Abstract
Whereas the inhibition of fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL), the respective major hydrolytic enzymes of N-arachidonoyl ethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), elicits no or partial substitution for Δ9-tetrahydrocannabinol (THC) in drug-discrimination procedures, combined inhibition of both enzymes fully substitutes for THC, as well as produces a constellation of cannabimimetic effects. The present study tested whether C57BL/6J mice would learn to discriminate the dual FAAH-MAGL inhibitor SA-57 (4-[2-(4-chlorophenyl)ethyl]-1-piperidinecarboxylic acid 2-(methylamino)-2-oxoethyl ester) from vehicle in the drug-discrimination paradigm. In initial experiments, 10 mg/kg SA-57 fully substituted for CP55,940 ((-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol), a high-efficacy CB1 receptor agonist in C57BL/6J mice and for AEA in FAAH (−/−) mice. Most (i.e., 23 of 24) subjects achieved criteria for discriminating SA-57 (10 mg/kg) from vehicle within 40 sessions, with full generalization occurring 1 to 2 hours postinjection. CP55,940, the dual FAAH-MAGL inhibitor JZL195 (4-nitrophenyl 4-(3-phenoxybenzyl)piperazine-1-carboxylate), and the MAGL inhibitors MJN110 (2,5-dioxopyrrolidin-1-yl 4-(bis(4-chlorophenyl)methyl)piperazine-1-carboxylate) and JZL184 (4-[Bis(1,3-benzodioxol-5-yl)hydroxymethyl]-1-piperidinecarboxylic acid 4-nitrophenyl ester) fully substituted for SA-57. Although the FAAH inhibitors PF-3845 ((N-3-pyridinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl]methyl]-1-piperidinecarboxamide) and URB597 (cyclohexylcarbamic acid 3′-(aminocarbonyl)-[1,1′-biphenyl]-3-yl ester) did not substitute for SA-57, PF-3845 produced a 2-fold leftward shift in the MJN110 substitution dose-response curve. In addition, the CB1 receptor antagonist rimonabant blocked the generalization of SA-57, as well as substitution of CP55,940, JZL195, MJN110, and JZL184. These findings suggest that MAGL inhibition plays a major role in the CB1 receptor-mediated SA-57 training dose, which is further augmented by FAAH inhibition.
Introduction
Cannabinoid CB1 (Devane et al., 1988; Matsuda et al., 1990) and CB2 receptors (Munro et al., 1993) and their endogenous ligands N-arachidonoyl ethanolamine (anandamide; AEA) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al., 1995; Sugiura et al., 1995) represent primary elements of the endocannabinoid system. This system modulates many physiologic processes, including pain (Hohmann et al., 2005; Kinsey et al., 2010; Woodhams et al., 2012; Ignatowska-Jankowska et al., 2014), memory (Hampson and Deadwyler, 1999), appetite (Kirkham and Tucci, 2006), and reward (Tsou et al., 1998; Marsicano and Lutz, 1999). The primary psychoactive constituent of Cannabis, Δ9-tetrahydrocannabinol (THC) (Gaoni and Mechoulam 1964) produces its psychotomimetic effects through CB1 receptors (Huestis et al., 2001) and induces dopamine release in the nucleus accumbens (Chen et al., 1991), although to a substantially lower magnitude than other abused drugs. Curiously, THC produces reinforcing effects in some (Gardner et al., 1988; Lepore et al., 1996; Justinova et al., 2003, 2005), but not all (Vlachou et al., 2007; Wiebelhaus et al., 2015), preclinical laboratory animal models. In contrast, THC serves as a reliable discriminative stimulus in the drug-discrimination paradigm (Henriksson et al., 1975; Järbe, 1989; Wiley et al., 1997; Vann et al., 2009), an assay that is highly predictive of drug psychoactivity in humans (Chait et al., 1988; Kamien et al., 1993; Lile et al., 2012).
Whereas THC elicits relatively long-lasting pharmacologic effects, AEA and 2-AG produce short-lived effects because of rapid hydrolysis by their respective primary catabolic enzymes fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996, 2001) and monoacylglycerol lipase (MAGL) (Di Marzo et al., 1999; Dinh et al., 2002). Accordingly, inhibitors of these enzymes elevate endocannabinoid brain levels and represent useful investigative tools. Although the selective FAAH inhibitors URB597 (Fu et al., 2005) and PF-3845 (Ahn et al., 2009) elevate AEA brain levels and produce antinociceptive effects, neither compound substitutes for THC (Gobbi et al., 2005; Wiley et al., 2014). Similarly, the MAGL inhibitor JZL184 elevates endogenous 2-AG brain levels and produces antinociception, but it only partially substitutes for THC (Long et al., 2009a,b; Wiley et al., 2014; Walentiny et al., 2015). Conversely, the dual FAAH-MAGL inhibitor JZL195 fully substitutes for THC, elicits a constellation of cannabimimetic effects (Long et al., 2009b; Wise et al., 2012; Hruba et al., 2015), and produces an increased magnitude of antinociceptive effects compared with single enzyme inhibition (Long et al., 2009b; Ghosh et al., 2015). Similarly, the dual FAAH-MAGL inhibitor SA-57 fully substitutes for THC in wild-type mice (Hruba et al., 2015).
As it has yet to be established whether an inhibitor of endocannabinoid hydrolysis can serve as the training drug in drug-discrimination procedures, the present study investigated whether mice will learn to discriminate SA-57 from vehicle. SA-57 inhibits FAAH much more potently than it inhibits MAGL or ABHD6, another serine hydrolase that degrades 2-AG, but to a much less extent than MAGL (Blankman et al., 2007). Thus, SA-57 possesses utility to investigate the consequences of maximally elevating brain AEA levels while dose-dependently increasing brain 2-AG levels (Niphakis et al., 2012). To select the SA-57 training dose, initial experiments examined its dose-effect relationship to substitute for the potent CB1 receptor agonist CP55,940 in C57BL/6J mice and AEA in FAAH (−/−) mice (to prevent rapid hydrolysis). Having established that mice learn to discriminate SA-57 from vehicle, we then assessed its dose-response relationship and time course. Because various substrates of FAAH (e.g., AEA, palmitoylethanolamide, and oleoylethanolamide and MAGL; e.g., 2-AG) bind CB1, CB2, TRPV1 (Smart et al., 2000), and peroxisome proliferator-activated receptor-α (PPARα) receptors (Lo Verme et al., 2005), we tested whether antagonists for these receptors would block the discriminative stimulus effects of SA-57. Additionally, we conducted an extensive series of drug substitution tests to gain further insight into the training dose of the SA-57 discriminative stimulus. Specifically, we tested whether CP55,940, as well as the noncannabinoid psychoactive drugs nicotine and diazepam, would substitute for the SA-57. As MAGL also plays a rate-limiting role in the production of arachidonic acid and prostanoids in brain (Nomura et al., 2011), we examined whether the COX-2 inhibitor valdecoxib, which reduces prostanoid synthesis but does not affect brain endocannabinoid levels, would substitute for SA-57. The final goal of the present study was to elucidate the degree to which relevant endocannabinoid hydrolytic enzyme inhibitors contribute to the SA-57 training dose. Accordingly, we investigated whether individual FAAH, MAGL, and ABHD6 inhibitors, as well as simultaneous inhibition of FAAH and MAGL, would substitute for SA-57.
Materials and Methods
Subjects
Male C57BL6/J mice (Jackson Laboratory; Bar Harbor, ME) and male FAAH (−/−) mice served as subjects. The FAAH (−/−) mice were backcrossed >14 generations on to a C57BL6/J background. The mice were 9–11 weeks of age at the beginning of training and were individually housed in a temperature-controlled (20–22°C) vivarium in accordance with Virginia Commonwealth University Institutional Animal Care and Use Committee guidelines. Mice were given water ad libitum and were food-restricted to 85%–90% of free-feed body weight, which was established during a 2-week period of ad libitum food every 6 months.
Drugs
SA-57, MJN110, KT182, KT195, and JZL195 were synthesized in the Cravatt Laboratory, as previously described (Long et al., 2009b; Niphakis et al., 2012, 2013; Hsu et al., 2013). N-arachidonoyl ethanolamine (AEA) was provided by Organix Inc. (Woburn, MA), and valdecoxib was provided by Sigma-Aldrich (Saint Louis, MO). CP55,940, JZL184, PF-3845, rimonabant, and SR144528 were generously supplied by the National Institute on Drug Abuse (NIDA) (Rockville, Maryland). Capsazepine was purchased from Cayman Chemical, and GW6471 was purchased from Tocris Bioscience (Ellisville, MO). Each compound was dissolved in a vehicle consisting of ethanol, emulphor-620 (Rhodia, Cranbury, NJ), and saline in a ratio of 1:1:18. All injections were given via the i.p. route of administration in a volume of 10 μl per 1 g of body weight.
Apparatus
Drug-discrimination was conducted in eight sound-attenuating operant conditioning boxes (18 × 18 × 18 cm) (MED Associates, St. Albans, VT). Each operant box contained two nose-poke apertures and a food dispenser delivering 14-mg food pellets to a receptacle chamber located between apertures. Computer software (MED-PC IV, MED Associates) was used to record nose pokes and to control stimulus presentations and food deliveries.
Drug-Discrimination Paradigm
Training.
Separate groups of mice were trained to discriminate each of the following three training drugs from vehicle. Groups 1 and 2 consisted of C57BL6/J mice (n = 8) trained to discriminate CP55,940 and FAAH (−/−) mice (n = 11) trained to discriminate AEA, respectively. The third group of mice consisted of three cohorts of C57BL6/J mice (n = 8/cohort) trained to discriminate SA-57 from vehicle. The treatment conditions for each cohort are described below in the Testing section as follows. The pretreatment times for the training drugs were 120 minutes for SA-57 and 30 minutes for CP55,940 and AEA. During each 15-minute training session, both nose-poke apertures were available, but only responses into the correct aperture associated with the appropriate training drug or vehicle resulted in food reinforcement. Each incorrect response reset the response requirement. Injections before training sessions were conducted (Monday–Friday) in a double-alternation sequence of drug (SA-57, CP55,940, or AEA) and vehicle (e.g., vehicle, vehicle, drug, drug).
Testing.
Test sessions were scheduled twice per week, with a minimum of 72 hours between test days. To be eligible for testing, subjects were required to meet the following three criteria on nine of the previous 19 consecutive training sessions: 1) correct completion of the first FR10 (i.e., first 10 consecutive responses into the appropriate aperture), 2) ≥80% correct responding, and 3) maintain response rates ≥10 responses/min. During the 15-minute test sessions, responses in either aperture resulted in the delivery of food reinforcement according to an FR10 schedule of reinforcement, without a limitation on the number of reinforcers earned within a session. Before conducting substitution tests, dose-response tests with SA-57, CP55,940, or AEA were conducted to characterize their generalization gradients to their respective discriminative stimulus. For time-course studies, animals were injected with SA-57 (10 mg/kg) and tested 0.25, 1, 2, 4, or 8 hours after injection. To assess whether CB1 receptors mediated the discriminative effects of SA-57 and the substitution of CP55,940, MJN110, JZL184, and JZL195, we used rimonabant (3 mg/kg; Rinaldi-Carmona et al., 1994). We also examined whether the CB2 receptor antagonist SR144528 (3 mg/kg; Rinaldi-Carmona et al., 1998), the TRPV1 receptor antagonist capsazepine (5 mg/kg; Kinsey et al., 2009), and the PPARα receptor antagonist GW6471 (2 mg/kg; Lo Verme et al., 2005) would block the discriminative stimulus effects of SA-57. Each antagonist was administered 15 minutes before injections of 10 mg/kg SA-57. The three cohorts of mice trained to discriminate SA-57 were used in the following experiments. All cohorts were included in the SA-57 acquisition curve. Cohort 1 was used in the time-course study, the MJN110 (0.25–5 mg/kg), KT182 (1 and 2 mg/kg), KT195 (40 mg/kg), valdecoxib (10 mg/kg), and MJN110 (2.5 mg/kg) + PF3845 (10 mg/kg) substitution studies; cohort 2 was used to test the psychoactive noncannabinoid drugs nicotine (1.5 mg/kg) and diazepam (10 mg/kg) and in substitution tests with JZL195 (2–20 mg/kg), JZL184 (4–100 mg/kg), PF3845 (10 and 30 mg/kg), and URB597 (10 mg/kg); and cohort 3 was used in the receptor antagonist experiments (rimonabant, SR144528, capsazepine, GW6471).
[3H]SR141716A Binding Assay.
Cerebella were dissected from adult male ICR mice, stored at −80°C, and membranes were prepared as described previously (Selley et al., 2004). Membrane protein (15 μg) was incubated with 0.94 nM [3H]SR141716A in assay buffer (50 mM Tris-HCl, pH 7.4, 3 mM MgCl2 and 0.2 mM EGTA) with 0.5% (wt/vol) bovine serum albumin (BSA) in the presence and absence of 5 μM unlabeled SR141716A to determine nonspecific and specific binding, respectively. The assay was incubated for 90 minutes at 30°C and terminated by rapid filtration under vacuum through Whatman GF/B glass fiber filters that were presoaked in Tris buffer containing 0.5% (wt/vol) BSA (Tris-BSA), followed by five washes with cold Tris-BSA. Bound radioactivity was determined by liquid scintillation spectrophotometry at 45% efficiency in ScintiSafe Econo 1 scintillation fluid after a 12-hour delay.
Data Analysis.
The percentage of drug appropriate responses and response rates (responses/min) were recorded for each experiment. Full substitution was defined as 80% or more nose pokes that occurred into aperture associated with the training drug. Partial substitution was defined as 20% or greater and less than 80% nose pokes in the training drug-paired aperture. Less than 20% nose pokes on the drug-paired aperture was defined as no substitution (Solinas et al., 2006). ED50 values (and 95% confidence intervals) for generalization or substitution were calculated using least-squares linear regression analysis. Behavioral data are depicted as mean ± S.E.M. The data were analyzed using one-way or two-way analysis of variance (ANOVA). Dunnett’s tests or Bonferroni’s post hoc analyses were used after a significant ANOVA for the response rate data. GraphPad Prism 6.0 statistical software (Graph Pad Software, Inc., La Jolla, CA) was used for data analysis.
Binding data were determined in triplicate and are reported as specific binding. Each competition data set was analyzed by one-way ANOVA to determine concentration dependence. Rimonabant competition curves were analyzed by nonlinear regression to determine IC50 and Hill coefficients using a four-parameter fit with GraphPad Prism 6.0. The IC50 values were then converted to Ki values using the Cheng-Prusoff equation.
Results
SA-57 Substitutes for CP55,940 in C57BL/6J Mice and AEA in FAAH (−/−) Mice.
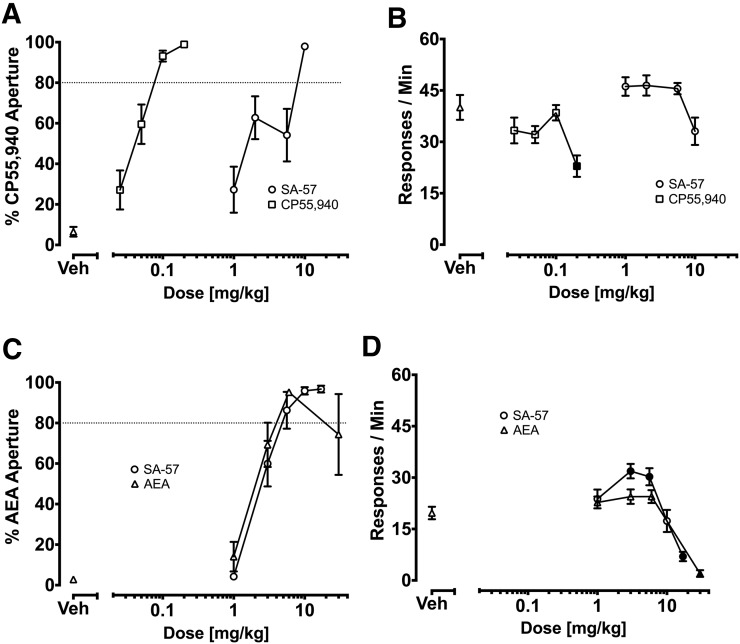
Figure 1 shows that SA-57 fully substituted for CP55,940 and AEA in mice trained to discriminate each of these drugs. C57BL/6J mice administered either CP55,940 or SA-57 completely occasioned the discriminative stimulus effects of CP55,940 (Fig. 1A). SA-57 did not affect response rates; however, CP55,940 significantly reduced response rates [F (4,55) = 4.7; P < 0.01], with 0.2 mg/kg yielding significant reductions in response rates compared with vehicle (Fig. 1B). In FAAH (−/−) mice trained to discriminate AEA (6 mg/kg) from vehicle, SA-57 also fully substituted for AEA (Fig. 1C). FAAH (−/−) mice administered AEA (1-30 mg/kg) or SA-57 (1–10 mg/kg) dose dependently selected the aperture associated with AEA (Fig. 1C). Both AEA [F (4, 50) = 27.5; P < 0.001] and SA-57 [F (5, 46) = 15.27; P < 0.001] significantly reduced response rates (Fig. 1D). The highest doses tested of AEA (i.e., 30 mg/kg) and SA-57 (i.e., 17 mg/kg) significantly depressed response rates compared with vehicle in FAAH (−/−) mice.
Fig. 1.
Effects of CP55,940, AEA, and SA-57 on the percentage of responses in training drug-paired apertures and response rates in C57BL/6J mice trained to discriminate CP55,940 (0.1 mg/kg; (A, B) or FAAH (−/−) mice trained to discriminate AEA (6 mg/kg; C, D). (A) Dose-dependent generalization of CP55,940 and dose-dependent substitution of SA-57 for the CP55,940-discriminative stimulus. The respective ED50 (95% confidence interval [CI]) values for CP55,940 generalization and SA-57 substitution in C57BL/6J mice were 0.04 (0.03–0.05) mg/kg and 2.4 (1.6–3.6) mg/kg. (B) CP55,940 (0.2 mg/kg), but not SA-57, significantly decreased rates of responding compared with vehicle. (C) Dose-dependent generalization of AEA and dose-dependent substitution of SA-57. The respective ED50 (95% CI) values for AEA and SA-57 in FAAH (−/−) mice were 2.7 (2.3-3.1) mg/kg and 3.1 (2.8–3.4) mg/kg. (D) SA-57 (17 mg/kg) and AEA (30 mg/kg) decreased rates of responding. Values represent mean ± S.E.M. Filled symbols indicate significant difference (P < 0.001) versus vehicle; n = 7-10 mice/group.
SA-57 Discriminative Stimulus.
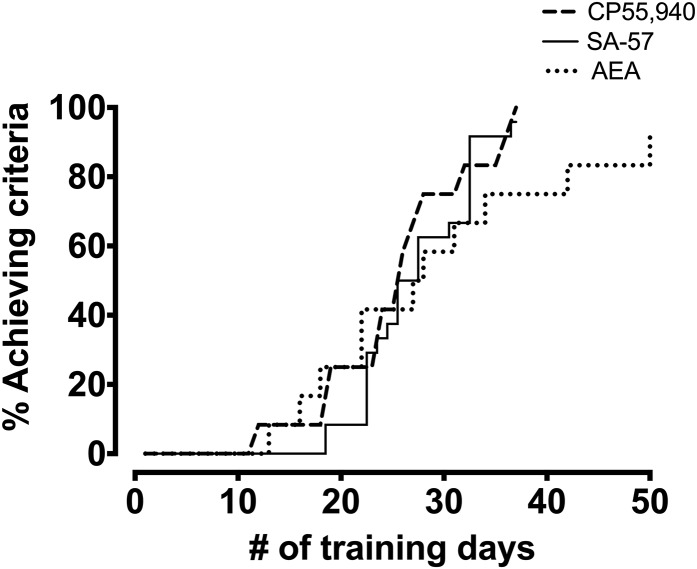
Because 10 mg/kg SA-57 fully substituted for CP55,940 in C57BL/6J mice and for FAAH (−/−) mice, this dose of SA-57 was selected as the training dose in three naïve cohorts of mice (n = 8 mice/group). As shown in Fig. 2, 50% of mice achieved the criteria to discriminate SA-57 from vehicle by the 27th training session, and 23 of 24 mice acquired the discrimination by day 40. The final mouse achieved criteria on day 74 of training but was excluded from subsequent experiments because of its substantial delay in acquisition. Similar rates of acquisition were found for CP55,940 in C57BL/6J mice and AEA in FAAH (−/−) mice.
Fig. 2.
Acquisition rates of SA-57 (10 mg/kg) in C57BL/6J mice, AEA (10 mg/kg) in FAAH (−/−) mice, and CP55,940 (0.1 mg/kg) in C57BL/6J mice trained in drug discrimination. Values represent the percentage of mice that achieved criteria (see text) across days. n = 24 mice for SA57, 12 for AEA, and 12 for CP55,940.
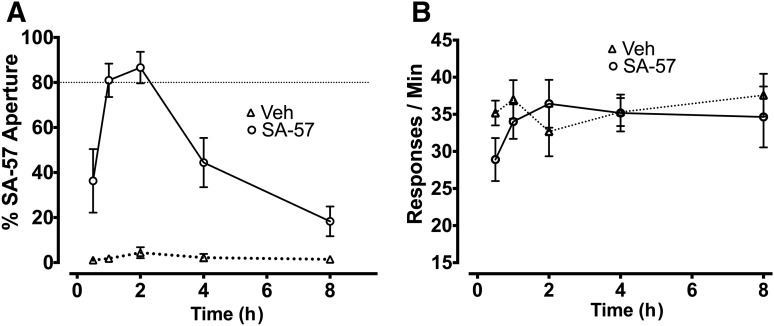
Figure 3 shows the time-course effects of 10 mg/kg SA-57 versus vehicle for selecting the aperture associated with SA-57 (Fig. 3A) and response rates (Fig. 3B). Whereas mice that received vehicle responded consistently on the vehicle-associated aperture at each of the time points, mice administered 10 mg/kg SA-57 selected the SA-57 aperture ≥80% at 1 and 2 hours postinjection, showed partial substitution at 0.25 and 4 hours and responded predominantly on the vehicle aperture 8 hours after injection. No differences were found in the rates of responding between mice injected with vehicle or SA-57 at any time point (Fig. 3B; P = 0.48).
Fig. 3.
Time-course effects for occasioning the 10 mg/kg SA-57 training dose. (A) Percentage of responses in the SA-57-associated aperture 0.25, 1, 2, 4, or 8 hours after an injection of vehicle or SA-57 (10 mg/kg). (B) SA-57 did not affect response rates at any time point after administration. Values represent mean ± S.E.M.; n = 7 mice/group.
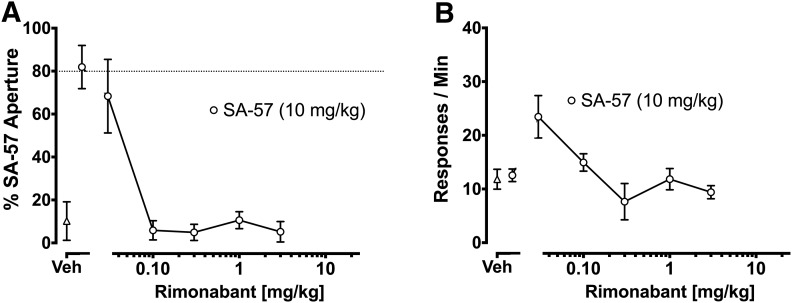
As shown in Fig. 4, the CB1 receptor antagonist rimonabant (0.03–3 mg/kg) significantly blocked the SA-57 training dose. In contrast, the CB2 receptor antagonist SR144528 (3 mg/kg), the TRPV1 receptor antagonist capsazepine (5 mg/kg), and the PPARα receptor antagonist GW6471 (2 mg/kg) did not block the SA-57 training dose (Table 1).
Fig. 4.
CB1 receptors play a necessary role in the SA-57 discriminative stimulus. (A) Rimonabant (0.3–3 mg/kg) significantly attenuated the SA-57 training dose. (B) Rimonabant doses (i.e., 0.1, 0.3, 1, 3 mg/kg) that blocked the SA-57 training dose did not reduce response rates. Values represent mean ± S.E.M.; n = 3–6 mice/group.
TABLE 1.
Cannaboid 1 receptor (CB1) receptors mediate the discriminative stimulus effects of the SA-57 (10 mg/kg) training dose
The CB1 receptor antagonist rimonabant (3 mg/kg) significantly blocked the discriminative stimulus effects of SA-57 (10 mg/kg) as well as substitution of CP55,940 (0.1 mg/kg). The CB2 receptor antagonist SR144528 (3 mg/kg), the TRPV1 receptor antagonist capsazepine (5 mg/kg), and the PPARα receptor antagonist GW6471 (2 mg/kg) did not block the SA-57 (10 mg/kg) discriminative stimulus. The vehicle-vehicle and rimonabant-vehicle conditions are the same as those used in Fig. 9. Values represent mean ± S.E.M. n = 6–8 mice/group.
| Drug | Antagonist | % SA-57 Substitution ± S.E.M. | Nose Pokes/min ± S.E.M. |
|---|---|---|---|
| Vehicle | Vehicle | 12.8 ± 9.4 | 38.9 ± 3 |
| Rimonabant | 4.0 ± 1.2 | 24.9 ± 3 | |
| SR144528 | 0.7 ± 0.3 | 36.6 ± 3.8 | |
| Capsazepine | 1.3 ± 0.4 | 20.1 ± 2.6 | |
| GW6471 | 0.3 ± 2.6 | 24.6 ± 3.1 | |
| SA-57 | Vehicle | 95.7 ± 1.7 | 27.3 ± 1.9 |
| Rimonabant | 3.4 ± 1.2 | 20.1 ± 2.5 | |
| SR144528 | 98 ± 1.5 | 30.5 ± 5.4 | |
| Capsazepine | 86 ± 12.2 | 15.7 ± 2.9 | |
| GW6471 | 96.5 ± 1.3 | 19.0 ± 2 | |
| CP55,940 | Vehicle | 82.5 ± 11 | 33.1 ± 3.3 |
| Rimonabant | 10.4 ± 5.7 | 20.7 ± 4.9 |
SA-57 Does Not Bind CB1 Receptors.
As the SA-57 discriminative stimulus required CB1 receptor activation, we next examined whether this compound interacts directly with CB1 receptors. Accordingly, we tested whether SA-57 would displace [3H]SR141716A binding in mouse cerebellar membranes. As shown in Fig. 5, rimonabant (i.e., unlabeled SR141716A) inhibited [3H]SR141716A binding in a concentration-dependent manner (P < 0.001, F = 17.36, df = 7), with a Ki value of 0.75 ± 0.16 nM and a Hill coefficient of 0.97 ± 0.08. In contrast, SA-57 (0.01–10 μM) did not inhibit [3H]SR141716A binding (P = 0.96; Fig. 5), indicating that this compound does not directly interact with CB1 receptors.
Fig. 5.
SA-57 does not compete with [3H]SR141716A binding to CB1 receptors in mouse cerebellum. Data represent mean [3H]SR141716A bound (pmol/mg) ± S.E.M. in the presence of varying concentrations of rimonabant or SA-57 (n = 3). Specific binding of [3H]SR141716A in the absence of competing ligand was 1.65 ± 0.26 pmol/mg. Similar results were obtained with [3H]CP55,940 binding in membranes prepared from Chinese hamster ovary cells stably expressing the mouse CB1 receptor, in which concentrations of up to 10 μM SA-57 did not affect binding (data not shown).
Substitution Tests in SA-57 Discriminating Mice.
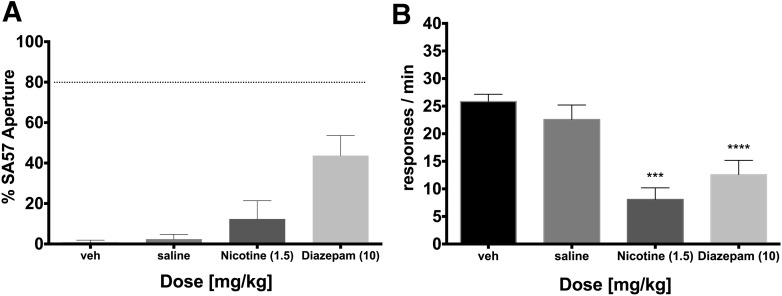
We next tested whether the noncannabinoid, psychoactive compounds nicotine and diazepam would substitute for SA-57. As shown in Fig. 6A, nicotine did not substitute for SA-57, but diazepam produced partial substitution. Both drugs significantly reduced response rates [Fig. 6B; F (3,28) = 14.01; P < 0.001], demonstrating that behaviorally active doses were reached.
Fig. 6.
Substitution experiments of noncannabinoid psychoactive drugs nicotine (1.5 mg/kg) and diazepam (10 mg/kg) for the SA-57 training dose. (A) Nicotine did not substitute, whereas diazepam partially substituted for the SA-57 training dose. (B) Nicotine (1.5 mg/kg) and diazepam (10 mg/kg) significantly reduced the rates of responding. Values represent mean ± S.E.M. Asterisks indicate significant difference (P < 0.05) versus vehicle; n = 7 to 8 mice/group.
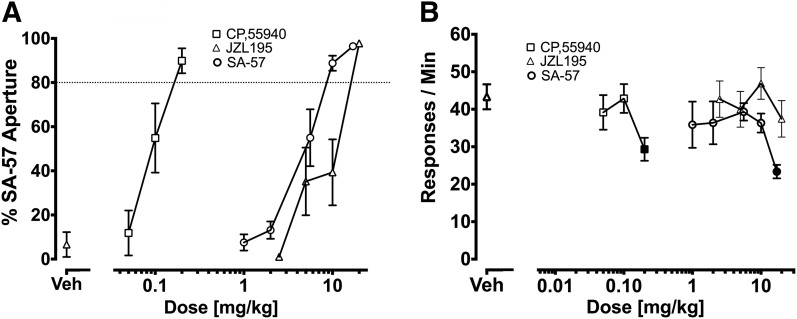
Figure 7 shows the dose-effect curves of the mixed CB1/CB2 receptor agonist CP55,940, the dual FAAH-MAGL inhibitor JZL195, and SA-57 in mice trained to discriminate SA-57 (10 mg/kg) from vehicle. CP55,940, JZL195, and SA-57 produced dose-related responding into the aperture associated with SA-57 (Fig. 7A). CP55,940 [F (3,28) = 2.99, P < 0.05] and SA-57 [F (4,42) = 2.78, P < 0.05], but not JZL195, reduced response rates (Fig. 7B).
Fig. 7.
Evaluation of the dose-response relationships of SA-57, CP55,940, and JZL195 to occasion the SA-57 (10 mg/kg) discriminative stimulus. (A) SA-57 produced dose-dependent generalization, and CP55,940 and JZL195 dose dependently substituted for SA-57. The respective ED50 (95% CI) values for CP55,940 substitution, JZL195, and SA-57 generalization were 0.096 (0.076–0.121) mg/kg, 6.2 (3.5–10.9) mg/kg, and 4.4 (3.5–5.4) mg/kg. (B) High doses of CP55,940 (0.2 mg/kg) or SA-57 (17 mg/kg) significantly reduced response rates. Values represent mean ± S.E.M. Filled symbols indicate significant difference (P < 0.05) versus vehicle; n = 7 or 8 mice/group.
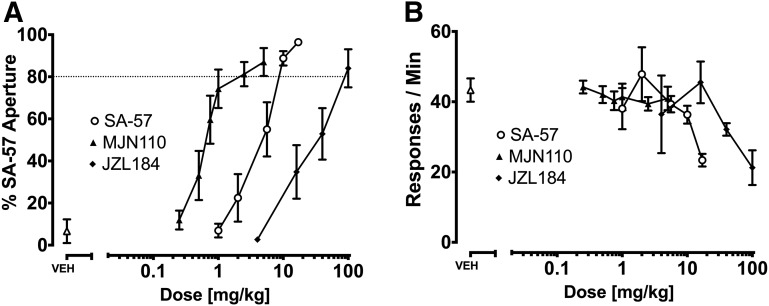
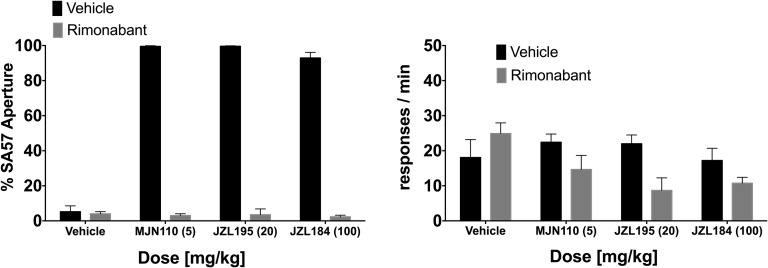
SA-57 generalized to itself in a dose-dependent fashion, and the MAGL inhibitors MJN110 and JZL184 dose dependently substituted for SA-57 (Fig. 8A). Although MJN110 did not affect response rates [F (6, 48) = 0.33, P = 0.92], the highest doses of SA-57 (17 mg/kg) [F (5, 42) = 3.391, P < 0.05] and JZL184 (100 mg/kg) [F (4, 18) = 3.985, P < 0.05] significantly reduced response rates (Fig. 8B). As shown in Fig. 9A, rimonabant (3 mg/kg) completely blocked substitution of MJN110 (5 mg/kg), JZL184 (100 mg/kg), and JZL195 (20 mg/kg) for the SA-57 training dose. Also, rimonabant significantly reduced rates of responding [Fig. 9B; F (1, 29) = 11.91, P < 0.01].
Fig. 8.
Evaluation of the dose-response relationships of SA-57, MJN110, and JZL184 to occasion the SA-57 (10 mg/kg) stimulus. (A) Dose-dependent generalization of SA-57 and dose-dependent substitution of MJN110 and JZL184. The respective ED50 (95% CI) values for MJN110 and JZL184 generalization and SA-57 substitution in C57BL/6J mice were 0.77 (0.53–1.1) mg/kg and 20.44 (11–37.97) mg/kg, and 4.39 (3.53–5.45) mg/kg. (B) SA-57 (17 mg/kg) and JZL184 (100 mg/kg) significantly decreased the rates of responding. Values represent mean ± S.E.M. **Significant difference (P < 0.001) versus vehicle; n = 7 or 8 mice/group.
Fig. 9.
Substitution of MJN110 (5 mg/kg), JZL184 (100 mg/kg), and JZL195 (20 mg/kg) for SA-57 (10 mg/kg) requires CB1 receptors. (A) Rimonabant (3 mg/kg) completely blocked MJN110, JZL184, and JZL195 substitution. (B) Rimonabant did affect response rates. Values represent mean ± S.E.M.; n = 7 or 8 mice/group.
In contrast, mice administered high doses of the FAAH inhibitors PF-3845 (10 and 30 mg/kg) and URB597 (10 mg/kg) selected the vehicle aperture (Table 2). Likewise, mice given high doses of the ABHD6 inhibitors KT182 (1 and 2 mg/kg) or KT195 (40 mg/kg), as well as mice given high dose of the selective cyclooxygenase-2 inhibitor valdecoxib (10 mg/kg), selected the vehicle aperture.
TABLE 2.
Fatty acid amide hydrolase (FAAH) inhibitors (PF-3845 and URB597), ABHD6 inhibitors (KT182 and KT195), and the cyclooxygenase-2 (COX2) selective inhibitor valdecoxib do not substitute for the discriminative stimulus effects of SA-57 (10 mg/kg) in C57BL/6J mice and do not affect response ratesa
| Enzyme | Drug (mg/kg) | % SA-57 Substitution | Nose Pokes/Min |
|---|---|---|---|
| mg/kg | ±S.E.M. | ±S.E.M. | |
| FAAH | Vehicle | 0.6 ± 0.5 | 47.5 ± 4.9 |
| PF-3845 (10) | 1.5 ± 0.5 | 34.4 ± 3.8 | |
| PF-3845 (30) | 0.7 ± 0.2 | 38.9 ± 3.5 | |
| URB597 (10) | 2.1 ± 1.0 | 36.9 ± 5.6 | |
| ABHD6 | Vehicle | 1.1 ± 0.6 | 46.5 ± 2.6 |
| KT182 (1) | 1.4 ± 0.7 | 41.1 ± 3.5 | |
| KT182 (2) | 1.4 ± 0.8 | 43.5 ± 2.4 | |
| KT195 (40) | 0.8 ± 0.3 | 38.1 ± 3.1 | |
| COX2 | Vehicle | 1.1 ± 0.6 | 46.5 ± 2.6 |
| Valdecoxib (10) | 1.1 ± 0.7 | 31.9 ± 3.9 |
Values represent mean ± S.E.M.; n = 7 or 8 mice/group.
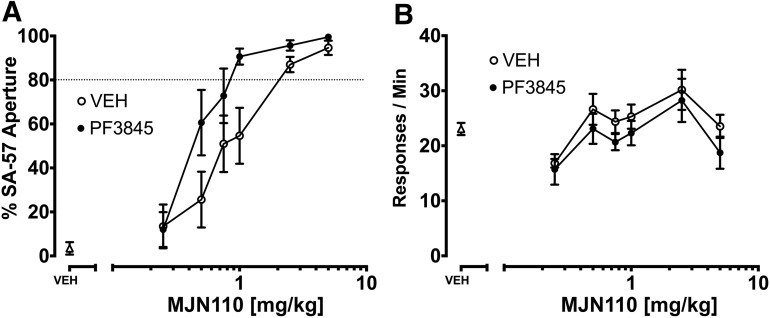
Because MAGL inhibitors, but not FAAH inhibitors, substituted for SA-57, we next examined whether full FAAH inhibition would elicit a leftward shift in the MAGL substitution dose-response curve. Accordingly, we tested the dose-response relationship of MJN110 with PF3845 (10 mg/kg) or vehicle for substitution in mice trained to discriminate SA-57 from vehicle. As shown in Fig. 10A, PF-3845 elicited a significant leftward shift in the MJN110 substitution dose-response curve [potency ratio (95% CL) = 1.84 (1.3–2.8)]. No significant changes were found for response rates (Fig. 10B).
Fig. 10.
The FAAH inhibitor PF-3845 augments the MJN110 substitution dose-response curve for SA-57 (10 mg/kg). (A) PF-3845 (10 mg/kg) produced a leftward shift of the MJN110 substitution dose-response curve. (B) None of the drug combinations significantly decreased the rates of responding. Values represent mean ± S.E.M.; n = 7 or 8 mice/group.
Discussion
The present study demonstrates that mice readily learn to discriminate the dual FAAH-MAGL inhibitor SA-57 from vehicle. Specifically, most (i.e., 23 of 24) subjects learned to discriminate the dual FAAH-MAGL inhibitor SA-57 from vehicle within 40 training sessions. The 10 mg/kg SA-57 training dose was previously demonstrated to produce significant increases in the brain levels of AEA and 2-AG (Wiebelhaus et al., 2015). As SA-57 fully blocks FAAH activity at lower doses (0.05–1 mg/kg) than those required to inhibit MAGL (1.25–12.5 mg/kg) (Niphakis et al., 2012) it provided a useful tool to examine the consequences of full FAAH inhibition while incrementally elevating brain 2-AG. The observation that 1 mg/kg SA-57, which produces maximal increases in endogenous AEA without detectable increases in 2-AG (Niphakis et al., 2012), did not generalize to the training dose (10 mg/kg SA-57) indicates that FAAH inhibition alone is not sufficient to occasion to the SA-57 training dose. Similarly, neither FAAH inhibitor (i.e., PF-3845 or URB597) substituted for SA-57. In contrast, the dual FAAH-MAGL inhibitor JZL195 and two MAGL inhibitors, MJN110 and JZL184, fully substituted for the SA-57 training dose, suggesting that MAGL inhibition alone may be sufficient for generalization to the 10 mg/kg SA-57 training dose. Interestingly, PF-3845 produced an approximately 2-fold leftward shift in the MJN110 substitution dose-response curve. The observation that rimonabant completely blocked the discriminative stimulus effects of SA-57 indicates that CB1 receptors play a necessary role in the subjective effects of SA-57. Similarly, rimonabant completely blocked the substitution of both MAGL inhibitors (MJN110 and JZL184) and the dual FAAH-MAGL inhibitor JZL195. These findings suggest that elevating endocannabinoid brain levels through the simultaneous blockade of FAAH and MAGL produces a CB1 receptor mediated interoceptive stimulus.
Consistent with previous studies reporting that SA-57 or the dual FAAH-MAGL inhibitor JZL195 substitute for the THC discriminative stimulus (Long et al., 2009b; Hruba et al., 2015; Walentiny et al., 2015), we found that SA-57 (10 mg/kg) fully substituted for the discriminative stimulus effects of the potent cannabinoid receptor agonist CP55,940 in C57BL/6J mice and the endogenous cannabinoid AEA (6 mg/kg) in FAAH (−/−) mice. The potency of SA-57 in producing a discriminative stimulus was similar to its potency in substituting for either CP55,940 or AEA. Furthermore, SA-57’s discriminative stimulus effects occurred at a training dose known to produce maximal increases in AEA and 2-AG (Niphakis et al., 2012). In addition, CP55,940 fully substituted for SA-57, an effect that was completely blocked by rimonabant, further implicating a pivotal role of CB1 receptors in these effects. Similarly, the dual FAAH-MAGL inhibitor JZL195 dose dependently substituted for SA-57. Time-course investigation revealed that SA-57 partially generalized at 0.5 hour, fully generalized at 1 and 2 hours, partially generalized at 4 hours, and by 8 hours, mice responded mostly on the aperture paired with vehicle.
It is noteworthy that MJN110 and JZL184 fully substituted for the discriminative stimulus effects of SA-57, whereas mice treated with a low dose of SA-57 (which inhibits FAAH and not MAGL), URB597, or PF-3845 selected the vehicle aperture. These findings suggest that MAGL inhibition represents a driving force underlying the SA-57 training dose; however, the observation that PF-3845 increased the potency of MJN110 to substitute for SA-57 suggests that FAAH inhibition increases the effectiveness of the discriminative stimulus produced by MAGL inhibition alone. The fact that 2-AG levels are approximately three orders of magnitude higher than AEA levels in wild-type mouse brain (Ahn et al., 2009; Long et al., 2009b) is consistent with the notion that MAGL inhibition elicits more prominent pharmacologic effects than those produced by FAAH inhibition. Moreover, as FAAH is expressed on the postsynaptic terminal (Gulyas et al., 2004) and MAGL (Dinh et al., 2002) is expressed on the pre-synaptic terminal, it is plausible that AEA and 2-AG activate distinct CB1 receptor–mediated neuronal circuits.
Because AEA and 2-AG bind CB1 and CB2 receptors, AEA also binds TRPV1 receptors, and other FAAH substrates (i.e., palmitoylethanolamide and oleoylethanolamide) bind PPARα receptors (Lo Verme et al., 2005), we examined whether selective antagonists for each of these receptors would block the discriminative stimulus effects of SA-57. Rimonabant, but not the other receptor antagonists, completely blocked the discriminative stimulus effects of SA-57. These findings indicate that CB1 receptor activation is required for the subjective effects of SA-57, whereas CB2, TRPV1, and PPARα receptors are dispensable. Moreover, the fact that SA-57 did not affect ligand binding to CB1 receptors in either a competitive or noncompetitive manner is consistent with the hypothesis that it increases brain endocannabinoid levels that then elicit a CB1 receptor-mediated discriminative stimulus.
The present study also assessed whether a variety of psychoactive noncannabinoid drugs would substitute for SA-57. Specifically, nicotine did not substitute for the SA-57 training dose, although it significantly reduced response rates. In contrast, diazepam partially substituted for SA-57, but it did so at a dose that reduced response rates. Similarly, diazepam partially substitutes for THC at high doses that produce motor impairment in the rat drug-discrimination paradigm (Wiley and Martin, 1999). Taken together, these studies suggest the possibility of a potential GABAergic component for CB1 receptor–mediated discriminative stimuli. In addition, because MAGL inhibition reduces brain levels of arachidonic acid as well as various prostanoids (Nomura et al., 2011), we tested whether the cyclooyygenase-2 inhibitor valdecoxib would substitute for SA-57; however, valdecoxib was devoid of action in this assay, suggesting that prostaglandins do not play a necessary role in the discriminative effects of SA-57.
It is noteworthy that the combined inhibition of FAAH and MAGL attenuates somatic signs of opioid withdrawal (Ramesh et al., 2011); however, simultaneous blockade of these enzymes also elicits other cannabimimetic effects, as assessed in the tetrad assay, including hypomotility, antinociception, catalepsy, and hypothermia (Long et al., 2009a; Anderson et al., 2014; Ghosh et al., 2015), as well as impaired performance in a Morris water maze spatial memory task (Wise et al., 2012). These effects of dual FAAH and MAGL inhibition are similar to those of THC, whereas single inhibition of either enzyme produces a decreased spectrum and magnitude of cannabimimetic effects. Specifically, the MAGL inhibitor JZL184 produces antinociception, hypomotility, and dysregulation of thermoregulation when challenged with manipulations that elicit hypothermia (Nass et al., 2015), whereas FAAH inhibition produces antinociception but not other cannabimimetic effects (Long et al., 2009b). However, drug-discrimination is more sensitive in detecting cannabimimetic effects compared with the tetrad assay. For example, THC is more potent in producing its discriminative stimulus effects than in eliciting the full set of tetrad effects (Long et al., 2009b; Marshell et al., 2014). Given that dual blockade of FAAH and MAGL significantly reduces locomotor activity (Long et al., 2009b) and SA-57 (10 mg/kg) completely inhibits FAAH and MAGL activity (Niphakis et al., 2012), the lack of any rate of suppressive effects of the training dose of SA-57 is interesting. Similarly, THC (5.6 mg/kg) reduces locomotor activity, but it does not reduce response rates in a drug-discrimination procedure (Wiley et al., 2005). This lack of apparent motor depression is consistent with the idea that rate-suppressive effects of drugs undergo tolerance throughout the course of drug-discrimination training (Solinas et al., 2006).
The results of the present study suggest that SA-57 serves as a discriminative stimulus at doses that produce increased levels of both AEA and 2-AG through a CB1 receptor mechanism of action, although elevated levels of 2-AG may be the main driving force for the SA-57 training dose. Although the brain regions mediating the discriminative stimulus effects of SA-57 and cannabinoid receptor agonists are unknown, it is noteworthy that endogenous cannabinoids and their receptors are located in neural pathways mediating the reinforcing effects of drugs of abuse (i.e., mesolimbic dopamine pathway) (Oleson and Cheer, 2012).
In conclusion, the present study demonstrates that the dual FAAH-MAGL inhibitor SA-57 serves as a reliable discriminative stimulus. The observations that rimonabant completely blocks the SA-57 training dose and that mice trained to discriminate SA-57, CP55,940, and AEA show symmetrical substitution strongly implicate the importance of the CB1 receptor in this novel interoceptive stimulus. Collectively, these findings raise the provocative possibility that FAAH and MAGL serve as dual brakes to prevent the psychoactive consequences of CB1 receptor overstimulation caused by elevated levels of AEA and 2-AG.
Acknowledgments
The authors thank the members of the Lichtman Laboratory for helpful discussions; the National Institute on Drug Abuse for supplying CP55,940, rimonabant, and SR144528; and Organix Inc. (Woburn, MA) for supplying anandamide.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- ABHD6
α/β-hydrolase domain 6
- AEA
N-arachidonoyl ethanolamine (anandamide)
- ANOVA
analysis of variance
- CB1
cannabinoid-1 receptor
- CB2
cannabinoid-2 receptor
- CP55,940
((-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol)
- FAAH
fatty acid amide hydrolase
- JZL184
4-[Bis(1,3-benzodioxol-5-yl)hydroxymethyl]-1-piperidinecarboxylic acid 4-nitrophenyl ester
- JZL195
4-nitrophenyl 4-(3-phenoxybenzyl)piperazine-1-carboxylate
- KT182
[4-[3′-(hydroxymethyl)[1,1′-biphenyl]-4-yl]-1H-1,2,3-triazol-1-yl](2-phenyl-1-piperidinyl)-methanone
- KT195
[4-(4′-methoxy[1,1′-biphenyl]-4-yl)-1H-1,2,3-triazol-1-yl](2-phenyl-1-piperidinyl)-methanone
- MAGL
monoacylglycerol lipase
- MJN110
2,5-dioxopyrrolidin-1-yl 4-(bis(4-chlorophenyl)methyl)piperazine-1-carboxylate
- PF-3845
N-3-pyridinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl]methyl]-1-piperidinecarboxamide
- SA-57
4-[2-(4-chlorophenyl)ethyl]-1-piperidinecarboxylic acid 2-(methylamino)-2-oxoethyl ester
- SR144,528
N-[(1S)-endo-1,3,3-trimethylbicyclo [2.2.1]heptan2-yl]-5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-1H-pyrazole-3-carboxamide
- URB597
cyclohexylcarbamic acid 3′-aminocarbonyl)-[1,1′-biphenyl]-3-yl ester
Authorship Contributions
Participated in research design: Owens, Ignatowska-Jankowska, Beardsley, Wiley, Selley, Cravatt, Lichtman.
Conducted experiments: Owens, Mustafa, Jali.
Contributed new reagents or analytic tools: Niphakis, Cravatt.
Performed data analysis: Owens, Mustafa, Selley, Lichtman.
Wrote or contributed to the writing of the manuscript: Owens, Ignatowska-Jankowska, Mustafa, Wiley, Beardsley, Selley, Lichtman.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA026449, R01DA032933, R01DA030404, P30DA033934, and T32DA007027].
References
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, et al. (2009) Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol 16:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WB, Gould MJ, Torres RD, Mitchell VA, Vaughan CW. (2014) Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine inflammatory pain model. Neuropharmacology 81:224–230 [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. (2007) A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 14:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. (1988) Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology (Berl) 94:206–212. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Lowinson JH, Gardner EL. (1991) Strain-specific facilitation of dopamine efflux by delta 9-tetrahydrocannabinol in the nucleus accumbens of rat: an in vivo microdialysis study. Neurosci Lett 129:136–180. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 98:9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, Kunos G. (1999) Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem 264:258–267. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 99:10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. (2005) Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology 48:1147–1153. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647. [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. (1988) Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl) 96:142–144. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Kinsey SG, Liu Q-S, Hruba L, McMahon LR, Grim TW, Merritt CR, Wise LE, Abdulla RA, Selley DE, et al. (2015) Full fatty acid amide hydrolase inhibition combined with partial monoacylglycerol lipase inhibition: augmented and sustained antinociceptive effects with reduced cannabimimetic side effects in mice. J Pharmacol Exp Ther 354:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, et al. (2005) Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA 102:18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. (2004) Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 20:441–458. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. (1999) Cannabinoids, hippocampal function and memory. Life Sci 65:715–723. [DOI] [PubMed] [Google Scholar]
- Henriksson BG, Johansson JO, Järbe TU. (1975) Delta 9-tetrahydrocannabinol produced discrimination in pigeons. Pharmacol Biochem Behav 3:771–774. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, et al. (2005) An endocannabinoid mechanism for stress-induced analgesia. Nature 435:1108–1112. [DOI] [PubMed] [Google Scholar]
- Hruba L, Seillier A, Zaki A, Cravatt BF, Lichtman AH, Giuffrida A, McMahon LR. (2015) Simultaneous inhibition of fatty acid amide hydrolase and monoacylglycerol lipase shares discriminative stimulus effects with Δ9-tetrahydrocannabinol in mice. J Pharmacol Exp Ther 353:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KL, Tsuboi K, Chang JW, Whitby LR, Speers AE, Pugh H, Cravatt BF. (2013) Discovery and optimization of piperidyl-1,2,3-triazole ureas as potent, selective, and in vivo-active inhibitors of α/β-hydrolase domain containing 6 (ABHD6). J Med Chem 56:8270–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. (2001) Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58:322–328. [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, O’ Neal ST, Walentiny DM, Wiley JL, et al. (2014) In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol 171:1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe T. (1989) Subjectively experienced cannabis effects in animals. Drug Dev Res 16:385–393. [Google Scholar]
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G. (2005) Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav 81:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. (2003) Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 169:135–140. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Hughes JR, Higgins ST, Smith BJ. (1993) Drug discrimination by humans compared to nonhumans: current status and future directions. Psychopharmacology (Berl) 111:259–270. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, Cravatt BF, Lichtman AH. (2010) Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J Pain 11:1420–1428 Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. (2009) Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 330:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Tucci SA. (2006) Endocannabinoids in appetite control and the treatment of obesity. CNS Neurol Disord Drug Targets 5:272–292. [DOI] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL. (1996) Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci 58:PL365–PL372. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. (2012) Separate and combined effects of the GABA(B) agonist baclofen and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend 126:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, et al. (2009a) Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, et al. (2009b) Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA 106:20270–20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 67:15–19. [DOI] [PubMed] [Google Scholar]
- Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, Fantegrossi WE. (2014) In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ9-THC in mice: inhalation versus intraperitoneal injection. Pharmacol Biochem Behav 124:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11:4213–4225. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65. [DOI] [PubMed] [Google Scholar]
- Nass SR, Long JZ, Schlosburg JE, Cravatt BF, Lichtman AH, Kinsey SG. (2015) Endocannabinoid catabolic enzymes play differential roles in thermal homeostasis in response to environmental or immune challenge. J Neuroimmune Pharmacol 10:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niphakis MJ, Cognetta AB, 3rd, Chang JW, Buczynski MW, Parsons LH, Byrne F, Burston JJ, Chapman V, Cravatt BF. (2013) Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci 4:1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niphakis MJ, Johnson DS, Ballard TE, Stiff C, Cravatt BF. (2012) O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci 3:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MCG, Ward AM, Hahn YK, Lichtman AH, Conti B, et al. (2011) Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF. (2012) A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med 2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, et al. (2011) Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther 339:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, et al. (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, et al. (1998) SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther 284:644–650. [PubMed] [Google Scholar]
- Selley DE, Cassidy MP, Martin BR, Sim-Selley LJ. (2004) Long-term administration of Delta9-tetrahydrocannabinol desensitizes CB1-, adenosine A1-, and GABAB-mediated inhibition of adenylyl cyclase in mouse cerebellum. Mol Pharmacol 66:1275–1284. [DOI] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. (2000) The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol 129:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. (2006) Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc 1:1194–1206. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411. [DOI] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. (2009) Discriminative stimulus properties of delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol 615:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G. (2007) Lack of evidence for appetitive effects of delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol 18:311–319. [DOI] [PubMed] [Google Scholar]
- Walentiny DM, Vann RE, Wiley JL. (2015) Phenotypic assessment of THC discriminative stimulus properties in fatty acid amide hydrolase knockout and wildtype mice. Neuropharmacology 93:237–242 Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebelhaus JM, Grim TW, Owens RA, Lazenka MF, Sim-Selley LJ, Abdullah RA, Niphakis MJ, Vann RE, Cravatt BF, Wiley JL, et al. (2015) Δ9-tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. J Pharmacol Exp Ther 352:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. (1997) Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav 58:1139–1143. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. (1999) Effects of SR141716A on diazepam substitution for delta9-tetrahydrocannabinol in rat drug discrimination. Pharmacol Biochem Behav 64:519–522. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Smith FL, Razdan RK, Dewey WL. (2005) Task specificity of cross-tolerance between Δ9-tetrahydrocannabinol and anandamide analogs in mice. Eur J Pharmacol 510:59–68. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Walentiny DM, Wright MJ, Jr, Beardsley PM, Burston JJ, Poklis JL, Lichtman AH, Vann RE. (2014) Endocannabinoid contribution to Δ9-tetrahydrocannabinol discrimination in rodents. Eur J Pharmacol 737:97–105 Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Long KA, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. (2012) Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS Chem Neurosci 3:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams SG, Wong A, Barrett DA, Bennett AJ, Chapman V, Alexander SPH. (2012) Spinal administration of the monoacylglycerol lipase inhibitor JZL184 produces robust inhibitory effects on nociceptive processing and the development of central sensitization in the rat. Br J Pharmacol 167:1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]