ABSTRACT
Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus are used in the fermentation of milk to produce yoghurt. These species normally metabolize only the glucose moiety of lactose, secreting galactose and producing lactic acid as the main metabolic end product. We used multiple serial selection steps to isolate spontaneous mutants of industrial strains of S. thermophilus and L. delbrueckii subsp. bulgaricus that secreted glucose rather than galactose when utilizing lactose as a carbon source. Sequencing revealed that the S. thermophilus strains had mutations in the galKTEM promoter, the glucokinase gene, and genes encoding elements of the glucose/mannose phosphotransferase system (PTS). These strains metabolize galactose but are unable to phosphorylate glucose internally or via the PTS. The L. delbrueckii subsp. bulgaricus mutants had mutations in genes of the glucose/mannose PTS and in the pyruvate kinase gene. These strains cannot grow on exogenous glucose but are proficient at metabolizing internal glucose released from lactose by β-galactosidase. The resulting strains can be combined to ferment milk, producing yoghurt with no detectable lactose, moderate levels of galactose, and high levels of glucose. Since glucose tastes considerably sweeter than either lactose or galactose, the sweetness of the yoghurt is perceptibly enhanced. These strains were produced without the use of recombinant DNA technology and can be used for the industrial production of yoghurt with enhanced intrinsic sweetness and low residual levels of lactose.
IMPORTANCE Based on a good understanding of the physiology of the lactic acid bacteria Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus, we were able, by selecting spontaneously occurring mutants, to change dramatically the metabolic products secreted into the growth medium. These mutants consume substantially more of the lactose, metabolize some of the galactose, and secrete the remaining galactose and most of the glucose back into the milk. This allows production of yoghurt with very low lactose levels and enhanced natural sweetness, because humans perceive glucose as sweeter than either lactose or galactose.
INTRODUCTION
Modern yoghurt production begins with pasteurization of milk, followed by inoculation and fermentation by commercially prepared starter cultures containing strains of the lactic acid bacteria Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus (1). In order to satisfy consumer demands regarding appearance, sweetness, texture, and flavor, a number of additional ingredients may be added, including natural or artificial colors, sucrose or artificial sweeteners, texturizing agents, and fruit preparations (2). Today, yoghurt may contain 5 to 12% (wt/vol) added sucrose in addition to the natural sugars (lactose and galactose) already present, resulting in sugar contents that rival those of soft drinks and tarnishing the image of yoghurt as a healthy food. In addition, the potential for gastrointestinal discomfort caused by residual lactose prevents some consumers from fully enjoying yoghurt (3).
The perception of sweetness by humans is mediated by specific receptors that are located in taste buds on the tongue and soft palate (4). These receptors react differently to different sugars, resulting in a wide range of perceived sweetness for various sugars. Robyt (5) presented a scheme showing that a taste panel rated glucose about 4 times as sweet as lactose, 2 times as sweet as galactose, and 80% as sweet as sucrose, on the basis of equal weights in solution.
Lactose, the primary sugar found in milk, is a disaccharide containing glucose and galactose. Lactic acid bacteria catabolize lactose, producing lactic acid as the main fermentation end product. Because most strains of S. thermophilus and all described strains of L. delbrueckii subsp. bulgaricus are unable to ferment galactose (6, 7), this sugar is secreted into the milk, resulting in dairy products containing significant amounts of galactose as well as high levels (30 to 40 mg/ml) of residual unfermented lactose.
We have used traditional bacterial genetic techniques to redirect the metabolic pathways in S. thermophilus and L. delbrueckii subsp. bulgaricus so that the bacteria consume the nonsweet sugars lactose and galactose and secrete the much sweeter glucose, thereby enhancing the sweetness of the product without increasing the calorie content. One interesting finding was that the glucose-secreting mutants, either alone or in combination, fermented considerably more lactose than the parent strains. This provides an opportunity to produce yoghurt or other dairy products with reduced levels of lactose, potentially allowing lactose-intolerant people to enjoy fermented dairy products.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are presented in Table 1. Wild-type S. thermophilus strains St1-WT and St2-WT and wild-type L. delbrueckii subsp. bulgaricus strains Lb1-WT and Lb2-WT were originally isolated from yoghurt and have a long history of use as industrial starter cultures. St1-WT is used for its robust acidification capabilities and St2-WT for its superior texturizing properties. Spontaneous mutants of these strains were isolated as described below. S. thermophilus strains were grown at 40°C using M17 medium (8) (Difco Laboratories) containing an appropriate carbon source. L. delbrueckii subsp. bulgaricus strains were grown at 40°C using MRS medium (9) (Oxoid) or a modified MRS medium-based medium, designated MRS-IM medium (E. Johansen, K. Sørensen, M. Curic-Bawden, and M. Junge, 2013, international patent application WO 2013160413 A1), containing an appropriate carbon source. Media were supplemented with sugars (sucrose, lactose, mannose, glucose, or galactose) at 2% (wt/vol).
TABLE 1.
Bacterial strains
| Strain | Properties | Source |
|---|---|---|
| Streptococcus thermophilus | ||
| St1-WT | Wild type | Chr. Hansen culture collection |
| St1-Gal+-1 | Galactose fermenting | Spontaneous mutant of St1-WT |
| St1-GS-1 | Glucose secreting | Spontaneous mutant of St1-Gal+-1 resistant to 20 mM 2-deoxyglucose |
| St1-GS-2 | Glucose secreting | Spontaneous mutant of St1-GS-1 resistant to 30 mM 2-deoxyglucose |
| St2-WT | Wild type | Chr. Hansen culture collection |
| St2-Gal+-1 | Galactose fermenting | Spontaneous mutant of St2-WT |
| St2-GS-1 | Glucose secreting | Spontaneous mutant of St2-Gal+-1 resistant to 20 mM 2-deoxyglucose |
| St2-GS-2 | Glucose secreting | Spontaneous mutant of St2-GS-1 resistant to 30 mM 2-deoxyglucose |
| Lactobacillus delbrueckii subsp. bulgaricus | ||
| Lb1-WT | Wild type | Chr. Hansen culture collection |
| Lb1-GS-1 | Glucose secreting | Spontaneous mutant of Lb1-WT resistant to 20 mM 2-deoxyglucose |
| Lb2-WT | Wild type | Chr. Hansen culture collection |
| Lb2-GS-1 | Glucose secreting | Spontaneous mutant of Lb2-WT resistant to 20 mM 2-deoxyglucose |
Mutant isolation.
Spontaneous mutants of S. thermophilus strains St1-WT and St2-WT that were able to ferment galactose were obtained by plating aliquots from fresh overnight cultures grown in M17 lactose broth on M17 galactose plates and incubating the plates for 24 to 48 h. Spontaneous mutants of S. thermophilus and L. delbrueckii subsp. bulgaricus that were resistant to 2-deoxyglucose were obtained by plating aliquots from fresh overnight cultures grown in M17 lactose broth or MRS lactose broth, as appropriate, on M17 galactose plates or MRS-IM lactose plates containing 2-deoxyglucose (at a moderate level [20 mM] or an elevated level [30 mM]).
Phenotypic stability.
The stability of the mutant phenotypes of the mutants St1-GS-2 and LB1-GS-1 was assessed following anaerobic growth at 40°C in nonselective medium (M17 sucrose and MRS-IM lactose, respectively) for at least 30 generations. Fresh saturated cultures were inoculated into fresh medium at 1.0% (vol/vol) and incubated overnight. After 3 subcultures, appropriate dilutions were plated on nonselective agar plates. Following growth, 96 single colonies of each strain were picked and inoculated into fresh broth in flat-bottomed low-well microtiter plates (Corning Costar). St1-GS-2 colonies were inoculated into plates containing M17 lactose and M17 glucose, while LB1-GS-1 colonies were inoculated into plates containing MRS-IM lactose and MRS-IM glucose. Growth was assessed spectrophotometrically following anaerobic incubation at 40°C for 20 h. Revertants grew equally well with lactose or glucose as an added carbon source, while mutants grew only with lactose.
Mutation identification.
Genomic DNA was purified from St1-WT, St2-WT, St1-Gal+-1, and St2-Gal+-1 using the DNeasy tissue kit (Qiagen, Venlo, Netherlands), according to the manufacturer's instructions. The galactokinase gene (galK) and upstream regions were amplified by PCR using the PCR master kit (Roche, Mannheim, Germany) and primers GALR1 (CCG ATG TCT AGT ATC CTC) and GALK3 (CCG GAA GGA CGT TAC CTC). PCR products were purified using the QIAquick gel extraction kit (Qiagen, Venlo, Netherlands), and the DNA sequences were determined by Macrogen (Seoul, Republic of Korea). The same protocol, using primers GK1F (CTT GGG TAA AAG GCT CTA) and GK1R (CGT TTT TCA ACA AAA AAG TGC TACC), was used to identify mutations in the glucokinase gene (glcK) in St1-GS-1 and St2-GS-1.
Total DNA for genome sequencing was purified as described above and sequenced at BGI (Beijing, China), and the genome sequence data were assembled and finished using CLC Genomics Workbench software (CLCBio, Århus, Denmark). S. thermophilus genome sequences were aligned using the annotated genome sequence of CNRZ1066 (GenBank accession no. NC_006449) (10) as a reference, using Mauve 2.3.1 software (11). After alignment, single-nucleotide polymorphisms (SNPs) were identified using Mauve 2.3.1 software. L. delbrueckii subsp. bulgaricus genome sequences were determined, aligned, and analyzed in the same way, using the annotated genome sequence of ATCC BAA-365 (GenBank accession no. NC_008529) (12) as a reference.
Enzyme activities.
Strains for enzyme assays were grown in M17 sucrose or MRS lactose, as appropriate, at 40°C. Cells from 10 ml of culture were harvested by centrifugation at an optical density at 600 nm (OD600) of 1, washed in 5 ml cold GLK buffer (5 mM MgCl2, 10 mM K2HPO4 [pH 7.2]), and resuspended in 500 μl cold GLK buffer. Cells were disrupted by the addition of 100 mg glass beads (150 to 212 μm, Sigma G1145) to 200 μl resuspended cells and oscillation at a frequency of 30 cycles/s for 6 min in a MM200 oscillating mill (Retsch, Haan, Germany). Cell debris and glass beads were removed by centrifugation, and total protein content was determined by the bicinchoninic acid (BCA) method (13). The glucokinase activity in the cell extracts was determined spectrophotometrically by a glucose-6-phosphate dehydrogenase (EC1.1.1.49):NADPH-coupled assay (14), essentially as described by Pool et al. (15). Cells from three parallel cultures of each strain were analyzed for each assay. One milliunit of glucokinase corresponds to the amount of enzyme that catalyzes the phosphorylation of 1.0 nmol of d-glucose per minute under the assay conditions.
The pyruvate kinase assay was carried out using the Sigma-Aldrich pyruvate kinase activity assay kit (MAK072-1KT; Sigma-Aldrich, St. Louis, MO, USA). The assay was performed essentially as described by the manufacturer but with the addition of glucose-6-phosphate to a final concentration of 8.3 mM, to activate pyruvate kinase. Cell extracts from three parallel cultures were prepared as described above, except that the cell pellet was washed in Tris buffer (20 mM Tris [pH 7.5]) before finally being resuspended in the pyruvate kinase assay buffer supplied with the kit. One milliunit of pyruvate kinase corresponds to the amount of enzyme that catalyzes the conversion of 1.0 nmol of phosphoenolpyruvate to pyruvate per minute under the assay conditions.
Acidification of milk and metabolic profiling.
Milk for acidification tests, referred to as B-milk, was made by reconstituting skim milk powder at a level of dry matter of 9.5% in distilled water and pasteurizing it at 99°C for 30 min, followed by cooling to 40°C. B-milk (100 ml in baby bottles) was inoculated with 1% (vol/vol) fresh overnight cultures grown in M17 galactose or M17 sucrose in the case of S. thermophilus and MRS-IM lactose in the case of L. delbrueckii subsp. bulgaricus. Mixtures, when used, contained 90% S. thermophilus/10% L. delbrueckii subsp. bulgaricus (vol/vol). Acidification was followed by determination of the changes in pH for 30 h at 40°C, using a CINAC logger and CINAC software version 4 (Alliance Instruments, Frepillon, France). Each acidification was performed in triplicate. Lactose, galactose, glucose, citrate, acetate, and lactate concentrations were quantified after 30 h of fermentation by using high-performance liquid chromatography (HPLC) and a Dionex CarboPac PA20 column (3 by 150 mm; Thermo Fisher Scientific, Waltham, MA, USA).
In experiments with St1-GS-2, the milk was supplemented with 0.01% (wt/vol) sucrose. To test whether other metabolites could be used to stimulate milk acidification, glutamine (1 mM), glutamate (1 mM), arginine (1 mM), glycine (1 mM), or betaine (2.5 or 5 mM) was added to the milk, either alone or together with sucrose (0.05%).
Yoghurt production and sensory analysis.
To make food-grade yoghurt suitable for sensory analysis, a milk base with 1.0% fat, 4.5% protein, and 0.1% sucrose was produced by mixing equal amounts of commercial milk with 0.5% and 1.5% fat (Arla Foods, Viby J, Denmark). The protein level was adjusted to 4.5% by adding skim milk powder (Arla Foods), and sucrose (Nordic Sugar, Copenhagen, Denmark) was added to a final concentration of 0.1%. The milk base was heat treated at 98°C for 5 min before being cooled to fermentation temperature and then was inoculated with 0.024% (vol/vol) preinoculation material (PIM) on a 3-liter scale. For mixtures, the ratio of the inoculation strains was 90% S. thermophilus/10% L. delbrueckii subsp. bulgaricus. PIM is a food-grade, certified, inoculation material used for the production of commercial starter cultures and is standardized and quality certified according to industry norms. Fermentation was performed at 43°C until the pH was 4.55. Following fermentation, additional sucrose was added to give a variety of final concentrations of added sugar (up to 5% [wt/vol]). Subsequently, sensory profiling of the yoghurts was performed by a panel of 10 trained assessors, all of whom were experienced in sensory evaluation of fermented milk products. Products were ranked by perceived sweetness. The sensory analyses were performed according to ISO international standard methodology and guidance protocols ISO 6564, ISO 8586-1, and ISO 8586-2 (16–18).
Nucleotide sequence accession numbers.
Whole-genome shotgun projects deposited in DDBJ/ENA/GenBank were assigned the following accession numbers: St1-WT, LVWV00000000; St1-GS-2, LVWW00000000; Lb1-WT, LVWX00000000; and Lb1-GS-1, LVWY00000000.
RESULTS
Mutant isolation.
Mutants appeared on the various selective plates at frequencies in the range of 10−6 to 10−8 mutants/viable cell; thus, we were able to isolate spontaneous mutants without the use of chemical or physical mutagenesis. All mutants described here are spontaneous mutants.
Galactose-fermenting mutants of St1-WT and St2-WT were obtained on M17 galactose plates. Two mutants, designated St1-Gal+-1 and St2-Gal+-1, were purified. Both strains fermented galactose considerably better than their respective mother strains (Table 2), and both strains grew and acidified milk comparably to other S. thermophilus strains (Fig. 1).
TABLE 2.
Growth of S. thermophilus strains on various carbon sources
| Carbon source | Growth of straina |
|||
|---|---|---|---|---|
| St1-WT | St1-Gal+-1 | St1-GS-1 | St1-GS-2 | |
| Glucose | +++ | +++ | ++ | − |
| Galactose | − | +++ | +++ | +++ |
| Mannose | − | − | − | − |
| Lactose | +++ | +++ | +++ | ++b |
| Sucrose | +++ | +++ | +++ | +++ |
Comparable results were obtained with St2-WT and mutants thereof.
This sample contained 0.01% sucrose in addition to lactose, to stimulate the initiation of growth.
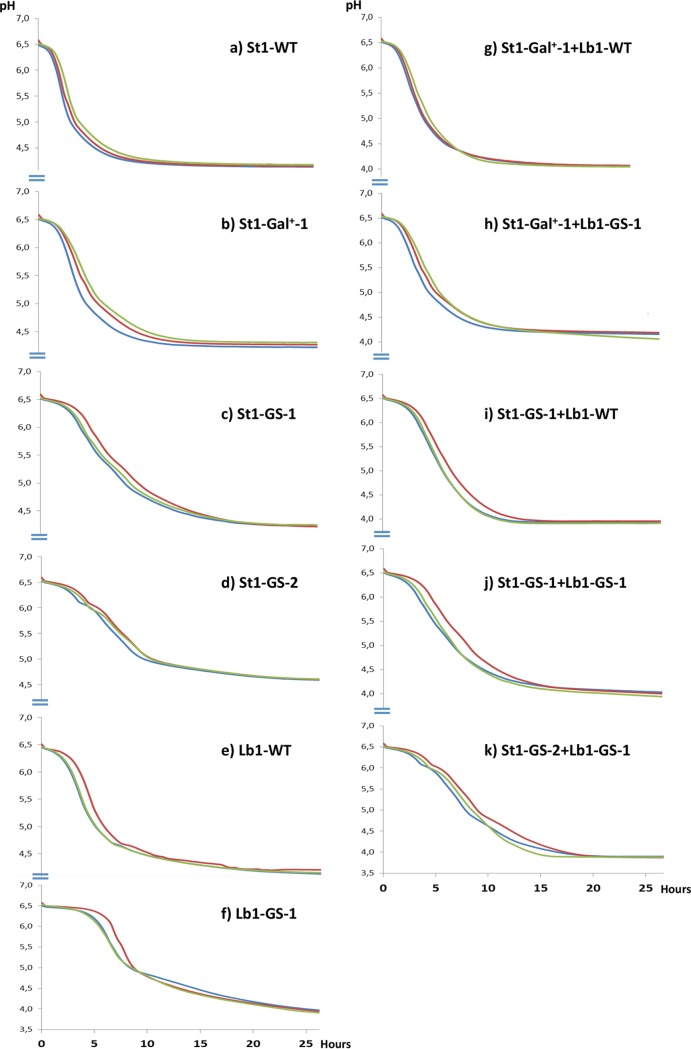
FIG 1.
Milk acidification curves. All experiments were performed in triplicate at 40°C in B-milk with cells pregrown in M17 sucrose or MRS-IM lactose, as appropriate. Each panel shows the three replicates in three different colors.
Mutants of St1-Gal+-1 and St2-Gal+-1 that are resistant to moderate levels of 2-deoxyglucose appeared on M17 galactose plates containing 2-deoxyglucose (20 mM). Mutants designated St1-GS-1 and St2-GS-1 were purified; both mutants showed impaired growth in M17 glucose broth but grew well with other carbon sources (Table 2).
Mutants of St1-GS-1 and St2-GS-1 that are resistant to elevated levels of 2-deoxyglucose were obtained on M17 galactose plates containing 2-deoxyglucose (30 mM). Mutants designated St1-GS-2 and St2-GS-2 were purified; those strains did not grow in M17 glucose broth (Table 2).
Mutants of L. delbrueckii subsp. bulgaricus Lb1-WT and Lb2-WT that are resistant to moderate levels of 2-deoxyglucose appeared on MRS-IM lactose plates containing 20 mM 2-deoxyglucose. Two mutants, designated Lb1-GS-1 and Lb2-GS-1, were purified; these strains did not grow in MRS-IM glucose broth but showed normal growth in MRS-IM lactose broth.
The stability of the mutant phenotypes was confirmed for St-1-GS-2 and Lb1-GS-1. No revertants were detected among 96 colonies tested after growth for at least 30 generations under nonselective conditions.
Mutant characterization.
Mutations were identified either by sequencing of the region of the chromosome deemed most likely to contain the mutation or by whole-genome sequencing. DNA sequencing of the proximal portion of the gal operon of the galactose-fermenting S. thermophilus strains St1-Gal+-1 and St2-Gal+-1 revealed that both strains have mutations in the promoter region (Fig. 2). DNA sequencing of the glucokinase gene (glcK) of the S. thermophilus mutants that were impaired in their ability to ferment glucose revealed that they both have missense mutations in glcK, i.e., T141I for St1-GS-1 and G249R for St2-GS-1. Neither mutant showed detectable glucokinase activity (limit of detection, 5 mU/mg protein), whereas the parental strains had specific activities of 122.6 ± 11.8 mU/mg protein and 125.8 ± 14.8 mU/mg protein, respectively.
FIG 2.
Promoter mutations in S. thermophilus. The S. thermophilus consensus intergenic region containing the promoter sequences for the galK and galR genes was aligned with the same regions of the gal-positive mutants St1-Gal+-1 and St2-Gal+-1. The −35, −10, and ribosomal binding site (RBS) regions are in bold type, and the start codon (ATG) is underlined. The point mutations present in the two mutants are highlighted with cyan.
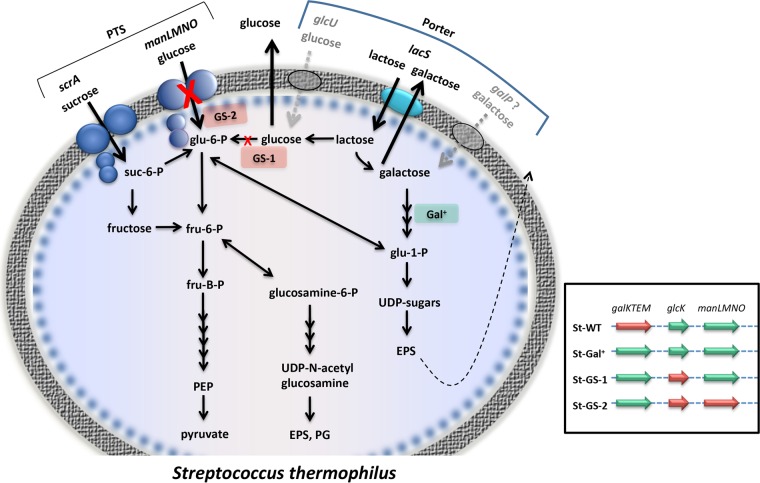
Whole-genome sequencing and analysis revealed that St1-GS-2 has, in addition to the mutation in glcK and the gal promoter mutation found in the parent strain St1-GS-1, a nonsense mutation (TAA) at codon 209 in the manM gene, encoding the IICman protein of the glucose/mannose phosphotransferase system (PTS). St2-GS-2 has a T79P change in the manN gene, encoding the IIDman protein of the same phosphotransferase system.
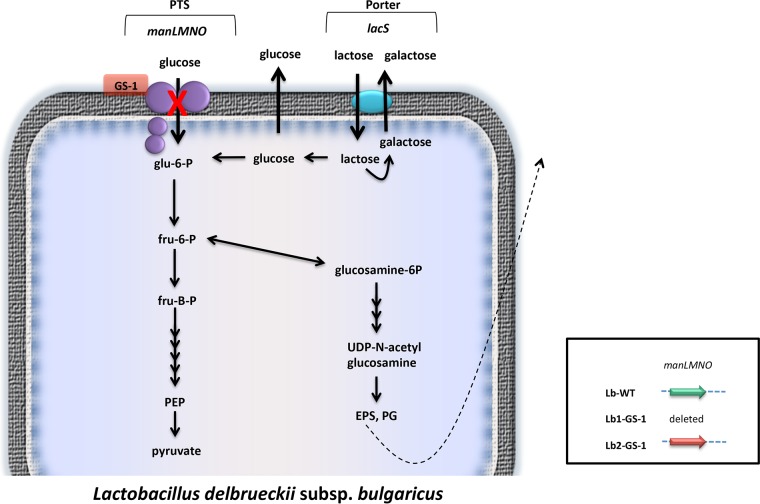
Whole-genome sequencing and analysis revealed that L. delbrueckii subsp. bulgaricus mutants that were unable to grow on glucose (Lb1-GS-1 and Lb2-GS-1) also have mutations in the glucose/mannose phosphotransferase system. Lb1-GS-1 has a deletion of the three genes encoding the IIABman, IICman, and IIDman proteins, while Lb2-GS-1 has a nonsense mutation in the gene encoding the IIABman protein, changing the serine codon (TCA) at position 312 to a stop codon (TAA). Furthermore, both strains contain additional mutations in the gene encoding pyruvate kinase, as follows: Lb1-GS-1, A438V; Lb2-GS-1, G69D. Enzyme assays showed only minor variations in pyruvate kinase activities; the parent strain Lb1-WT showed 24.8 ± 5.1 mU/mg protein, while the mutant Lb1-GS-1 demonstrated 22.9 ± 1.8 mU/mg protein.
Acidification of milk.
The S. thermophilus glcK mutants had delays of 2 to 3 h in the onset of acidification when grown in milk (Fig. 1). However, the acidification speed and final pH were almost the same as for the mother strain. During characterization of the triple mutants St1-GS-2 and St2-GS-2, we observed that they were unable to acidify milk or had a very long lag phase before the initiation of acidification (data not shown). However, if the mutants were pregrown in M17 sucrose or as little as 0.01% (wt/vol) sucrose was added to the milk, the delay of acidification was significantly reduced and was comparable to that of the parental glcK mutants. The final pH was somewhat higher, however (Fig. 1).
The glucose/mannose transport mutants of L. delbrueckii subsp. bulgaricus had delays in the onset of milk acidification of 3 to 4 h, and the rate of acidification was also reduced. The final pH was similar to that observed with acidification by the wild-type strains, however (Fig. 1). Normal initiation of acidification was observed when a mixture containing 10% L. delbrueckii subsp. bulgaricus mutant Lb1-GS-1 and 90% S. thermophilus triple mutant St1-GS-2 was inoculated into milk (Fig. 1).
Sugar and acid contents of fermented milk.
The levels of lactose, glucose, galactose, and lactate in milk fermented by the various strains and combinations presented in Fig. 1 were determined by HPLC and are shown in Table 3. The levels of citrate were identical in milk and all fermentations (1.8 ± 0.1 mg/ml), while no acetate was detectable in any of the samples (detection limit, 0.4 mg/ml).
TABLE 3.
Sugar and lactate contents of milk after 30 h of fermentation
| Strain(s) | Level (mg/ml)a |
|||
|---|---|---|---|---|
| Lactose | Galactose | Glucose | Lactate | |
| None (milk alone) | 47.6 ± 0.0 | BDL | BDL | BDL |
| St1-WT | 32.4 ± 0.5 | 6.5 ± 0.1 | BDL | 7.2 ± 0.1 |
| St1-Gal+-1 | 34.1 ± 0.3 | 4.2 ± 0,1 | 0.8 ± 0.1 | 6.8 ± 0.4 |
| St1-GS-1 | 14.4 ± 0.5 | 9.6 ± 0.1 | 11.1 ± 0.1 | 7.2 ± 0.2 |
| St1-GS-2 | 1.1 ± 0.2 | 16.0 ± 0.0 | 22.9 ± 0.0 | 5.3 ± 0.1 |
| Lb1-WT | 24.5 ± 0.7 | 10.5 ± 0.1 | 2.1 ± 0.2 | 6.9 ± 0.3 |
| Lb1-GS-1 | 6.6 ± 0.6 | 17.5 ± 0.5 | 8.6 ± 0.6 | 7.9 ± 0.2 |
| St1-Gal+-1 + Lb1-WT | 31.8 ± 0.8 | 6.8 ± 0.6 | 0.9 ± 0.2 | 8.1 ± 0.3 |
| St1-Gal+-1 + Lb1-GS-1 | 31.7 ± 0.7 | 6.0 ± 1.2 | 1.5 ± 0.7 | 7.2 ± 0.4 |
| St1-GS-1 + Lb1-WT | 14.5 ± 0.9 | 12.3 ± 0.1 | 8.0 ± 0.5 | 9.0 ± 0.5 |
| St1-GS-1 + Lb1-GS-1 | 7.5 ± 0.8 | 13.8 ± 0.7 | 12.7 ± 0.4 | 8.6 ± 0.5 |
| St1-GS-2 + Lb1-GS-1 | BDL | 20.2 ± 0.1 | 19.1 ± 0.6 | 9.3 ± 0.3 |
Each value represents the average of samples from the three replicates presented in Fig. 1. BDL, below detection limit.
Taste testing of yoghurt.
Tasting of yoghurt by a trained taste panel revealed that the yoghurt produced using the combination of St1-GS-2 and Lb1-GS-1 was perceptibly sweeter than the corresponding yoghurt produced using the wild-type strains in the absence of added sucrose. Addition of various amounts of sucrose to the two yoghurts showed that the sweetness engendered by the mutant strains was able to replace approximately 10 g of added sucrose per liter of yoghurt while maintaining the same perceived sweetness. Thus, yoghurt produced with the wild-type strains with 5% (wt/vol) added sucrose was not distinguishable from yoghurt produced with the mutant strains with 4% (wt/vol) added sucrose.
DISCUSSION
Most strains of S. thermophilus, including St1-WT and St2-WT, are unable to ferment galactose despite having intact galKTEM genes encoding the required enzymes for the Leloir pathway (19, 20) (Table 2). The most likely reason for this phenotype is insufficient transcription due to a defective promoter (19). The spontaneous galactose-fermenting mutants designated St1-Gal+-1 and St2-Gal+-1 have changes in the galK promoter region (Fig. 2). The G-to-A mutation in St1-Gal+-1 creates a −10 region, i.e., TACAAT, which is closer to the consensus sequence TATAAT. An identical mutation was described by Vaughan et al. (19), who designated this type of mutation class III. The mutation in St2-Gal+-1 is a C-to-A change between the −10 region and the galK transcription initiation site. This mutation, which replaces a CG base pair with an AT base pair, might increase the “opening potential” for the −9 to +3 region and thereby create a stronger promoter (21).
Mutants of bacteria, including lactic acid bacteria, that are resistant to 2-deoxyglucose often contain mutations that render them unable to metabolize glucose (22, 23). The mutants St1-GS-1 and St2-GS-1 are impaired in their ability to ferment glucose but retain the ability to ferment sucrose, galactose, and lactose (Table 2). Neither mutant produces detectable levels of glucokinase activity, due to missense mutations in the glucokinase gene (glcK). Thus, these mutants are resistant to 2-deoxyglucose and fail to ferment glucose because they cannot phosphorylate these substrates.
Whole-genome sequencing of the mutants St1-GS-2 and St2-GS-2, which were isolated from St1-GS-1 and St2-GS-1, respectively, revealed that these strains have mutations in elements of the glucose/mannose phosphotransferase system. As a consequence, these strains are further impaired in glucose metabolism, being unable to phosphorylate glucose and 2-deoxyglucose during transport into the cell. Growth of these mutants on lactose results in the galactose moiety being catabolized and the glucose moiety being secreted into the medium. This contrasts with the normal situation, in which glucose is fermented and galactose is secreted (6).
The sugar contents of milk fermented by various strains are shown in Table 3. The mutation imparting the ability to ferment galactose in S. thermophilus St1-Gal+-1 results in a slight reduction in the galactose level and a slight increase in the glucose level of the fermented milk. Knocking out the ability to phosphorylate glucose (St1-GS-1) changes the metabolism significantly; considerably more lactose is consumed and significant levels of glucose and higher levels of galactose are excreted. Eliminating the ability to transport glucose (St1-GS-2) results in further enhancement of the consumption of lactose and the excretion of even higher levels of both galactose and glucose. Sugar metabolism in these strains is illustrated in Fig. 3. L. delbrueckii subsp. bulgaricus strains are naturally unable to ferment galactose (7), due to the absence of the gal genes (24); consequently, the isolation of galactose-fermenting mutants is not possible.
FIG 3.
Sugar metabolism in S. thermophilus. Black arrows, active enzyme reactions; dotted gray arrows, putative enzyme reactions; red Xs, enzyme reactions inactivated by mutations; dotted black arrow, externalization of exopolysaccharide. The indicated genes are as follows: scrA, sucrose PTS component II; manLMNO, glucose/mannose PTS IIABCD; lacS, lactose permease; glcU, glucose permease; and galP, putative galactose permease. PTS, phosphotransferase system; EPS, exopolysaccharide; PG, peptidoglycan; PEP, phosphoenolpyruvate; glu-1-P, glucose-1-phosphate; glu-6-P, glucose-6-phosphate; fru-6-P, fructose-6-phosphate; fru-B-P, fructose-1,6-biphosphate; suc-6-P, sucrose-6-phosphate. (Inset) Green arrows, active genes; red arrows, inactive genes. Genes are as follows: galKTEM, galactose operon; glcK, glucokinase; manLMNO, and glucose/mannose PTS IIABCD.
Mutants of L. delbrueckii subsp. bulgaricus Lb1-WT and Lb2-WT resistant to 2-deoxyglucose are unable to grow on MRS-IM glucose plates but retain the ability to ferment lactose. The mutants have mutations in the glucose/mannose phosphotransferase system, and they are resistant to 2-deoxyglucose and fail to grow with glucose as the sole added carbon source because they cannot transport these substrates. Growth with lactose is unaffected, as this sugar enters the cell via the lactose permease (24) and the glucose released internally through cleavage by β-galactosidase is readily available for catabolism. Furthermore, both strains contain additional mutations in the gene encoding pyruvate kinase; however, enzyme assays showed only minor variations in pyruvate kinase activities. Thus, the metabolic implication of these mutations is likely minor. It is striking, however, that two independently isolated strains have a second-site mutation in the same gene.
Deletion of the L. delbrueckii subsp. bulgaricus glucose transporter (Lb1-GS-1) results in the consumption of considerably more lactose and the secretion of glucose and higher levels of galactose, compared with the respective wild-type strain, when the bacteria are grown in milk (Table 3). Sugar metabolism in these strains is illustrated in Fig. 4.
FIG 4.
Sugar metabolism in L. delbrueckii subsp. bulgaricus. Black arrows, active enzyme reactions; red X, enzyme reaction inactivated by mutation; dotted black arrow, externalization of exopolysaccharide. The indicated genes are as follows: manLMNO, glucose/mannose PTS IIABCD; lacS, and lactose permease. PTS, phosphotransferase system; EPS, exopolysaccharide; PG, peptidoglycan; PEP, phosphoenolpyruvate; glu-6-P, glucose-6-phosphate; fru-6-P, fructose-6-phosphate; fru-B-P, fructose-1,6-biphosphate. (Inset) Green arrow, active genes; red arrow, inactive genes; manLMNO, glucose PTS IIABCD gene.
Yoghurt is traditionally made with a mixture of S. thermophilus and L. delbrueckii subsp. bulgaricus (1). Interactions between various mutants growing in milk were assessed following cofermentations (Fig. 1; Table 3). Some mutants show a delay in the initiation of acidification when inoculated alone but not during coculture, indicating that the well-known protocooperation seen when these two species are mixed is still efficient with the mutants.
Fermentation of milk by a galactose-fermenting S. thermophilus strain combined with the L. delbrueckii subsp. bulgaricus wild-type strain (St1-Gal+-1 plus Lb1-WT) results in accumulation of low levels of glucose. Replacing the L. delbrueckii subsp. bulgaricus wild-type strain with a glucose-secreting mutant (St1-Gal+-1 plus Lb1-GS-1) does not result in significantly altered glucose accumulation, even though Lb1-GS-1 produces increased amounts of glucose when used alone in a fermentation. This indicates that the glucose excreted by Lb1-GS-1 is consumed by St1-Gal+-1. This can be prevented by replacing St1-Gal+-1 with St1-GS-1, which is unable to phosphorylate glucose (St1-GS-1 plus Lb1-GS-1), or with St1-GS-2, which is additionally unable to transport glucose (St1-GS-2 plus Lb1-GS-1).
Combining a glucose-secreting S. thermophilus strain with a wild-type L. delbrueckii subsp. bulgaricus strain (St1-GS-1 plus Lb1-WT) results in increased levels of galactose and reduced levels of glucose in the milk, compared to the S. thermophilus mutant alone, indicating that some of the glucose excreted by the mutant is consumed by the L. delbrueckii subsp. bulgaricus strain. This is prevented by the use of Lb1-GS-1. Thus, glucose consumption by either species can be prevented by mutation of the respective glucose transporter genes.
Combining the glucose-secreting triple mutant S. thermophilus St1-GS-2 with the L. delbrueckii subsp. bulgaricus mutant Lb1-GS-1, which is unable to take up glucose from the milk, results in a fermented dairy product with high levels of glucose and galactose but no detectable lactose (Table 3). Similar results were obtained by combining St2-GS-2 and Lb2-GS-2 (data not shown).
Lactose fermentation by S. thermophilus and L. delbrueckii subsp. bulgaricus begins with the transport of lactose across the cell membrane. In S. thermophilus, this is mediated by LacS, which predominantly uses an exchange mechanism in which one molecule of galactose is excreted for every lactose molecule transported into the cell (25). Alternatively, if a sufficient proton motive force is present, lactose transport can be driven by a lactose/proton symport reaction (25). Since the lactose permease of L. delbrueckii subsp. bulgaricus is very similar to that of S. thermophilus (26), it is highly likely the same mechanisms are used in the two species. The exact mechanism of glucose export from the cell has not been determined but it is unlikely that it is via the lactose exchange mechanism, since the LacS protein does not interact with glucose (27). Facilitated diffusion has been suggested as a mechanism for sugar efflux in members of both Streptococcus and Lactobacillus genera (28); we favor the concept that the efflux of glucose observed in our mutants is by a similar mechanism, although the specific proteins involved have not been identified.
Lactose is cleaved to glucose and galactose by a cytoplasmic β-galactosidase in both species. Galactose is excreted and glucose is phosphorylated by glucokinase to glucose-6-phosphate, which is further metabolized by glycolysis. Glucokinase mutants of S. thermophilus (St1-GS-1 and St-GS-2) derived from strains that are able to ferment galactose (St1-Gal+-1 and St2-Gal+-1) are still able to grow on lactose, indicating that the cells have alternative ways of producing glucose-6-phosphate; these include phosphoglucomutase, whose normal role is the conversion of glucose-6-phosphate to glucose-1-phosphate by a reversible reaction, and transport via the glucose/mannose phosphotransferase system (Fig. 1). The reason why milk fermented with the galactose-fermenting mutants contains only low levels of glucose (Table 3) is proposed to be because much of the excreted glucose is rapidly transported back into the cell by the glucose/mannose phosphotransferase system. The resulting glucose-6-phosphate can be metabolized by glycolysis. Evidence for this is the fact that a triple mutant (St-GS-2) in which the glucose/mannose phosphotransferase system is inactivated results in the accumulation of higher levels of glucose than the double mutant parent (St1-GS-1) when grown in milk (Table 3).
We compared the abilities of the S. thermophilus strains to grow in M17 broth supplemented with different carbohydrates (Table 2) (identical results were obtained with St2-WT and derivatives). The pattern observed is as expected for glucose and galactose and is fully consistent with the genotypes. All strains are able to utilize sucrose and none of the strains can use mannose as a carbon source, as observed previously (29, 30).
Several of our observations are inconsistent with the work of Cochu et al. (30). This may reflect differences in the specific strains studied. Indeed, ATCC 19258, the strain studied by Cochu et al. (30), differs from most other S. thermophilus strains, including St1-WT and St2-WT, in the sequence of the manL gene encoding the IIABman protein of the glucose/mannose phosphotransferase system (PTS). The consensus manL gene encodes a IIABman protein with N51 and A256, whereas ATCC 19258 has K51 and V256 in these positions. Position 51 is part of the active site of the protein. The same differences are also seen in two S. thermophilus strains, i.e., M17PTZA496 (GenBank accession no. AZJT01000000) (31) and TH985 (GenBank accession no. AZTM01000000) (32), isolated from Italian dairy sources.
The triple mutants St1-GS-2 and St2-GS-2 could not acidify milk and grew poorly in M17 lactose (Table 2) without prior growth in the presence of sucrose or the addition of a low level of sucrose to the final growth medium. Hutkins et al. (33) suggested that resting cells of S. thermophilus are energy depleted. We speculate that the role of sucrose is to provide an initial source of energy, allowing cells to exit the stationary phase. Lactose cannot play this role because transport of one molecule of lactose requires the export of one molecule of galactose (25), thereby depriving these glucokinase-negative mutants of a fermentable carbon source. Once a sufficient proton motive force has been generated by sucrose metabolism, however, lactose can be transported with the concomitant export of a proton (25) and the galactose moiety metabolized, allowing continued growth. None of the other potential energy sources tested (glutamine, glutamate, arginine, glycine, and betaine) stimulated milk acidification by the triple mutants.
L. delbrueckii subsp. bulgaricus does not have the genes for galactose metabolism (7, 24), and strains of this species cannot be mutated to use galactose. Thus, the only sugars available to this species in milk fermented by glucose-secreting S. thermophilus strains are lactose and glucose. Knocking out the glucose/mannose phosphotransferase results in strains unable to transport glucose but still able to catabolize the intracellular glucose released by the cleavage of lactose.
The lactic acid bacterium Lactococcus lactis has, in addition to two distinct phosphotransferase systems (mannose PTS and cellobiose PTS) that can transport glucose, a non-PTS glucose permease designated GlcU (34). Since glucokinase-negative S. thermophilus strains with mutations in either manM or manN are unable to grow on glucose, the man operon encodes the only active PTS capable of transporting glucose in these strains. A homologue of glcU is present in S. thermophilus (A. R. Neves, personal communication) but, since the glucose-secreting mutants are glucokinase deficient, transport of glucose by GlcU would not lead to glucose catabolism. The L. delbrueckii subsp. bulgaricus glucose-secreting mutants have an intact glucokinase gene but are unable to grow on externally supplied glucose; this suggests that there is no non-PTS transport system active in these strains and that the glucose/mannose phosphotransferase system is the sole active PTS capable of transporting glucose in this species.
A number of studies have analyzed the interaction between S. thermophilus and L. delbrueckii subsp. bulgaricus during yoghurt production and have demonstrated sharing of a number of nutrients (35, 36). Our results show that glucose can be secreted by both species and also taken up by both species, suggesting that this is another nutrient that these organisms can potentially share.
Pool et al. (15) modified a strain of L. lactis so that it was unable to transport or to ferment glucose, resulting in a glucose-excreting phenotype. This was accomplished by deleting the glucokinase gene (glk) and the genes for two different glucose phosphotransferase systems (ptnABCD and ptcBA). Since deletion of all three systems was required to render the strain unable to grow on exogenous glucose, these mutants could not have been obtained by traditional selection or screening methods. Instead, the genes were deleted using recombinant DNA technology. Because the method of deleting the genes did not leave behind any undesirable genetic content, the strain would satisfy a stringent definition of a food-grade genetically modified organism (37). However, the use of recombinant DNA technology in the food industry is controversial, and genetically modified lactic acid bacteria for yoghurt production have not been brought to market. The reasons are numerous and include a general skepticism among consumers, the burdensome regulatory landscape, and the labeling requirements imposed as part of consumer information in some parts of the world (38). Classic mutants, on the other hand, can be used without additional regulatory approval and without requiring specific labeling.
We have estimated that use of a combination of the S. thermophilus and L. delbrueckii subsp. bulgaricus mutants described here would allow yoghurt manufacturers to reduce the amount of added sugar by up to 10 g per liter while still providing consumers with the desired taste experience. This would have a modest effect on the caloric content of the yoghurt (eliminating less than 10 cal per 200-ml serving) but would represent cost savings for the manufacturers. An additional benefit is that yoghurt produced with these strains has very low levels of lactose, providing consumers who experience digestive problems following the consumption of yoghurt with a natural alternative.
Metabolic rerouting does not necessarily require the use of recombinant DNA technology (38, 39). Based on a good understanding of the physiology of the lactic acid bacteria S. thermophilus and L. delbrueckii subsp. bulgaricus, we were able to change dramatically the metabolic products secreted into the growth medium. This was done by selecting spontaneous mutants under specific conditions that allowed growth only of strains with the desired phenotype. When growing in milk, the wild-type bacteria normally consume only a fraction of the lactose present, metabolize the glucose moiety, and secrete most of the galactose back into the milk. The mutants described here consume substantially more of the lactose, metabolize some of the galactose, and secrete the remaining galactose and most of the glucose back into the milk. This allows production of yoghurt with very low lactose levels and enhanced natural sweetness, because humans perceive glucose as being considerably sweeter than either lactose or galactose.
ACKNOWLEDGMENTS
We are grateful to Ditte Christiansen and Minna Wernegren for excellent technical assistance, to Silja Kej Diemer and Sussi Pleinert for upscaling and PIM production, to Gaelle Buchhorn, Nanna Christensen, and Janne Holm Andersen for the milk acidifications and yoghurt production, to the members of the Chr. Hansen taste panel for tasting of the various yoghurts, and to Mette Bertelsen and Helle Simonsen for the sugar and organic acid analyses. Figure 3 and Fig. 4 were inspired by Hols et al. (6). We thank Ana Rute Neves and Patrick Derkx for helpful discussions and critical reading of the manuscript.
All authors are employed by Chr. Hansen, either in the United States or in Denmark; some of the authors are shareholders in Chr. Hansen A/S, and some of the authors are inventors on patent applications related to this work.
Funding Statement
This work was funded by Chr. Hansen A/S.
REFERENCES
- 1.Codex Alimentarius Commission. 2000. Codex standard for yoghurt (yogurt) and sweetened yoghurt (sweetened yogurt), p 42–43. Secretariat of the Joint FAO/WHO Food Standards Programme (ed), Codex alimentarius, vol 12. Milk and milk products, 2nd ed Food and Agriculture Organisation of the United Nations, Rome, Italy. [Google Scholar]
- 2.Baglio E. 2014. The industry of yoghurt: formulations and food additives, p 33–57. In Parisi S. (ed), Chemistry and technology of yoghurt fermentation. Springer, London, United Kingdom. [Google Scholar]
- 3.Silanikove N, Leitner G, Merin U. 2015. The interrelationships between lactose intolerance and the modern dairy industry: global perspectives in evolutional and historical backgrounds. Nutrients 7:7312–7331. doi: 10.3390/nu7095340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernstrom J, Munger S, Sclafani A, de Araujo I, Roberts A, Molinary S. 2012. Mechanisms for sweetness. J Nutr 142:1134S–1142S. doi: 10.3945/jn.111.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robyt J. 1998. Essentials of carbohydrate chemistry, p 142–146. Springer-Verlag, New York, NY. [Google Scholar]
- 6.Hols P, Hancy F, Fontaine L, Grossiord B, Prozzi D, Leblond-Bourget N, Decaris B, Bolotin A, Delorme C, Ehrlich SD, Guédon E, Monnet V, Renault P, Kleerebezem M. 2005. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol Rev 29:435–463. doi: 10.1016/j.fmrre.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Turner K, Martley F. 1983. Galactose fermentation and classification of thermophilic lactobacilli. Appl Environ Microbiol 45:1932–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terzaghi B, Sandine W. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol 29:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Man J, Rogosa M, Sharpe M. 1960. A medium for the cultivation of lactobacilli. J Appl Bacteriol 23:130–135. doi: 10.1111/j.1365-2672.1960.tb00188.x. [DOI] [Google Scholar]
- 10.Bolotin A, Quinquis B, Renault P, Sorokin A, Ehrlich SD, Kulakauskas S, Lapidus A, Goltsman E, Mazur M, Pusch GD, Fonstein M, Overbeek R, Kyprides N, Purnelle B, Prozzi D, Ngui K, Masuy D, Hancy F, Burteau S, Boutry M, Delcour J, Goffeau A, Hols P. 2004. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat Biotechnol 22:1554–1558. doi: 10.1038/nbt1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darling A, Mau B, Blattner F, Perna N. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, Shakhova V, Grigoriev I, Lou Y, Rohksar D, Lucas S, Huang K, Goodstein DM, Hawkins T, Plengvidhya V, Welker D, Hughes J, Goh Y, Benson A, Baldwin K, Lee JH, Diaz-Muniz I, Dosti B, Smeianov V, Wechter W, Barabote R, Lorca G, Altermann E, Barrangou R, Ganesan B, Xie Y, Rawsthorne H, Tamir D, Parker C, Breidt F, Broadbent J, Hutkins R, O'Sullivan D, Steele J, Unlu G, Saier M, Klaenhammer T, Richardson P, Kozyavkin S, Weimer B, Mills D. 2006. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A 103:15611–15616. doi: 10.1073/pnas.0607117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith P, Krohn R, Hermanson G, Mallia A, Gartner F, Provenzano M, Fujimoto E, Goeke N, Olson B, Klenk D. 1985. Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 14.Porter EV, Chassy BM, Holmlund CE. 1982. Purification and kinetic characterization of a specific glucokinase from Streptococcus mutans OMZ70 cells. Biochim Biophys Acta 709:178–186. doi: 10.1016/0167-4838(82)90459-9. [DOI] [PubMed] [Google Scholar]
- 15.Pool W, Neves A, Kok J, Santos H, Kuipers O. 2006. Natural sweetening of food products by engineering Lactococcus lactis for glucose production. Metab Eng 8:456–464. doi: 10.1016/j.ymben.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.International Organization for Standardization. 1985. Sensory analysis–methodology–flavour profile methods. International standard ISO 6564. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 17.International Organization for Standardization. 1993. Sensory analysis–general guidance for the selection, training and monitoring of assessors, part 1: selected assessors. International standard ISO 8586-1. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 18.International Organization for Standardization. 2008. Sensory analysis–general guidance for the selection, training and monitoring of assessors, part 2: expert sensory assessors. International standard ISO 8586-2. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 19.Vaughan E, van den Bogaard P, Catzeddu P, Kuipers O, de Vos W. 2001. Activation of silent gal genes in the lac-gal regulon of Streptococcus thermophilus. J Bacteriol 183:1184–1194. doi: 10.1128/JB.183.4.1184-1194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vin F, Rådstrom P, Herman L, De Vuyst L. 2005. Molecular and biochemical analysis of the galactose phenotype of dairy Streptococcus thermophilus strains reveals four different fermentation profiles. Appl Environ Microbiol 71:3659–3667. doi: 10.1128/AEM.71.7.3659-3667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana H, Ishihama A. 1985. Correlation between the rate of productive transcription initiation and the strand-melting property of Escherichia coli promoters. Nucleic Acids Res 13:9031–9042. doi: 10.1093/nar/13.24.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youderian P, Lawes M, Creighton C, Cook JC, Saier M Jr. 1999. Mutations that confer resistance to 2-deoxyglucose reduce the specific activity of hexokinase from Myxococcus xanthus. J Bacteriol 181:2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chervaux C, Ehrlich S, Maguin E. 2000. Physiological study of Lactobacillus delbrueckii subsp. bulgaricus strains in a novel chemically defined medium. Appl Environ Microbiol 66:5306–5311. doi: 10.1128/AEM.66.12.5306-5311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Guchte M, Penaud S, Grimaldi C, Barbe V, Bryson K, Nicolas P, Robert C, Oztas S, Mangenot S, Couloux A, Loux V, Dervyn R, Bossy R, Bolotin A, Batto J-M, Walunas T, Gibrat J-F, Bessières P, Weissenbach J, Ehrlich SD, Maguin E. 2006. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc Natl Acad Sci U S A 103:9274–9279. doi: 10.1073/pnas.0603024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poolman B. 2002. Transporters and their roles in LAB cell physiology. Antonie Van Leeuwenhoek 82:147–164. doi: 10.1023/A:1020658831293. [DOI] [PubMed] [Google Scholar]
- 26.Leong-Morgenthaler P, Zwahlen M, Hottinger H. 1991. Lactose metabolism in Lactobacillus bulgaricus: analysis of the primary structure and expression of the genes involved. J Bacteriol 173:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veenhoff L, Poolman B. 1999. Substrate recognition at the cytoplasmic and extracellular binding site of the lactose transport protein of Streptococcus thermophilus. J Biol Chem 274:33244–33250. doi: 10.1074/jbc.274.47.33244. [DOI] [PubMed] [Google Scholar]
- 28.Sutrina S, Reizer J, Saier M Jr. 1988. Inducer expulsion in Streptococcus pyogenes: properties and mechanism of the efflux reaction. J Bacteriol 170:1874–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman J, Stark P. 1938. The fermentation of disaccharides by Streptococcus thermophilus. J Bacteriol 36:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cochu A, Vadeboncoeur C, Moineau S, Frenette M. 2003. Genetic and biochemical characterization of the phosphoenolpyruvate:glucose/mannose phosphotransferase system of Streptococcus thermophilus. Appl Environ Microbiol 69:5423–5432. doi: 10.1128/AEM.69.9.5423-5432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treu L, Vendramin V, Bovo B, Campanaro S, Corich V, Giacomini A. 2014. Genome sequences of Streptococcus thermophilus strains MTH17CL396 and M17PTZA496 from fontina, an Italian PDO cheese. Genome Announc 2(1):e00067-14. doi: 10.1128/genomeA.00067-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treu L, Vendramin V, Bovo B, Campanaro S, Corich V, Giacomini A. 2014. Genome sequences of four Italian Streptococcus thermophilus strains of dairy origin. Genome Announc 2(2):e00126-14. doi: 10.1128/genomeA.00126-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutkins R, Morris H, McKay L. 1985. Galactose transport in Streptococcus thermophilus. Appl Environ Microbiol 50:772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro R, Neves AR, Fonseca LL, Pool WA, Kok J, Kuipers OP, Santos H. 2009. Characterization of the individual glucose uptake systems of Lactococcus lactis: mannose-PTS, cellobiose-PTS and the novel GlcU permease. Mol Microbiol 71:795–806. doi: 10.1111/j.1365-2958.2008.06564.x. [DOI] [PubMed] [Google Scholar]
- 35.Herve-Jimenez L, Guillouard I, Guedon E, Boudebbouze S, Hols P, Monnet V, Maguin E, Rul F. 2009. Post-genomic analysis of Streptococcus thermophilus co-cultivated in milk with Lactobacillus delbrueckii subsp. bulgaricus: involvement of nitrogen, purine and iron metabolisms. Appl Environ Microbiol 75:2062–2073. doi: 10.1128/AEM.01984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sieuwerts S, Molenaar D, van Hijum SAFT, Beerthuyzen M, Stevens MJA, Janssen PWM, Ingham CJ, de Bok FAM, de Vos WM, van Hylckama Vlieg JET. 2010. Mixed-culture transcriptome analysis reveals the molecular basis of mixed-culture growth in Streptococcus thermophilus and Lactobacillus bulgaricus. Appl Environ Microbiol 76:7775–7784. doi: 10.1128/AEM.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansen E. 1999. Genetic engineering: modification of bacteria, p 917–921. In Robinson R, Batt C, Patel P (ed), Encyclopedia of food microbiology. Academic Press, London, United Kingdom. [Google Scholar]
- 38.Johansen E, Øregaard G, Sørensen K, Derkx P. 2015. Modern approaches for isolation, selection and improvement of bacterial strains for fermentation applications, p 227–248. In Holzapfel W. (ed), Advances in fermented foods and beverages: improving quality, technologies and health benefits. Woodhead Publishing Ltd., Cambridge, United Kingdom. [Google Scholar]
- 39.Derkx P, Janzen T, Sørensen K, Christensen J, Stuer-Lauridsen B, Johansen E. 2014. The art of strain improvement of industrial lactic acid bacteria without the use of recombinant DNA technology. Microb Cell Fact 13(Suppl 1):S5. doi: 10.1186/1475-2859-13-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]