:2121–2135. [DOI] [PubMed] [Google Scholar]
- Raff H, Hong JJ, Oaks MK, Widmaier EP. (2003) Adrenocortical responses to ACTH in neonatal rats: effect of hypoxia from birth on corticosterone, StAR, and PBR. Am J Physiol Regul Integr Comp Physiol 284:R78–R85. [DOI] [PubMed] [Google Scholar]
- Rogatsky I, Trowbridge JM, Garabedian MJ. (1997) Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol Cell Biol 17:3181–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Bhave S, Gregg E, Uht R. (2013) Dexamethasone induces a putative repressor complex and chromatin modifications in the CRH promoter. Mol Endocrinol 27:1142–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoener JA, Baig R, Page KC. (2006) Prenatal exposure to dexamethasone alters hippocampal drive on hypothalamic-pituitary-adrenal axis activity in adult male rats. Am J Physiol Regul Integr Comp Physiol 290:R1366–R1373. [DOI] [PubMed] [Google Scholar]
- Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. (1996) Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol 271:H2183–H2189. [DOI] [PubMed] [Google Scholar]
- Sundberg M, Savola S, Hienola A, Korhonen L, Lindholm D. (2006) Glucocorticoid hormones decrease proliferation of embryonic neural stem cells through ubiquitin-mediated degradation of cyclin D1. J Neurosci 26:5402–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassin H, Flavin M, Espinás ML, Grange T. (2001) Glucocorticoid-induced DNA demethylation and gene memory during development. EMBO J 20:1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Xiong F, Li Y, Zhang L. (2013) Hypoxia inhibits cardiomyocyte proliferation in fetal rat hearts via upregulating TIMP-4. Am J Physiol Regul Integr Comp Physiol 304:R613–R620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Pontén A, Fleischmann BK, Jovinge S. (2010) Cardiomyocyte cell cycle control and growth estimation in vivo--an analysis based on cardiomyocyte nuclei. Cardiovasc Res 86:365–373. [DOI] [PubMed] [Google Scholar]
- Xiao Y, He J, Gilbert RD, Zhang L. (2000) Cocaine induces apoptosis in fetal myocardial cells through a mitochondria-dependent pathway. J Pharmacol Exp Ther 292:8–14. [PubMed] [Google Scholar]
- Zou W, Yang S, Zhang T, Sun H, Wang Y, Xue H, Zhou D. (2015) Hypoxia enhances glucocorticoid-induced apoptosis and cell cycle arrest via the PI3K/Akt signaling pathway in osteoblastic cells. J Bone Miner Metab 33:615–624. [DOI] [PubMed] [Google Scholar]