Abstract
Hepatic multidrug resistance–associated protein 2 (MRP2) provides the biliary elimination pathway for many xenobiotics. Disruption of this pathway contributes to retention of these compounds and may ultimately lead to adverse drug reactions. MRP2 mislocalization from the canalicular membrane has been observed in nonalcoholic steatohepatitis (NASH), the late stage of nonalcoholic fatty liver disease, which is characterized by fat accumulation, oxidative stress, inflammation, and fibrosis. MRP2/Mrp2 mislocalization is observed in both human NASH and the rodent methionine and choline–deficient (MCD) diet model, but the extent to which it impacts overall transport capacity of MRP2 is unknown. Pemetrexed is an antifolate chemotherapeutic indicated for non–small cell lung cancer, yet its hepatobiliary elimination pathway has yet to be determined. The purpose of this study was to quantify the loss of Mrp2 function in NASH using an obligate Mrp2 transport substrate. To determine whether pemetrexed is an obligate Mrp2 substrate, its cumulative biliary elimination was compared between wild-type and Mrp2−/− rats. No pemetrexed was detected in the bile of Mrp2−/− rats, indicating pemetrexed is completely reliant on Mrp2 function for biliary elimination. Comparing the biliary elimination of pemetrexed between MCD and control animals identified a transporter-dependent decrease in biliary excretion of 60% in NASH. This study identifies Mrp2 as the exclusive biliary elimination mechanism for pemetrexed, making it a useful in vivo probe substrate for Mrp2 function, and quantifying the loss of function in NASH. This mechanistic feature may provide useful insight into the impact of NASH on interindividual variability in response to pemetrexed.
Introduction
Precision medicine seeks to ensure that patients receive therapeutic intervention at the appropriate dose, and drives the need to understand interindividual variability in drug response. Although the field of pharmacogenomics provides valuable insights, it is clearly necessary to identify nongenetic factors that also influence the pharmacokinetic profiles of therapeutics via enzyme and transporter proteins. Nonalcoholic fatty liver disease is the most common chronic liver disorder in the world, with an estimated global prevalence of 25% (Younossi et al., 2015). As the early nonalcoholic fatty liver disease stage of steatosis worsens to nonalcoholic steatohepatitis (NASH), there is an alarmingly high number of transcriptomic changes that accompany the histopathologic progression, many of which are instrumental in dictating the fate of drugs (Lake et al., 2011). One striking example is the global downregulation of hepatic uptake transporters, accompanied by a selectively increased expression of basolateral and canalicular efflux transporters (Hardwick et al., 2011; Lake et al., 2011; Clarke et al., 2014). Multidrug resistance–associated protein 2 (MRP2, encoded by ABCC2), a vital canalicular efflux transporter, exhibits a unique post-translational misregulation event that results in partial mislocalization from the canalicular membrane (Hardwick et al., 2011). The resultant decrease in canalicular efflux is a novel mechanism for a NASH-induced increase in exposure, which may explain a number of idiosyncratic adverse drug reactions (ADRs) in patient populations (Hardwick et al., 2014; Dzierlenga et al., 2015). While canalicular efflux may be limited by MRP2 mislocalization, immunohistochemistry indicates that not all MRP2 protein is mislocalized. Furthermore, many MRP2 substrates may be effluxed by compensatory transporters such as breast cancer resistance protein (BCRP) or bile salt export pump. Therefore, the direct impact of MRP2 mislocalization on the altered pharmacokinetic profiles of its substrates has not been quantified.
Pemetrexed is an antifolate that has become one of the most highly used therapeutic interventions for nonsquamous, non–small cell lung cancer, typically in combination with cisplatin (Pérez-Moreno et al., 2014). As an antimetabolite, as in methotrexate, pemetrexed functions by blocking natural folate cofactors, such as thymidylate synthase and dihydrofolate reductase, to inhibit purine and pyrimidine synthesis, inducing cell cycle arrest and cell death (Paz-Ares et al., 2003). Although phase II studies revealed a manageable hematologic toxicity profile for a chemotherapeutic, more recent case studies have revealed higher hepatic enzyme levels and renal tubular toxicities, suggesting a more complex toxicity profile (Paz-Ares et al., 2003; Glezerman et al., 2011). Characterization of the pemetrexed transport profile and the role of MRP2 has been largely neglected, as pemetrexed is often categorized readily as an antifolate compound, which are mostly effluxed promiscuously by many other transporters of the ATP-binding cassette family (Assaraf, 2006; Vlaming, et al., 2009). One study, however, indicated that methotrexate and pemetrexed harbor differing affinities for specific MRP transporters with a partial overlap in the brain (Li et al., 2013). Even though Mrp2 was not found to transport methotrexate in the brain, it is a well-known determinant of the hepatobiliary fate of methotrexate (Vlaming et al., 2008). Because of the structural similarity of pemetrexed to methotrexate, we hypothesized that pemetrexed may also be a substrate for hepatic MRP2. The purposes of this study were to identify the canalicular transport pathway for pemetrexed, to determine the effect of NASH on pemetrexed distribution, and to quantify how much of the NASH-induced disposition change can be attributed to MRP2 dysfunction.
Materials and Methods
Animals.
Male Mrp2−/− and Sprague-Dawley wild-type control rats (n = 3) weighing 200–250 g were obtained from SAGE Laboratories (St. Louis, MO). Additional Sprague Dawley rats (n = 10) were obtained from Harlan (Indianapolis, IN). All animals were acclimated in 12-hour light/dark cycles in a University of Arizona Association for Assessment and Accreditation of Laboratory Animal Care–certified animal facility for at least 1 week before experiments and were allowed water and standard chow ad libitum. Handling, care, maintenance, and testing of the animals were in accordance with the policies and recommendations of the National Institutes of Health guidelines for the handling and use of laboratory animals. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Arizona. Rats from Harlan were fed either a control diet with methionine and choline or a methionine and choline–deficient (MCD) diet (Dyets Inc., Bethlehem, PA; n = 5 each) ad libitum for 8 weeks prior to the study.
Disposition.
Rats were anesthetized with an intraperitoneal bolus dose of urethane (Sigma-Aldrich, St. Louis, MO) in saline (1 g/kg). The disposition of pemetrexed in blood and bile was assessed via cannulation surgeries of the jugular vein for drug administration, carotid artery for blood collection, and bile duct for bile collection. After an intravenous injection of 10 mg/kg pemetrexed disodium (AK Scientific, Inc., Union City, CA) in saline, blood and bile were collected for 120 minutes. The livers were harvested and slices prepared for histologic analysis and placed in 10% neutral-buffered formalin for at least 24 hours followed by 70% ethanol until paraffin embedding by the University of Arizona Histology Service Laboratory. The remaining tissue was snap-frozen in liquid nitrogen. All samples were stored at –80°C for later analysis.
Quantification.
Five microliters plasma was diluted in 20 μl 100% acetonitrile spiked with 100 μg/l (ppb) of the internal standard, DAMPA (Toronto Research Chemicals, Toronto, Canada), and centrifuged for 10 minutes at 13,000 rpm for protein precipitation. The supernatants were diluted in the mobile phase (10% acetonitrile) at 1:100. Bile samples were diluted 1:1000 in DAMPA-spiked mobile phase. Twenty microliters per sample was injected into an Agilent Poroshell 120 EC C-18 (3.0 × 50 mm) column with a 2.7-μm particle size (Agilent Technologies, Santa Clara, CA). An Agilent 1260 liquid chromatograph with an Agilent Poroshell EC-120 C-18 column (3.0 × 50 mm; 2.7 μm) was used for separation of the analytes. The column was maintained at 30°C and the binary pump with dual-eluent separation using 0.1% formic acid in water (A) and acetonitrile (B) at a flow rate of 300 μl/min (graded from 20–100% B) was performed. The organic phase (B) was held at 20% for 1 minute followed by a linear increase to 100% at 11 minutes. This was then held for 1 minute before being returned to the initial 20% condition at 13 minutes. An equilibration time of 2 minutes was used at the end of the run. The eluent from the column was delivered to an Agilent 6490 triple quadrupole mass spectrometer operated with the jet stream technology in positive electrospray ionization mode. The compounds were analyzed in the multiple reaction monitoring mode with two transitions and quantified with isotope dilution technique. Data processing was performed using the Agilent MassHunter (Ver.06.00) software.
Protein Preparations.
Whole-cell lysate preparations of rat liver tissue were prepared from 300 mg of tissue homogenized in NP40 buffer [20 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 1% Nonidet P-40 and 2 mM EDTA with 1 Protease Inhibitor Cocktail Tablet (Roche; Indianapolis, IN) per 25 ml] at 4°C. Homogenized tissue was agitated at 4°C for 2 hours and centrifuged at 10,000g for 30 minutes. The supernatant was measured for protein concentration using the Pierce BCA Protein Quantitation Assay (Thermo Fisher Scientific, Waltham, MA) per the manufacturer’s recommendations.
Immunoblot Protein Analysis.
Whole-cell liver lysate (50 µg) was separated by SDS-PAGE on 7.5% polyacrylamide gels, and the proteins were transferred to a polyvinylidene difluoride membrane, followed by blocking of nonspecific sites with 5% nonfat dry milk in Tris-buffered saline/Tween-20 pH 7.4. The primary antibody against Mrp2, M8316 (Sigma Aldrich, St. Louis, MO), was diluted to 1:500 in blocking solution and incubated with the membrane overnight at 4°C. Relative protein expression was determined using image processing and analysis with ImageJ software (National Institutes of Health, Bethesda, MD) and normalized to the housekeeping protein Erk2, C14 (Santa Cruz Biotechnology, Dallas, TX).
Branched Chain DNA Analysis.
Total RNA was extracted from rat liver using RNAzol B reagent (Tel-Test, Inc., Friendswood, TX) per the manufacturer’s protocol. RNA concentrations were determined using a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Fisher Scientific). RNA (10 μg) was allowed to hybridize to specific oligonucleotide probes for Mrp2 and Mrp3 diluted in lysis buffer overnight at 53°C in a 96-well plate format with signal amplification steps occurring the following day. Substrate solution, lysis buffer, capture hybridization buffer, amplifier, and label probe used for the assay were obtained from the QuantiGene Discovery Kit (Affymetrix; Santa Clara, CA). Luminescence was measured with a Quantiplex 320 bDNA Luminometer interfaced with Quantiplex Data Management Software (v5.02; Bayer, Walpole, MA). A background reading from a well consisting of all reagents except RNA was subtracted from sample readings to determine expression above background level.
Immunohistochemistry.
Immunohistochemical staining for Mrp2 was performed on formalin-fixed, paraffin-embedded samples. Liver sections were deparaffinized in xylene and rehydrated in ethanol, followed by antigen retrieval with Tris-EDTA buffer pH 9.0. Endogenous peroxidase activity was blocked with 0.3% (v/v) hydrogen peroxide in methanol for 20 minutes. Mrp2 staining was performed by overnight incubation (4°C) with Mrp2, M8316 (Sigma Aldrich, St. Louis, MO), followed by the MACH3 staining kit (Biocare Medical, Concord, CA) per the manufacturer’s protocol. All slides were imaged with a Leica DM4000B microscope and a DFC450 camera (Leica Microsystems, Buffalo Grove, IL).
Statistical Analysis.
Statistical comparisons between wild-type and Mrp2−/− (n = 3) or control and MCD (n = 5) groups were made using an unpaired two-way Student’s t test on GraphPad Prism (v5.0, La Jolla, CA), with significance defined as P ≤ 0.05.
Results
Histopathologic Status of Livers in Study Groups.
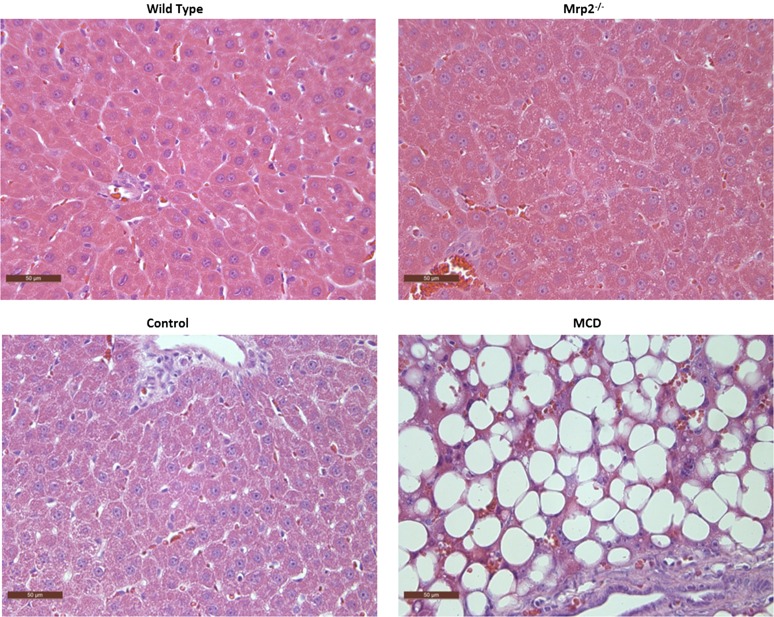
Histologic evaluation was performed for each of the four groups, which included Mrp2−/− and its wild-type control, and MCD and its dietary control, using hematoxylin and eosin–stained liver sections under light microscopy at 40× magnification (Fig. 1). As seen in previous studies, the group that had received the 8-week MCD diet exhibited the classic hallmarks of NASH, including macrovesicular steatosis, fibrosis, and inflammation, which serve to recapitulate the human NASH pathology (George et al., 2003; Kleiner et al., 2005; Canet et al., 2014). The Mrp2−/− group and both control groups exhibited healthy hepatic histology, noted by the absence of NASH hallmarks.
Fig. 1.
Liver histopathology across experimental groups. Hematoxylin and eosin–stained liver sections from a representative Mrp2−/− rat and its wild-type control indicate healthy liver pathology. The representative MCD-fed rat demonstrates the characteristic features of NASH, including steatosis and inflammation. Rats fed control diet had healthy livers with no evidence of steatosis. Original magnification, ×40.
Impact of NASH on Mrp2 Expression and Localization.
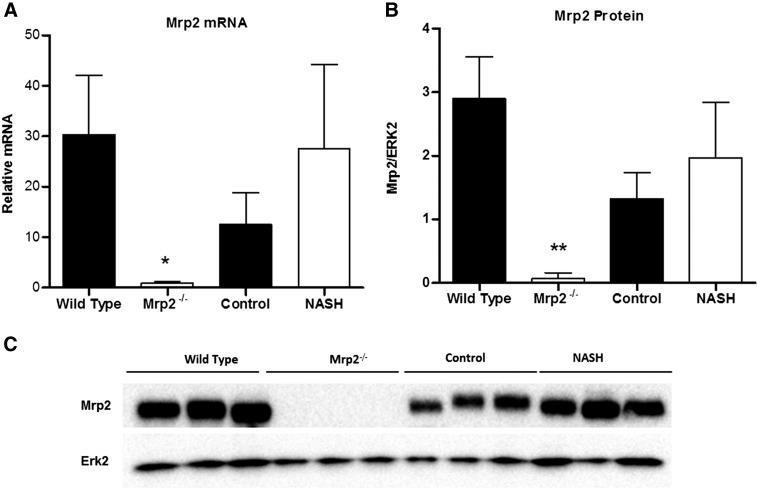
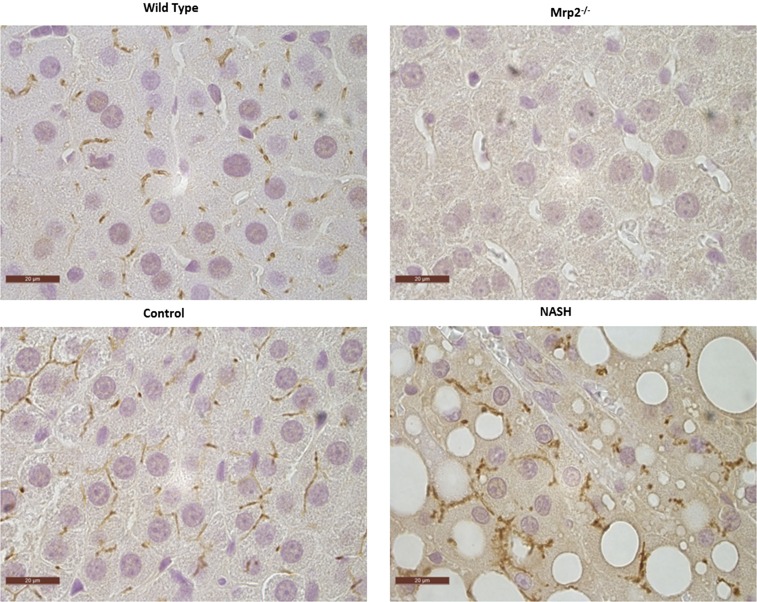
bDNA analysis revealed an absence of Mrp2 mRNA expression in the Mrp2−/− group, as expected. This result was substantiated by the absence of Mrp2 protein expression in the Mrp2−/− group, measured by Western blot (Fig. 2). There was no significant change in Mrp2 mRNA or protein expression in this MCD cohort compared with control. Immunohistochemistry staining of formalin-fixed paraffin-embedded (FFPE) liver slices also revealed an absence of Mrp2 protein in the Mrp2−/− group (Fig. 3). Specific Mrp2 staining indicated proper canalicular localization in both wild-type and dietary control groups; however, the MCD group demonstrated the characteristic mislocalization reported previously (Hardwick et al., 2012).
Fig. 2.
Mrp2−/−- and NASH-induced alterations in Mrp2 expression. (A) Relative mRNA levels of wild-type, Mrp2−/−, control, and NASH rats were assessed by branched-chain DNA analysis. (B) Relative protein expression of Mrp2 was measured by Western blot analysis, normalized to Erk2, with representative blots (C). Graphs represent mean ± S.D. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 (Student’s t test). Wild-type and Mrp2−/−, n = 3; control, n = 4, and MCD, n = 5.
Fig. 3.
Mrp2−/−- and NASH-induced alterations in Mrp2 localization. Representative immunohistochemical staining of Mrp2 in FFPE samples from wild-type, Mrp2−/−, control and NASH rats are shown herein. Original magnification, ×100.
Role of Mrp2 in Pemetrexed Disposition.
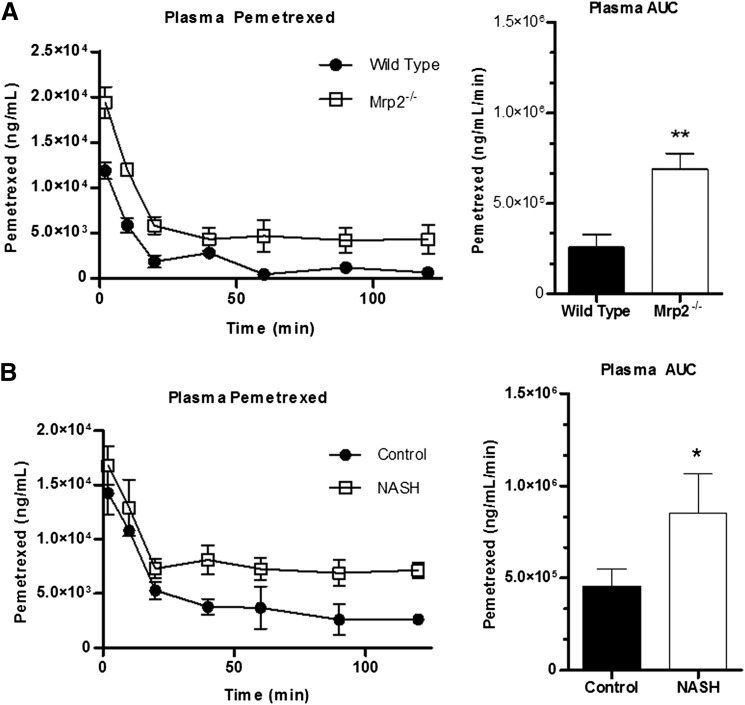
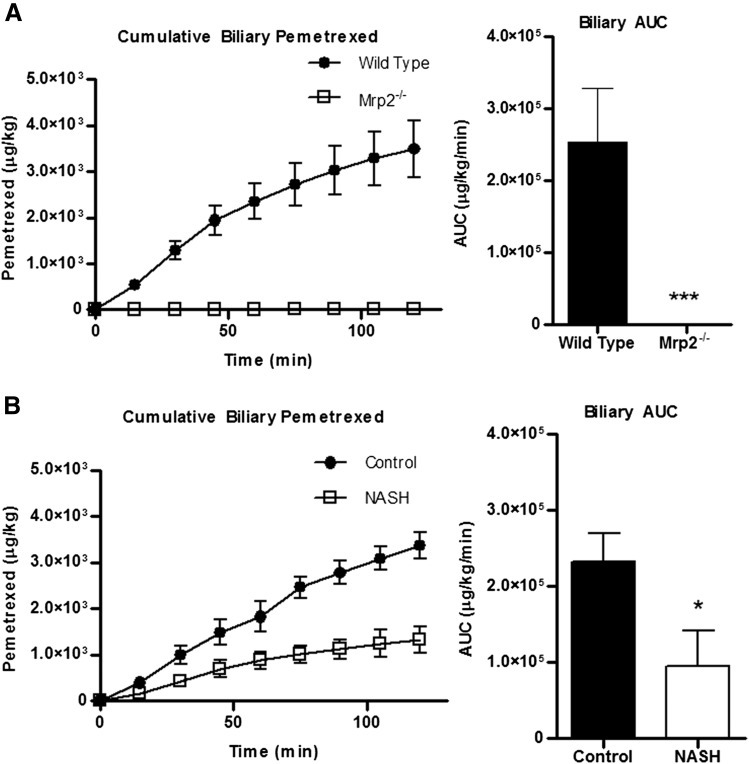
The disposition of pemetrexed in the four groups was measured in the plasma and bile over the course of 120 minutes. Differences in the plasma levels of pemetrexed in both Mrp2−/− and MCD experimental groups compared with their respective controls is noted as early as 2 minutes, and the experimental groups maintained an increased exposure level throughout the course of the study (Fig. 4, A and B). The plasma area under the curves (AUCs) for both time courses confirmed a significant increase in the systemic exposure to pemetrexed in both Mrp2−/− and MCD groups. To further identify the role of canalicular transport, we measured the total biliary excretion of pemetrexed over the course of the study (Fig. 5). The Mrp2−/− group exhibited a complete absence of pemetrexed in the bile compared with the wild-type control group, as indicated by the lack of biliary excretion in the cumulative biliary excretion over time profile and 0% biliary AUC (Fig. 5A). The MCD group, in contrast, still exhibited biliary excretion of pemetrexed, although there was a distinct decrease in the AUC compared with the control counterpart, by approximately 60% (Fig. 5B).
Fig. 4.
Effects of Mrp2 knockout or NASH on systemic exposure to pemetrexed. Plasma concentration of pemetrexed over 120 minutes after intravenous administration of 10 mg/kg pemetrexed to wild-type (closed circles) and Mrp2−/− (open squares) rats (A) or control (closed circles) and MCD (open squares) rats (B). Corresponding plasma AUC graphs represent means ± S.D. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 (Student’s t test). Wild-type and Mrp2−/−, n = 3; control, n = 4, and MCD, n = 5.
Fig. 5.
Effects of Mrp2 knockout or NASH on biliary elimination of pemetrexed. Cumulative biliary excretion of pemetrexed over 120 minutes after intravenous administration of 10 mg/kg pemetrexed to wild-type (closed circles) and Mrp2−/− (open squares) rats (A) or control (closed circles) and MCD (open squares) rats (B). Corresponding bile AUC graphs represent means ± S.D. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 (Student’s t test). Wild-type and Mrp2−/−, n = 3; control, n = 4, and MCD, n = 5.
Discussion
This study revealed that not only is pemetrexed a substrate for Mrp2, but it is an obligatory substrate of Mrp2 for biliary elimination, as illustrated by the complete loss of biliary elimination of pemetrexed in the knockout animals. Furthermore, NASH rats exhibited a 60% decrease in biliary elimination of pemetrexed, implying at least partial functionality of Mrp2 in this group. Both Mrp2−/− and NASH rats had a significantly increased systemic retention, as illustrated by the plasma AUCs. The apparent greater difference between the Mrp2−/− group and its wild-type counterpart compared with the NASH group and its healthy counterpart may be directly explained by the amount of functional, membrane-localized Mrp2 in the NASH rats, contributing to the partial biliary elimination.
Identification of a novel obligatory Mrp2 in vivo probe substrate is useful because most therapeutic compounds and metabolites boast overlapping specificity, rendering it difficult to quantify the functional loss of Mrp2 transport activity owing to mislocalization. Because the changes in biliary excretion among the four groups result from Mrp2 activity alone, we identify a 60% decrease in the overall hepatic transport capacity of Mrp2 in the MCD model of NASH. With pemetrexed acting as a probe substrate, NASH-induced dysfunction of Mrp2 can be quantified independent of the effect on other biliary efflux transporters. This is the first study to link the immunohistochemical visualization of Mrp2 mislocalization to a quantifiable decrease in function.
Prior to this study, there was limited information regarding the mechanism of hepatobiliary transport of pemetrexed. As pemetrexed has been found to be transported by renal OAT3, it is likely taken up into hepatocytes by similar mechanisms (Kurata et al., 2014). Specifics regarding the biliary efflux of pemetrexed for excretion remain elusive, although it was generally accepted to be mediated by one or more ATP-binding cassette transporters, as are other antifolate therapeutics (Assaraf, 2006). The hepatic efflux of methotrexate, a similar compound, is well characterized to be mediated by canalicular MRP2 and BCRP, or sinusoidal MRP3 (Vlaming et al., 2008, 2009). Our laboratory has documented that MCD-induced changes in rat hepatic transporter regulation, specifically the mislocalization of Mrp2 and Pgp, and the upregulation of Mrp3, are capable of altering the disposition of methotrexate such that it modifies the profile of dose-limiting toxicities (Hardwick et al., 2014). In the case of methotrexate, significantly less methotrexate and the 7-hydroxy metabolite were eliminated into the feces as a result of the mechanistic shift from biliary to renal excretion. The results from this study indicate that, unlike methotrexate, which has overlapping specificity for Bcrp, Mrp2, and even Pgp, pemetrexed is completely dependent on Mrp2 for biliary efflux. This is evident by the lack of compensatory transport into the bile observed in the Mrp2−/− group. This was surprising considering neither Bcrp nor Mrp2 play a role in the transport of pemetrexed across the blood-brain barrier (Li et al., 2013).
One possible source of variability in plasma concentrations between the Mrp2 knockouts and NASH may be the impact of other transporters involved in pemetrexed hepatobiliary disposition, which is not established to date but may be speculated. For instance, Mrp3, a sinusoidal efflux transporter often harbors overlapping substrate specificity with Mrp2. It is possible that pemetrexed is also a substrate for hepatic Mrp3, although this is not definitively established. If pemetrexed is a substrate for Mrp3, it would be the most likely route for sinusoidal efflux following Mrp2 compromise. We have previously published a NASH-induced increase in MRP3/Mrp3 expression in the human and MCD rat model (Hardwick et al., 2011, 2012; Canet et al., 2014; Dzierlenga et al., 2015). Mrp3 induction is also well-reported in other instances of Mrp2 deficiency, including TR– Mrp2–naturally deficient rats as well as the SAGE Mrp2−/− used in this study (Zamek-Gliszczynski et al., 2006, 2013). These instances of induction are probably a result of the same compensatory processes, and thus place the Mrp2−/− and MCD experimental groups on a similar plane with respect to Mrp3 expression and function. Another source that may contribute to the disposition changes of pemetrexed in NASH is the hepatic uptake process, which may be mediated by OAT3 and OATP2B1, although more transporters of organic anions may be involved (Visentin et al., 2012; Li et al., 2013). OATP2B1/Oatp2b1 does not exhibit a change in mRNA expression in either human NASH or MCD mice, although MCD does decrease the protein expression of other organic anion–transporting polypeptide isoforms 1b2, 1a1, and 1a4, which is consistent with mRNA expression (Canet et al., 2014; Clarke et al., 2014). Eisai hyperbilirubinemic rats, another model of Mrp2 genetic deficiency, exhibit incidental decreases in uptake of organic anions, which contribute to their systemic retention (Kosaka et al., 2015). Mrp2−/− rats may also exhibit this alteration.
The overall impact of these results, demonstrating approximately 60% decrease in Mrp2 functionality, underscores the importance of the altered regulation of MRP2/Mrp2 in the disease state. Numerous therapeutics rely on MRP2/Mrp2 for proper biliary elimination and, whereas many have compensatory mechanisms for excretion, some, like pemetrexed, are fully dependent on the function of MRP2/Mrp2. Translation of these direct results to a clinical population would, of course, be dependent on whether pemetrexed is also an obligatory substrate of human MRP2, as well as on the extent of MRP2 dysfunction as a result of mislocalization in the human disease. Although it may be that pemetrexed is an obligatory substrate for human MRP2, there have been instances of differential specificity between species, and total hepatic levels of MRP2/Mrp2 protein as well (Li et al., 2009). For instance, bisphenol-A glucuronide has distinct profiles in rodents and humans (Mazur et al., 2012). There are, however, precedents for Mrp2/Mrp3 studies of NASH completed in rodent MCD models that correlate to the human condition more reliably. For instance, the altered disposition of morphine glucuronide in NASH rats, which shifted biliary elimination to plasma retention (Dzierlenga et al., 2015), was nearly identical to results in a clinical study of NASH patients (Ferslew et al., 2015). These matched studies suggest that the MCD rat model of NASH not only recapitulates the transporter misregulation that occurs in the human condition, but may also be used to reliably predict changes in disposition that may lead to human ADRs. This lends credence to the assertion that MRP2/Mrp2 mislocalization, which occurs in both human NASH and the MCD model, may lead to a substantial decrease in overall hepatic transport capacity of MRP2 in humans as well.
Translation of these results to clinical populations, as they pertain specifically to pemetrexed therapy, is further challenged by the differences in the pharmacokinetic profile of pemetrexed between rodents and humans. For instance, liver plays a major role in pemetrexed excretion in rodents, but renal excretion of pemetrexed in humans is responsible for elimination of about 80% of the initial dose (Rinaldi et al., 1999). Preclinical pharmacokinetic studies indicate differences in renal-to-fecal elimination ratios among species, with larger species tending toward renal elimination, and smaller species tending toward fecal elimination (Woodland et al., 1997). However, the propensity of rats to favor biliary excretion of pemetrexed facilitates its utility as a direct probe for Mrp2 transport capacity in this rodent model.
This specific characterization of transporter misregulation in NASH may serve to better predict outcomes for NASH patients receiving therapeutics that are MRP2 substrates. By having accurate transport and pharmacokinetic profiles for drugs, and by understanding the impact NASH has on these processes, it will be more feasible to enact precision medicine for the ultimate prevention of ADRs. The data presented herein highlight the importance of fully characterizing transport processes for therapeutics such as pemetrexed, and in understanding the contribution of disease as an interindividual variable in drug response.
Abbreviations
- ADR
adverse drug reaction
- AUC
area under the curve
- Bcrp
breast cancer resistance protein
- DAMPA
4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoic acid
- MCD
methionine and choline–deficient
- MRP
multidrug resistance–associated protein
- NASH
nonalcoholic steatohepatitis
Authorship Contributions
Participated in research design: Dzierlenga, Clarke, Li, Snyder, Cherrington.
Conducted experiments: Dzierlenga, Clarke, Klein, Anumol.
Contributed new reagents or analytic tools: Snyder.
Performed data analysis: Dzierlenga, Anumol.
Wrote or contributed to the writing of the manuscript: Dzierlenga, Cherrington.
Footnotes
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences Toxicology Training [Grant ES007091] and the National Institutes of Health [Grants HD062489 and ES019487].
References
- Assaraf YG. (2006) The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat 9:227–246. [DOI] [PubMed] [Google Scholar]
- Canet MJ, Hardwick RN, Lake AD, Dzierlenga AL, Clarke JD, Cherrington NJ. (2014) Modeling human nonalcoholic steatohepatitis-associated changes in drug transporter expression using experimental rodent models. Drug Metab Dispos 42:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Hardwick RN, Lake AD, Canet MJ, Cherrington NJ. (2014) Experimental nonalcoholic steatohepatitis increases exposure to simvastatin hydroxy acid by decreasing hepatic organic anion transporting polypeptide expression. J Pharmacol Exp Ther 348:452–458 Department of Pharmacology and Toxicology, University of Arizona, Tucson, Arizona. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierlenga AL, Clarke JD, Hargraves TL, Ainslie GR, Vanderah TW, Paine MF, Cherrington NJ. (2015) Mechanistic basis of altered morphine disposition in nonalcoholic steatohepatitis. J Pharmacol Exp Ther 352:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferslew BC, Johnston CK, Tsakalozou E, Bridges AS, Paine MF, Jia W, Stewart PW, Barritt AS, 4th, Brouwer KL. (2015) Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther 97:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. (2003) Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J Hepatol 39:756–764. [DOI] [PubMed] [Google Scholar]
- Glezerman IG, Pietanza MC, Miller V, Seshan SV. (2011) Kidney tubular toxicity of maintenance pemetrexed therapy. Am J Kidney Dis 58:817–820. [DOI] [PubMed] [Google Scholar]
- Hardwick RN, Clarke JD, Lake AD, Canet MJ, Anumol T, Street SM, Merrell MD, Goedken MJ, Snyder SA, Cherrington NJ. (2014) Increased susceptibility to methotrexate-induced toxicity in nonalcoholic steatohepatitis. Toxicol Sci 142:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. (2011) Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Street SM, Canet MJ, Cherrington NJ. (2012) Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab Dispos 40:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu Y-C, Torbenson MS, Unalp-Arida A, et al. Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Watanabe T, Susukida T, Aoki S, Sekine S, Kume T, Ito K. (2015) Key determinants of the circulatory exposure of organic anions: differences in hepatic uptake between multidrug resistance-associated protein 2 (Mrp2)-deficient rats and wild-type rats. Xenobiotica 45:556–562. [DOI] [PubMed] [Google Scholar]
- Kurata T, Iwamoto T, Kawahara Y, Okuda M. (2014) Characteristics of pemetrexed transport by renal basolateral organic anion transporter hOAT3. Drug Metab Pharmacokinet 29:148–153. [DOI] [PubMed] [Google Scholar]
- Lake AD, Novak P, Fisher CD, Jackson JP, Hardwick RN, Billheimer DD, Klimecki WT, Cherrington NJ. (2011) Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Agarwal S, Elmquist WF. (2013) Brain efflux index to investigate the influence of active efflux on brain distribution of pemetrexed and methotrexate. Drug Metab Dispos 41:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang Y, Hua F, Lai Y. (2009) Absolute difference of hepatobiliary transporter multidrug resistance-associated protein (MRP2/Mrp2) in liver tissues and isolated hepatocytes from rat, dog, monkey, and human. Drug Metab Dispos 37:66–73. [DOI] [PubMed] [Google Scholar]
- Mazur CS, Marchitti SA, Dimova M, Kenneke JF, Lumen A, Fisher J. (2012) Human and rat ABC transporter efflux of bisphenol a and bisphenol a glucuronide: interspecies comparison and implications for pharmacokinetic assessment. Toxicol Sci 128:317–325. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L, Bezares S, Tabernero JM, Castellanos D, Cortes-Funes H. (2003) Review of a promising new agent--pemetrexed disodium. Cancer 97(8, Suppl)2056–2063. [DOI] [PubMed] [Google Scholar]
- Pérez-Moreno MA, Galván-Banqueri M, Flores-Moreno S, Villalba-Moreno A, Cotrina-Luque J, Bautista-Paloma FJ. (2014) Systematic review of efficacy and safety of pemetrexed in non-small-cell-lung cancer. Int J Clin Pharm 36:476–487. [DOI] [PubMed] [Google Scholar]
- Rinaldi DA, Kuhn JG, Burris HA, Dorr FA, Rodriguez G, Eckhardt SG, Jones S, Woodworth JR, Baker S, Langley C, et al. (1999) A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol 44:372–380. [DOI] [PubMed] [Google Scholar]
- Visentin M, Chang M-H, Romero MF, Zhao R, Goldman ID. (2012) Substrate- and pH-specific antifolate transport mediated by organic anion-transporting polypeptide 2B1 (OATP2B1-SLCO2B1). Mol Pharmacol 81:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaming MLH, Pala Z, van Esch A, Wagenaar E, de Waart DR, van de Wetering K, van der Kruijssen CMM, Oude Elferink RPJ, van Tellingen O, Schinkel AH. (2009) Functionally overlapping roles of Abcg2 (Bcrp1) and Abcc2 (Mrp2) in the elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate in vivo. Clin Cancer Res 15:3084–3093. [DOI] [PubMed] [Google Scholar]
- Vlaming MLH, Pala Z, van Esch A, Wagenaar E, van Tellingen O, de Waart DR, Oude Elferink RPJ, van de Wetering K, Schinkel AH. (2008) Impact of Abcc2 (Mrp2) and Abcc3 (Mrp3) on the in vivo elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate. Clin Cancer Res 14:8152–8160. [DOI] [PubMed] [Google Scholar]
- Woodland JM, Barnett CJ, Dorman DE, Gruber JM, Shih C, Spangle LA, Wilson TM, Ehlhardt WJ. (1997) Metabolism and disposition of the antifolate LY231514 in mice and dogs. Drug Metab Dispos 25:693–700. [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. (2015) Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology DOI: 10.1002/hep.28431 (published ahead of print). [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Goldstein KM, Paulman A, Baker TK, Ryan TP. (2013) Minor compensatory changes in SAGE Mdr1a (P-gp), Bcrp, and Mrp2 knockout rats do not detract from their utility in the study of transporter-mediated pharmacokinetics. Drug Metab Dispos 41:1174–1178. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Humphreys JE, Tian X, Nezasa K, Brouwer KLR. (2006) Differential involvement of Mrp2 (Abcc2) and Bcrp (Abcg2) in biliary excretion of 4-methylumbelliferyl glucuronide and sulfate in the rat. J Pharmacol Exp Ther 319:459–467. [DOI] [PubMed] [Google Scholar]