ABSTRACT
6-Chloro-2-benzoxazolinone (CDHB) is a precursor of herbicide, insecticide, and fungicide synthesis and has a broad spectrum of biological activity. Pigmentiphaga sp. strain DL-8 can transform CDHB into 2-amino-5-chlorophenol (2A5CP), which it then utilizes as a carbon source for growth. The CDHB hydrolase (CbaA) was purified from strain DL-8, which can also hydrolyze 2-benzoxazolinone (BOA), 5-chloro-2-BOA, and benzamide. The specific activity of purified CbaA was 5,900 U · mg protein−1 for CDHB, with Km and kcat values of 0.29 mM and 8,500 s−1, respectively. The optimal pH for purified CbaA was 9.0, the highest activity was observed at 55°C, and the inactive metal-free enzyme could be reactivated by Mg2+, Ni2+, Ca2+, or Zn2+. Based on the results obtained for the CbaA peptide mass fingerprinting and draft genome sequence of strain DL-8, cbaA (encoding 339 amino acids) was cloned and expressed in Escherichia coli BL21(DE3). CbaA shared 18 to 21% identity with some metal-dependent hydrolases of the PF01499 family and contained the signature metal-binding motif Q127XXXQ131XD133XXXH137. The conserved amino acid residues His288 and Glu301 served as the proton donor and acceptor. E. coli BL21(DE3-pET-cbaA) resting cells could transform 0.2 mM CDHB into 2A5CP. The mutant strain DL-8ΔcbaA lost the ability to degrade CDHB but retained the ability to degrade 2A5CP, consistent with strain DL-8. These results indicated that cbaA was the key gene responsible for CDHB degradation by strain DL-8.
IMPORTANCE 2-Benzoxazolinone (BOA) derivatives are widely used as synthetic intermediates and are also an important group of allelochemicals acting in response to tissue damage or pathogen attack in gramineous plants. However, the degradation mechanism of BOA derivatives by microorganisms is not clear. In the present study, we reported the identification of CbaA and metabolic pathway responsible for the degradation of CDHB in Pigmentiphaga sp. DL-8. This will provide microorganism and gene resources for the bioremediation of the environmental pollution caused by BOA derivatives.
INTRODUCTION
6-Chloro-2-benzoxazolinone (CDHB) is the precursor of fenoxaprop-p-ethyl (FE) and metamifop synthesis (1). FE is a postemergence aryloxyphenoxy propionate (AOPP) herbicide that is used to control annual and perennial weeds in crops, such as soybean, turf, and wheat (2, 3). Many studies have demonstrated that FE can be hydrolyzed to fenoxaprop acid (FA) and further transformed into CDHB and 2-(4-hydroxyphenoxy)-propionic acid (HPP) by soil microorganisms (4–6). Recently, the hydrolysis of FE to FA by several esterases from different microorganisms has been reported (7–9). However, the underlying genes and the metabolic pathway responsible for the degradation of CDHB in microorganisms are unknown.
CDHB is a type of 2-benzoxazolinone (BOA) derivative. BOA is widely used as a synthetic intermediate of related derivatives and is an important group of allelochemicals that respond to tissue damage or pathogen attack in gramineous plants, such as corn, rye, and wheat (10–14). BOA is a defensive compound that inhibits the growth and development of microorganisms and plants and also deters insect feeding (15, 16). When BOA and its related products are exuded as active plant metabolites from the roots into the soil, they can be absorbed and translocated into other crop plants to cause physiological disturbances that can even include declines in crop yields (17). This phenomenon has been demonstrated in various test plants, such as Lactuca sativa (18), Lepidium sativum (19), Avena sativa (20) and Cucumis sativus (21). Therefore, reducing the inhibitory effect of BOA derivatives on economic crops through the use of microbial metabolic processes is imperative.
CDHB is highly toxic to microorganisms and is difficult to degrade (13). Pigmentiphaga sp. strain DL-8 was isolated from an enriched FE-degrading consortium, W1 (6), and could mineralize CDHB. In the present study, we report the identification of CbaA and the metabolic pathway responsible for CDHB degradation in Pigmentiphaga sp. DL-8.
MATERIALS AND METHODS
Chemicals and media.
CDHB was purchased from Qingdao Vochem Co. Ltd. (Shandong, China), 2A5CP and BOA were purchased from Sigma-Aldrich (Shanghai, China), and the other chemical reagents were purchased from Sinopharm Chemical Reagent Co. Ltd. (Beijing, China). The molecular reagents were purchased from TaKaRa Co. Ltd. All chemicals used in this study were of analytical grade or higher purity. The stock solutions of the abovementioned aromatic compounds (1% [wt/vol]) were prepared in methanol and sterilized by membrane filtration (pore size, 0.22 μm). Minimal salts medium (MSM) and Luria-Bertani (LB) medium were used to culture the strains in this study (22).
Strains, plasmids, and primers.
The strains and plasmids used in this study are listed in Table 1. E. coli strains and Pigmentiphaga sp. DL-8 (CCTCC M 2014057) (6) were routinely grown aerobically at 37°C in LB broth or on LB agar. The genes were amplified from the genomic DNA of strain DL-8 using the primers listed in Table 2 with PrimeSTAR high-sensitivity (HS) DNA polymerase.
TABLE 1.
Strains and plasmids used in this study
| Strains or plasmid | Characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| Pigmentiphaga sp. | ||
| DL-8 | Wild-type CDHB degrader, Smr NDr | This lab |
| DL-8ΔcbaA | cbaA insertion mutant of DL-8, Smr NDr Gmr | This study |
| Escherichia coli | ||
| BL21(DE3) | Host strain for expression vector pET-29a(+) | TaKaRa |
| SM10λpir | Host strain for suicide vector pJQ200SK | 40 |
| DH5α | Host strain for cloning vectors | TaKaRa |
| HB101(pRK600) | Conjugation helper strain, Cmr | This lab |
| Plasmids | ||
| pMD19-T | T-A clone vectors, Ampr | TaKaRa |
| pJQ200SK | Suicide vector, Gmr | 40 |
| pET-29a(+) | Expression vector, Kmr | TaKaRa |
| pET-cbaA | pET-29a(+) derivative carrying cbaA, Kmr | This study |
| pJQ-cbaA | pJQ200SK derivative carrying cbaA, Gmr | This study |
Smr, streptomycin resistant; NDr, nalidixic acid resistant; Gmr, gentamicin resistant, Cmr, chloramphenicol resistant; Ampr, ampicillin resistant; Kmr, kanamycin resistant.
TABLE 2.
Oligonucleotide primers used for PCR
| Primer | Sequence (5′ to 3′)a | Purpose |
|---|---|---|
| cbaA-F | CATATGACTAAAAAATACCTAGTTG | Expression of cbaA |
| cbaA-R | AAGCTTTCAGTGGTGGTGGTGGTGGTG GTAGATCACGAAAGGTCGAGCAG | |
| cbaA-TYF | GAGCTCAATCGCTGCGTGTGGCTTGA | Homologous sequence of cbaA |
| cbaA-TYR | CTGCAGTTTTTGAGAGCTCCAATTAA | |
| H137A-F | GGCGAACGGCACCGCATTCGACTC | H137A mutation |
| H137A-R | GAGTCGAATGCGGTGCCGTTCGCC | |
| H288A-F | AGCGAATGCTCAAAAATTCTTG | H288A mutation |
| H288A-R | CAAGAATTTTTGAGCATTCGCT | |
| E301A-F | CATTATCGCGAATATGGATTTC | E301A mutation |
| E301A-R | GAAATCCATATTCGCGATAATG |
Restriction sites are underlined.
Growth and degradation experiments.
Strain DL-8 cells precultured in LB medium were harvested by centrifugation (Allegra X-22R centrifuge, F0630 rotor; Beckman Coulter, USA) at 3,140 × g for 5 min, washed with sterilized MSM, and resuspended in MSM to an optical density at 600 nm (OD600) of 1.0 (∼2.6 × 108 cells·ml−1). The suspension was used as the inoculum for the biodegradation experiments described below. For all experiments, the cells were inoculated at a 5% (vol/vol) concentration into 20 ml of MSM (pH 7.0) containing 0.2 mM CDHB and then incubated at 37°C and 180 rpm on a rotary shaker, unless otherwise stated. Medium without inoculation was used as the control. The degradation of strain DL-8 toward aromatic pollutants was assessed using the method described above. All degradation experiments consisted of three replicates.
Determination of biodegradation kinetics.
The bacterial suspension was inoculated into 250 flasks containing 100 ml of MSM with 0.2 mM CDHB or 2A5CP to obtain a final cell density of 1.0 × 106 to 2.0 × 106 CFU ml−1. The flasks were incubated on a rotary shaker at 180 rpm at 37°C. At regular intervals, 5-ml samples were collected from each flask and used to determine the CDHB concentration by high-performance liquid chromatography (HPLC). Cell counts were performed using the plate dilution technique with LB plates, and colonies were counted after 72 h of incubation at 37°C.
Identification of CDHB degradation metabolites.
Strain DL-8 was inoculated into a 1,000-ml Erlenmeyer flask (2% [vol/vol]) containing 300 ml of MSM supplemented with 0.2 mM CDHB and cultivated as described above. The CDHB concentration was monitored at 6-h intervals using HPLC, and the metabolites were analyzed by high-pressure liquid chromatography-mass spectrometry (HPLC-MS), as described below.
The samples were freeze-dried, dissolved in 1 ml of methanol, and filtered through a 0.22-μm-pore-size Millipore membrane. For the HPLC analysis, a separation column (internal diameter, 4.6 mm; length, 250 mm) filled with Kromasil 100-5-C18 was used. The mobile phase was methanol:water (80:20 [vol/vol]), and the flow rate was 0.8 ml min−1. The detection wavelength was 240 nm, and the injection volume was 20 μl. The MS analyses were performed in electrospray ionization (ESI) mode with an Agilent G6410B triple quad mass spectrometer. The metabolites were confirmed by standard MS and ionized by electrospray with a positive polarity. Characteristic fragment ions were detected using second-order MS.
Purification of CbaA and zymogram analysis.
Cells grown in LB broth without the addition of any inductive compounds were harvested by centrifugation at 2,180 × g at 4°C. All purification steps were performed at temperatures below 4°C to avoid any potential enzyme denaturation. The cells of strain DL-8 (10 g [wet weight]) were disrupted by ultrasonic disruption (sonicator 201 M; Kubota, Japan) for 10 min. The cell extracts were fractionated using ammonium sulfate, and the precipitate (60 to 80% saturation) was harvested by centrifugation at 12,580 × g for 25 min. The precipitates were dissolved in 50 ml of 20 mM buffer A (glycine-NaOH [pH 9.0]) containing 60% ammonium sulfate. The enzyme possessing CbaA activity was subjected to butyl-650M hydrophobic interaction chromatography (1.6 cm by 25 cm; Toyopearl, Japan) and eluted with a 60% to 0% linear concentration gradient of ammonium sulfate. The eluate was collected in 1-ml samples and assessed for CbaA activity. Fractions with relatively high CbaA activity were pooled and dialyzed against buffer A. The dialyzed enzyme preparation was applied to a Toyopearl DEAE-650M column (1.6 cm by 12.5 cm) and eluted with 0 to 1 M NaCl. The fractions containing CbaA activity were pooled, dialyzed against buffer A, and concentrated with a Millipore Centriprep centrifugal filter concentrator (23). The proteins were electrophoresed on a 10% native PAGE gel and visualized by staining with Coomassie brilliant blue R-250 (24). The monomer molecular weight of CbaA was determined by 10% SDS-PAGE after boiling for 5 min.
After native electrophoresis, the gel was sliced into two parts. One part was stained with 0.1% Coomassie brilliant blue R-250 to analyze the protein purity, and the other part was applied for zymogram analysis (25). The gel slice with CbaA was incubated in a 20 mM buffer A agar plate containing 2 mM CDHB. After incubation at 37°C for 30 min, the gel was stained with 40 mM potassium hexacyanoferrate (III) and 20 mM 4-aminoantipyrene solution (26); the CbaA band became visible when it turned red, as described below. Then, the stained gels were compared with the zymogram to determine the position of CbaA (25). The corresponding band in the native PAGE gel was sliced for peptide fingerprint analysis. Protein sample homogeneity was assessed by size-exclusion chromatography (SEC) using a Superdex 200 10/300 GL column connected to an Äkta purifier system (GE Healthcare). The column was equilibrated with buffer A, and the protein was eluted with the same buffer at a flow rate of 0.4 ml min−1. The standard proteins used were phosphatase B (97 kDa), bovine serum albumin (66 kDa), ovalbumin (44 kDa), and carbonic anhydrase (29 kDa).
The CbaA activity was determined using a discontinuous method to measure the release of 2A5CP during CDHB hydrolysis. A reaction mixture containing 2 mM CDHB and 10 μl of the enzyme solution diluted in 3 ml of buffer A was incubated at 55°C for 10 min. After incubation, 30 μl of 0.2 M potassium hexacyanoferrate (III) and 30 μl of 0.1 M 4-aminoantipyrene were added to the reaction mixture. The 2A5CP reacted with 4-aminoantipyrene to form a red compound with a maximum absorption at 545 nm (27). One unit of CbaA activity was defined as the amount of enzyme needed to release 1 μmol 2A5CP per minute. The 2A5CP dioxygenase activity in strain DL-8 was also determined using the same detection method to measure the decrease of 2A5CP in buffer B (20 mM Tris-HCl [pH 7.0]) at 37°C for 10 min.
Biochemical properties of purified CbaA. (i) Substrate specificity.
The substrate specificity of CbaA was determined using CDHB, BOA, benzamide, formanilide, acetanilide, isatin, p-nitrophenyl esters, and triglycerides with different carbon chain lengths. The enzymatic activities toward CDHB, BOA, formanilide, acetanilide, and isatin were assessed in buffer A at 55°C for 10 min and detected using the 4-aminoantipyrene chromogenic method described above. The enzymatic activities for benzamide were detected using Nessler's reagent colorimetry (28). The esterase activities against different p-nitrophenyl esters and triglycerides were assessed by titrating the produced fatty acids (29, 30).
CbaA activity at different concentrations (0.1 to 1 mM) of the test substrates was determined as described above. The kinetic constants Km and kcat were calculated using a Lineweaver-Burk plot (31). All determinations were performed based on three replicates, and a control experiment without CbaA was conducted under the same conditions. R version 3.1.1 (Vanderbilt University, USA) was used for the statistical analysis. The one-way analysis of variance (ANOVA) test was used, and a P value of <0.05 was deemed significant.
(ii) Effects of temperature and pH on CbaA activity and stability.
The optimal reaction pH was assessed using several buffers with various pH values at 55°C. The following buffers were used: 20 mM citrate buffer (pH 3.0 to 6.0), 20 mM phosphate-buffered saline (PBS) (pH 6.0 to 8.0), and 20 mM glycine-NaOH buffer (pH 8.0 to 12.0). The effect of the temperature on CbaA activity was determined using the optimal pH at temperatures ranging from 20 to 70°C. To measure the pH stability, the enzyme was incubated at 4°C for 1 h in different buffers, and the residual activity was determined under the enzyme assay conditions described above. The thermal stability of CbaA was assessed by incubating the enzyme preparations at different temperatures for 30 min and measuring the remaining activity under the enzyme assay conditions described above. Nonheated enzyme was used as the control (100%). All determinations were performed using three replicates.
(iii) Effects of metal ions and chemicals on CbaA activity.
Purified CbaA was treated with 1 mM or 5 mM EDTA for 5 h at 4°C and then dialyzed against buffer A to remove the EDTA. The activities were assayed as described above and compared to the activity of an untreated enzyme solution incubated under the same conditions. For reactivation, the metal-free enzyme was incubated with divalent metal ions (Ba2+, Cu2+, Co2+, Cr2+, Ni2+, Zn2+, Ca2+, Mg2+, and Mn2+) at a final concentration of 1 mM for 10 min, and the remaining activity was determined. The activity prior to EDTA treatment was used as the control (100%).
The procedure used to assess the metal ions was also used to determine the effects of chemical agents (Triton X-100, SDS, phenylmethylsulfonyl fluoride [PMSF], dimethylformamide [DMF], β-mercaptoethanol, dithiothreitol [DTT], and cetyltrimethylammonium bromide [CTAB]) and organic reagents (methanol, ethanol, methanol, acetone, acetonitrile, isopropanol, and dimethyl sulfoxide [DMSO]). The activity in the absence of any additives was used as the control (100%).
Protein fragment fingerprint analysis by mass spectrometry.
Using the electrospray ionization-quadrupole time of flight tandem mass spectrometry (ESI-Q-TOF MS/MS) technique, an array of peptide masses was recorded from the enzymatic digestion of the protein isolated from a native gradient gel (Bo-Yuan Biological Technology Co. Ltd.). These masses were searched in the Mascot website databases (Matrix Science, Boston, MA) to identify the amino acid sequences of the peptide fragments (32). The resulting peptide fragments were analyzed by searching the draft genome sequence of strain DL-8, as described below. The downstream metabolic gene cluster was also searched in the genome and subjected to a BLAST search against the cnb clusters from Comamonas sp. strain CNB-1 and Pseudomonas putida ZWL73 (33–36).
Genome sequencing, assembly, and annotation.
The DNA manipulations were performed according to standard protocols described by Sambrook and Russell (22). The genome of strain DL-8 was sequenced using an Illumina HiSeq 2000 system (Han-Yu Biological Technology Co. Ltd.) (37). The DNA was sequenced as a mixture of shotgun and 350-bp paired-read fragments to provide both uniform genome coverage and a paired-read assembly. Sequencing reads were assembled using the SOAPdenovo (version 1.05) method (http://soap.genomics.org.cn/soapdenovo.html). De novo gene prediction was conducted using the Glimmer (version 3.0) system (http://ccb.jhu.edu/software/glimmer/index.shtml). The BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) combined with sequences from the KEGG, COG, Swiss-Prot, and nonredundant protein databases was used to accomplish the functional annotation, using an E value cutoff of 1 E−5.
For the phylogenetic analysis, all protein sequences were first aligned using the Clustal X (version 2.1) program (38) and then imported into the MEGA (version 5.0) software (39) to construct a phylogenetic tree using the neighbor-joining method. Distances were calculated using the Kimura two-parameter distance model. Confidence values for the branches of the phylogenetic tree were determined using bootstrap analyses based on 1,000 resamplings.
Functional verification of the CbaA coding gene cbaA.
Homologous recombination was used to disrupt cbaA in strain DL-8. The homologous arms were amplified with the primer pair cbaA-TYF and cbaA-TYR (cbaA-TYF/R) (Table 2) and then cloned into the suicide plasmid pJQ200SK (40) to generate pJQ-cbaA. pJQ-cbaA was delivered into strain DL-8 via conjugal transfer, and the transconjugates were selected on LB plates supplemented with streptomycin and gentamicin. The cbaA-disrupted mutant, designated strain DL-8ΔcbaA, was verified by PCR amplification. The ability to degrade CDHB and 2A5CP was assessed as described below.
cbaA was heterologously expressed in E. coli BL21(DE3). cbaA was amplified using the primers cbaA-F and cbaA-R with a carboxyl-terminal 6×His tag and inserted into pET29a(+) to produce the pET-cbaA plasmid. The recombinant plasmid was transformed into E. coli BL21(DE3) and designated E. coli BL21(DE3-pET-cbaA). E. coli BL21(DE3-pET-cbaA) was grown in LB medium supplemented with 50 μg/ml kanamycin at 37°C to an optical density at 600 nm (OD600) of 0.5 to 0.6. Protein expression was induced at 18°C with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.2 mM (22). SDS-PAGE was used to detect the expression of the recombinant enzyme CbaA. Recombinant CbaA was purified with Ni2+-nitrilotriacetic acid (NTA) resin (Qiagen, Valencia, CA, USA) with 0 to 300 mM imidazole in buffer A (41). The ability of the enzyme to degrade CDHB was tested in a resting cell assay, as described below.
Biotransformation of CDHB by resting cells.
To perform the resting cell assay, the tested cells [from strains DL-8, DL-8ΔcbaA, BL21(DE3-pET29a), and BL21(DE3-pET-cbaA)] were washed twice with sterilized MSM and then suspended in MSM to a final OD600 of 2.0. CDHB was added to the cell suspensions to a final concentration of 0.2 mM, and the suspensions were incubated at 150 rpm and 37°C for 72 h. Each treatment consisted of three replicates. The samples were analyzed by HPLC, as described above.
Site-directed mutagenesis of the active-site residues.
Overlap PCR was used to construct the point mutations (42). The forward and reverse primers (flanking primers) were cbaA-F and cbaA-R, respectively. The internal primer pairs H127A-F/R, H288A-F/R, and E301A-F/R are shown in Table 2. All PCR assays were performed using the PrimeSTAR HS DNA polymerase (TaKaRa Co. Ltd.) and a standard site-directed mutagenesis protocol (42). The PCR products were gel purified and cloned into the NdeI and HindIII sites of the DNA pET-29a(+) plasmid, as described above. The substitutions were confirmed by DNA sequencing. The effects of the substitutions on CDHB degradation were tested using the resting cell assay, as described above. The 4-aminoantipyrene chromogenic method was used to evaluate 2A5CP (26).
Accession number(s).
The nucleotide sequences of the Pigmentiphaga sp. strain DL-8 16S rRNA and cbaA genes were deposited in the GenBank database under accession numbers KJ155792 and KR732327, respectively.
RESULTS AND DISCUSSION
Degradation of CDHB and other aromatic compounds by strain DL-8.
The growth of strain DL-8 on liquid MSM supplemented with 0.2 mM CDHB and its ability to degrade CDHB are shown in Fig. 1A. The growth curve showed a steady increase in the bacterial population after a short lag phase (approximately 24 h). After incubation for 72 h, approximately 98% of the 0.2 mM CDHB that was initially added was degraded by strain DL-8; correspondingly, the cell density increased from 1.5 × 106 to 4.2 × 106 CFU ml−1. The increase in the cell density appears to be extremely low, most likely because CDHB is highly toxic to various microbes (see Fig. S1 in the supplemental material). No significant change in the CDHB concentration was observed in cultures that were not inoculated with strain DL-8. Thus, we concluded that strain DL-8 was able to degrade CDHB and utilize it as a sole carbon source for growth.
FIG 1.

Degradation dynamics of CDHB (A) and 2A5CP (B) by strain DL-8 and the growth curves. Error bars represent the standard errors for three replicates.
Pigmentiphaga sp. strain DL-8 showed a broad substrate spectrum toward aromatic pollutants. This strain could also completely mineralize BOA derivatives (BOA, 5-chloro-BOA, and 5-chloro-6-hydroxy-BOA), aromatic acids (phthalic acid, salicylic acid, gentisic acid, protocatechuic acid, and benzoic acid) and aminophenol derivatives (AP, 2A5CP, 2A4CP, and 2-nitrophenol) and utilize them as carbon sources for growth; however, the strain could not completely degrade phenol, catechol, or hydroquinone. Few reports have investigated the degradation of environmental pollutants by strains of the Pigmentiphaga genus. Several strains have been found to be highly effective for the biodegradation of acetamiprid (43, 44), 2,6-naphthalenedisulfonate (45), and the azo dye carboxy-orange I (46). Strain DL-8 was isolated for its ability to degrade BOA compounds, which have been less well studied due to their high biological toxicity.
CDHB metabolite identification.
To clarify the CDHB degradation pathway of strain DL-8, the intermediate metabolites produced during CDHB degradation were analyzed and identified by HPLC-MS. A biodegradation metabolite of CDHB with a retention time (tR) of 1.79 min was identified as 2-amino-5-chlorophenol (2A5CP), which showed a molecular ion at m/z 144 ([M-H]+), with fragment ions at m/z 125.8 ([M-OH-H]+), 108.9 ([M-Cl-H]+), and 89.9 ([M-OH-Cl-H]+) (in agreement with the 2A5CP standard) (Fig. 2A). 2A5CP was detected only at the highest concentration of 10 μM, and no other intermediates were detected. The 2A5CP substrate was also completely degraded by strain DL-8 within 24 h, with cell densities that increased from 1.5 × 106 to 5.3 × 106 CFU ml−1 (Fig. 1B). The metabolic pathway and molecular mechanism of 2A5CP biodegradation have been reported in Comamonas sp. CNB-1 and Pseudomonas putida ZWL73 (33–36). The putative whole-gene cluster for 2A5CP degradation could be found in the genome of strain DL-8 (shown below). The proposed metabolic pathway for CDHB is shown in Fig. 3A.
FIG 2.
Second-order mass spectra of the CDHB metabolite (A) and the red pigment (B). The inserts in the mass spectra indicate that the color of the liquid MSM supplemented with 0.5 mM 2A5CP gradually deepened with increasing CAPO concentration.
FIG 3.
Genetic organization and functional prediction of the cba and cnb genes involved in CDHB degradation in Pigmentiphaga sp. strain DL-8. (A) Potential metabolic pathway of CDHB. (B) Genetic organization of the cba gene and cnb gene cluster involved in CDHB degradation. TCA, tricarboxylic acid.
During CDHB degradation by strain DL-8, a red pigment accumulated when the CDHB concentration was >0.3 mM (Fig. 2B). The red pigment showed a molecular ion at m/z 245.9 ([M-H]+), with fragment ions at m/z 218.9 ([M-C2H3]+) and 190.9 ([M-C2H3-NH2-O]+), and was identified as 9-chloro-2-amino-3H-phenoxazin-3-one (CAPO) by HPLC-MS (Fig. 2B).
The metabolic pathway by which BOA is detoxified in Fusarium verticillioides has been explored in recent decades (see Fig. S2 in the supplemental material). BOA metabolism in F. verticillioides involves hydrolysis by Fdb1 to produce 2-aminophenol (2AP), which is malonylated by Fdb2 to produce nontoxic N-(2-hydroxyphenyl) malonamic acid (HPMA) (47, 48). When the modification is prevented due to a genetic mutation (FDB2), nontoxic 2-acetamidophenol (HPAA) may accumulate as a result of acetylation (49). During the BOA detoxification process, 2AP can also be spontaneously oxidized to produce 2-amino-3H-phenoxazin-3-one (APO), which is highly toxic to F. verticillioides (50) and exhibits greater allelopathic phytotoxicity toward barnyard grass (Echinochloa crus-galli) than BOA (51, 52). Doratomyces stemonitis can utilize APO as a sole carbon source, but the utilization of this product seems to have a high energy demand (53). Strain DL-8 may promote the formation of CAPO with high concentrations (>0.5 mM) of 2A5CP (Fig. 2B). After its formation, CAPO cannot be further degraded by strain DL-8, and the color of the liquid MSM supplemented with 0.5 mM 2A5CP gradually deepens with increasing CAPO concentration (Fig. 2B).
Purification of CbaA from strain DL-8.
The cell extract from strain DL-8 also transformed CDHB into 2A5CP. According to the degradation pathway, we deduced that CDHB was hydrolyzed to 2A5CP by a hydrolase (Fig. 3A). The CbaA was a constitutive enzyme in strain DL-8, and the transformation product was also identified as 2A5CP by HPLC-MS. When cultivated in LB medium, strain DL-8 showed CbaA activity (up to 300 U · mg protein−1) without the addition of any inductive compounds. CbaA was purified by ammonia sulfate precipitation and ion exchange and hydrophobic interaction chromatography. A summary of the CbaA purification process is shown in Table 3. CbaA was eluted with 45% ammonium sulfate by butyl-650M hydrophobic interaction chromatography and 0.8 M NaCl by the DEAE column. After the 3-step purification process, CbaA was purified 18.9-fold with a 4% recovery. Native PAGE and SEC were used to analyze the purified CbaA to verify its purity and determine its natural molecular weight. The purified CbaA exhibited a single band with CbaA activity on a native PAGE gel (Fig. 4A). The molecular mass of the CbaA under nondenaturing conditions was calculated to be 75 kDa by gel filtration chromatography (Fig. 4). After boiling for 5 min, purified CbaA exhibited a single band on the SDS-PAGE gel, with a molecular mass of ∼37 kDa (see Fig. S3 in the supplemental material). Thus, we deduced that the CbaA naturally existed as a homodimer.
TABLE 3.
Purification of CbaA from strain DL-8
| Purification step from DL-8 | Total protein (mg) | Total activity (U) | Sp act (U mg−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude extract of DL-8 | 1,150 | 343,000 | 300 | 1.0 | 100 |
| Ammonium sulfate precipitation | 249 | 265,000 | 1,100 | 3.6 | 77 |
| Hydrophobic chromatography | 26 | 70,000 | 2,700 | 9.0 | 20 |
| DEAE-Sepharose chromatography | 2.3 | 13,000 | 5,900 | 18.9 | 4 |
FIG 4.
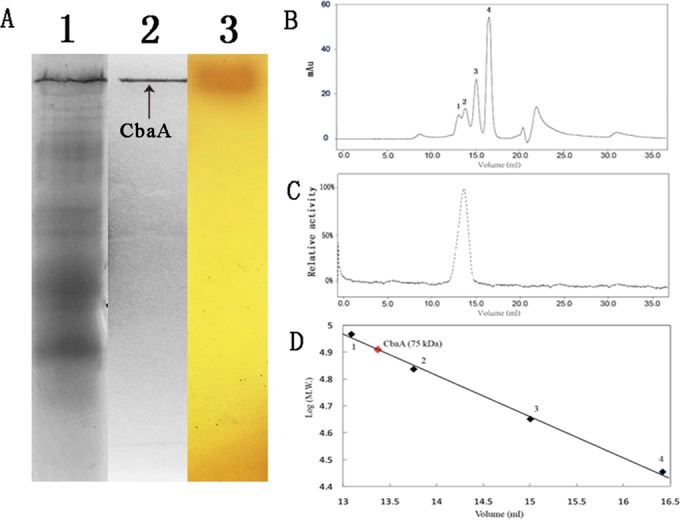
Zymogram analysis and molecular weight measurement of CbaA in strain DL-8 by native PAGE and gel filtration chromatography. (A) Native PAGE analysis of CbaA; lane 1, crude extract of strain DL-8; lane 2, purified CbaA; lane 3, potassium hexacyanoferrate (III) and 4-aminoantipyrene solution staining the 20 mM buffer A agar plate containing 2 mM CDHB. (B) A mixture of four proteins was used as the molecular weight marker. Protein 1, phosphatase B (97 kDa); protein 2, bovine serum albumin (66 kDa); protein 3, ovalbumin (44 kDa); protein 4, carbonic anhydrase (29 kDa). (C) Activity distribution of CbaA on the Superdex 200 10/300 GL column. (D) Molecular mass determination of CbaA (75 kDa, red dot).
We deduced that CDHB was first transformed into the transitory intermediate 2-amino-5-chlorophenyl hydrogen carbonate (2A5CPHC) by CbaA. 2A5CPHC was spontaneously decarboxylated to 2A5CP via rapid hydrolysis during the aqueous phase (Fig. 3A). Similar reactions occur in many chemical and biochemical processes. Smith and Bucher (54) reported that chlorpropham was transformed into carbamic acid when the temperature was >45°C; however, carbamic acid is unstable and readily decarboxylates to yield m-chloroaniline (54). Sphingomonas sp. strain CF06 could also convert carbofuran to carbofuran-7-phenol and methylcarbamic acid (MA), and MA was spontaneously and rapidly hydrolyzed to methylamine and CO2 (55).
Biochemical properties of purified CbaA. (i) Substrate specificity.
The substrate specificities of CbaA were determined with CDHB, BOA, benzamide, formanilide, acetanilide, isatin, p-nitrophenyl esters, and triglycerides with different carbon chain lengths (Table 4). The specific activities of CbaA were 5,900, 3,800, and 1,700 U · mg protein−1 for CDHB, BOA, and benzamide, respectively. CbaA showed no activity on p-nitrophenyl esters and triglycerides. The most suitable substrate for CbaA was CDHB, with Km and kcat values of 0.29 mM and 8,500 s−1, respectively. The amide substrate spectrum of CbaA was very narrow, and the enzyme could not hydrolyze formanilide, acetanilide, and isatin.
TABLE 4.
Substrate specificity and apparent kinetic constants of CbaA
| Substrate | Sp act (U mg−1) | Km (mM) | kcat (s−1) | Catalytic efficiency (kcat/Km [μM s−1]) |
|---|---|---|---|---|
| CDHB | 5,900 | 0.29 | 8,500 | 29.3 |
| BOA | 3,800 | 0.31 | 5,900 | 19.0 |
| Benzamide | 1,700 | 0.76 | 2,600 | 3.4 |
| Formanilide | NAa | |||
| Acetanilide | NA | |||
| Isatin | NA | |||
| 4-Nitrophenyl butyrate | NA | |||
| 4-Nitrophenyl acetate | NA | |||
| Glyceryl triacetate | NA | |||
| Glyceryl tributyrate | NA |
NA, no activity for the chemical structure.
(ii) Effects of temperature and pH on CbaA activity and stability.
CbaA exerted high levels of activity at pH 8.5 to 10.0, with an optimum pH of 9.0 (Fig. 5A). Little activity was detected at a pH below 7.0, but CbaA retained >80% of its activity after storage at pH 6.0 to 11.0 for 1 h (Fig. 5A). CbaA was stable and retained >90% residual activity for 30 min at temperatures of <60°C but was unstable at temperatures of >60°C. The enzyme was active at 35 to 70°C, with the highest activity at 55°C (Fig. 5B). These results suggested that CbaA was a mesophilic enzyme.
FIG 5.

Effects of pH and temperature on CbaA enzymatic activity and stability. (A) Determination of the optimal pH (black) and pH stability (red). (B) Effect of temperature on CbaA (black) and the determination of thermal stability (red). Error bars represent the standard errors for three replicates.
(iii) Effect of metal ions and chemical agents on CbaA enzymatic activity.
Because the CbaA amino acid sequence showed identity with the metal-dependent hydrolases described below, the effect of the metal ions on the CbaA enzymatic activity was determined. As shown in Table 3, EDTA did not inhibit the enzymatic activity at a final concentration of 1 mM, whereas the activity was strongly inhibited by incubation with 5 mM EDTA; the result indicated that the CbaA activity represented a divalent metal ion-dependent hydrolase. The metal-free enzyme was prepared by incubation with 5 mM EDTA for 5 h. Reactivation of the metal-free enzyme was attempted by incubation with 1 mM divalent metal ions for 10 min. Incubation in the presence of Zn2+, Ni2+, Ca2+, and Mg2+ resulted in complete reactivation to 135.8% ± 8.6%, 189.5% ± 3.3%, 119.8% ± 5.2%, and 102.1% ± 6.8%, respectively. The Cu2+, Ba2+, and Fe2+ ions had little effect on the activity, whereas Cr2+, Co2+, and Mn2+ inhibited the activity at a final concentration of 1 mM.
The effects of various chemical agents on the enzyme activity were also assessed (Table 5). DTT strongly inhibited CbaA enzyme activity, whereas isopropanol, Triton X-100, and CTAB slightly reduced its activity. In addition to isopropanol, other organic reagents had no significant influence on the enzyme activity. These data suggest that CbaA was a metal-dependent hydrolase and that disulfide bonds played a crucial role in the activity of this enzyme.
TABLE 5.
Effect of metal ions and chemical agents on the CbaA enzymatic activity
| Additive | Concn | Relative activity (%)b |
||
|---|---|---|---|---|
| No EDTA | 1 mM EDTAa | 5 mM EDTA | ||
| No addition | 100.0 ± 1.5 | |||
| Metal ions | ||||
| Mg2+ (MgCl2) | 1 mM | 92.7 ± 1.8 | 102.1 ± 6.8 | |
| Cu2+ (CuCl2) | 1 mM | 109.1 ± 4.6 | 21.2 ± 4.7 | |
| Ca2+ (CaCl2) | 1 mM | 93.1 ± 3.2 | 119.8 ± 5.2 | |
| Ni2+ (NiCl2) | 1 mM | 134.0 ± 5.6 | 189.5 ± 3.3 | |
| Zn2+ (ZnCl2) | 1 mM | 96.6 ± 0.9 | 135.8 ± 8.6 | |
| Ba2+ (CrCl2) | 1 mM | 102.3 ± 2.4 | 3.6 ± 5.2 | |
| Mn2+ (MnCl2) | 1 mM | 41.2 ± 7.7 | 9.6 ± 3.1 | |
| Fe2+ (FeCl2) | 1 mM | 95.5 ± 6.8 | 23.6±.9 | |
| Co2+ (CoCl2) | 1 mM | 39.9 ± 6.1 | 5.6 ± 7.9 | |
| Cr2+ (CrCl2) | 1 mM | 67.1 ± 4.5 | 13.2±.6 | |
| EDTA | 95.5 ± 3.6 | 8.3 ± 0.8 | ||
| Organic solvents | ||||
| Acetone | 10% | 82.4 ± 3.3 | ||
| Methanol | 10% | 86.6 ± 5.8 | ||
| Acetonitrile | 10% | 80.3 ± 4.6 | ||
| Ethanol | 10% | 85.2 ± 2.9 | ||
| Isopropanol | 10% | 67.7 ± 6.3 | ||
| Surfactants | ||||
| TritonX-100 | 20 mg ml−1 | 57.7 ± 2.9 | ||
| SDS | 1% | 80.3 ± 1.3 | ||
| CTAB | 5% | 52.7 ± 3.7 | ||
| Enzyme inhibitors | ||||
| PMSF | 5% | 86.6 ± 1.8 | ||
| DTT | 10 mM | 4.8 ± 2.3 | ||
| DMF | 5% | 107.9 ± 5.7 | ||
CbaA was previously treated with 1 mM or 5 mM EDTA for 5 h at 4°C prior to the metal addition.
Values are means ± standard deviations from three experiments.
Identification of key genes in CDHB degradation.
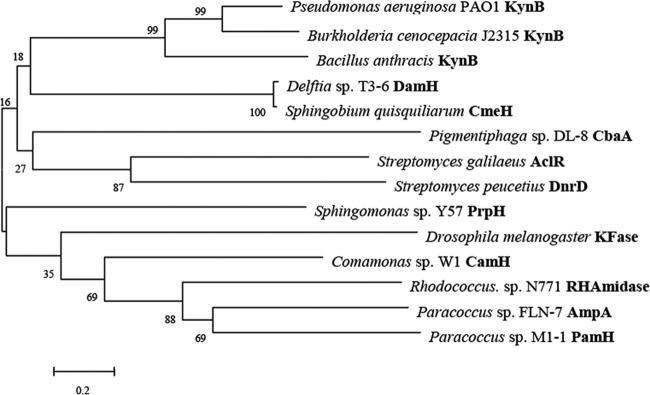
Peptide mass fingerprints were used as queries against the draft genome database of strain DL-8 (Mascot search). The results showed that the band on the native PAGE gel belonged to an open reading frame (ORF) that consisted of 1,020 bp and encoded a protein of 339 amino acids (identified as CbaA) in the DL-8 genome. The BLAST analysis showed that CbaA shared 10 to 15% identity with amidases, polyketide cyclases, and kynurenine formamidases (Fig. 6).
FIG 6.
Phylogenetic tree of CbaA and related amidases constructed by the neighbor-joining method. CbaA was aligned with the following proteins (accession numbers): CbaA (KP307927), paKynB (Q9I234), BcKynB (B4E9I9), BaKynB (Q81PP9), AcIR (Q8VWA2), DnrD (Q54808), PrpH (AEO21835.1), KFase (AAF52391), RhAmidase (3A1K), DamH (KF638270), CmeH (KF294465), AmpA (JQ388838), and PamH (AKF33439).
Further analysis showed that CbaA shared 18 to 21% identity with proteins from the cyclase family (PF04199) in the PDB database, such as the Zn2+-dependent kynurenine formamidase from Bacillus anthracis (PDB accession numbers 4COB and 4CZ1), the metal-dependent amide hydrolase (PDB accession numbers 1R61 and 2B0A), and the Mn2+-dependent isatin hydrolase from Labrenzia aggregata (PDB accession no. 4J0N) (Fig. 7). Isatin is the metabolic intermediate of indole acetic acid and has a chemical structure similar to that of BOA (56), but CbaA showed no activity on isatin. The sequences of typical PF01499 family members contain a signature motif, HXGTHXDXPXH (e.g., H73XG75T76H77XD79XP81XH83, His212, and Glu229 in isatin hydrolase 4J0N; H50XG51T52H53XD55XP57XH59, His161, and Glu173 in kynurenine formamidase 4CZ1; and H40XG42T43H44XD46XP48XH50, His136, and Glu154 in metal-dependent hydrolase 2B0A [57, 58]), which is replaced by QXXXQXDXXXH in some putative cyclases, including those of the PF04199 family (57). The motif Q101XXXQ105XD107XXXH111 and His281 and Glu294 in the Zn2+-dependent trans-dienelactone hydrolase protein trans-dienelactone hydrolase (DLH) (19% amino acid sequence identity with CbaA) from Pseudomonas reinekei MT1 were identified (58).
FIG 7.
Multiple alignments of the amino acid sequences of CbaA and other relative proteins in the PDB database. The arrows represent conserved amino acid residues; the box represents the metal-binding region.
CbaA contains the Q127XXXQ131XD133XXXH137 motif and the highly conserved amino acid residues His288 and Glu301 (Fig. 7). The motif HXGTHXDXPXH or QXXXQXDXXXH may be the direct metal-binding region, and His137 and His288 may serve the proton donor/acceptor (59). Interestingly, various divalent metal ions (Zn2+, Ni2+, Ca2+, and Mg2+) could completely reactivate metal-free CbaA, whereas Mn2+ inhibited its activity at a concentration of 1 mM, as described above (Table 5). However, a promoting effect of Mn2+ on the metal-dependent hydrolase has been reported (57, 58). Thus, CbaA with BOA derivative hydrolase activity was identified as a novel member of the PF01499 family based on the hydrolytic product, substrate specificity, and amino acid sequence analyses (Fig. 6 and 7 and Table 4). The BOA hydrolase gene FDB1 from Fusarium spp., which catalyzes the first step in the degradation of the BOA derivatives, was recently cloned and reported (60). However, the CbaA in this study from strain DL-8 shared only 10% identity with the γ-lactamase FDB1 from Fusarium species.
The 2A5CP metabolic gene cluster (cnbR, cnbCαCβ, cnbD, cnbE, cnbF, cnbG, cnbH, and cnbI) surrounded by two IS1071 transposable elements was discovered and analyzed in the DL-8 genome by Local-BLAST (Fig. 3B). The 2A5CP metabolic gene cluster located in the genome of strain DL-8 shares 99% identity with the cluster from Comamonas sp. strain CNB-1 and Pseudomonas putida strain ZWL73 (33–36). The activity of the 2A5CP dioxygenase in strain DL-8 was also determined at 37°C by measuring the decrease in 2A5CP (see Fig. S4A in the supplemental material). 2A5CP was rapidly transformed into a transiently stable yellow product by cell extracts. This compound exhibited an absorption maximum at 395 nm and was spontaneously converted into a dead-end product with maxima at 226 and 272 nm (Fig. S4B). The yellow and dead-end products were identified as 2-aminomuconic semialdehyde (2AS) and 5-chloropicolinic acid (5CPA) in many studies (61, 62). Thus, we deduced that the 2A5CP gene cluster was involved in CDHB downstream metabolism.
Cloning, expression, and functional verification of the cbaA gene.
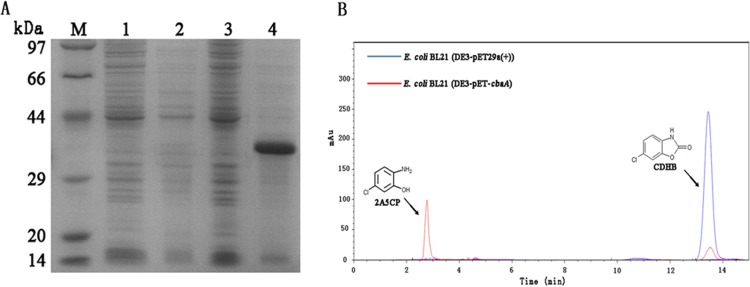
Overexpression of CbaA in E. coli BL21(DE3) was successful, as revealed by SDS-PAGE (Fig. 8A). The molecular mass of the denatured CbaA enzyme was approximately 37 kDa, which was in good agreement with the molecular mass deduced from the amino acid sequence. However, CbaA formed large amounts of insoluble protein, with slight hydrolase activity in the IPTG-induced E. coli BL21(DE3-pET-cbaA). The specific activity of the crude extract from E. coli BL21(DE3-pET-cbaA) was 1.4 U · mg protein−1 for CDHB, which indicated that the amount of the soluble CbaA protein was negligible in the supernatant (the specific activity was 298 U · mg protein−1 for CDHB in strain DL-8). We attempted to purify recombinant CbaA using Ni2+-NTA resin; however, the CbaA content was too low to be combined with the Ni2+-NTA resin. E. coli BL21(DE3-pET-cbaA) resting cells could transform 0.2 mM CDHB into 2A5CP, as detected by HPLC (Fig. 8B). In contrast, the control strain E. coli BL21(DE3) harboring pET-29a(+) was unable to transform CDHB. We attempted to optimize expression conditions (temperature, induction time, and IPTG concentration) and added different concentrations of bivalent metal ions (Zn2+, Ni2+, Ca2+, and Mg2+) during the induction process. However, the recombinant CbaA formed an insoluble protein with negligible hydrolase activity under different conditions. Additionally, the Saccharomyces cerevisiae, Bacillus subtilis, and Pseudomonas putida expression systems were applied to achieve soluble CbaA expression. Unfortunately, neither the CbaA protein band on the SDS-PAGE gel nor CDHB hydrolytic activity were detected in these recombinant cells.
FIG 8.
Expression and functional verification of recombinant CbaA. (A) Analysis of the expression of recombinant CbaA. Lane M, protein molecular weight marker; lane 1, total supernatant proteins from induced BL21(DE3) harboring pET-29a(+); lane 2, total insoluble proteins from induced BL21(DE3) harboring pET-29a(+); lane 3, total supernatant proteins from induced BL21(DE3) harboring pET-cbaA; lane 4, total insoluble proteins from induced BL21(DE3) harboring pET-cbaA. (B) Functional verification of recombinant CbaA by HPLC. The blue line indicates the E. coli BL21(DE3-pET-29a(+)) resting cells, and the red line indicates the E. coli BL21(DE3-pET-cbaA) resting cells.
To verify the function of cbaA in CDHB biodegradation, a 606-bp DNA fragment was amplified by PCR using primer pair cbaA-TYF/R as a homologous recombination arm to disrupt cbaA in strain DL-8. The cbaA-disrupted mutant was designated DL-8ΔcbaA. Strain DL-8ΔcbaA lost the ability to degrade CDHB, whereas the ability to degrade 2A5CP was consistent with strain DL-8. These data indicate that cbaA was the key gene responsible for CDHB degradation by strain DL-8.
Site-directed mutation of conserved amino acids in CbaA.
To investigate the functions of the conserved amino acid residues His137, His288, and Glu301, the residues were substituted with alanine by site-directed mutagenesis. Resting E. coli BL21(DE3) cells harboring the wild-type (WT) cbaA and three mutant genes were inoculated into liquid MSM containing 0.2 mM CDHB. E. coli BL21(DE3) resting cells harboring the WT cbaA converted 16% of the CDHB to 2A5CP in 24 h; 2A5CP was detected using potassium hexacyanoferrate (III) and 4-aminoantipyrene (see Fig. S5 in the supplemental material). Three substitutions (His137Ala, His288Ala, and Glu301Ala) resulted in the loss of the ability to transform CDHB into 2A5CP compared with the WT CbaA (Fig. S5). The results were in agreement with other reports concerning metal-dependent hydrolases and demonstrated that His137, His288, and Glu301 were involved in CDHB catalysis (57, 58).
In conclusion, we identified and characterized the novel metal-dependent hydrolase CbaA that catalyzed the initial step of CDHB degradation from Pigmentiphaga sp. strain DL-8. This enzyme demonstrated amidase activities for the 2-benzoxazolinone derivatives. These characteristics together with the broad pH and temperature tolerance of the enzyme position CbaA as a beneficial agent for environmental remediation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported financially by the Major State Basic Research Development Program of China (973 program) (grant 2015CB150502), the Natural Science Foundation of China (grants 31570059, 31400098, and 31270095), the National Science and Technology Support Program (grants 2012BAD14B02 and 20142X08011-003), and the 863 program (grant 2013AA102804).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00532-16.
REFERENCES
- 1.Moon JK, Keum YS, Hwang EC, Park BS, Chang HR, Li QX, Kim JH. 2007. Hapten syntheses and antibody generation for a new herbicide, metamifop. J Agric Food Chem 55:5416–5422. doi: 10.1021/jf070358f. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Yu S, Han L, Sun S, Zhi Y, Li W. 2011. Residues and dissipation of the herbicide fenoxaprop-p-ethyl and its metabolite in wheat and soil. Bull Environ Contam Toxicol 87:50–53. doi: 10.1007/s00128-011-0239-6. [DOI] [PubMed] [Google Scholar]
- 3.Cocker KM, Moss SR, Coleman JOD. 1999. Multiple mechanisms of resistance to fenoxaprop-p-ethyl in United Kingdom and other european populations of herbicide-resistant Alopecurus myosuroides (black-grass). Pestic Biochem Phys 65:169–180. doi: 10.1006/pest.1999.2439. [DOI] [Google Scholar]
- 4.Hoagland RE, Zablotowicz RM. 1998. Biotransformations of fenoxaprop-ethyl by fluorescent Pseudomonas strains. J Agric Food Chem 46:4759–4765. doi: 10.1021/jf980637q. [DOI] [Google Scholar]
- 5.Gennari M, Vincenti M, Nègre M, Ambrosoli R. 1995. Microbial metabolism of fenoxaprop-ethyl. Pestic Sci 44:299–303. doi: 10.1002/ps.2780440314. [DOI] [Google Scholar]
- 6.Dong WL, Hou Y, Xi XD, Wang F, Li ZK, Ye XF, Huang Y, Cui ZL. 2015. Biodegradation of fenoxaprop-ethyl by an enriched consortium and its proposed metabolic pathway. Int Biodeter Biodegr 97:159–167. doi: 10.1016/j.ibiod.2014.10.009. [DOI] [Google Scholar]
- 7.Hou Y, Tao J, Shen W, Liu J, Li J, Li Y, Cao H, Cui Z. 2011. Isolation of the fenoxaprop-ethyl (FE)-degrading bacterium Rhodococcus sp. T1, and cloning of FE hydrolase gene feh. FEMS Microbiol Lett 323:196–203. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Liu Y, Mao DM, Liu XM, Li SP, Jiang JD. 2013. An essential esterase (BroH) for the mineralization of bromoxynil octanoate by a natural consortium of Sphingopyxis sp. strain OB-3 and Comamonas sp. strain 7D-2. J Agric Food Chem 61:11550–11559. doi: 10.1021/jf4037062. [DOI] [PubMed] [Google Scholar]
- 9.Nie ZJ, Hang BJ, Cai S, Xie XT, He J, Li SP. 2011. Degradation of Cyhalofop-butyl (CyB) by Pseudomonas azotoformans strain QDZ-1 and cloning of a novel gene encoding CyB-hydrolyzing esterase. J Agric Food Chem 59:6040–6046. doi: 10.1021/jf200397t. [DOI] [PubMed] [Google Scholar]
- 10.Girre C, Lucas D, Hispard E, Menez C, Dally S, Menez JF. 1994. Assessment of cytochrome P4502E1 induction in alcoholic patients by chlorzoxazone pharmacokinetics. Biochem Pharmacol 47:1503–1508. doi: 10.1016/0006-2952(94)90524-X. [DOI] [PubMed] [Google Scholar]
- 11.Kim TJ, Chang HS, Kim JS, Hwang IT, Hong KS, Kim DW, Chung BJ. 2003. Metamifop: mechanism of herbicidal activity and selectivity in rice and barnyardgrass. Crop Sci Technol 2003:833–838. [Google Scholar]
- 12.Altuntas I, Delibas N, Doguc DK, Ozmen S, Gultekin F. 2003. Role of reactive oxygen species in organophosphate insecticide phosalone toxicity in erythrocytes in vitro. Toxicol In Vitro 17:153–157. doi: 10.1016/S0887-2333(02)00133-9. [DOI] [PubMed] [Google Scholar]
- 13.Niemeyer HM. 2009. Hydroxamic acids derived from 2-hydroxy-2H-1,4 -benzoxazin-3-(4H)-one: key defense chemicals of cereals. J Agric Food Chem 57:1677–1696. doi: 10.1021/jf8034034. [DOI] [PubMed] [Google Scholar]
- 14.Niemeyer HM. 1988. Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the Gramineae. Phytochemistry 27:3349–3358. doi: 10.1016/0031-9422(88)80731-3. [DOI] [Google Scholar]
- 15.Glenn AE, Meredith FI, Morrison WH III, Bacon CW. 2003. Identification of intermediate and branch metabolites resulting from biotransformation of 2-benzoxazolinone by Fusarium verticillioides. Appl Environ Microbiol 69:3165–3169. doi: 10.1128/AEM.69.6.3165-3169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Moreiras AM, Oliveros-Bastidas A, Reigosa MJ. 2010. Reduced photosynthetic activity is directly correlated with 2-(3H)-benzoxazolinone accumulation in lettuce leaves. J Chem Ecol 36(2):205–209. doi: 10.1007/s10886-010-9750-1. [DOI] [PubMed] [Google Scholar]
- 17.Chiapusio G, Pellissier F, Gallet C. 2004. Uptake and translocation of phytochemical 2-benzoxazolinone (BOA) in radish seeds and seedlings. J Exp Bot 55:1587–1592. doi: 10.1093/jxb/erh172. [DOI] [PubMed] [Google Scholar]
- 18.Barnes JP, Putnam AR. 1987. Role of benzoxazinones in allelopathy by rye (Secale cereale L.). J Chem Ecol 13:889–906. doi: 10.1007/BF01020168. [DOI] [PubMed] [Google Scholar]
- 19.Barnes JP, Putnam AR, Burke BA, Aasen AJ. 1987. Isolation and characterization of allelochemicals in rye herbage. Phytochemistry 26:1385–1390. doi: 10.1016/S0031-9422(00)81818-X. [DOI] [Google Scholar]
- 20.Friebe A, Wieland I, Schulz M. 1996. Tolerance of Avena sativa to the allelochemical benzoxazolinone. Degradation of BOA by root-colonizing bacteria. Angew Bot 70:150–154. [Google Scholar]
- 21.Chase WR, Nair MG, Putnam AR. 1991. 2,2′-oxo-1,1′-azobenzene: selective toxicity of rye (Secale cereale L.) allelochemicals to weed and crop species: II. J Chem Ecol 17:9–19. doi: 10.1007/BF00994418. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Russell D. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 23.Boden R, Borodina E, Wood AP, Kelly DP, Murrell JC, Schäfer H. 2011. Purification and characterization of dimethylsulfide monooxygenase from Hyphomicrobium sulfonivorans. J Bacteriol 193:1250–1258. doi: 10.1128/JB.00977-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Ying H, Zhou J, Li ZK, Huang Y, Cui ZL. 2014. Purification of an amide hydrolase DamH from Delftia sp. T3-6 and its gene cloning, expression, and biochemical characterization. Appl Microbiol Biot 17:7491–7499. [DOI] [PubMed] [Google Scholar]
- 26.Norwitz G, Keliher PN. 1980. Effect of acidity and alkalinity on the distillation of phenol: interferences of aromatic amines and formaldehyde with the 4-aminoantipyrine spectro-photometric method for phenol. Anal Chim Acta 119:99–111. doi: 10.1016/S0003-2670(00)00034-9. [DOI] [Google Scholar]
- 27.Kotresha D, Vidyasagar G. 2008. Isolation and characterization of phenol-degrading Pseudomonas aeruginosa MTCC 4996. World J Microb Biotechnol 24:541–547. doi: 10.1007/s11274-007-9508-2. [DOI] [Google Scholar]
- 28.Lin YM, Li LY, Hu JW, Huang XF, Zhou C, Jia M, Li ZB. 2014. Photometric determination of ammonia nitrogen in slaughterhouse wastewater with Nessler's reagent: effects of different pretreatment methods. Adv Mater 955:1241–1244. [Google Scholar]
- 29.Liang WQ, Wang ZY, Li H, Wu PC, Hu JM, Luo N, Liu YH. 2005. Purification and characterization of a novel pyrethroid hydrolase from Aspergillus niger ZD11. J Agric Food Chem 53:7415–7420. doi: 10.1021/jf051460k. [DOI] [PubMed] [Google Scholar]
- 30.Rashamuse K, Magomani V, Ronneburg T, Brady D. 2009. A novel family VIII carboxylesterase derived from a leachate metagenome library exhibits promiscuous β-lactamase activity on nitrocefin. Appl Microbiol Biotechnol 83:491–500. doi: 10.1007/s00253-009-1895-x. [DOI] [PubMed] [Google Scholar]
- 31.Dowd JE, Riggs DS. 1965. A comparison of estimates of Michaelis-Menten kinetic constants from various linear transformations. J Biol Chem 240:863–869. [PubMed] [Google Scholar]
- 32.Dong W, Hou Y, Li S, Wang F, Zhou J, Li Z, Cui Z. 2015. Purification, cloning, expression, and biochemical characterization of a monofunctional catalase, KatP, from Pigmentiphaga sp. DL-8. Protein Expres Purif 108:54–61. doi: 10.1016/j.pep.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Ma YF, Wu JF, Wang SY, Jiang CY, Zhang Y, Qi SW, Liu SJ. 2007. Nucleotide sequence of plasmid pCNB1 from Comamonas strain CNB-1 reveals novel genetic organization and evolution for 4-chloronitrobenzene degradation. Appl Environ Microbiol 73:4477–4483. doi: 10.1128/AEM.00616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JF, Jiang CY, Wang BJ, Ma YF, Liu ZP, Liu SJ. 2006. Novel partial reductive pathway for 4-chloronitrobenzene and nitrobenzene degradation in Comamonas sp. strain CNB-1. Appl Environ Microbiol 72:1759–1765. doi: 10.1128/AEM.72.3.1759-1765.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhen D, Liu H, Wang SJ, Zhang JJ, Zhao F, Zhou NY. 2006. Plasmid-mediated degradation of 4-chloronitrobenzene by newly isolated Pseudomonas putida strain ZWL73. Appl Microbiol Biotechnol 72:797–803. doi: 10.1007/s00253-006-0345-2. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Y, Wu JF, Liu H, Wang SJ, Liu SJ, Zhou NY. 2006. Characterization of genes involved in the initial reactions of 4-chloronitrobenzene degradation in Pseudomonas putida ZWL73. Appl Microbiol Biotechnol 73:166–171. doi: 10.1007/s00253-006-0441-3. [DOI] [PubMed] [Google Scholar]
- 37.Ansorge WJ. 2009. Next-generation DNA sequencing techniques. New Biotechnol 25:195–203. doi: 10.1016/j.nbt.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Larkin M, Blackshields G, Brown N, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 39.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quandt J, Hynes MF. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 41.Dong W, Jiang S, Shi K, Wang F, Li S, Zhou J, Cui Z. 2015. Biodegradation of fenoxaprop-p-ethyl (FE) by Acinetobacter sp. strain DL-2 and cloning of FE hydrolase gene afeH. Bioresour Technol 186:114–121. doi: 10.1016/j.biortech.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 42.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Wang X, Zheng J, Wang G, Hong Q, Li S, Jiang J. 2013. Biodegradation of acetamiprid by Pigmentiphaga sp. D-2 and the degradation pathway. Int Biodeter Biodegr 85:95–102. doi: 10.1016/j.ibiod.2013.03.038. [DOI] [Google Scholar]
- 44.Wang G, Yue W, Liu Y, Li F, Xiong M, Zhang H. 2013. Biodegradation of the neonicotinoid insecticide acetamiprid by bacterium Pigmentiphaga sp. strain AAP-1 isolated from soil. Bioresour Technol 138:359–368. doi: 10.1016/j.biortech.2013.03.193. [DOI] [PubMed] [Google Scholar]
- 45.Uchihashi K, Misawa T, Takeo M, Negoro S. 2003. Mutational analysis of the metabolism of 2,6-naphthalenedisulfonate by Pigmentiphaga sp. NDS-2. J Biosci Bioeng 95:476–482. doi: 10.1016/S1389-1723(03)80048-8. [DOI] [PubMed] [Google Scholar]
- 46.Kulla HG, Krieg R, Zimmermann T, Leisinger T. 1984. Experimental evolution of azo dye-degrading bacteria, p 663–667. In Klug MJ, Reddy CA (ed), Current perspectives in microbial ecology. American Society for Microbiology, Washington, DC. [Google Scholar]
- 47.Friebe A, Vilich V, Hennig L, Kluge M, Sicker D. 1998. Detoxification of benzoxazolinone allelochemicals from wheat by Gaeumannomyces graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and Fusarium culmorum. Appl Environ Microbiol 64:2386–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glenn AE, Hinton DM, Yates IE, Bacon CW. 2001. Detoxification of corn antimicrobial compounds as the basis for isolating Fusarium verticillioides and some other Fusarium species from corn. Appl Environ Microbiol 67:2973–2981. doi: 10.1128/AEM.67.7.2973-2981.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glenn AE, Gold SE, Bacon CW. 2002. Fdb1 and Fdb2, Fusarium verticillioides loci necessary for detoxification of preformed antimicrobials from corn. Mol Plant Microbe Interact 15:91–101. doi: 10.1094/MPMI.2002.15.2.91. [DOI] [PubMed] [Google Scholar]
- 50.Bacon CW, Hinton DM, Glenn AE, Macías FA, Marin D. 2007. Interactions of Bacillus mojavensis and Fusarium verticillioides with a benzoxazolinone (BOA) and its transformation product, APO. J Chem Ecol 33:1885–1897. doi: 10.1007/s10886-007-9347-5. [DOI] [PubMed] [Google Scholar]
- 51.Fomsgaard IS, Mortensen AG, Carlsen SC. 2004. Microbial transformation products of benzoxazolinone and benzoxazinone allelochemicals-a review. Chemosphere 54:1025–1038. doi: 10.1016/j.chemosphere.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 52.Glenn AE, Bacon CW. 2009. FDB2 encodes a member of the arylamine N-acetyltransferase family and is necessary for biotransformation of benzoxazolinones by Fusarium verticillioides. J Appl Microbiol 107:657–671. doi: 10.1111/j.1365-2672.2009.04246.x. [DOI] [PubMed] [Google Scholar]
- 53.Voloshchuk N, Knop M, Colby T, Kombrink E, Hennig L, Hofmann D, Schulz M. 2007. How Doratomyces stemonitis copes with benzoxazolin-2(3H)-one (BOA), its derivatives and detoxification products. Chemoecology 17:1–12. doi: 10.1007/s00049-006-0350-z. [DOI] [Google Scholar]
- 54.Smith MJ, Bucher G. 2012. Tools to study the degradation and loss of the N-phenyl carbamate chlorpropham-A comprehensive review. Environ Int 49:38–50. doi: 10.1016/j.envint.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Feng X, Ou LT, Ogram A. 1997. Plasmid-mediated mineralization of carbofuran by Sphingomonas sp. strain CF06. Appl Environ Microbiol 63:1332–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan LJ, Liu JB, Xiao XG. 2014. Biooxidation of indole and characteristics of the responsible enzymes. Afr J Biotechnol 10:9855–19863. [Google Scholar]
- 57.Qin Y, Shen X, Wang N, Ding X. 2015. Characterization of a novel cyclase-like gene family involved in controlling stress tolerance in rice. J Plant Physiol 181:30–41. doi: 10.1016/j.jplph.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Marín M, Pieper DH. 2009. Novel metal-binding site of Pseudomonas reinekei MT1 trans-dienelactone hydrolase. Biochem Biophys Res Commun 390:1345–1348. doi: 10.1016/j.bbrc.2009.10.151. [DOI] [PubMed] [Google Scholar]
- 59.Bjerregaard AK, Sommer T, Jensen JK, Jochimsen B, Etzerodt M, Morth JP. 2014. A proton wire and water channel revealed in the crystal structure of isatin hydrolase. J Biol Chem 289:21351–21359. doi: 10.1074/jbc.M114.568824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kettle AJ, Carere J, Batley J, Benfield AH, Manners JM, Kazan K, Gardiner DM. 2015. A γ-lactamase from cereal infecting Fusarium spp. catalyses the first step in the degradation of the benzoxazolinone class of phytoalexins. Fungal Genet Biol 83:1–9. doi: 10.1016/j.fgb.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Katsivela E, Wray V, Pieper DH, Wittich RM. 1999. Initial reactions in the biodegradation of 1-chloro-4-nitrobenzene by a newly isolated bacterium, strain LW1. Appl Environ Microbiol 65:1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu JF, Sun CW, Jiang CY, Liu ZP, Liu SJ. 2005. A novel 2-aminophenol 1,6-dioxygenase involved in the degradation of p-chloronitrobenzene by Comamonas strain CNB-1: purification, properties, genetic cloning and expression in Escherichia coli. Arch Microbiol 183:1–8. doi: 10.1007/s00203-004-0738-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.