ABSTRACT
Nitrate reduction to nitrite in oil fields appears to be more thermophilic than the subsequent reduction of nitrite. Concentrated microbial consortia from oil fields reduced both nitrate and nitrite at 40 and 45°C but only nitrate at and above 50°C. The abundance of the nirS gene correlated with mesophilic nitrite reduction activity. Thauera and Pseudomonas were the dominant mesophilic nitrate-reducing bacteria (mNRB), whereas Petrobacter and Geobacillus were the dominant thermophilic NRB (tNRB) in these consortia. The mNRB Thauera sp. strain TK001, isolated in this study, reduced nitrate and nitrite at 40 and 45°C but not at 50°C, whereas the tNRB Petrobacter sp. strain TK002 and Geobacillus sp. strain TK003 reduced nitrate to nitrite but did not reduce nitrite further from 50 to 70°C. Testing of 12 deposited pure cultures of tNRB with 4 electron donors indicated reduction of nitrate in 40 of 48 and reduction of nitrite in only 9 of 48 incubations. Nitrate is injected into high-temperature oil fields to prevent sulfide formation (souring) by sulfate-reducing bacteria (SRB), which are strongly inhibited by nitrite. Injection of cold seawater to produce oil creates mesothermic zones. Our results suggest that preventing the temperature of these zones from dropping below 50°C will limit the reduction of nitrite, allowing more effective souring control.
IMPORTANCE Nitrite can accumulate at temperatures of 50 to 70°C, because nitrate reduction extends to higher temperatures than the subsequent reduction of nitrite. This is important for understanding the fundamentals of thermophilicity and for the control of souring in oil fields catalyzed by SRB, which are strongly inhibited by nitrite.
INTRODUCTION
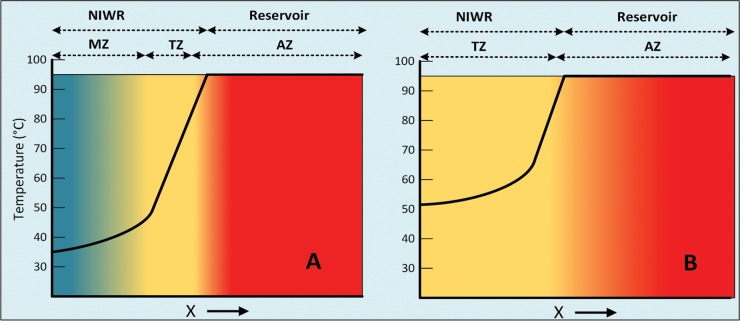
Deep oil fields harbor anaerobic, thermophilic microbial communities, which include sulfate-reducing, fermenting, and methanogenic Archaea and Bacteria (1–3). Although the temperature limit for microbial life is above 110°C, there is good geochemical evidence to suggest that microbial activity in oil fields stops at 80 to 90°C (4). Hence, deep oil fields with a resident temperature above 90°C are likely to be sterile. However, such fields may gain lower-temperature habitats once oil is produced by water injection. For example, in the Terra Nova field, offshore from Newfoundland, Canada, continuous injection of cold seawater (4°C) into a 95°C oil-bearing zone located at 3,200 to 3,700 m below the sea floor gives rise to a near-injection wellbore region (NIWR) in which the temperature increases from 35 to 95°C. Hence, the NIWR comprises successive mesothermic (35 to 45°C), thermogenic (45 to 85°C), and abiotic (85 to 95°C) zones, as indicated in Fig. 1A. Because seawater has a high sulfate concentration, its injection will stimulate the growth of mesophilic sulfate-reducing bacteria (mSRB), thermophilic sulfate-reducing bacteria (tSRB), and thermophilic sulfate-reducing Archaea (tSRA), causing the production of sulfide in the NIWR, a process referred to as souring (3, 5, 6). Since souring increases corrosion risk (7, 8), the sulfide concentrations in produced fluids are carefully monitored, with remedial measures being taken when these exceed a defined threshold. Measures include the use of biocides (9–11) or the injection of nitrate (3, 12–14).
FIG 1.
Hypothetical temperature profiles as a function of radial distance x from the injection well for a high-temperature oil field, such as Terra Nova, injected with cold (4°C) seawater, which heats up to 35°C during its 3,000-m downward transport (A), and with hot produced water or heated seawater (B). The near-injection wellbore region (NIWR) is the region in which the temperature increases from that of the incoming injection water (35°C) to that of the reservoir (95°C). Mesothermic (MZ) (35 to 45°C), thermogenic (TZ) (45 to 85°C), and abiotic (AZ) (85 to 95°C, the reservoir temperature) zones are indicated.
Although nitrate is not typically present as an electron acceptor in oil fields, its large-scale injection to control souring will induce growth of mesophilic nitrate-reducing bacteria (mNRB) and thermophilic nitrate-reducing bacteria (tNRB). Several mechanisms act in the control of souring by the activity of NRB, including the reduction of nitrate to nitrite, which is a strong inhibitor of dissimilatory sulfite reductase (Dsr), the enzyme that produces sulfide (15). However, many NRB reduce nitrite further through denitrification to N2 or through dissimilatory nitrate reduction to ammonium (DNRA) (16–19), causing the inhibition of SRB by nitrite to be only transient.
Although oil field tSRB and tSRA isolates can grow at temperatures of up to 105°C under laboratory conditions, oil field tNRB are less thermophilic, with maximal growth temperatures of up to 70°C. In a seminal bioreactor study on control of souring by tSRB consortia from hot Alaskan oil reservoirs with nitrate at 60°C, Reinsel et al. (13) found that nitrate was reduced to nitrite but that nitrite was not reduced further. As a result, souring control was observed with a low concentration of continuously injected nitrate (0.71 mM) under these conditions. Likewise, tNRB enrichments from the Barrancas field in Argentina, grown on nitrate and volatile fatty acids (VFA) (a mixture of acetate, propionate, and butyrate), accumulated nitrite at 60 but not at 37°C (2). These results suggest that in oil field consortia, reduction of nitrate to nitrite extends to higher temperatures than the subsequent reduction of nitrite to nitrogen or ammonium. We have since found this for other high-temperature oil fields, including for Terra Nova. Activities of tNRB and tSRB from such fields are best characterized by concentrating samples by up to 50-fold prior to their use as inocula. Using this approach we were also able to demonstrate the presence of tNRB in samples from the low-temperature (30°C) Medicine Hat Glauconitic C (MHGC) field in Alberta, Canada, and these were used to delineate the temperature limits of tNRB activities in the present study.
MATERIALS AND METHODS
Study site and sample collection.
Samples of injection water (14IW), produced water (18PW), and source water (22SW) were obtained monthly from the MHGC oil field near Medicine Hat, Alberta, Canada. This heavy oil-producing field has been described before (20). The oil in the produced water-oil mixture is separated by treatment with demulsifier at 80°C and transferred to storage tanks, while the deoiled produced water is mixed with source water (also referred to as makeup water) and reinjected as injection water (see Fig. S1 in the supplemental material). Application of nitrate for control of souring has been in operation since 2007 (20). Samples were collected in sterile 1-liter Nalgene bottles filled to the brim to exclude air during transportation. Following arrival at the University of Calgary within half a day from collection, samples were immediately transferred to a Coy anaerobic hood with an atmosphere of 90% N2 and 10% CO2 (N2-CO2). Methods for chemical analysis of oil field water samples have been described elsewhere (20, 21).
Incubation of concentrated cell suspensions with nitrate and oil organics.
Coleville synthetic brine medium K (CSBK) (22) with sodium nitrate (10 mM) as the electron acceptor, 3 mM VFA (3 mM [each] acetate, propionate, and butyrate) as the electron donor, and an N2-CO2 headspace was used for cultivation of mNRB and tNRB. For cultivation of tNRB, water samples (20 ml) were concentrated up to 20-fold by centrifugation at 15,000 × g for 20 min in an Avanti JE centrifuge (Beckman Coulter). Each pellet was then suspended in 1 ml of the supernatant, whereas the remaining supernatant was filtered through an 0.2-μm nylon membrane filter (Pall Life Science, NY). The nylon filters were then added to the suspended pellets. The combined cell suspensions were added to 60-ml serum bottles closed with butyl rubber stoppers containing 19 ml of CSBK with nitrate and VFA. Duplicate samples were incubated in the dark at 40, 45, 50, 55, 60, 65, 70, and 75°C without shaking. Sterile controls without inoculation were identically treated and incubated at 40 or 70°C. BTEX compounds (benzene [34 mM], toluene [28 mM], ethylbenzene [24 mM], m-xylene [24 mM], o-xylene [24 mM], and p-xylene [24 mM] in 0.5 ml of 2,2,4,4,6,8,8-heptamethylnonane [HMN]) were also used as electron donors at either 30 or 60°C. The concentrations of nitrate and nitrite were determined with high-performance liquid chromatography (HPLC), using a Waters 1515 HPLC instrument equipped with a Waters 2489 UV/visible detector and an IC-PAK Anion HC 4.6- by 150-mm column (Waters, Japan), as described elsewhere (23). The concentrations of ammonium were quantified using the indophenol method (24). Nitrite was also monitored by chemical reaction using the Griess-Ilosvays reagent (Sigma-Aldrich) (25).
Community analysis.
DNA was extracted at the end of incubation when no more reduction of nitrate or nitrite was observed. Equal volumes of samples (500 μl) were taken and centrifuged at 17,000 × g for 5 min to pellet the cells. DNA was isolated using the FastDNA extraction kit for soil (MP Biomedicals), according to the manufacturer's instructions. Centrifuged samples not subjected to incubation were also analyzed. DNA was quantified with a Qubit fluorimeter (Invitrogen) using the Quant-iT double-stranded DNA (dsDNA) HS assay kit (Invitrogen). 16S rRNA genes were amplified through a two-step PCR using nonbarcoded universal 16S forward primer (926Fw, AAACTYAAAKGAATTGACGG) and reverse primer (1392R, ACGGGCGGTGTGTRC) for 25 cycles followed by amplicon purification and PCR using barcoded primers 454T_RA_X and 454T_FwB, which have 926Fw and 1392R as their 3′ ends, for 10 cycles, as described elsewhere (26). The purified PCR product (100 ng) was subjected to pyrosequencing at the McGill University Genome Quebec Innovation Centre, Montreal, using a Genome Sequencer FLX instrument and a GS FLX titanium series XLR70 kit (Roche Diagnostics Corporation). Sequences were subjected to quality control and bioinformatics analysis using Phoenix 2 (27).
Enumeration of NRB.
Aliquots (100 μl) of concentrated MHGC samples were serially diluted in triplicate in CSBK medium (900 μl) containing 10 mM nitrate and 3 mM VFA in 48-well microtiter plates. These were incubated anaerobically at 40°C or 60°C for 7 days in jars flushed with N2-CO2. Wells with turbidity due to microbial growth or with color change due to formation of nitrite as assayed with the Griess-Ilosvays reagent were scored positive. Most probable numbers (MPNs) were derived from the data using appropriate statistical tables (28).
Isolation and identification of NRB strains.
NRB enrichments obtained at 40 to 70°C were plated on CSBK medium with 3 mM VFA and 10 mM nitrate, solidified with 15 g/liter of agar. The plates were incubated at the same temperature as used for the enrichment in jars flushed with N2-CO2. Individual colonies were picked and grown in CSBK medium with 10 mM nitrate and 3 mM VFA. The grown isolates were phylogenetically identified by Sanger sequencing of 1,500-bp 16S rRNA gene amplicons obtained with primers 27F and 1525R (29) at the Core DNA Services Laboratory of the University of Calgary. Duplicate samples from selected isolates were incubated at 20, 30, 40, 45, 50, 55, 60, 65, 70, and 75°C. Samples (1 ml) were periodically withdrawn to monitor growth as optical density at 600 nm (OD600). The concentrations of nitrate and nitrite were quantified in the supernatants obtained by centrifugation at 17,000 × g for 5 min.
Quantitative PCR (qPCR).
The presence of nirS for heme c- and heme d1-containing nitrite reductase and of nirK for copper- and heme d1-containing nitrite reductase in 18PW consortia and in pure isolates was determined by PCR of 0.4 ng of DNA using PCR conditions and primers NirS2F/NirS3R (30) and PCR conditions and primers GnirK2F/GnirK2R (31), respectively. 16S rRNA genes were amplified with primers 16SrRNA-1055F/1392R (32). After confirming the presence of nirS and 16S rRNA genes by agarose gel electrophoresis of the PCR products, their abundance was quantified with the SsoFast EvaGreen Supermix kit (Bio-Rad) using a CFX96 real-time PCR detection system C1000 thermal cycler (Bio-Rad) according to the manufacturer's instructions. Calibration curves, made with purified PCR products of target genes serially diluted over a range of 8 orders of magnitude, were used to determine gene copy numbers.
Thermophilic nitrite reduction by deposited strains.
Fourteen strains of tNRB and thermophilic nitrate-reducing Archaea (tNRA) were obtained from the DSMZ culture collection, Braunschweig, Germany. Following cultivation in the suggested medium at the optimum temperature for each strain, cultures were transferred to CSBK with 10 mM nitrate and either 3 mM VFA, 10 mM acetate, 20 mM succinate, or 20 mM lactate. Incubation was done at the optimum temperature reported for each strain from 55 to 85°C.
Accession number(s).
The 16S rRNA gene amplicon sequence reads of samples incubated at 40, 45, 50, 55, 60, 65, and 70°C and prior to incubation were deposited in the Sequence Read Archive (SRA) at NCBI under accession numbers SRX1435999, SRX1436001, SRX1436002, SRX1435957, SRX1435982, SRX1435996, SRX1435997, and SRX1435998, respectively. The 16S rRNA gene sequences of Thauera sp. strain TK001, Petrobacter sp. strain TK002, and Geobacillus sp. strain TK003, isolated in this study, were deposited in GenBank with accession numbers KU057961, KU057962, and KU057963, respectively. Partial nirK sequences, representing PCR amplicons of pure cultures, were deposited in GenBank with accession numbers KX139464 to KX139468.
RESULTS
Activity of tNRB in water samples from the MHGC field.
The physicochemical properties of water samples obtained from the MHGC field are indicated in Table S1 in the supplemental material. When microbial activities of these samples were analyzed by injecting 5% (vol/vol) into CSBK medium with 10 mM nitrate and 3 mM VFA at 37 or 60°C, activity was observed only for the mesophilic incubations with nitrate (results not shown). However, incubations with 20-fold-concentrated inocula indicated the presence of both mNRB and tNRB activity (Fig. 2; see Fig. S2 in the supplemental material).
FIG 2.
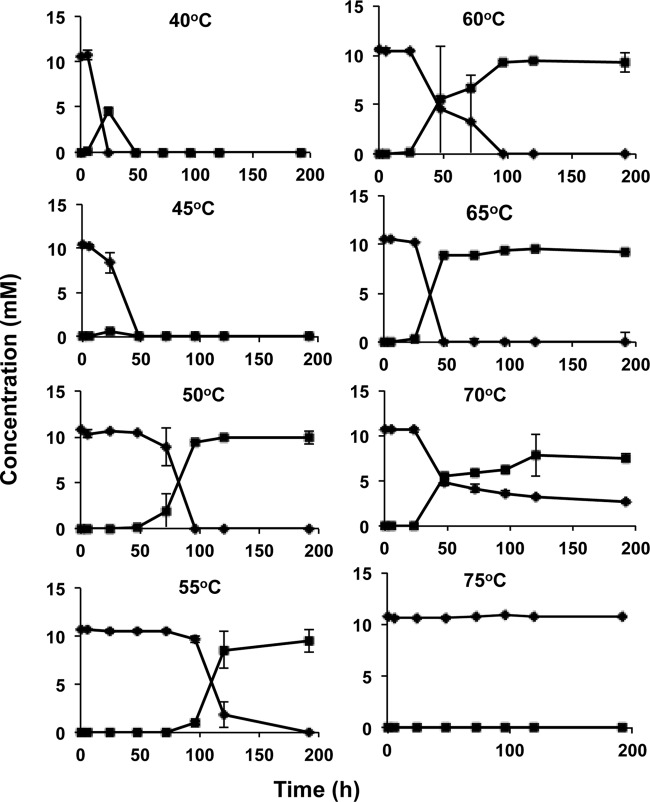
Reduction of nitrate (◆) to nitrite (■) in CSBK medium with VFA as electron donor by concentrated 18PW consortia at different temperatures. Data shown are averages from duplicate incubations. No reduction of nitrate was seen at 40 or 70°C in the absence of inoculation.
Temperature dependence of reduction of nitrate and nitrite by MHGC microbial consortia.
Concentrated microbial consortia of 18PW-produced water and of 14IW injection water (18PW and 14IW consortia) showed rapid and complete reduction of 10 mM nitrate with 3 mM VFA as the electron donor within 24 h with transient formation of nitrite at 40 and 45°C (Fig. 2; see Fig. S2 in the supplemental material). Similar to what has been found elsewhere (32), ammonium was not a significant product of nitrite reduction. At 50 to 65°C 18PW samples reduced nitrate to nitrite within 200 h. Nitrite was not reduced further, even with extended incubation times up to 720 h. The use of either 1 or 5 mM nitrate gave the same results, indicating that the lack of nitrite reduction was not caused by nitrite toxicity (results not shown). At 70°C, partial reduction of 7.5 mM nitrate to nitrite was observed, whereas no nitrate reduction was observed at 75°C (Fig. 2). These data indicate that the 18PW consortia had temperature limits of 70°C for reduction of nitrate to nitrite and of 50°C for subsequent reduction of nitrite. Concentrated 14IW consortia, incubated at or above 50°C, reduced nitrate partially at 60°C only (see Fig. S2 in the supplemental material). Neither mNRB nor tNRB activity was observed in the 22SW source water sample. Abiotic reduction of nitrate was not observed at either 40 or 70°C. These results indicate the presence of high mNRB activity in both the produced water and injection water, whereas tNRB activity was higher in the produced water than in the injection water. This was confirmed by determination of MPNs of mNRB at 40°C (5 × 105 ml−1 and 3 × 107 ml−1 for 18PW and 14IW, respectively) and of tNRB at 60°C (45 ml−1 and 20 ml−1 for 18PW and 14IW, respectively).
NRB in the MHGC field use low-molecular-weight oil components, especially toluene, as electron donors for nitrate reduction (33, 34). Concentrated 18PW reduced 10 mM nitrate at 40°C with toluene and ethylbenzene, but not with benzene or xylenes, as the electron donor (see Fig. S3 in the supplemental material). With toluene and ethylbenzene, 3.5 and 1.3 mM nitrite remained, respectively (Fig. S3), because insufficient electron donor was added to reduce all nitrate to N2 (as explained in the legend to Fig. S3). Nitrate reduction was not observed at 60°C with any of these BTEX compounds.
Change in community composition as a function of temperature.
Microbial community analysis indicated that mesophilic enrichments (40 and 45°C) of 18PW were dominated by Proteobacteria, whereas thermophilic enrichments (50 to 70°C) were dominated by Firmicutes. The dominant mesophilic genera were Thauera and Pseudomonas, whereas the dominant thermophilic genera were Geobacillus and Petrobacter (Table 1). Petrobacter was prominent only at 50°C, whereas Geobacillus was strongly represented at all temperatures from 50 to 70°C (Table 1). In addition to Geobacillus, Anoxybacillus (Table 1, 60°C only) and Pseudomonas appeared as significant community members under thermophilic conditions. Proteobacteria (Pseudomonas) and Euryarchaeota (Methanoculleus, Methanolinea, and Methanosaeta) dominated the 18PW community not subjected to incubation. The microbial community composition of 14IW incubated at 40, 45, and 60°C, conditions where nitrate reduction was observed (see Fig. S2 in the supplemental material), was similar to that of 18PW incubated at these same temperatures (see Fig. S4 in the supplemental material). At 60°C, in addition to Geobacillus, Ignavibacteria were a major community component. Cultured representatives of this class are thermophilic, facultatively anaerobic heterotrophs found in oil fields (35).
TABLE 1.
Microbial community composition as the fraction of total quality-controlled pyrosequencing reads of 18PW incubated in CSBK medium with 3 mM VFA and 10 mM nitrate at different temperatures
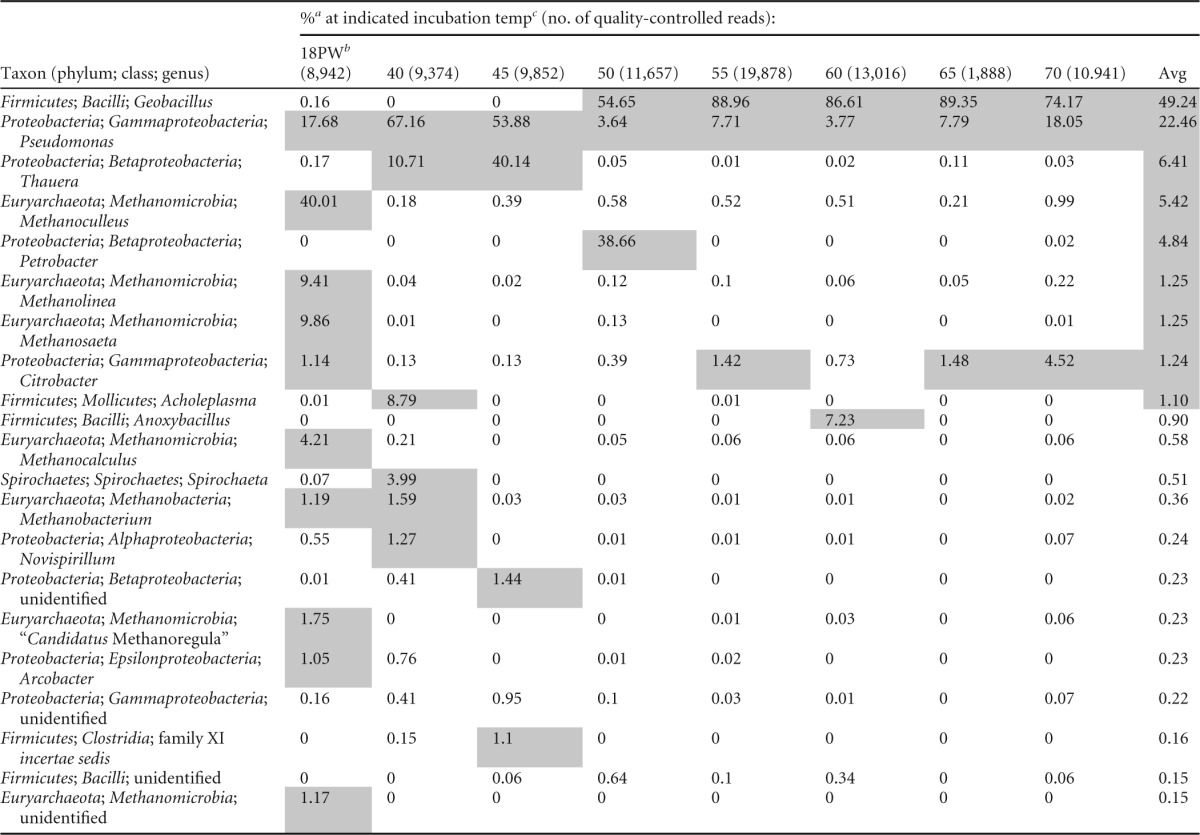
a Fractions in excess of 1% are shaded.
b Community composition prior to incubation.
c Temperature shown in °C.
Identification and physiological properties of isolated NRB strains.
Plating of NRB enrichments obtained at 40 to 70°C yielded three different bacterial isolates able to grow and reduce nitrate at different temperatures. 16S rRNA gene sequence analysis indicated that isolates TK001 (40°C), TK002 (55°C), and TK003 (70°C) had 99% sequence identity to Thauera aminoaromatica S2 (36), 99% to Petrobacter succinatimandens 4BONT (37), and 99% to Geobacillus kaustophilus HTA426 (38), respectively.
Thauera sp. TK001 grew and reduced nitrate from 20 to 45°C (Fig. 3A; see Fig. S5 in the supplemental material). The optimum growth temperature for this strain was near 30°C. It did not grow or reduce nitrate at or above 50°C. Nitrite did not accumulate at any growth temperature. Petrobacter sp. TK002 grew and reduced nitrate to nitrite from 50 to 60°C, with the optimum growth temperature being 55°C (Fig. 3B; see Fig. S5 in the supplemental material). Growth and nitrate reduction were not observed below 50°C or above 60°C. The accumulated nitrite was not reduced further. Geobacillus sp. TK003 grew and reduced nitrate to nitrite within a wider range of temperatures, from 45 to 70°C, with the optimum growth temperature being near 65°C (Fig. 3C; see Fig. S5 in the supplemental material). Similar to the case for Petrobacter sp. TK002, the accumulated nitrite was not reduced further. None of the isolates were able to use BTEX compounds as electron donors to reduce nitrate. These results indicated that Thauera sp. TK001 can be categorized as an mNRB, whereas Petrobacter sp. TK002 and Geobacillus sp. TK003 are tNRB, with Petrobacter sp. TK002 being a moderate tNRB. The isolated mNRB and tNRB reflected the community property of being able to reduce nitrite at temperatures of 45°C and lower but not at temperatures of 50°C or higher (Fig. 2 and 3; see Fig. S2 in the supplemental material).
FIG 3.
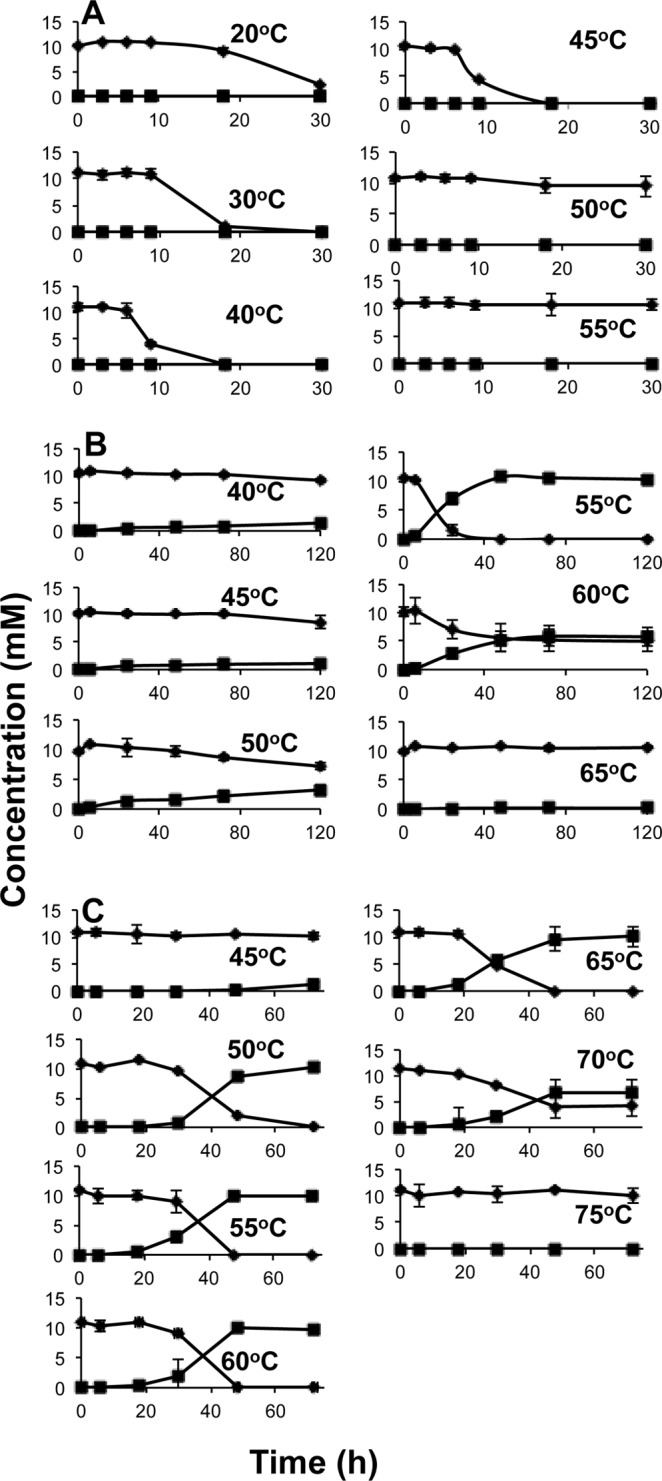
Temperature-dependent reduction of nitrate to nitrite by Thauera sp. TK001 (A), Petrobacter sp. TK002 (B), and Geobacillus sp. TK003 (C) in CSBK medium with 10 mM nitrate and 3 mM VFA. The symbols represent the concentrations of nitrate (◆) and of nitrite (■). Data are averages from duplicate incubations. Data for growth (OD600) of these cultures can be found in Fig. S5 in the supplemental material.
Abundance of nitrite reductase as a function of temperature.
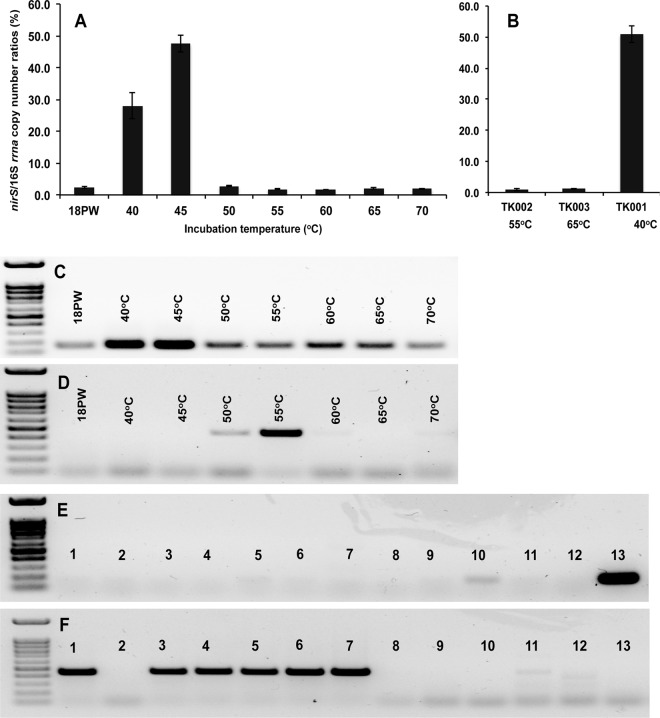
The ratio of nirS to 16S rRNA gene copies was determined for 18PW samples incubated at different temperatures. An increased ratio was observed for samples incubated at 40 and 45°C compared to samples incubated at 50 to 70°C (Fig. 4A). The ratios of nirS to 16S rRNA gene copies in samples incubated at 40 and 45°C were 28 and 48%, respectively, whereas they were maximally 3% for samples incubated at higher temperatures. The higher fraction of nirS gene copies at 40 and 45°C and the lower fraction at 50 to 70°C correlate with the presence and absence of nitrite reduction at these temperatures (Fig. 2). The fraction of nirS gene copies was also low for isolates with no nitrite reductase activity (0.9% for Petrobacter sp. TK002 and 1.1% for Geobacillus sp. TK003), whereas a fraction of 51% of nirS to 16S rRNA gene copies was detected for Thauera sp. TK001 (Fig. 4B). The low fractions of nirS to 16S rRNA genes in TK002 and TK003 indicate that the gene for cytochrome cd1 type nitrite reductase (NirS) was absent from these pure cultures. No amplification of nirK genes with primers GnirK2F and GnirK2R was detected in the TK001, TK002, and TK003 isolates, whereas genes were found in 18PW enrichments at 50 and 55°C only, but not at higher temperature (Fig. 4D and F). The nirK genes detected at 50 and 55°C did not contribute to the reduction of nitrite (Fig. 2). Hence, NirS was the dominant nitrite reductase in denitrification at 40 and 45°C (Fig. 4A and C). Its gene was not present at higher temperature, where nirK was found but was not active.
FIG 4.
Detection of nirS and nirK genes in 18PW and pure cultures of tNRB. (A) Ratio of nirS/16S rRNA gene copies in incubations with VFA and nitrate at the indicated temperatures of 18PW. (B) Ratio of nirS/16S rRNA gene copies in incubations with VFA and nitrate of isolates Thauera sp. TK001, Petrobacter sp. TK002, and Geobacillus sp. TK003 obtained in this study. (C) Detection of nirS genes by PCR with primers NirS2F and NirS3R in incubations of 18PW at 40 to 70°C, as indicated; qPCR detection of nirS in these incubations is shown in panel A. (D) Detection of nirK genes by PCR with primers GnirK2F and GnirK2R in incubations of 18PW as for panel C. (E) Detection of nirS genes by PCR with primers NirS2F and NirS3R in the following pure cultures: 1, Geobacillus thermoleovorans DSM5366; 2, G. kaustophilus DSM7263; 3, G. stearothermophilus DSM22; 4, G. thermodenitrificans DSM465; 5, G. toebii DSM14590; 6, G. thermocatenulatus DSM730; 7, G. subterraneus DSM13552; 8, G. galactosidasius DSM18751; 9, Tepidiphilus succinatimandens DSM15512; 10, Ammonifex degensii DSM10501; 11, Petrobacter (Tepidiphilus) sp. strain TK002; 12, Geobacillus sp. strain TK003; and 13, Thauera sp. TK001. (F) Detection of nirK genes by PCR with primers GnirK2F and GnirK2R in pure cultures as for panel E.
Thermophilic nitrite reduction by deposited pure cultures.
Because thermophilic nitrite reduction at temperatures of or above 50°C has been described in the literature, we compared the reduction of nitrate and nitrite by 14 deposited strains of tNRB and some thermophilic Archaea with that by Petrobacter sp. TK002 and Geobacillus sp. strain TK003. These were grown at 55, 60, or 65°C, depending on the optimum growth temperature reported for each strain. Because 3 mM VFA (3 mM [each] acetate, propionate, and butyrate), 10 mM acetate, 20 mM succinate, or 20 mM lactate was added, sufficient electron donor was present to reduce 10 mM nitrate to 5 mM N2 in all cases. A strain was scored positive for nitrite reduction when its concentration reached a maximum and then declined. By this criterion, G. kaustophilus DSM7263 reduced nitrate with all four electron donors and nitrite with VFA, succinate, and lactate, but not with acetate (see Fig. S6 in the supplemental material). Geobacillus sp. TK003 and Ammonifex degensii reduced nitrate but not nitrite with acetate, VFA and lactate, whereas Petrobacter sp. TK002 reduced nitrate with all four electron donors, but did not reduce nitrite (see Fig. S6 in the supplemental material), a phenotype shared with Geobacillus thermoleovorans DSM5366, Geobacillus stearothermophilus DSM22, and Geobacillus thermocatenulatus DSM730. Tepidiphilus succinatimandens DSM15512 (formerly Petrobacter succinatimandens) reduced nitrate and nitrite with VFA or succinate but not with acetate or lactate. Nitrate reduction was not observed for Thermus thermophilus DSM579 at 75°C, Aquifex pyrophilus DSM6858 at 80°C, Thermovibrio ruber DSM14644 at 80°C, and Pyrobaculum aerophilum DSM7523 at 95°C (results not shown).
Overall it appeared that for 12 isolates with four electron donors, thermophilic nitrate reduction was observed for 40/48 incubations tested, whereas thermophilic nitrite reduction was observed for only 9/48 incubations tested (Table 2). This indicates that thermophilic nitrite reduction in defined media is more the exception than the rule. Thermophilic nitrite reduction with acetate was not observed for any of the 12 isolates. The distribution of the nirK gene, found in many Geobacillus spp. (31), was determined by PCR (Fig. 4F). Its presence or absence did not correlate well with the reduction of nitrite in defined media; e.g., G. thermoleovorans, G. stearothermophilus, G. thermodenitrificans, and G. thermocatenulatus were positive for nirK but did not reduce nitrite, whereas G. kaustophilus and G. galactosidasius were negative for nirK but reduced nitrite with at least one of the four electron donors tested. G. toebii and G. subterraneus were positive for nirK and displayed nitrite reduction, whereas Geobacillus sp. TK003 was negative for nirK and nitrite reduction (Fig. 4E; Table 2). Of the isolates listed in Table 2, A. degensii was weakly positive for nirS (Fig. 4E, lane 10), whereas the mesophilic Thauera sp. TK001 was strongly positive for nirS (Fig. 4E, lane 13). Sanger sequencing of PCR products confirmed these to represent nirK of Geobacillus and nirS of Thauera, as indicated in Table S2 in the supplemental material.
TABLE 2.
Summary of the results shown in Fig. S6 in the supplemental material for reduction of nitrate and nitrite with acetate, VFA, succinate, or lactate as the electron donor by 12 tNRB
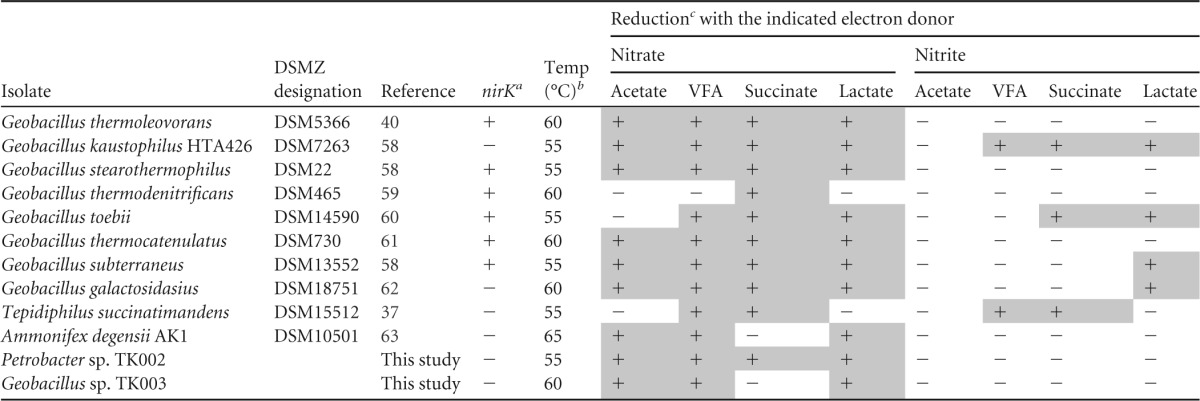
a Presence (+) or absence (−) of the nirK gene, as judged by PCR (Fig. 4F).
b Optimum temperature for growth used for incubations.
c Positive values are shaded.
DISCUSSION
The main result of our study is that the reduction of nitrate to nitrite by tNRB consortia and pure cultures from oil fields is more common than the subsequent reduction of nitrite. This may cause nitrite to accumulate in oil fields injected with nitrate at 50 to 70°C. Our results agree with those reported by Reinsel et al. (13), who demonstrated that tNRB consortia from a high-temperature Alaskan oil field did not reduce nitrite, allowing control of tSRB-mediated H2S production by injection of low concentrations of nitrate in a bioreactor at 60°C. However, these results may seem at odds with literature on tNRB pure cultures, which are capable of complete denitrification at high temperature (39, 40).
Members of the genus Geobacillus, phylum Firmicutes, are thermophilic, spore-forming, facultative nitrate reducing anaerobes, which grow from 45 to 75°C (41). Nazina et al. (42), using a medium with 0.2 g liter−1 of yeast extract at 60°C, found that of 9 Geobacillus spp. capable of reducing nitrate to nitrite, only 4 were able to produce gas from nitrate, indicating reduction of nitrite to N2 or N2O. In contrast, Verbaendert et al. (31) found that all of 23 strains of G. thermodenitrificans, G. stearothermophilus, G. kaustophilus, and G. toebii had the nirK gene and were able to reduce nitrite at 55°C. However, consistent reduction of nitrite was obtained only when minimal medium with nitrate and succinate was amended with 1% Trypticase soy broth (TSB). Using the same primer pair, we found nirK to be present in these strains, except in G. kaustophilus (Fig. 4F; Table 2). However, nitrite reduction in defined medium without added yeast extract or TSB with succinate as the sole electron donor was found only in incubations with G. kaustophilus and G. toebii (Table 2). Analysis of the genome of G. thermodenitrificans NG80-2 (39) indicated the presence of the complete denitrification pathway with genes for nitrate (narGHJI), nitrite (nirK), NO (norZ), and N2O (nosZSYF) reductase. However, only the narGHJI genes were identified in the genome of G. thermoleovorans (40), which we found to be positive for nirK (Fig. 4F) but to lack the ability to reduce nitrite in defined medium with any of the four electron donors tested (Table 2). Nara et al. (43) demonstrated complete denitrification by Geobacillus sp. strain TDN01 in a continuous-culture bioreactor fed with high concentrations (100 mM) of succinate and nitrate. The lack of NirK nitrite reductase activity in defined medium cultures of strains which evidently have the gene (Table 2) and the presence of NirK activity in rich media (31) indicate that nirK expression may be upregulated by as-yet-unknown rich medium components.
Petrobacter succinatimandens 4BONT, which was recently renamed Tepidiphilus succinatimandens comb. nov. (44), is a moderate thermophile (55°C) isolated from a petroleum reservoir (37). Strain 4BONT reduced nitrate to nitrite and N2O using succinate as an electron donor. However, Tepidiphilus thermophilus reduced nitrate only to nitrite (44). Thermophilic denitrifiers, which reduce nitrate to nitrite only, are thus not at all rare and include Petrobacter sp. TK002 and Geobacillus sp. TK003, which were isolated from the MHGC field in this study and were found to lack the nirS and nirK genes, as judged by PCR assays (Fig. 4E and F).
In contrast, mesophilic species like Thauera spp. generally have the complete denitrification pathway. However, the pattern of gene expression can differ, with some species expressing all denitrification genes at once (rapid complete onset [RCO]) and others exhibiting progressive onset (PO) of expression of denitrification genes (45). Nitrite transiently accumulates in PO, but not in RCO species. Thauera sp. TK001 did not show transient nitrite accumulation, indicating an RCO phenotype (Fig. 3A). However, some mNRB in the 18PW consortium had the PO phenotype, because transient accumulation of nitrite was observed at 40°C (Fig. 2). Thauera spp. exist in diverse environments, biodegrading multiple compounds, including aromatic hydrocarbons, such as toluene, benzoate, p-cresol, and phenylacetate (36). Strain TK001 grew with VFA but not with toluene. Thauera spp. use NirS nitrite reductase (45). Other mNRB include Pseudomonas spp. (46). Species of this genus can also be thermophiles, allowing its presence in 18PW consortia from 40 to 70°C with a minimum at 50°C (Table 1).
The higher numbers and activity of tNRB in produced water 18PW than in injection water 14IW (Fig. 2; see Fig. S2 in the supplemental material) suggests that these originated from the subsurface and not from the high-temperature (80°C) oil-water separator (see Fig. S1 in the supplemental material), since higher tNRB activity is expected in 14IW in that case. The tNRB in 18PW may be derived from deeper, hotter subsurface layers connected to the low-temperature MHGC reservoir, which is at 780 m below the surface. The presence of high fractions and activity of denitrifying mNRB in MHGC produced waters has been shown before (33, 47). Toluene and ethylbenzene were also used as electron donors to reduce nitrate (see Fig. S3 in the supplemental material). Although we did not detect nitrate reduction with xylenes, the use of m-xylene as an electron donor for nitrate reduction with accumulation of nitrite under mesophilic conditions has been reported previously (34, 48).
A small fraction of the taxon Geobacillus was detected in the community composition of 18PW prior to enrichment (Table 1). These thermophiles can survive under lower-temperature conditions, as shown for other cold environments (49–51). This has led to the suggestion that thermophiles in oil fields originated from the low concentrations present in seawater injected to promote oil recovery (1, 52–54) and, conversely, that low numbers of thermophiles in cold marine sediments may have originated from seeps from hot oil fields, where they may be part of the indigenous microbial community (49, 53, 55–57).
Hence, mNRB and tNRB may colonize zones in the NIWR with the appropriate temperature range (Fig. 1A) when this is injected with cold seawater harboring low numbers of these microbes. Unfortunately, when injected seawater is amended with nitrate, a fraction of this will be reduced in the mesophilic zone to N2 and will not contribute to souring control by inhibition of SRB. Assuming an upper temperature limits of 70°C for reduction of nitrate to nitrite and 50°C for reduction of nitrite to N2, as found here for oil field environments, we may expect improved performance of nitrate as a souring control agent if the temperature of any part of the NIWR is not allowed to drop below 50°C, as shown in Fig. 1B. This can be achieved, for instance, by reinjection of hot produced water, which is not yet commonly done in offshore seawater flooded reservoirs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported through a Natural Sciences and Engineering Research Council (NSERC) Industrial Research Chair awarded to G.V., which was also supported by Baker Hughes, BP, Computer Modeling Group Ltd., ConocoPhillips, Intertek, Dow Microbial Control, Enbridge, Enerplus Corporation, Oil Search Limited, Shell Global Solutions International, Suncor Energy Inc., Yara Norge, and Alberta Innovates—Energy and Environment Solutions (AIEES).
We are grateful to Rhonda Clark for administrative support, to Yin Shen and Dongshan An for technical assistance, and to Baker Hughes and Enerplus Corporation for providing the field samples used in this study. Discussions with Phil Stemler on the Terra Nova field were much appreciated.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00599-16.
REFERENCES
- 1.Dahle H, Garshol F, Madsen M, Birkeland NK. 2008. Microbial community structure analysis of produced water from a high-temperature North Sea oil-field. Antonie Van Leeuwenhoek 93:37–49. doi: 10.1007/s10482-007-9177-z. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A, An D, Cavallaro A, Voordouw G. 2014. Souring in low-temperature surface facilities of two high-temperature Argentinian oil fields. Appl Microbiol Biotechnol 98:8017–8029. doi: 10.1007/s00253-014-5843-z. [DOI] [PubMed] [Google Scholar]
- 3.Voordouw G. 2011. Production-related petroleum microbiology: progress and prospects. Curr Opin Biotechnol 22:401–405. doi: 10.1016/j.copbio.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Magot M. 2005. Indigenous microbial communities in oil fields, p 21–34. In Ollivier B, Magot M (ed), Petroleum microbiology. ASM Press, Washington, DC. [Google Scholar]
- 5.Lysnes K, Bodtker G, Torsvik T, Bjornestad EO, Sunde E. 2009. Microbial response to reinjection of produced water in an oil reservoir. Appl Microbiol Biotechnol 83:1143–1157. doi: 10.1007/s00253-009-2015-7. [DOI] [PubMed] [Google Scholar]
- 6.Gieg LM, Jack TR, Foght JM. 2011. Biological souring and mitigation in oil reservoirs. Appl Microbiol Biotechnol 92:263–282. doi: 10.1007/s00253-011-3542-6. [DOI] [PubMed] [Google Scholar]
- 7.Hubert C, Nemati M, Jenneman G, Voordouw G. 2005. Corrosion risk associated with microbial souring control using nitrate or nitrite. Appl Microbiol Biotechnol 68:272–282. doi: 10.1007/s00253-005-1897-2. [DOI] [PubMed] [Google Scholar]
- 8.Tang K, Baskaran V, Nemati M. 2009. Bacteria of the sulphur cycle: an overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem Eng J 44:73–94. doi: 10.1016/j.bej.2008.12.011. [DOI] [Google Scholar]
- 9.Whitham TS, Gilbert PD. 1993. Evaluation of a model biofilm for the ranking of biocide performance against sulphate-reducing bacteria. J Appl Bacteriol 75:529–535. doi: 10.1111/j.1365-2672.1993.tb01590.x. [DOI] [Google Scholar]
- 10.Fraise AP. 2002. Biocide abuse and antimicrobial resistance—a cause for concern? J Antimicrob Chemother 49:11–12. doi: 10.1093/jac/49.1.11. [DOI] [PubMed] [Google Scholar]
- 11.Telang AJ, Ebert S, Foght JM, Westlake DWS, Voordouw G. 1998. Effects of two diamine biocides on the microbial community from an oil field. Can J Microbiol 44:1060–1065. doi: 10.1139/w98-105. [DOI] [Google Scholar]
- 12.Myhr S, Lillebo BL, Sunde E, Beeder J, Torsvik T. 2002. Inhibition of microbial H2S production in an oil reservoir model column by nitrate injection. Appl Microbiol Biotechnol 58:400–408. doi: 10.1007/s00253-001-0881-8. [DOI] [PubMed] [Google Scholar]
- 13.Reinsel MA, Sears JT, Stewart PS, McInerney MJ. 1996. Control of microbial souring by nitrate, nitrite or glutaraldehyde injection in a sandstone column. J Ind Microbiol 17:128–136. doi: 10.1007/BF01570056. [DOI] [Google Scholar]
- 14.Larsen J. 2002. Downhole nitrate applications to control sulfate reducing bacteria activity and reservoir souring, paper 02025. Corrosion 2002. NACE International, Houston, TX. [Google Scholar]
- 15.Karkhoff-Schweizer RR, Huber DP, Voordouw G. 1995. Conservation of the genes for dissimilatory sulfite reductase from Desulfovibrio vulgaris and Archaeoglobus fulgidus allows their detection by PCR. Appl Environ Microbiol 61:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zumft WG. 1997. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong LF, Sobey MN, Smith CJ, Rusmana I, Phillips W, Stott A, Osborn AM, Nedwell DB. 2011. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnol Oceanogr 56:279–291. doi: 10.4319/lo.2011.56.1.0279. [DOI] [Google Scholar]
- 18.Mancinelli RL, McKay CP. 1983. Effects of nitric oxide and nitrogen dioxide on bacterial growth. Appl Environ Microbiol 46:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg EM, van Dongen U, Abbas B, van Loosdrecht MC. 2015. Enrichment of DNRA bacteria in a continuous culture. ISME J 9:2153–2161. doi: 10.1038/ismej.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voordouw G, Grigoryan AA, Lambo A, Lin S, Park HS, Jack TR, Coombe D, Clay B, Zhang F, Ertmoed R, Miner K, Arensdorf JJ. 2009. Sulfide remediation by pulsed injection of nitrate into a low temperature Canadian heavy oil reservoir. Environ Sci Technol 43:9512–9518. doi: 10.1021/es902211j. [DOI] [PubMed] [Google Scholar]
- 21.Folarin Y, An D, Caffrey S, Soh J, Sensen CW, Voordouw J, Jack T, Voordouw G. 2013. Contribution of make-up water to the microbial community in an oilfield from which oil is produced by produced water re-injection. Int Biodeterior Biodegradation 81:44–50. doi: 10.1016/j.ibiod.2012.07.017. [DOI] [Google Scholar]
- 22.Callbeck CM, Agrawal A, Voordouw G. 2013. Acetate production from oil under sulfate-reducing conditions in bioreactors injected with sulfate and nitrate. Appl Environ Microbiol 79:5059–5068. doi: 10.1128/AEM.01251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigoryan AA, Cornish SL, Buziak B, Lin S, Cavallaro A, Arensdorf JJ, Voordouw G. 2008. Competitive oxidation of volatile fatty acids by sulfate- and nitrate-reducing bacteria from an oil field in Argentina. Appl Environ Microbiol 74:4324–4335. doi: 10.1128/AEM.00419-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.APHA. 1980. Standard methods for the examination of water and waste water, p 439–440. American Public Health Association, Washington, DC. [Google Scholar]
- 25.Qu Y, Spain JC. 2010. Biodegradation of 5-nitroanthranilic acid by Bradyrhizobium sp. strain JS329. Appl Environ Microbiol 76:1417–1422. doi: 10.1128/AEM.02816-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An D, Caffrey SM, Soh J, Agrawal A, Brown D, Budwill K, Dong X, Dunfield PF, Foght J, Gieg LM, Hallam SJ, Hanson NW, He Z, Jack TR, Klassen J, Konwar KM, Kuatsjah E, Li C, Larter S, Leopatra V, Nesbø CL, Oldenburg T, Pagé AP, Ramos-Padron E, Rochman FF, Saidi-Mehrabad A, Sensen CW, Sipahimalani P, Song YC, Wilson S, Wolbring G, Wong M-L, Voordouw G. 2013. Metagenomics of hydrocarbon resource environments indicates aerobic taxa and genes to be unexpectedly common. Environ Sci Technol 47:10708–10717. doi: 10.1021/es4020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soh J, Dong X, Caffrey SM, Voordouw G, Sensen CW. 2013. Phoenix 2: a locally installable large-scale 16S rRNA gene sequence analysis pipeline with Web interface. J Biotechnol 167:393–403. doi: 10.1016/j.jbiotec.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Voordouw G. 2015. Primers for dsr genes and most probable number method for detection of sulfate-reducing bacteria in oil reservoirs, p 1–9. In McGenity T, Timmis K, Nogales B (ed), Hydrocarbon and lipid microbiology protocols. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 29.Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. 2008. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol 74:2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braker G, Fesefeldt A, Witzel K-P. 1998. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl Environ Microbiol 64:3769–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbaendert I, Hoefman S, Boeckx P, Boon N, De Vos P. 2014. Primers for overlooked nirK, qnorB, and nosZ genes of thermophilic Gram-positive denitrifiers. FEMS Microbiol Ecol 89:162–180. doi: 10.1111/1574-6941.12346. [DOI] [PubMed] [Google Scholar]
- 32.Harms G, Layton AC, Dionisi HM, Gregory IR, Garrett VM, Hawkins SA, Robinson KG, Sayler GS. 2003. Real-time PCR quantification of nitrifying bacteria in a municipal wastewater treatment plant. Environ Sci Technol 37:343–351. doi: 10.1021/es0257164. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal A, Park Hs Nathoo S, Gieg LM, Jack TR, Miner K, Ertmoed R, Benko A, Voordouw G. 2012. Toluene depletion in produced oil contributes to souring control in a field subjected to nitrate injection. Environ Sci Technol 46:1285–1292. doi: 10.1021/es203748b. [DOI] [PubMed] [Google Scholar]
- 34.Lambo AJ, Noke K, Larter SR, Voordouw G. 2008. Competitive, microbially-mediated reduction of nitrate with sulfide and aromatic oil components in a low-temperature, western Canadian oil reservoir. Environ Sci Technol 42:8941–8946. doi: 10.1021/es801832s. [DOI] [PubMed] [Google Scholar]
- 35.Podosokorskaya OA, Kadnikov VV, Gavrilov SN, Mardanov AV, Merkel AY, Karnachuk OV, Ravin NV, Bonch-Osmolovskaya EA, Kublanov IV. 2013. Characterization of Melioribacter roseus gen. nov., sp. nov., a novel facultatively anaerobic thermophilic cellulolytic bacterium from the class Ignavibacteria, and a proposal of a novel bacterial phylum Ignavibacteriae. Environ Microbiol 15:1759–1771. doi: 10.1111/1462-2920.12067. [DOI] [PubMed] [Google Scholar]
- 36.Mechichi T, Stackebrandt E, Gad'on N, Fuchs G. 2002. Phylogenetic and metabolic diversity of bacteria degrading aromatic compounds under denitrifying conditions, and description of Thauera phenylacetica sp. nov., Thauera aminoaromatica sp. nov., and Azoarcus buckelii sp. nov. Arch Microbiol 178:26–35. [DOI] [PubMed] [Google Scholar]
- 37.Salinas MB, Fardeau ML, Cayol JL, Casalot L, Patel BK, Thomas P, Garcia JL, Ollivier B. 2004. Petrobacter succinatimandens gen. nov., sp. nov., a moderately thermophilic, nitrate-reducing bacterium isolated from an Australian oil well. Int J Syst Evol Microbiol 54:645–649. doi: 10.1099/ijs.0.02732-0. [DOI] [PubMed] [Google Scholar]
- 38.Takami H, Takaki Y, Chee G-J, Nishi S, Shimamura S, Suzuki H, Matsui S, Uchiyama I. 2004. Thermoadaptation trait revealed by the genome sequence of thermophilic Geobacillus kaustophilus. Nucleic Acids Res 32:6292–6303. doi: 10.1093/nar/gkh970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng L, Wang W, Cheng JS, Ren Y, Zhao G, Gao CX, Tang Y, Liu XQ, Han WQ, Peng X, Liu RL, Wang L. 2007. Genome and proteome of long-chain alkane degrading Geobacillus thermodenitrificans NG80-2 isolated from a deep-subsurface oil reservoir. Proc Natl Acad Sci U S A 104:5602–5607. doi: 10.1073/pnas.0609650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molha D, Hack C, Marchant R. 2007. The complete denitrification pathway of Geobacillus thermoleovorans. BMC Syst Biol 1:P21. doi: 10.1186/1752-0509-1-S1-P21. [DOI] [Google Scholar]
- 41.Hussein AH, Lisowska BK, Leak DJ. 2015. The genus Geobacillus and their biotechnological potential. Adv Appl Microbiol 92:1–48. doi: 10.1016/bs.aambs.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Nazina TN, Sokolova DS, Grigoryan AA, Shestakova NM, Mikhailova EM, Poltaraus AB, Tourova TP, Lysenko AM, Osipov GA, Belyaev SS. 2005. Geobacillus jurassicus sp. nov., a new thermophilic bacterium isolated from a high-temperature petroleum reservoir, and the validation of the Geobacillus species. Syst Appl Microbiol 28:43–53. doi: 10.1016/j.syapm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Nara K, Iwata K, Matsui T, Shigeno T, Omori T. 2009. Functional analysis of the thermophilic denitrifying bacterium Geobacillus sp. strain TDN01 in continuous culture. J Gen Appl Microbiol 55:87–92. doi: 10.2323/jgam.55.87. [DOI] [PubMed] [Google Scholar]
- 44.Poddar A, Lepcha RT, Das SK. 2014. Taxonomic study of the genus Tepidiphilus: transfer of Petrobacter succinatimandens to the genus Tepidiphilus as Tepidiphilus succinatimandens comb. nov., emended description of the genus Tepidiphilus and description of Tepidiphilus thermophilus sp. nov., isolated from a terrestrial hot spring. Int J Syst Evol Microbiol 64:228–235. doi: 10.1099/ijs.0.056424-0. [DOI] [PubMed] [Google Scholar]
- 45.Liu B, Mao Y, Bergaust L, Bakken LR, Frostegard A. 2013. Strains in the genus Thauera exhibit remarkably different denitrification regulatory phenotypes. Environ Microbiol 15:2816–2828. doi: 10.1111/1462-2920.12142. [DOI] [PubMed] [Google Scholar]
- 46.Chayabutra C, Ju LK. 2000. Degradation of n-hexadecane and its metabolites by Pseudomonas aeruginosa under microaerobic and anaerobic denitrifying conditions. Appl Environ Microbiol 66:493–498. doi: 10.1128/AEM.66.2.493-498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shartau SL, Yurkiw M, Lin S, Grigoryan AA, Lambo A, Park HS, Lomans BP, van der Biezen E, Jetten MS, Voordouw G. 2010. Ammonium concentrations in produced waters from a mesothermic oil field subjected to nitrate injection decrease through formation of denitrifying biomass and anammox activity. Appl Environ Microbiol 76:4977–4987. doi: 10.1128/AEM.00596-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alain K, Harder J, Widdel F, Zengler K. 2012. Anaerobic utilization of toluene by marine alpha- and gammaproteobacteria reducing nitrate. Microbiology 158:2946–2957. doi: 10.1099/mic.0.061598-0. [DOI] [PubMed] [Google Scholar]
- 49.Hubert C, Loy A, Nickel M, Arnosti C, Baranyi C, Brüchert V, Ferdelman T, Finster K, Christensen FM, Rosa de Rezende J, Vandieken V, Jørgensen BB. 2009. A constant flux of diverse thermophilic bacteria into the cold Arctic seabed. Science 325:1541–1544. doi: 10.1126/science.1174012. [DOI] [PubMed] [Google Scholar]
- 50.Jones B, Renaut RW, Rosen MR. 2003. Silicified microbes in a geyser mound: the enigma of low-temperature cyanobacteria in a high-temperature setting. Palaios 18:87–109. doi:. [DOI] [Google Scholar]
- 51.Ren HY, Zhang XJ, Song ZY, Rupert W, Gao GJ, Guo SX, Zhao LP. 2011. Comparison of microbial community compositions of injection and production well samples in a long-term water-flooded petroleum reservoir. PLoS One 6:e23258. doi: 10.1371/journal.pone.0023258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basso O, Lascourrèges J-F, Jarry M, Magot M. 2005. The effect of cleaning and disinfecting the sampling well on the microbial communities of deep subsurface water samples. Environ Microbiol 7:13–21. doi: 10.1111/j.1462-2920.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- 53.Magot M, Ollivier B, Patel BK. 2000. Microbiology of petroleum reservoirs. Antonie Van Leeuwenhoek 77:103–116. doi: 10.1023/A:1002434330514. [DOI] [PubMed] [Google Scholar]
- 54.Stetter KO, Huber R, Blochl E, Kurr M, Eden RD, Fielder M, Cash H, Vance I. 1993. Hyperthermophilic Archaea are thriving in deep North-Sea and Alaskan oil-reservoirs. Nature 365:743–745. doi: 10.1038/365743a0. [DOI] [Google Scholar]
- 55.Gittel A, Sorensen KB, Skovhus TL, Ingvorsen K, Schramm A. 2009. Prokaryotic community structure and sulfate reducer activity in water from high-temperature oil reservoirs with and without nitrate treatment. Appl Environ Microbiol 75:7086–7096. doi: 10.1128/AEM.01123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lharidon S, Reysenbach AL, Glenat P, Prieur D, Jeanthon C. 1995. Hot subterranean biosphere in a continental oil-reservoir. Nature 377:223–224. doi: 10.1038/377223a0. [DOI] [Google Scholar]
- 57.Nesbo CL, K SS, Dahle H, Haverkamp TH, Birkeland NK, Sokolova T, Kublanov I, Zhaxybayeva O. 2015. Evidence for extensive gene flow and Thermotoga subpopulations in subsurface and marine environments. ISME J 9:1532–1542. doi: 10.1038/ismej.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nazina TN, Tourova TP, Poltaraus AB, Novikova EV, Grigoryan AA, Ivanova AE, Lysenko AM, Petrunyaka VV, Osipov GA, Belyaev SS, Ivanov MV. 2001. Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int J Syst Evol Microbiol 51:433–446. doi: 10.1099/00207713-51-2-433. [DOI] [PubMed] [Google Scholar]
- 59.Manachini PL, Mora D, Nicastro G, Parini C, Stackebrandt E, Pukall R, Fortina MG. 2000. Bacillus thermodenitrificans sp. nov., nom. rev. Int J Syst Evol Microbiol 50:1331–1337. doi: 10.1099/00207713-50-3-1331. [DOI] [PubMed] [Google Scholar]
- 60.Sung MH, Kim H, Bae JW, Rhee SK, Jeon CO, Kim K, Kim JJ, Hong SP, Lee SG, Yoon JH, Park YH, Baek DH. 2002. Geobacillus toebii sp. nov., a novel thermophilic bacterium isolated from hay compost. Int J Syst Evol Microbiol 52:2251–2255. doi: 10.1099/00207713-52-6-2251. [DOI] [PubMed] [Google Scholar]
- 61.Tomita K, Ikeda N, Ueno A. 2003. Isolation and characterization of a thermophilic bacterium, Geobacillus thermocatenulatus, degrading nylon 12 and nylon 66. Biotechnol Lett 25:1743–1746. doi: 10.1023/A:1026091711130. [DOI] [PubMed] [Google Scholar]
- 62.Poli A, Guven K, Romano I, Pirinccioglu H, Guven RG, Euzeby JPM, Matpan F, Acer O, Orlando P, Nicolaus B. 2012. Geobacillus subterraneus subsp. aromaticivorans subsp nov, a novel thermophilic and alkaliphilic bacterium isolated from a hot spring in Sırnak, Turkey. J Gen Appl Microbiol 58:437–446. doi: 10.2323/jgam.58.437. [DOI] [PubMed] [Google Scholar]
- 63.Huber R, Rossnagel P, Woese CR, Rachel R, Langworthy TA, Stetter KO. 1996. Formation of ammonium from nitrate during chemolithoautotrophic growth of the extremely thermophilic bacterium Ammonifex degensii gen. nov. sp. nov. Syst Appl Microbiol 19:40–49. doi: 10.1016/S0723-2020(96)80007-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.