Abstract
Toll-like receptor 4 (TLR4) signaling is implied in opioid reinforcement, reward, and withdrawal. Here, we explored whether TLR4 signaling is involved in the acute psychomotor-stimulating effects of heroin, 6-acetylmorphine (6-AM), and morphine as well as whether there are differences between the three opioids regarding TLR4 signaling. To address this, we examined how pretreatment with (+)-naloxone, a TLR4 active but opioid receptor (OR) inactive antagonist, affected the acute increase in locomotor activity induced by heroin, 6-AM, or morphine in mice. We also assessed the effect of pretreatment with (−)-naloxone, a TLR4 and OR active antagonist, as well as the pharmacokinetic profiles of (+) and (−)-naloxone in the blood and brain. We found that (−)-naloxone reduced acute opioid-induced locomotor activity in a dose-dependent manner. By contrast, (+)-naloxone, administered in doses assumed to antagonize TLR4 but not ORs, did not affect acute locomotor activity induced by heroin, 6-AM, or morphine. Both naloxone isomers exhibited similar concentration versus time profiles in the blood and brain, but the brain concentrations of (−)-naloxone reached higher levels than those of (+)-naloxone. However, the discrepancies in their pharmacokinetic properties did not explain the marked difference between the two isomers’ ability to affect opioid-induced locomotor activity. Our results underpin the importance of OR activation and do not indicate an apparent role of TLR4 signaling in acute opioid-induced psychomotor stimulation in mice. Furthermore, there were no marked differences between heroin, 6-AM, and morphine regarding involvement of OR or TLR4 signaling.
Introduction
Heroin is rapidly metabolized to 6-acetylmorphine (6-AM) and further to morphine (for review, see Rook et al., 2006), acting primarily through its active metabolites. Morphine has been considered the main metabolite responsible for heroin’s pharmacological effects (Way et al., 1965), but the role of 6-AM as a predominant mediator of early heroin effects has gained increasing focus (Umans and Inturrisi, 1981; Inturrisi et al., 1983; Andersen et al., 2009; Boix et al., 2013; Raleigh et al., 2013; Schlosburg et al., 2013; Bogen et al., 2014; Gottås et al., 2014). Heroin is more potent (van Ree et al., 1978; Hubner and Kornetsky, 1992) and has a greater addictive potential than morphine. Consequently, it could be asked whether this may be the result of neurobiological effects of heroin and/or 6-AM that are different from those of morphine.
We previously showed that the acute psychomotor-stimulating effects of heroin, 6-AM, and morphine in mice do not seem to depend on different µ-opioid receptor (OR) subtypes or binding sites (Eriksen et al., 2014). This contrasts with previous findings for the analgesic effects of these opioids (Rossi et al., 1996; Brown et al., 1997; Schuller et al., 1999; Walker et al., 1999; Pan et al., 2009). Another possible distinction between the effects of heroin/6-AM and morphine could be linked to differences in their action through non-ORs such as Toll-like receptor 4 (TLR4) and hence their activation of central immune responses. Previous studies implied that TLR4 signaling contributes to opioid reinforcement, reward, and withdrawal (Hutchinson et al., 2010, 2012; Theberge et al., 2013). As opposed to ORs, which are stereoselective in their binding properties and thus preferentially bind (−)-isomers of their antagonists such as naloxone (Iijima et al., 1978), TLR4 signaling can be antagonized by both (+) and (−)-isomers of naloxone (and naltrexone) (Hutchinson et al., 2008, 2010; Wang et al., 2016). Blockade of TLR4 signaling by (+)-naloxone was previously reported to suppress morphine-induced striatal dopamine release and conditioned placed preference (CPP) and to reduce remifentanil self-administration in rats (Hutchinson et al., 2012).
Administration of heroin, 6-AM, or morphine to mice induces a marked increase in locomotor activity (Andersen et al., 2009; Eriksen et al., 2014), which can be used as a model of the psychomotor-stimulating properties of these drugs. To our knowledge, TLR4 signaling has not yet been investigated for involvement in opioid-induced psychomotor activation. Therefore, this study aimed to explore whether TLR4 signaling is involved in the acute psychomotor-stimulating effects of heroin, 6-AM, and morphine, as well as if there are differences between the three opioids regarding TLR4 signaling. To address this aim, we examined how pretreatment with (+)-naloxone affected the acute increase in locomotor activity induced by heroin, 6-AM, or morphine in mice. Furthermore, to provide a context for interpreting the results, (−)-naloxone antagonism of locomotor activity induced by heroin, 6-AM, and morphine was also assessed, as were the pharmacokinetic profiles of (+)-naloxone and (−)-naloxone in the blood and brain.
Materials and Methods
Animals.
342 adult male C57BL/6J-Bom mice (Taconic, Bomholt, Denmark) weighing 21–29 g were used in the experiments. Mice were used for either locomotion or pharmacokinetic experiments. After arrival, the animals were housed four to eight per cage under standard conditions, with free access to commercial mouse pellets and water. The animals arrived at least 5 days prior to the experiments, and all experiments were performed during the light period of a 12-hour light/dark cycle. The experiments were approved by the Norwegian Animal Research Authority and were performed in conformity with the laws and regulations controlling experiments and procedures on live animals in Norway.
Drugs.
Heroin hydrochloride and 6-AM hydrochloride were purchased from Lipomed AG (Arlesheim, Switzerland). Morphine hydrochloride was from Norsk Medisinaldepot AS (Oslo, Norway). (−)-Naloxone hydrochloride was acquired from Sigma-Aldrich (Saint Louis, MO). (+)-Naloxone hydrochloride was synthesized (Iijima et al., 1978) by Dr. Kenner C. Rice (National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD). The commercial suppliers reported purities > 98% for all compounds, and the purity (>98%) of (+)-naloxone was determined by the accepted chemical standards of nuclear magnetic resonance, mass spectral, and combustion analysis as well as chromatographic homogeneity. The compounds were dissolved in 0.9% saline and administered as s.c. injections in total volumes of 0.01 ml/g mouse. All injection solutions were analyzed by liquid chromatography (LC)–tandem mass spectrometry (MS/MS) for verification of compound concentrations (data not shown). Doses are reported as free base.
Chemicals, Reagents, and Solutions.
Oxycodone-d6 (100%), supplied by Cerilliant Corporation (Round Rock, TX), was the internal standard for chemical analysis. Disodium tetraborate-10-hydrate was supplied by Chemi-Teknik AS (Oslo, Norway). Ethyl acetate, sodium hydroxide, and n-heptane were purchased from Merck Millipore Corporation (Darmstadt, Germany). Methanol (LC-MS Chromasolv) and ammonium formate were obtained from Sigma-Aldrich. Formic acid (98%) was acquired from VWR International AS (Oslo, Norway). Type 1 water was prepared by a Milli-Q Advantage A10 Water Purification System (Merck Millipore Corporation). Human whole blood was supplied by the blood bank at Oslo University Hospital, Ullevål (Oslo, Norway), and homogenized rat brain tissue (2 ml 5 mM ammonium formate buffer, pH 3.1/g tissue) was from rats housed in the animal department at the Norwegian Institute of Public Health (Oslo, Norway). Two stock solutions of (−)-naloxone hydrochloride (Sigma-Aldrich) were prepared in methanol and identified as calibrator or quality control (QC). The (−)-naloxone hydrochloride was from the same vendor, but from different batches. Calibrator and QC working stock solutions were prepared by dilution of the stock solutions in 5 mM ammonium formate buffer (pH 3.1). Seven calibrators were prepared in human whole blood or rat brain homogenate fortified with working stock solution in the concentration range of 0.001–12 µM. Three QC samples with concentrations of 0.0012, 0.8, and 10 µM were prepared independently. Calibrators and QC samples prepared from (−)-naloxone were used to quantify both (−)-naloxone and (+)-naloxone in the samples, since the chromatograms from pilot experiments demonstrated that both compounds eluted at the exact same time and gave equal signal strength (data not shown). Possible differences between human and mouse blood and rat and mouse brain were not evaluated specifically with respect to recoveries and matrix effects.
Locomotor Activity.
Locomotor activity was tested using a VersaMax optical animal activity monitoring system (AccuScan Instruments Inc., Columbus, OH). The 40 × 40-cm Plexiglas chambers, equipped with infrared beams spaced at 2.5-cm intervals, were divided into four 20 × 20 cm equal quadrants by two perpendicular transparent Plexiglas walls. Mice were randomly assigned to treatment group, and two animals were tested simultaneously in each chamber using two nonadjacent quadrants. After 60 minutes of habituation in the activity chamber, mice were gently removed and injected (subcutaneously) with (−)-naloxone (0.01, 0.1, or 1 mg/kg, equivalent to 0.025, 0.25, and 2.5 µmol/kg, respectively), (+)-naloxone (0.01, 0.1, 1, 10, or 50 mg/kg, equivalent to 0.025, 0.25, 2.5, 25, and 125 µmol/kg, respectively) (antagonist pretreatment) or saline (control pretreatment) in another room. The mice were then placed in their respective home cages for 5 minutes. Thereafter, heroin (3.5 µmol/kg, equivalent to 1.47 mg/kg), 6-AM (4 µmol/kg, equivalent to 1.63 mg/kg), morphine (30 µmol/kg, equivalent to 11.16 mg/kg), or saline was administered subcutaneously (t = 0), and each mouse was immediately returned to the same activity chamber as used for habituation. Locomotor activity (horizontal distance traveled) was measured for 4 hours. The agonist doses were chosen, based on results from previous experiments (Andersen et al., 2009; Eriksen et al., 2014) and pilot studies, for their ability to induce robust and almost equal maximal locomotor activity without apparent narcotic effects such as staggering and incoherent running (Eriksen et al., 2014). Six to eight animals were used for each group except for the saline plus saline, (−)-naloxone plus saline, and (+)-naloxone plus saline control groups, in which three or four animals were used.
Pharmacokinetics.
Each mouse was randomized to a treatment group and given a bolus injection of (−)-naloxone (0.1 or 1 mg/kg, s.c.) or (+)-naloxone (0.1, 1, or 10 mg/kg, s.c.). At given times after injection (0.1 mg/kg: 2, 5, 10, 15, 20, 30, 45, 60, or 90 minutes; 1 or 10 mg/kg: 15 minutes; n = 4–6 at each sample point), the mice were anesthetized with isoflurane and blood was collected by heart puncture using a syringe containing 80 µl heparin (100 IU/ml). Immediately after blood sampling, cervical dislocation was performed and the brain (except cerebellum) was rapidly removed, washed in ice-cold 0.9% saline, blotted on a filter paper, and homogenized (2 ml/g tissue) in ice-cold 0.9% saline. All samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis by ultra-performance liquid chromatography (UPLC)–MS/MS the same day.
Calibrator or QC samples were prepared by adding 100 µl calibrator or QC working stock solution to an aliquot of blank whole blood or brain tissue homogenate (100 µl) in test tubes. Blood or brain homogenate samples (200 µl) were transferred to test tubes. The internal standard (50 µl, 0.56 µM oxycodone-d6) was added to all samples, followed by immediate mixing on a multitube vortexer. Borate buffer (pH 11, 100 µl) and ethyl acetate/heptane mixture (1.2 ml, 4:1 v/v) were added to the samples before approximately 1 minute of mixing. Samples were shaken mechanically for 10 minutes, followed by centrifugation at 4°C for 10 minutes at 4500 rpm. The organic phase (approximately 950 µl) was transferred to 5-ml glass tubes, evaporated until dryness at 40°C, and reconstituted in 5 mM ammonium formate buffer (pH 3.1, 100 µl). The glass tubes were shaken in a multitube vortexer and centrifuged at 4°C for 10 minutes at 4500 rpm before the supernatant was transferred to autosampler vials.
UPLC-MS/MS.
Chromatographic separations were performed on an Acquity UPLC system (Waters Corporation, Milford, MA), with an Acquity UPLC HSS T3 C18 column (2.1 × 100 mm, 1.8 µm) and an Acquity UPLC HSS T3 C18 VanGuard Pre-Column (2.1 × 5 mm, 1.8 µm). Gradient elution over 5 minutes with a mobile phase consisting of 10 mM ammonium formate buffer (pH 3.1) (A) and methanol (B) at a flow rate of 0.5 ml/min was performed. The gradient program was as follows: 90% A at 0 → 0.5 minutes, 90% → 70% A at 0.5 → 1.5 minutes, 70% → 0% A at 1.5 → 1.6 minutes, 0% A at 1.6–3.6 minutes, 0% → 90% A at 3.6 → 3.7 minutes, and 90% A at 3.7 → 5.0 minutes. The column temperature was held at 65°C and the injection volume was 0.1 µl [samples from mice injected with 10 mg/kg (+)-naloxone] or 4 µl [samples from mice injected with 0.1 or 1 mg/kg (−)-naloxone or (+)-naloxone]. All analyses were performed with a Waters TQS tandem mass spectrometer with an electrospray ionization source. Detection with electrospray ionization–MS/MS was carried out in the multiple reaction monitoring mode using positive ionization, with two transitions for (+)-and (−)-naloxone (328.2 > 310.1 and 328.2 > 253.1) and one transition for oxycodone-d6 (322.0 > 262.1). Retention times were 1.72 and 1.84 minutes for naloxone and oxycodone-d6, respectively. The limit of quantification was set as the lowest calibrator concentration at which the difference (residual) between the concentration predicted by the calibration curve and the actual concentration at the calibration points was no more than ±20% (over six assays). Quantification of the compounds was executed with TargetLynx using MassLynx software (version 4.2; Waters). Peak heights were used for calculations.
Data Analysis.
Locomotor activity is presented as run distance (in centimeters) versus time curves at 5-minute intervals (means) or as the maximal distance run in centimeters in a 5-minute interval (means + S.E.M.) for the full test period. Kinetica (version 5.1; Thermo Fischer Scientific Inc., Waltham, MA) was used to fit a one-compartment, extravascular model to the mean blood and brain concentrations of (−)-naloxone and (+)-naloxone obtained in vivo to calculate the area under the curve (AUC) from time 0 to the last sample time point. In addition, the estimated maximum concentrations (Cmax), the estimated time to reach Cmax (Tmax), the absorption constant (Ka), the elimination constant (Ke), and the half-life during elimination (t1/2) were calculated for each antagonist in blood. Statistically significant differences between treatment groups were evaluated by the Mann–Whitney U test using the IBM SPSS Statistics 22 software package (SPSS Inc., Chicago, IL).
Results
Locomotor Activity.
Mice administered heroin, 6-AM, or morphine in doses of 3.5, 4, or 30 µmol/kg, respectively, demonstrated a clear and comparable increase in locomotor activity compared with those receiving only saline (P < 0.01, saline data not shown) (Figs. 1 and 2, saline-pretreated groups are the same in both figures).
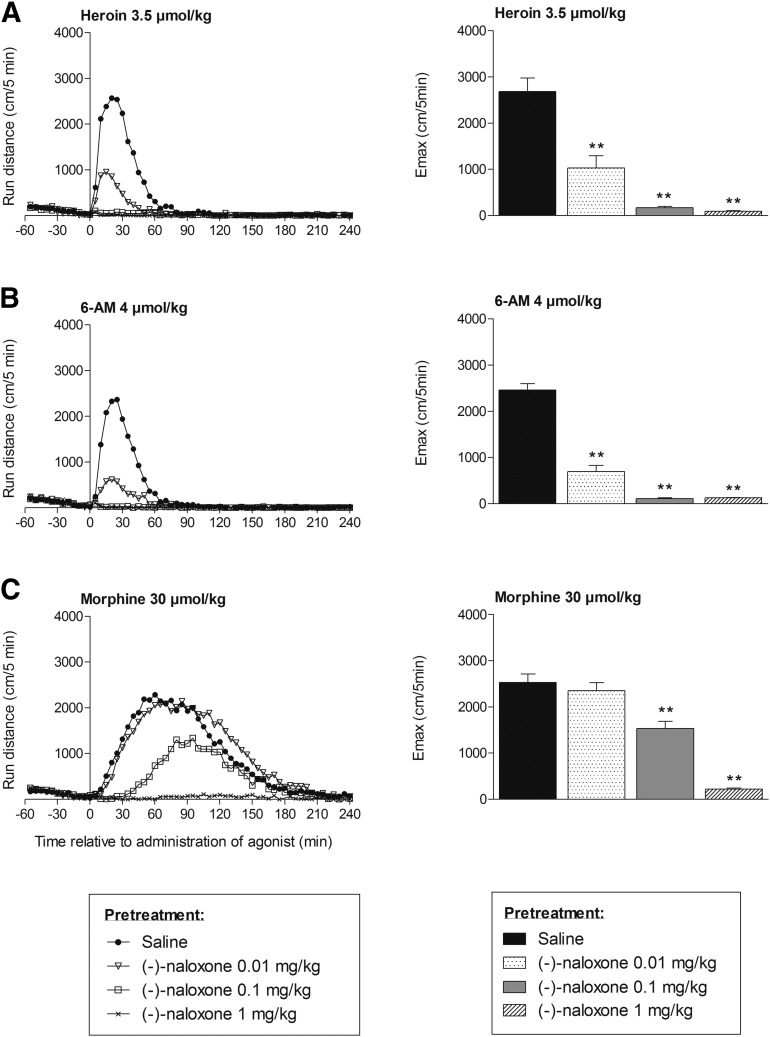
Fig. 1.
Locomotor activity after subcutaneous administration of heroin (A), 6-AM (B), and morphine (C) to mice pretreated with saline or (−)-naloxone (0.01, 0.1, or 1 mg/kg, s.c.). Locomotor activity is given as run distance in centimeters versus time curves at 5-minute intervals (means) (left) and as the maximal distance run (Emax) in centimeters in a 5-minute interval (means + S.E.M.) (right). n = 6–8 in each group. **P < 0.01 (Mann–Whitney U test compared with the saline-pretreated group).
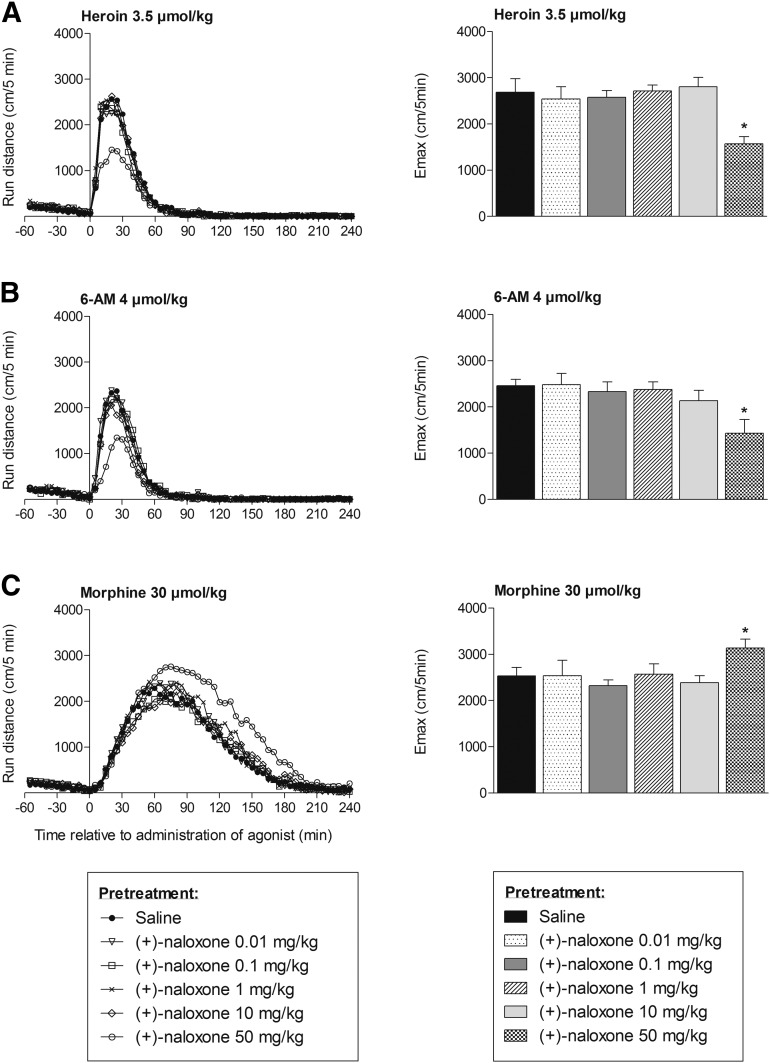
Fig. 2.
Locomotor activity after subcutaneous administration of heroin (A), 6-AM (B), and morphine (C) to mice pretreated with saline or (+)-naloxone (0.01, 0.1, 1, 10, or 50 mg/kg, s.c.). Locomotor activity is given as run distance in centimeters versus time curves at 5-minute intervals (means) (left) and as the maximal distance run (Emax) in centimeters in a 5-minute interval (means + S.E.M.) (right). n = 6–8 in each group. *P < 0.05 (Mann–Whitney U test compared with the saline-pretreated group).
Pretreatment with (−)-naloxone significantly reduced locomotor activity induced by heroin, 6-AM, or morphine in a dose-dependent manner. (−)-Naloxone in doses of 0.1 and 1 mg/kg almost completely blocked (>94% reduction in maximal distance run) locomotor activity induced by heroin and 6-AM (P < 0.01). The lowest dose of (−)-naloxone (i.e., 0.01 mg/kg) significantly reduced the maximal locomotor activity induced by heroin and 6-AM by 62% and 72%, respectively (P < 0.01) (Fig. 1, A and B). Pretreatment with the highest dose of (−)-naloxone (i.e., 1 mg/kg) effectively blocked (91% reduction in maximal distance run) morphine-induced locomotor activity (P < 0.01), whereas the 0.1-mg/kg dose revealed a complete blockade of locomotor activity for only the first 30 minutes after morphine administration. Thereafter, locomotor activity increased until approximately 90 minutes after morphine administration, displaying an almost 40% reduction of the maximal distance run (P < 0.01), before it declined. The lowest dose of (−)-naloxone showed no effect on morphine-induced locomotor activity (Fig. 1C).
Pretreatment with (+)-naloxone in doses ranging from 0.01 to 10 mg/kg did not reveal any significant effect on locomotor activity induced by heroin, 6-AM, or morphine (Fig. 2). Mice administered a higher 50-mg/kg (+)-naloxone dose in combination with heroin demonstrated a clear reduction in locomotor activity (41% reduction in maximal distance run, P < 0.05, Fig. 2A), as did mice given this dose of (+)-naloxone in combination with 6-AM (39% reduction in maximal distance run, P < 0.05, Fig. 2B). Pretreatment with 50 mg/kg (+)-naloxone did not attenuate morphine-induced locomotor activity. Instead, the maximal morphine-induced locomotor activity was increased by 24% (P < 0.05). The increase in locomotor activity was, however, first evident from approximately 60 minutes after administration of morphine (Fig. 2C).
The administration of saline, (−)-naloxone, or (+)-naloxone alone did not induce any effect on locomotor activity (data not shown).
Pharmacokinetics.
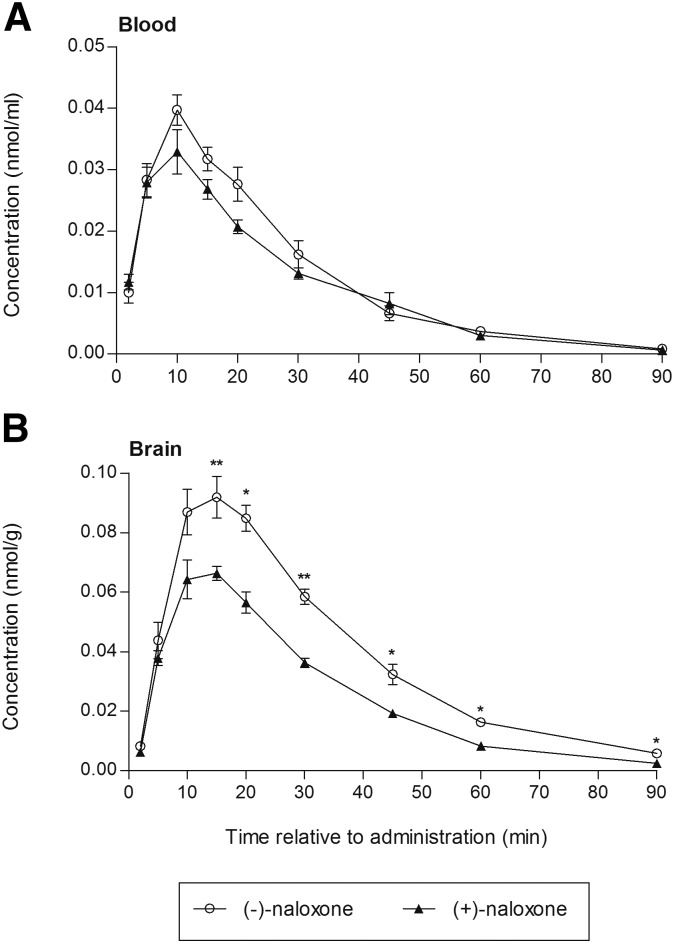
Blood and brain concentrations of both naloxone isomers were analyzed to ensure that the discrepancy between the effect of (−)-naloxone and (+)-naloxone on opioid-induced locomotor activity after equal isomer doses was not a result of pharmacokinetic differences. Figure 3 shows blood and brain concentration versus time profiles of (−)-naloxone and (+)-naloxone measured from 2 to 90 minutes after a subcutaneous injection of 0.1 mg/kg. The concentration profiles in the brain paralleled the profiles seen in the blood. However, the maximal concentration of both isomers reached higher levels in the brain and occurred somewhat later than in the blood. The brain concentrations of (−)-naloxone were significantly higher than the brain concentrations of (+)-naloxone from 15 minutes after administration and throughout the experimental period of 90 minutes (P < 0.05). No marked concentration differences were observed between the two antagonists in the blood. These findings were also reflected by the calculated AUCs, which revealed that the AUCs for (+)-naloxone (AUCblood = 0.94 nmol/ml·min, AUCbrain = 2.28 nmol/g·min) were approximately 90% and 65% of the calculated AUCs for (−)-naloxone (AUCblood = 1.05 nmol/ml·min, AUCbrain = 3.53 nmol/g·min) in the blood and brain, respectively. Other calculated pharmacokinetic parameters are listed in Table 1.
Fig. 3.
Concentration versus time profiles of (−)-naloxone and (+)-naloxone in the blood (A) and brain (B) of mice after a subcutaneous injection of 0.1 mg/kg. Values are given as means ± S.E.M. n = 4–6 at each sample point. *P < 0.05; **P < 0.01 (Mann–Whitney U test between treatment groups).
TABLE 1.
Pharmacokinetic characteristics of (−)-naloxone and (+)-naloxone in the blood after a subcutaneous injection of 0.1 mg/kga
| Parameter | (−)-Naloxone | (+)-Naloxone |
|---|---|---|
| AUC (µM·min) | 1.05 | 0.94 |
| Cmax (µM) | 0.04 | 0.03 |
| Tmax (min) | 10.89 | 9.33 |
| Ka (min−1) | 0.09 | 0.19 |
| Ke (min−1) | 0.09 | 0.05 |
| t1/2 (min) | 7.67 | 12.82 |
All parameters are calculated by the Kinetica software based on a one-compartment extravascular model fitted to the mean blood concentrations of (−)-naloxone and (+)-naloxone obtained in vivo (n = 4–6 in each group).
AUC, area under the curve; Cmax, maximum concentration; Ka, absorption constant; Ke, elimination constant; t1/2, half-life during elimination; Tmax, estimated time to reach Cmax.
The antagonist concentrations measured in the blood and brain were below 10% of the maximal concentrations 60 and 90 minutes after administration, respectively (Fig. 3). The brain concentrations measured 15 minutes after administration of 0.1 mg/kg of the antagonists, as well as the brain concentrations 15 minutes after injection of higher doses of (−)-naloxone (1 mg/kg) or (+)-naloxone (1 or 10 mg/kg), are listed in Table 2. Administration of (−)-naloxone and (+)-naloxone in doses of 1 mg/kg gave brain levels of the compounds that were approximately 10 times the concentrations seen after injection of 0.1 mg/kg. Similarly, the brain concentration of (+)-naloxone after administration of 10 mg/kg was approximately 100 times the concentration seen after injection of 0.1 mg/kg.
TABLE 2.
Concentrations of (−)-naloxone and (+)-naloxone in the brain 15 minutes after a subcutaneous injection (n = 4–6 in each group)
Values are means ± S.E.M.
| Drug | Dose | Concentration in Brain |
|---|---|---|
| mg/kg | nmol/g | |
| (−)-Naloxone | 0.1 | 0.092 ± 0.007a |
| 1 | 0.832 ± 0.025 | |
| (+)-Naloxone | 0.1 | 0.066 ± 0.002a |
| 1 | 0.736 ± 0.043 | |
| 10 | 6.933 ± 0.554 |
Values from results presented in Fig. 1.
Discussion
We found that (−)-naloxone (a TLR4 and OR active antagonist) inhibited the acute increase in locomotor activity evoked by heroin, 6-AM, or morphine in a dose-dependent manner. Administration of 1 mg/kg (−)-naloxone completely abolished the opioid-induced locomotor activity. By contrast, (+)-naloxone (a TLR4 active, but OR inactive antagonist) in doses up to 10 mg/kg did not affect the acute increase in locomotor activity induced by the same opioids. Pretreatment with 50 mg/kg (+)-naloxone reduced locomotor activity evoked by heroin or 6-AM, but not by morphine. The pharmacokinetic analysis revealed that (+) and (−)-naloxone exhibited similar concentration versus time profiles in the blood and brain, but the brain concentrations of (+)-naloxone reached only 72% (maximal concentration) and 65% (AUC) of the concentrations of (−)-naloxone.
Wang et al. (2016) recently showed that (+) and (−)-naloxone (and naltrexone) antagonize TLR4 with similar potency in vitro. In vivo, (−)-naloxone demonstrated a higher potency than (+)-naloxone in blocking antianalgesia induced by lipopolysaccharides (TLR4 agonists) (Wu et al., 2006). It can therefore be assumed that (−)-naloxone is at least as efficient as (+)-naloxone in antagonizing the activation of TLR4 signaling by opioid agonists. Here, we demonstrate a clear dose response of (−)-naloxone, whereby 0.01 mg/kg reduced locomotor activity induced by heroin and 6-AM and 1 mg/kg abolished the locomotion induced by all three opioids (Fig. 1). By contrast, administration of (+)-naloxone in doses up to 10 mg/kg showed no effect on locomotion induced by the same opioids (Fig. 2). Our results therefore suggest that the opioid-induced locomotor activity in this study was mediated through ORs rather than TLR4. The pharmacokinetic analysis of the two naloxone isomers further revealed that, although (−)-naloxone reached higher brain concentrations than (+)-naloxone after administration of equal doses, the discrepancies in their pharmacokinetic properties cannot explain the marked difference between the two isomers’ ability to affect opioid-induced locomotor activity. This statement holds true because the observation that brain concentrations after higher doses of the isomers [up to 10 mg/kg for (+)-naloxone] largely corresponded with the concurrent increase in doses (Table 2).
The lack of an antagonistic effect on opioid-induced locomotor activity after pretreatment with up to 10 mg/kg (+)-naloxone apparently contrasts with previous studies. Hutchinson et al. (2012) reported a profound effect of 1 mg/kg (+)-naloxone on opioid reward, as measured by morphine-induced CPP and elevation of extracellular dopamine concentrations in the nucleus accumbens (NAc) shell in rats. Although engaging the mesocorticolimbic dopamine system is considered important for both drug-induced psychomotor activation and reward behaviors (Wise and Bozarth, 1987; Robinson and Berridge, 2001), dopamine-dependent and dopamine-independent (i.e., not involving increased dopamine release in the NAc) mechanisms are implicated in opioid-induced locomotor activity (Kalivas et al., 1983). This may explain why opioid effects on locomotor activity and CPP can differ (Vindenes et al., 2008; Vindenes et al., 2009) and also suggests that the opioid-induced locomotor activity observed in our study may include processes not involving elevated dopamine levels in the NAc. However, further experiments are necessary to corroborate this hypothesis, and the possible contribution of TLR4 signaling in dopamine-dependent and dopamine-independent processes is not fully elucidated. For instance, although Hutchinson et al. (2012) reported that 1 mg/kg (+)-naloxone blunted morphine-induced elevations of dopamine in the NAc shell, it cannot be excluded that opioid-induced dopamine elevations in other brain locations (e.g., the NAc core) may be dependent on ORs rather than TLR4. Our findings may be supported by results from Theberge et al. (2013), demonstrating that chronic delivery of (+)-naltrexone or acute injections of (+)-naloxone/naltrexone (in doses up to 30 mg/kg) did not interfere with acquisition or maintenance of heroin self-administration. Thus, acute and more chronic effects of opioids seem to engage various neuropharmacological mechanisms in the mesocorticolimbic system, of which ORs and TLR4 may have different importance.
In our study, 0.1 mg/kg (−)-naloxone inhibited locomotor activity induced by all three opioids. Thus, we assumed that even if (−)-naloxone may be somewhat more potent than (+)-naloxone in antagonizing TLR4 signaling (Wu et al., 2006), increasing the (+)-naloxone dose 100 times (from 0.1 to 10 mg/kg) would be sufficient to reveal a possible effect of (+)-naloxone. Still, a 500 times higher dose (50 mg/kg) of (+)-naloxone was also included in the study. This high dose of (+)-naloxone inhibited locomotion induced by heroin and 6-AM, whereas morphine-induced locomotion was not decreased (Fig. 2). This distinction between the effect of (+)-naloxone on locomotor activity induced by heroin/6-AM and morphine may suggest that heroin/6-AM involves non-ORs/targets such as TLR4 in a different manner than morphine. However, a more likely explanation is a nonspecific effect of high doses of (+)-naloxone on ORs, since high concentrations of (+)-naloxone/naltrexone will also antagonize ORs, although with approximately 2500–10,000 times lower binding affinities (Iijima et al., 1978; Theberge et al., 2013). By extrapolating from Table 2, we can calculate that brain concentrations after 50 mg/kg (+)-naloxone were approximately 3500–5000 times higher than after 0.01 mg/kg (−)-naloxone, indicating possible OR antagonism. Furthermore, pretreatment with 0.01 mg/kg (−)-naloxone significantly inhibited the locomotor response to heroin and 6-AM, but not to morphine. It is therefore possible that the difference observed between inhibition of heroin/6-AM– and morphine–induced locomotor activity by a high dose of (+)-naloxone can be related to OR effects. These discrepancies between naloxone antagonism of heroin/6-AM– and morphine–induced locomotion cannot be simply explained by differences in brain levels of active agonists after opioid administration, at least not for the first 15–20 minutes. From previous experiments conducted in our laboratory (Andersen et al., 2009), we can extrapolate and roughly calculate the brain concentrations of the different opioids at given times after administration. From these calculations, no major difference in brain concentrations of 6-AM and morphine is evident the first 15–20 minutes after opioid administration, even though the morphine dose administered (30 µmol/kg) was approximately 8.5 and 7.5 times higher than the heroin (3.5 µmol/kg) and 6-AM (4 µmol/kg) doses, respectively. Moreover, differences in binding affinities to µORs between 6-AM and morphine may contribute to the lack of inhibitory effect on morphine-induced locomotor activity after 0.01 mg/kg (−)-naloxone [and 50 mg/kg (+)-naloxone], since Frölich et al. (2011) reported higher binding affinity to human µORs for morphine than 6-AM in [3H]-naloxone competition displacement experiments.
Another aspect to consider in deducing a possible discrepancy in TLR4 involvement between heroin/6-AM– and morphine–induced locomotor activity is the brief half-lives and thus the short-lived presence of both naloxone isomers in the brain (approximately 15% and less than 10% of maximal concentrations 60 and 90 minutes after administration, respectively). This should be considered when interpreting the locomotor activity data, especially the morphine data, since the duration of increased locomotor activity and the time to reach maximal distance run are quite different for heroin, 6-AM, and morphine (20, 25, and 60 minutes, respectively, in saline-pretreated animals in this study). Accordingly, heroin– and 6-AM–induced maximal locomotor activity in mice pretreated with 0.1 mg/kg (−)-naloxone was well within a time frame in which brain concentrations of the antagonist were still considerably high. In contrast, morphine had its locomotor activity peak at a time at which the antagonist concentration in brain was quite low. Hence, a total blockade of morphine-induced locomotor activity was revealed the first 30 minutes after administration of 0.1 mg/kg (−)-naloxone. Thereafter, the locomotor activity increased until it reached maximal activity after approximately 90 minutes (Fig. 1C, left). The brief presence of the antagonists in the brain also affects how the effect of 50 mg/kg (+)-naloxone on morphine-induced locomotor activity should be interpreted. By inspection of the locomotor activity curve (Fig. 2C, left), an increase in locomotor activity first becomes evident at 60 minutes and is most pronounced 90 minutes after morphine administration, thus after most of (+)-naloxone has disappeared from the brain. We have no explanation for the increase in morphine-induced locomotor activity after 50 mg/kg (+)-naloxone. Administration of this dose was repeated in different experiments to see whether this was a random effect, but the increased activity was confirmed.
In conclusion, this study shows that pretreatment with (+)-naloxone, in doses assumed to antagonize TLR4 but not ORs, does not affect acute locomotor activity induced by heroin, 6-AM, or morphine in mice. Our results from locomotor activity experiments utilizing (+)-naloxone as a TLR4 antagonist and (−)-naloxone as both an OR and TLR4 antagonist therefore underpin the importance of OR activation but do not indicate an apparent role of TLR4 signaling in acute opioid-induced psychomotor stimulation in mice. Furthermore, there were no marked differences between heroin, 6-AM, and morphine regarding involvement of TLR4 signaling. These findings add novel information for evaluating the role of TLR4 signaling in processes related to mechanisms underlying opioid reinforcement, reward, and addiction.
Acknowledgments
The authors thank Synne Steinsland and Ritva Karinen for valuable assistance with the pharmacokinetic experiments.
Abbreviations
- 6-AM
6-acetylmorphine
- AUC
area under curve
- Cmax
maximum concentration
- CPP
conditioned place preference
- Ka
absorption constant
- Ke
elimination constant
- LC
liquid chromatography
- MS/MS
tandem mass spectrometry
- NAc
nucleus accumbens
- OR
opioid receptor
- QC
quality control
- TLR4
Toll-like receptor 4
- Tmax
time to reach Cmax
- UPLC
ultra-performance liquid chromatography
Authorship Contributions
Participated in research design: Eriksen, Andersen, Boix, Vindenes, Mørland.
Conducted experiments: Eriksen, Andersen, Bergh.
Performed data analysis: Eriksen, Boix, Bergh.
Wrote or contributed to the writing of the manuscript: Eriksen, Andersen, Boix, Bergh, Vindenes, Rice, Huestis, Mørland.
Footnotes
This research was supported in part by the Research Council of Norway [Grant 196621/V50] and by the Intramural Research Program of the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse.
References
- Andersen JM, Ripel A, Boix F, Normann PT, Mørland J. (2009) Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a prodrug for the mediator 6-monoacetylmorphine in vivo. J Pharmacol Exp Ther 331:153–161. [DOI] [PubMed] [Google Scholar]
- Bogen IL, Boix F, Nerem E, Mørland J, Andersen JM. (2014) A monoclonal antibody specific for 6-monoacetylmorphine reduces acute heroin effects in mice. J Pharmacol Exp Ther 349:568–576. [DOI] [PubMed] [Google Scholar]
- Boix F, Andersen JM, Mørland J. (2013) Pharmacokinetic modeling of subcutaneous heroin and its metabolites in blood and brain of mice. Addict Biol 18:1–7. [DOI] [PubMed] [Google Scholar]
- Brown GP, Yang K, King MA, Rossi GC, Leventhal L, Chang A, Pasternak GW. (1997) 3-Methoxynaltrexone, a selective heroin/morphine-6beta-glucuronide antagonist. FEBS Lett 412:35–38. [DOI] [PubMed] [Google Scholar]
- Eriksen GS, Andersen JM, Boix F, Mørland J. (2014) 3-Methoxynaltrexone is not a selective antagonist for the acute psychomotor stimulating effects of heroin and 6-monoacetylmorphine in mice. Pharmacol Biochem Behav 122:82–88. [DOI] [PubMed] [Google Scholar]
- Frölich N, Dees C, Paetz C, Ren X, Lohse MJ, Nikolaev VO, Zenk MH. (2011) Distinct pharmacological properties of morphine metabolites at G(i)-protein and β-arrestin signaling pathways activated by the human μ-opioid receptor. Biochem Pharmacol 81:1248–1254. [DOI] [PubMed] [Google Scholar]
- Gottås A, Boix F, Øiestad EL, Vindenes V, Mørland J. (2014) Role of 6-monoacetylmorphine in the acute release of striatal dopamine induced by intravenous heroin. Int J Neuropsychopharmacol 17:1357–1365. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Kornetsky C. (1992) Heroin, 6-acetylmorphine and morphine effects on threshold for rewarding and aversive brain stimulation. J Pharmacol Exp Ther 260:562–567. [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, et al. (2012) Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32:11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, et al. (2008) Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci 28:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, et al. (2010) Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 24:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima I, Minamikawa J, Jacobson AE, Brossi A, Rice KC. (1978) Studies in the (+)-morphinan series. 5. Synthesis and biological properties of (+)-naloxone. J Med Chem 21:398–400. [DOI] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. (1983) Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci 33 (Suppl 1):773–776. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Widerlöv E, Stanley D, Breese G, Prange AJ., Jr (1983) Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J Pharmacol Exp Ther 227:229–237. [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. (2009) Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci USA 106:4917–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK, Pentel PR. (2013) Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J Pharmacol Exp Ther 344:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (2001) Incentive-sensitization and addiction. Addiction 96:103–114. [DOI] [PubMed] [Google Scholar]
- Rook EJ, Huitema AD, van den Brink W, van Ree JM, Beijnen JH. (2006) Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. Curr Clin Pharmacol 1:109–118. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Brown GP, Leventhal L, Yang K, Pasternak GW. (1996) Novel receptor mechanisms for heroin and morphine-6 beta-glucuronide analgesia. Neurosci Lett 216:1–4. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AAK, Stowe GN, Edwards S, Janda KD, Koob GF. (2013) Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci USA 110:9036–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, et al. (1999) Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci 2:151–156. [DOI] [PubMed] [Google Scholar]
- Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC, Watkins LR, et al. (2013) Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry 73:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umans JG, Inturrisi CE. (1981) Pharmacodynamics of subcutaneously administered diacetylmorphine, 6-acetylmorphine and morphine in mice. J Pharmacol Exp Ther 218:409–415. [PubMed] [Google Scholar]
- van Ree JM, Slangen JL, de Wied D. (1978) Intravenous self-administration of drugs in rats. J Pharmacol Exp Ther 204:547–557. [PubMed] [Google Scholar]
- Vindenes V, Handal M, Ripel A, Thaulow CH, Vindenes HB, Boix F, Mørland J. (2008) Different time schedules affect conditioned place preference after morphine and morphine-6-glucuronide administration. Pharmacol Biochem Behav 89:374–383. [DOI] [PubMed] [Google Scholar]
- Vindenes V, Ripel A, Handal M, Boix F, Mørland J. (2009) Interactions between morphine and the morphine-glucuronides measured by conditioned place preference and locomotor activity. Pharmacol Biochem Behav 93:1–9. [DOI] [PubMed] [Google Scholar]
- Walker JR, King M, Izzo E, Koob GF, Pasternak GW. (1999) Antagonism of heroin and morphine self-administration in rats by the morphine-6beta-glucuronide antagonist 3-O-methylnaltrexone. Eur J Pharmacol 383:115–119. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, Watkins LR. (2016) Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol 173:856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way EL, Young JM, Kemp JW. (1965) Metabolism of heroin and its pharmacologic implications. Bull Narc 17:25–33. [Google Scholar]
- Wise RA, Bozarth MA. (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492. [PubMed] [Google Scholar]
- Wu HE, Sun HS, Cheng CW, Terashvili M, Tseng LF. (2006) dextro-Naloxone or levo-naloxone reverses the attenuation of morphine antinociception induced by lipopolysaccharide in the mouse spinal cord via a non-opioid mechanism. Eur J Neurosci 24:2575–2580. [DOI] [PubMed] [Google Scholar]