ABSTRACT
In this study, we characterized Gly5M, originating from a marine bacterium, as a novel β-1,3-1,6-endoglucanase in glycoside hydrolase family 5 (GH5) in the Carbohydrate-Active enZyme database. The gly5M gene encodes Gly5M, a newly characterized enzyme from GH5 subfamily 47 (GH5_47) in Saccharophagus degradans 2-40T. The gly5M gene was cloned and overexpressed in Escherichia coli. Through analysis of the enzymatic reaction products by thin-layer chromatography, high-performance liquid chromatography, and matrix-assisted laser desorption ionization–tandem time of flight mass spectrometry, Gly5M was identified as a novel β-1,3-endoglucanase (EC 3.2.1.39) and bacterial β-1,6-glucanase (EC 3.2.1.75) in GH5. The β-1,3-endoglucanase and β-1,6-endoglucanase activities were detected by using laminarin (a β-1,3-glucan with β-1,6-glycosidic linkages derived from brown macroalgae) and pustulan (a β-1,6-glucan derived from fungal cell walls) as the substrates, respectively. This enzyme also showed transglycosylase activity toward β-1,3-oligosaccharides when laminarioligosaccharides were used as the substrates. Since laminarin is the major form of glucan storage in brown macroalgae, Gly5M could be used to produce glucose and laminarioligosaccharides, using brown macroalgae, for industrial purposes.
IMPORTANCE In this study, we have discovered a novel β-1,3-1,6-endoglucanase with a unique transglycosylase activity, namely, Gly5M, from a marine bacterium, Saccharophagus degradans 2-40T. Gly5M was identified as the newly found β-1,3-endoglucanase and bacterial β-1,6-glucanase in GH5. Gly5M is capable of cleaving glycosidic linkages of both β-1,3-glucans and β-1,6-glucans. Gly5M also possesses a transglycosylase activity toward β-1,3-oligosacchrides. Due to the broad specificity of Gly5M, this enzyme can be used to produce glucose or high-value β-1,3- and/or β-1,6-oligosaccharides.
INTRODUCTION
Marine macroalgae have been considered as potential sources of carbohydrates for the production of biofuels and biologically based products (1). Among the marine macroalgae, over 70 million tons of brown macroalgae are produced annually (2). The major carbon storage compounds of brown algae are alginate, laminarin, fucoidan, and mannitol (3). Laminarin is a linear polysaccharide composed of d-glucose with β-1,3-glycosidic linkages in the main backbone and degrees of polymerization (DPs) of 20 to 25 (4), with some 6-O-branching from d-glucose in the main chain and some β-1,6-glycosidic linkages as branches; the β-1,3-glucan/β-1,6-glucan ratio is approximately 3:1, depending on the species of brown macroalgae (5). Among brown macroalgae, the main sources of laminarin are Laminaria and Saccharina spp. For instance, Laminaria hyperborean and Saccharina latissima contain laminarin at up to 32% of dry weight (6), and 84% of total carbohydrates are laminarin in Fucus vesiculosus (7). Unlike other marine polysaccharides, such as alginate and agar, from which monomeric sugars are not fermentable by terrestrial microorganisms (8, 9), the final hydrolysis product of laminarin is glucose, which can be directly fermented by microorganisms. Therefore, laminarin is an ideal substrate for the production of biofuels and biologically based products.
For the efficient saccharification of laminarin, glycoside hydrolases (GHs) can be used to cleave the glycosidic linkages of laminarin. The β-1,3-glucanases are classified into two major groups on the basis of their different modes of action. The β-1,3-endoglucanases hydrolyze the internal β-1,3 linkages and produce various oligosaccharides (10, 11). The β-1,3-exoglucanases release glucose from the nonreducing ends (12, 13). There are three types of β-1,3-endoglucanases; β-1,3-endoglucanase (EC 3.2.1.39) requires at least two adjacent β-1,3 linkages, whereas β-1,3-1,4-endoglucanase (EC 3.2.1.6) hydrolyzes both β-1,3 and β-1,4 linkages adjacent to the reducing terminal, and lichenase (EC 3.2.1.73) degrades only β-1,4 linkages adjacent to the reduced terminal side of the β-1,3 linkage in β-1,3-1,4-glucans (14). Based on the divergence of amino acid sequences, the β-1,3-endoglucanases originating from plants are categorized as being in glycoside hydrolase family 17 (GH17), whereas those from bacteria are categorized as being in GH16.
Bacterial β-1,3-glucanases were isolated from a variety of microorganisms, such as Bacillus circulans (15), Paenibacillus (16), and Thermotoga maritima (17), and characterized. In addition to these microorganisms, the marine bacterium Saccharophagus degradans 2-40T, which is known as a superdegrader in the marine environment, utilizes at least 10 complex polysaccharides (18). Many enzymes for depolymerization of complex polysaccharides, including cellulases, xylanases, agarases, and alginate lyases, were characterized in S. degradans 2-40T (19–24). However, enzymes depolymerizing laminarin in S. degradans 2-40T have not been identified and characterized. In this study, we cloned, expressed, and characterized a novel β-1,3-endoglucanase, Gly5M, from S. degradans 2-40T. To our knowledge, Gly5M is a novel β-1,3-endoglucanase from GH5 in the Carbohydrate-Active enZyme (CAZy) database, as well as the newly characterized enzyme in GH5 subfamily 47 (GH5_47).
MATERIALS AND METHODS
Cloning of gly5M from S. degradans 2-40T.
S. degradans 2-40T (ATCC 43961) was cultured for 12 h at 30°C in minimal broth containing 23 g/liter Instant Ocean sea salt (Aquarium Systems), 50 mM Tris-HCl, 2 g/liter glucose, 1 g/liter yeast extract, and 0.5 g/liter ammonium chloride. Cells were harvested by centrifugation (4,000 × g) for 15 min, and the genomic DNA was obtained by using a commercial DNA isolation kit (Qiagen). The target gene, gly5M, was amplified by PCR using Solg 2× Taq PCR Smart Mix 2 (Solgent); the primers used were 5′-GCGGGATCCATGAGAGAAAAACTACTGCGCG-3′ (forward) and 5′-GCGCTCGAGGTGGTGGTGGTGGTGGTGGTCAACTGCTTCAACACTCCA-3′ (reverse), with the underlined regions indicating BamHI and XhoI restriction sites. A sequence encoding 6 histidines was added to the reverse primer for purification of the recombinant protein by using a HisTrap column (GE Healthcare). The PCR product and pET28a+ vector (Novagen) were both double digested with BamHI and XhoI, the fragments were ligated using T4 DNA ligase (BioLabs), and the resulting plasmid, harboring the gly5M gene, was transformed into Escherichia coli BL21(DE3).
Expression and purification of recombinant proteins.
Recombinant Escherichia coli BL21(DE3) harboring the gly5M gene was cultured in a shaking incubator in Luria-Bertani broth (BD) containing 50 mg/liter kanamycin, at 37°C and 200 rpm, until the absorbance at 600 nm of the culture broth reached 0.6. Gene expression was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) (Amresco) at 16°C and 180 rpm for 18 h. Cells were harvested by centrifugation at 4,000 × g for 15 min, disrupted by ultrasonication, and centrifuged at 16,000 × g for 1 h. The cell-free supernatant was passed through a HisTrap column (GE Healthcare), following the manufacturer's protocol. The purified recombinant protein was concentrated using an Amicon ultracentrifugal filter unit (molecular weight cutoff value of 50,000, UFC903024; Millipore). The concentration of the purified proteins was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce).
Determination of Gly5M enzymatic activity.
To determine Gly5M activity, 1.05 nmol of Gly5M was incubated for 30 min at 40°C with 100 μl of 20 mM Tris-HCl buffer (pH 6.0) containing 2% (wt/vol) laminarin (TCI) or pustulan (InvivoGen). The reaction was quenched using boiling water. The relative enzyme activity was measured by using the dinitrosalicylic acid (DNS) method, with d-glucose as the standard. One unit of Gly5M was defined as the amount of enzyme required to induce the release of 1 μmol of the reducing sugar per min under the enzymatic reaction conditions described above.
Screening for substrate specificity of Gly5M.
To determine the specificity of Gly5M, 1% (wt/vol) levels of various glycans, including laminarin, pustulan, curdlan (Wako), β-glucan from barley (Sigma-Aldrich), carboxymethylcellulose (Sigma-Aldrich), and xylan (Sigma-Aldrich), were used as the substrates and were incubated for 30 min at 40°C with 10.5 μM Gly5M in 20 mM Tris-HCl buffer (pH 6.0). The released reducing sugars were quantified by the DNS method, as described above.
Characterization of Gly5M.
To determine the optimum temperature for the enzyme activity of Gly5M, the enzyme was incubated for 30 min with 2% (wt/vol) laminarin in 20 mM Tris-HCl buffer (pH 6.0), at temperatures ranging from 20°C to 70°C, the reactions were quenched by immersion in a boiling water bath, and the products were quantified by using the DNS assay. To determine the optimum pH for Gly5M activity, the enzyme was incubated for 30 min at 40°C with 2% (wt/vol) laminarin in buffers with different pH values, such as 20 mM glycine-HCl (pH 2.0 to 4.0), 20 mM sodium acetate (pH 4.0 to 6.0), 20 mM Tris-HCl (pH 6.0 to 9.0), and 20 mM glycine-NaOH (pH 9.0 to 10.0). To avoid the possible effect of different pH values on the DNS assay, the 2-cyanoacetamide method was used for the quantification of reaction products (25). The kinetic parameters (Vmax, Km, and kcat) of Gly5M toward laminarin were determined from the Lineweaver-Burk plot, in which the enzyme activity was measured using laminarin at different concentrations, ranging from 0.45% to 9.1% (wt/vol), under the optimized pH and temperature conditions determined as described above.
Analysis of enzymatic reaction products by thin-layer chromatography and high-performance liquid chromatography.
To visualize the Gly5M reaction process, thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) were used to monitor the progress of the enzymatic reactions over time. For the TLC analysis, reaction products were developed on a silica gel 60 plate (Merck) with a mobile phase consisting of n-butanol/acetic acid/water (3:2:2, by volume) and then visualized using the orcinol reagent (26), followed by heating of the TLC plate at 130°C for 5 min. The reaction products were also analyzed with an Agilent 1100 HPLC system (Agilent) equipped with a gel permeation-ligand exchange column (KS-802; Shodex) and a refractive index detector (Agilent). The HPLC analysis was performed at 80°C with distilled water as the mobile phase, at a flow rate of 0.5 ml/min. Laminaribiose, laminaritriose, laminaritetraose, laminaripentaose, and laminarihexaose (all purchased from Megazyme) were used as the standards.
Analysis of enzymatic reaction products by matrix-assisted laser desorption ionization–tandem time of flight mass spectrometry.
The enzymatic reaction products of Gly5M incubated with laminarin or pustulan were analyzed by matrix-assisted laser desorption ionization–tandem time of flight mass spectrometry (MALDI–TOF/TOF MS), using an ultrafleXtreme MALDI–TOF/TOF MS system (Bruker Daltonics). For the sample preparation, the purified reaction products were dissolved in water, and 1 μl of the solubilized reaction products was spotted onto a stainless steel target plate, followed by the addition of 0.3 μl of 0.01 M NaCl and 0.5 μl of 50 g/liter 2,5-dihydroxybenzoic acid in 50% (vol/vol) acetonitrile. The spot was rapidly dried under vacuum for homogeneous crystallization. The samples were analyzed by MALDI–TOF/TOF MS as described previously (27). Raw MS data were processed using FlexAnalysis software (version 3.3; Bruker Daltonics). All MS peaks were deconvoluted and a list of all neutral masses in the samples was generated, with the abundances represented by mass spectral peak intensities.
RESULTS
Overexpression of Gly5M in E. coli BL21(DE3).
The full-length sequence of gly5M was inserted into the pET28a+ vector in frame with a sequence encoding a 6-His tag at the C terminus. To avoid the formation of inclusion bodies at 37°C, protein production was induced at 16°C for 18 h using 0.5 mM IPTG. The molar mass of the expressed recombinant Gly5M was 95 kDa, according to the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) findings (data not shown), consistent with its theoretical molar mass (94.87 kDa).
Substrate specificity of Gly5M.
To determine Gly5M substrate specificity, several glycans with different glycosidic linkages, including laminarin, pustulan, curdlan, barley β-glucan, Avicel, and carboxymethyl cellulose, were subjected to the enzymatic reaction. Gly5M showed the greatest activity with laminarin (data not shown), and it hydrolyzed pustulan (a β-1,6-glucan from fungal cell walls) with 55.6% relative activity, compared to its activity with laminarin (data not shown); this is functionally different from most β-1,3-glucanases, which show activity only with laminarin. Unlike some β-glucanases from Streptomyces (28), Gly5M did not hydrolyze curdlan (data not shown), which is a water-insoluble β-1,3-glucan, probably owing to the highly triple-helix structure of curdlan. This characteristic is similar to that of most β-1,3-glucanases, which have much lower levels of activity toward curdlan than toward laminarin (10). Gly5M showed no activity toward Avicel, barley β-glucan, carboxymethyl cellulose, or xylan (data not shown), which indicated that it has no hydrolytic activity at β-1,4-glycosidic linkages. The study of the effects of various cations on the activity of Gly5M showed that heavy metal cations such as Ni2+, Cu2+, Fe2+, and Co2+ were strong inhibitors of Gly5M (Table 1).
TABLE 1.
Effects of various cations on Gly5M activity
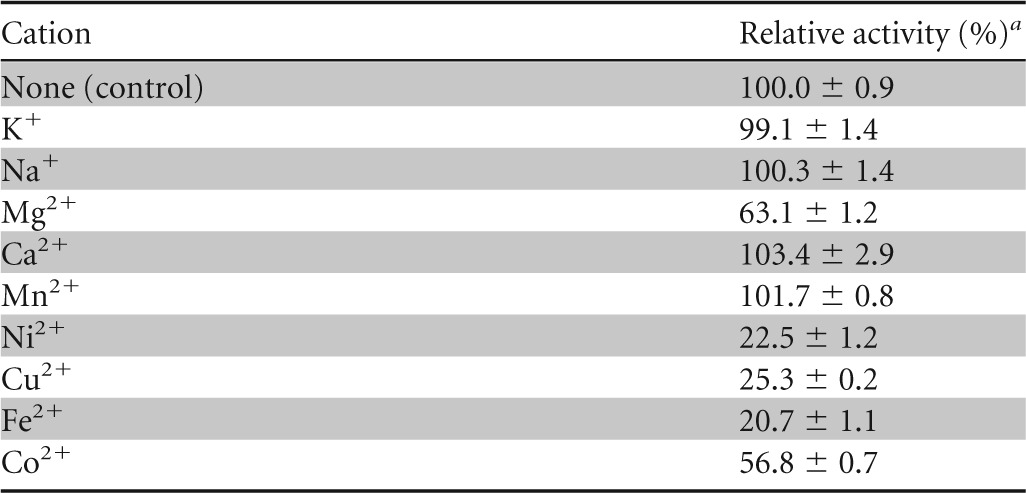
a The activity without metal ions was set as 100%. Experimental data are means ± standard deviations from triplicate experiments.
Optimal pH and temperature for Gly5M.
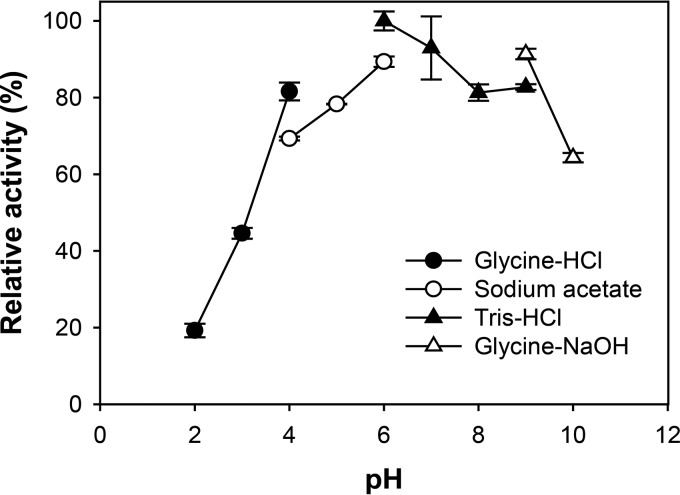
To investigate the effects of pH and temperature on Gly5M enzymatic activity, 2% (wt/vol) laminarin was incubated with 10.5 μM Gly5M at various pH values (pH 2.0 to 10.0) and temperatures (20 to 70°C), and the highest enzymatic activity was obtained at pH 6.0 in 20 mM Tris-HCl buffer (Fig. 1). Gly5M displayed relative enzymatic activities of less than 20% at pH 2.0 in 20 mM glycine-HCl and more than 60% at pH 10.0 in 20 mM glycine-NaOH. Therefore, pH 6.0 was determined to be the optimum pH for Gly5M enzymatic activity for subsequent experiments.
FIG 1.
Effects of pH on Gly5M activity. Enzymatic reactions were performed for 30 min at 40°C in 20 mM glycine-HCl (pH 2.0 to 4.0), sodium acetate (pH 4.0 to 6.0), Tris-HCl (pH 6.0 to 9.0), or glycine-NaOH (pH 9.0 to 10.0).
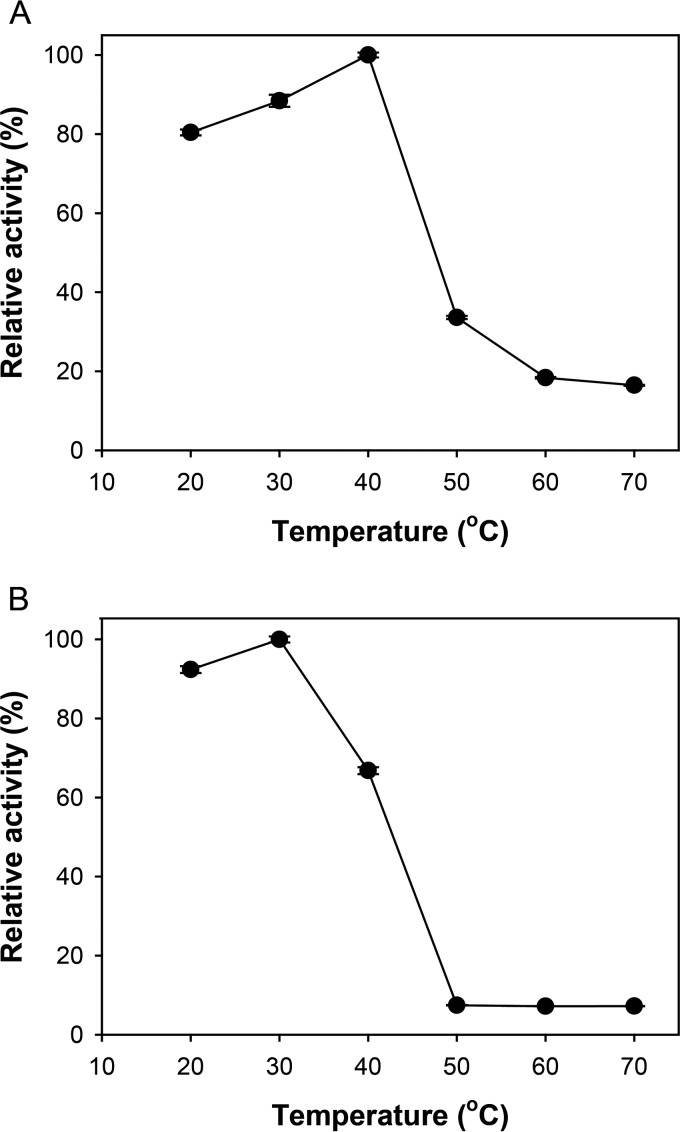
The optimal temperature for Gly5M enzymatic activity was determined to be approximately 40°C at pH 6.0 (Fig. 2A). The enzyme presented 80% relative activity at 20°C, but the relative activity decreased significantly when the reaction temperature was higher than 40°C (Fig. 2A). In the thermal stability test, incubation at temperatures higher than 40°C for 1 h resulted in more than 60% activity loss, compared to the activity of Gly5M incubated at 40°C (Fig. 2B). To prevent Gly5M activity loss, the optimum reaction conditions were set at pH 6.0 and 30°C.
FIG 2.
Relative activity of Gly5M at different temperatures (A); relative activity of 30-min-preincubated Gly5M at different temperatures, measured to determine the thermal stability of Gly5M (B). Enzymatic activity was measured by reactions performed for 30 min at pH 6.0 and 40°C, using laminarin as the substrate.
Kinetic parameters of Gly5M hydrolysis of laminarin.
To determine the kinetic parameters of Gly5M with laminarin, enzymatic reactions were performed using laminarin as the substrate, at pH 6.0 and 40°C in 20 mM Tris-HCl buffer. From the Lineweaver-Burk plot (data not shown), Km, Vmax, and kcat were determined to be 10.4 g/liter, 10.6 U/mg, and 16.8 s−1, respectively.
Modes of action of Gly5M with laminarin and pustulan.
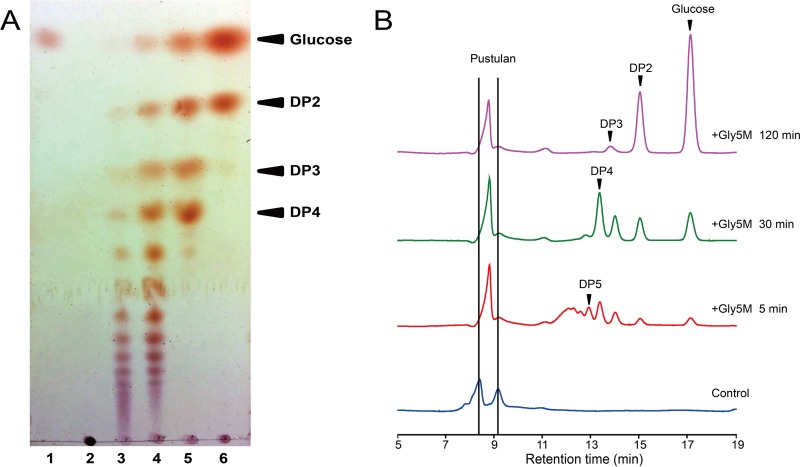
The reaction products of Gly5M hydrolysis of laminarin or pustulan were analyzed. The progress of the enzymatic reaction was monitored through analysis of the reaction products by TLC and HPLC. When laminarin was used as the substrate, higher-DP oligosaccharides were mainly formed after the initial 5 min, and lower-DP oligosaccharides, such as DP2 to DP5 and glucose, were formed in larger amounts as the reaction progressed further (Fig. 3A and B). Accordingly, the abundance of oligosaccharides higher than DP5 decreased as the reaction progressed, resulting in an increase in glucose and oligosaccharides with DPs lower than 5 (Fig. 3B). These results indicated that Gly5M randomly hydrolyzes the β-1,3-glycosidic linkages of various DPs from laminarin. The mode of action was similar when pustulan was used as the substrate. The formation of higher-DP oligosaccharides was predominant in the early period of the reaction, and the formation of lower-DP oligosaccharides followed (Fig. 4). The final products from the hydrolysis of pustulan by Gly5M were glucose and gentiobiose, with DP2 linked with a β-1,6-glycosidic bond.
FIG 3.
TLC (A) and HPLC (B) analyses of Gly5M enzymatic reaction products using laminarin as the substrate. The reactions were performed at 30°C in 20 mM Tris-HCl (pH 6.0). Lane 1, glucose; lane 2, laminarin; lane 3, laminarin plus Gly5M for 5 min; lane 4, laminarin plus Gly5M for 30 min; lane 5, laminarin plus Gly5M for 60 min; lane 6, laminarin plus Gly5M for 120 min. The control contained substrate but lacked enzyme, and no incubation was performed.
FIG 4.
TLC (A) and HPLC (B) analyses of Gly5M enzymatic reaction products using pustulan as the substrate. The reactions were performed at 30°C in 20 mM Tris-HCl (pH 6.0). Lane 1, glucose; lane 2, pustulan; lane 3, pustulan plus Gly5M for 5 min; lane 4, pustulan plus Gly5M for 30 min; lane 5, pustulan plus Gly5M for 60 min; lane 6, pustulan plus Gly5M for 120 min. The control contained substrate but lacked enzyme, and no incubation was performed.
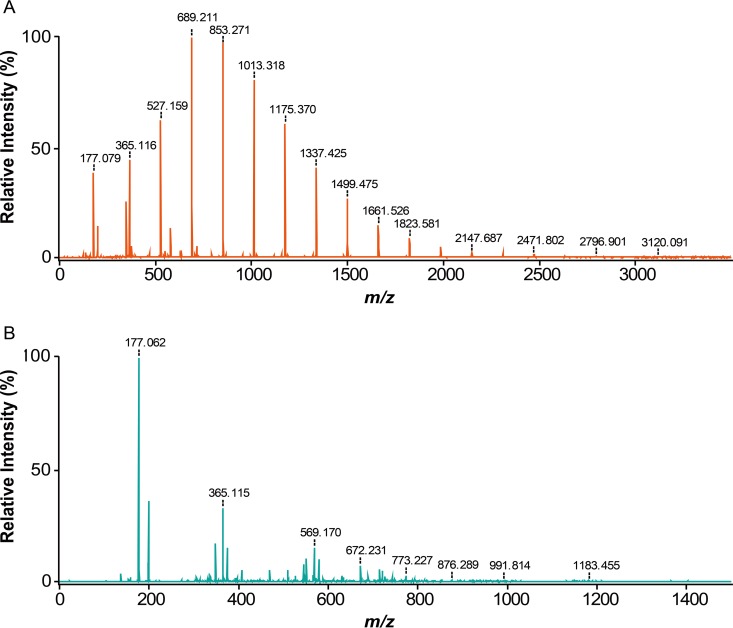
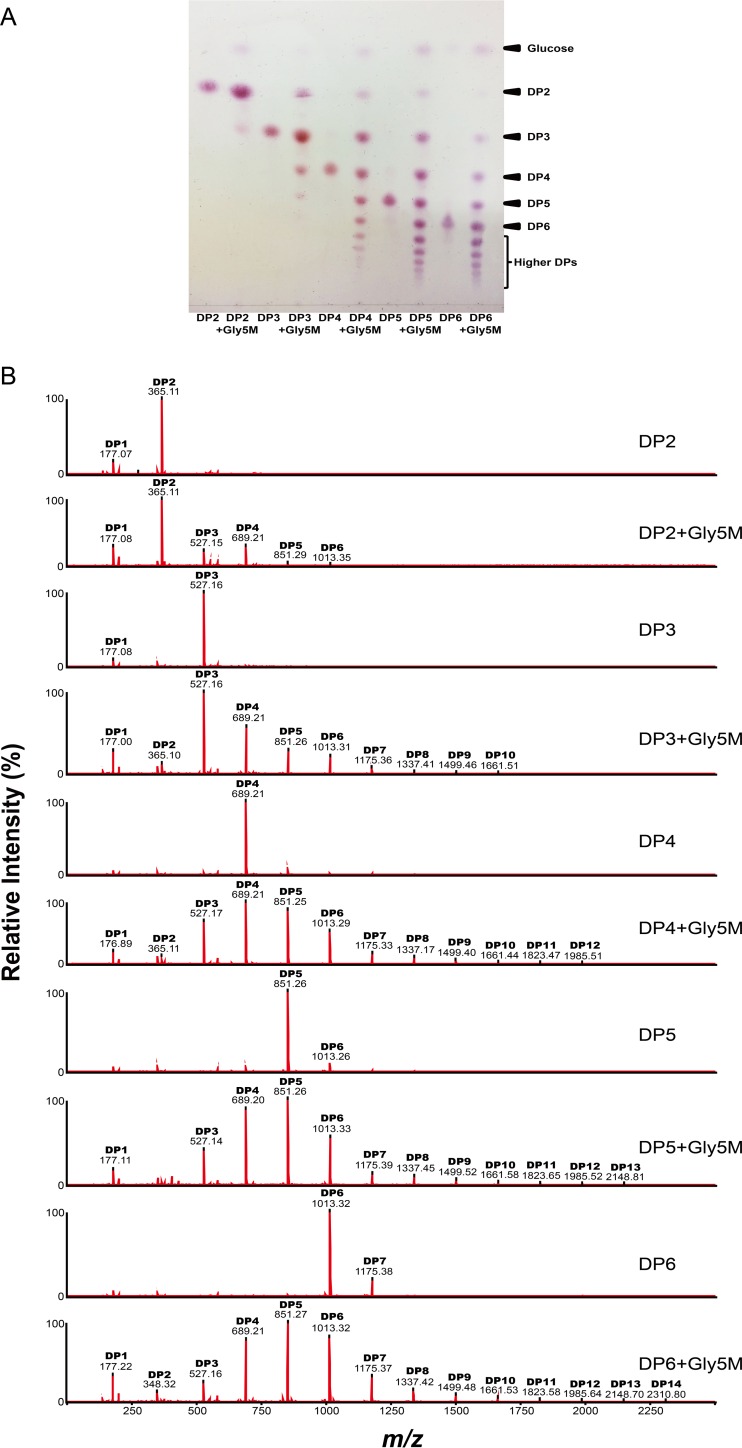
To investigate further the modes of action of Gly5M, the enzymatic reaction products of Gly5M incubated with laminarin, pustulan, and laminarioligosaccharides for 2 h were analyzed by MALDI–TOF/TOF MS. The reaction products obtained with laminarin as the substrate showed that Gly5M primarily hydrolyzed laminarin into glucose and oligosaccharides ranging up to DP17 but with a major fraction of oligosaccharides with DP4 and DP5, according to the peak intensity (Fig. 5A). When pustulan was used as the substrate, glucose was found to be the major product, while oligosaccharides with DP2 to DP8 were also detected (Fig. 5B). Interestingly, when laminarioligosaccharides with DP2 to DP6 were used as the substrates for Gly5M, transglycosylative enzyme activity was detected in addition to the hydrolytic enzyme activity (Fig. 6A). Oligosaccharides that had higher DPs than the substrates (i.e., laminarioligosaccharides with DP2 to DP6) were detected in all five reactions using DP2, DP3, DP4, DP5, and DP6 individually as the substrates. When laminaribiose and laminaritriose were used as the substrates, products with DPs higher than those of the substrates were formed due to the transglycosylative activity of Gly5M (Fig. 6B). These results suggested that Gly5M uses laminaribiose and laminaritriose as the building blocks for its transglycosylative mode of action.
FIG 5.
MALDI–TOF/TOF MS analyses of Gly5M enzymatic reaction products using laminarin (A) and pustulan (B) as the substrates.
FIG 6.
TLC (A) and MALDI–TOF/TOF MS (B) analyses of Gly5M enzymatic reaction products using laminarioligosaccharides as the substrates.
DISCUSSION
In this study, we characterized Gly5M, a novel β-1,3-1,6-endoglucanase originating from a marine bacterium, S. degradans 2-40T. Gly5M is a newly identified β-1,3-endoglucanase and bacterial β-1,6-endoglucanase in GH5. Moreover, among the reported β-1,3-endoglucanases, Gly5M is a novel enzyme with β-1,3-glucanase, β-1,6-glucanase, and transglycosylase activities. In this study, Gly5M cleaved the β-1,3-glycosidic linkages of laminarin, producing β-1,3-linked laminarioligosaccharides and glucose from laminarin. Gly5M also cleaved the β-1,6-glycosidic linkages of pustulan, randomly hydrolyzing the β-1,6-glucan and leading to the formation of β-1,6-linked gentiooligosaccharides and glucose. Moreover, Gly5M transglycosylation activity was also observed when laminarioligosaccharides were used as the substrates, resulting in the formation of laminarioligosaccharides with higher molecular weights than the substrates.
According to the similarity search with the deduced amino acid sequences of other proteins from GenBank, Gly5M is composed of a GH5 catalytic domain and a carbohydrate-binding module 6 (CBM6) domain. For the hydrolysis of β-1,3-glucans, represented by laminarin, β-1,3-endoglucanases (EC 3.2.1.39) and β-1,3-glucosidases or β-1,3-exoglucanases (EC 3.2.1.58) are needed. β-1,3-Endoglucanases in GH16, GH17, GH55, GH64, GH81, and GH128 require at least two adjacent β-1,3-glycosidic linkages in their substrates. However, β-1,3-glucosidases or β-1,3-exoglucanases (EC 3.2.1.58) in the GH5, GH7, GH12, GH16, and GH17 families act on the nonreducing end of β-1,3-glucans to release glucose (29). GH5 is one of the largest GH families, previously known as cellulase family A (30). To date, the amino acid sequences of more than 5,000 GH5 enzymes are available in the CAZy database. Currently, GH5 contains 21 experimentally characterized enzymes with EC numbers, including 19 GH5 enzymes from S. degradans 2-40T (Table 2). The GH5 enzymes share the canonical retaining mechanism, involving a covalent glycosyl-enzyme intermediate (31, 32). Therefore, the GH5 enzymes have both hydrolysis and transglycosylation activities.
TABLE 2.
GH5 enzymes from S. degradans 2-40T
| Gene name | GenBank accession no. | Subfamily | EC no. of subfamily | Reference(s) |
|---|---|---|---|---|
| cel5A | ABD82260.1 | GH5_2 | 3.2.1.4, 3.2.1.32 | 36 |
| cel5B | ABD81750.1 | NCa | NAb | |
| cel5C | ABD79589.1 | GH5_4 | 3.2.1.4, 3.2.1.8, 3.2.1.73, 3.2.1.151 | 36 |
| cel5D | ABD81896.1 | GH5_4 | 3.2.1.4, 3.2.1.8, 3.2.1.73, 3.2.1.151 | 36 |
| cel5E | ABD82186.1 | NC | NA | |
| cel5F | ABD80834.1 | GH5_26 | 3.2.1.4, 3.2.1.73 | 36, 46 |
| cel5G | ABD82496.1 | GH5_2 | 3.2.1.4, 3.2.1.32 | 36 |
| cel5H | ABD82494.1 | GH5_2 | 3.2.1.4, 3.2.1.32 | 36 |
| cel5I | ABD82675.1 | GH5_53 | 3.2.1.74 | 36 |
| cel5J | ABD81754.1 | GH5_2 | 3.2.1.4, 3.2.1.32 | 36 |
| gly5K | ABD82250.1 | GH5_46 | NA | |
| gly5l | ABD82253.1 | GH5_47 | 3.2.1.39 | This study |
| gly5M | ABD82280.1 | GH5_47 | 3.2.1.39 | This study |
| man5N | ABD79328.1 | GH5_8 | 3.2.1.78 | 36 |
| man5O | ABD79918.1 | GH5_8 | 3.2.1.78 | 36 |
| man5P | ABD79773.1 | GH5_7 | 3.2.1.25, 3.2.1.78 | 36 |
| man5Q | ABD81801.1 | NC | NA | |
| gly5R | ABD80383.1 | NC | NA | |
| gly5S | ABD81545.1 | GH_10 | 3.2.1.78 | 36 |
NC, not classified.
NA, not applicable.
To our knowledge, before this study, no enzyme from GH5 was shown to exhibit β-1,3-endoglucanase activity. Gly5M belongs to GH5 and consists of a GH5 catalytic domain and a CBM6 domain. Gly5M was functionally predicted by CAZy to be an endo-(1,3 or 1,4)-β-glucanase (19), and all 7 conserved amino acid residues of GH5 exist in Gly5M. Cel5A, which is from S. degradans 2-40T like Gly5M, consists of two GH5 catalytic domains and three CBM6 domains; however, the substrate specificity of Cel5A is not well known (19). The CBM6 domain functions as a carbohydrate-binding module (CBM) and exists widely in cellulase, while some CBMs also bind laminarin by presenting a unique ligand-binding surface that can recognize the nonreducing end of 1,3-linked glucans (33), such as laminarinases from Bacillus halodurans C-125 (34) and Streptomyces sioyaensis (35). Gly5L, which is also from S. degradans 2-40T (Table 2) and consists of a GH5 domain and a CBM6 domain, showed β-1,3-endoglucanase activity like that of Gly5M in our preliminary study (data not shown). Since CBM6 has not been found in any characterized β-1,3-exoglucanases in GH5 to date, CBM6 may play an important role in conferring β-1,3-endoglucanase activity on GH5 enzymes. A phylogenetic tree was drawn for Gly5M (GenBank accession no. ABD82280.1) and all other characterized bacterial β-1,3-endoglucanases (EC 3.2.1.39) (except for the GH17 β-1,3-endoglucanases from eukaryotes) in the CAZy database (http://www.cazy.org) (Fig. 7). This phylogenetic tree showed that Gly5M is the only β-1,3-endoglucanase found in GH5. GH5 enzymes are categorized into 53 subfamilies (36), and Gly5M was categorized into GH5_47. Gly5L (GenBank accession no. ABD82253.1), which presents 44% similarity with Gly5M, also belongs to GH5_47. Therefore, it is suggested that GH5_47 enzymes function as β-1,3-endoglucanases, which represents a novel activity for the entire GH5.
FIG 7.
Phylogenetic analysis of Gly5M (GenBank accession no. ABD82280.1) and other characterized bacterial β-1,3-glucanases (EC 3.2.1.39) in the CAZy database. GH16 sequences (from Thermotoga maritima MSB8 [GenBank accession no. AAD35118.1], from Bacillus circulans [GenBank accession no. AAA22474.1], from Flavobacterium johnsoniae UW101 [GenBank accession no. ABQ07185.2], from Streptomyces sioyaensis [GenBank accession no. AAF31438.1], from Thermotoga petrophila RKU-1 [GenBank accession no. ABQ46917.1], from Nocardiopsis sp. F96 [GenBank accession no. BAE54302.1], from Paenibacillus sp. CCRC 17245 [GenBank accession no. ABJ15796.1], from Pseudomonas sp. PE2 [GenBank accession no. BAC16331.1], from Flavobacterium sp. 4221 [GenBank accession no. ABW02990.1], from Thermotoga neapolitana [GenBank accession no. CAA88008.1], from Streptomyces sp. S27 [GenBank accession no. ACO94508.1], from Bacillus circulans [GenBank accession no. AAC60453.1], from Cellulosimicrobium cellulans [GenBank accession no. AAC44371.1], and from Lysobacter enzymogenes [GenBank accession no. AAN77504.1]), GH64 sequences (from Cellulosimicrobium cellulans [GenBank accession no. AAA25520.1], from Arthrobacter sp. YCWD3 [GenBank accession no. BAA04892.1], from Streptomyces matensis [GenBank accession no. BAA34349.1], and from Lysobacter enzymogenes [GenBank accession no. AAN77503.1]), GH81 sequences (from Bacillus halodurans C-125 [GenBank accession no. BAB03955.1] and from Thermobifida fusca YX [GenBank accession no. AAZ56163.1]), and a GH55 sequence (from Arthrobacter sp. NHB-10 [GenBank accession no. BAF52916.1]) were aligned by using the UniProt Align tool (http://www.uniprot.org/align). The phylogenetic tree was inferred from the alignments with the minimum linkage method and was drawn by using MAFFT (version 7) (http://mafft.cbrc.jp/alignment/software).
The common broad specificity of β-1,3-glucanases is the cleavage action on β-1,3-1,4-glucans through the β-1,3-1,4-glucanase activity (37). However, this catalytic activity was not observed for Gly5M in this study. Gly5M not only presented β-1,3-glucanase activity but also showed β-1,6-glucanase activity, which is a rare broad specificity of β-1,3-glucanases (38). In this study, Gly5M showed 55.6% relative activity with a β-1,6-glucan, pustulan, compared to its activity with laminarin. Gly5M effectively hydrolyzes pustulan to glucose and gentiobiose as the main products. Gly5M activity toward β-1,6-glycosidic linkages was much higher than that of some β-1,6-glucan-specific β-1,6-glucanases (39, 40).
Many glycoside hydrolases with the canonical retaining mechanism were shown to possess transglycosylation activity in addition to their hydrolytic activity (41–43). In this study, Gly5M exhibited transglycosylation activity toward β-1,3-oligosaccharides (Fig. 7). According to our results, glucose released from the nonreducing end of the substrate is transferred to the O-3 position of the substrate itself. Therefore, the substrate, a laminarioligosaccharide, can serve as both donor and receptor, leading to the formation of oligosaccharides with DPs higher than that of the substrate. Some β-1,3-1,4-endoglucanases can act as a glucan synthase by transferring oligosaccharides with different DPs to sugar acceptors (44). An β-1,3-endoglucanase, EgCel17A from Euglena gracilis, catalyzed such transglycosylations but, ultimately, all of the intermediates were degraded to glucose, laminaribiose, and laminaritriose (45). In this study, Gly5M was suggested to recognize laminarioligosaccharides as both donors and acceptors for the synthesis of high-DP β-1,3-glucan oligosaccharides. In general, enzyme-catalyzed synthesis of oligosaccharides is a useful method because it allows the selective formation of well-defined oligosaccharides in the absence of any protecting groups. Therefore, Gly5M could be used as an oligosaccharide-producing catalyst.
In this study, along with its transglycosylase activity, Gly5M was identified as a β-1,3-1,6-endoglucanase acting on both laminarin and pustulan. Gly5M is a novel glycoside hydrolase and is newly identified as being in GH5 in the CAZy database. Considering the utilization of a marine complex polysaccharide, laminarin, as a resource for fuels or functional oligosaccharides, Gly5M can be considered a suitable enzyme for the production of glucose and multiple laminarioligosaccharides.
ACKNOWLEDGMENTS
This work was financially supported by the Advanced Biomass R&D Center of Korea (grant 2011-0031359), funded by the Korean government. K.H.K. acknowledges the support for page charges by Korea University BK21 PLUS for the School of Life Sciences and Biotechnology. Experiments were carried out using the facilities of the Institute of Biomedical Science and Food Safety at the Food Safety Hall, Korea University.
REFERENCES
- 1.Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. 2007. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 2.Roesijadi G, Jones SB, Snowden-Swan LJ, Zhu Y. 2010. Macroalgae as a biomass feedstock: a preliminary analysis. Publication PNNL-19944. Pacific Northwest National Laboratory, Richland, WA: http://www.pnl.gov/main/publications/external/technical_reports/PNNL-19944.pdf. [Google Scholar]
- 3.Anastasakis K, Ross AB, Jones JM. 2011. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel 90:598–607. doi: 10.1016/j.fuel.2010.09.023. [DOI] [Google Scholar]
- 4.Nelson TE, Lewis BA. 1974. Separation and characterization of the soluble and insoluble components of insoluble laminaran. Carbohydr Res 33:63–74. doi: 10.1016/S0008-6215(00)82940-7. [DOI] [PubMed] [Google Scholar]
- 5.Kadam SU, Tiwari BK, O'Donnell CP. 2015. Extraction, structure and biofunctional activities of laminarin from brown algae. Int J Food Sci Technol 50:24–31. doi: 10.1111/ijfs.12692. [DOI] [Google Scholar]
- 6.Schiener P, Black KD, Stanley MS, Green DH. 2015. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J Appl Phycol 27:363–373. doi: 10.1007/s10811-014-0327-1. [DOI] [Google Scholar]
- 7.Rioux L-E, Turgeon SL, Beaulieu M. 2007. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr Polym 69:530–537. doi: 10.1016/j.carbpol.2007.01.009. [DOI] [Google Scholar]
- 8.Yun EJ, Lee S, Yun EJ, Kim HT, Pelton JG, Kim S, Ko HJ, Choi I-G, Kim KH. 2015. The novel catabolic pathway of 3,6-anhydro-l-galactose, the main component of red macroalgae, in a marine bacterium. Environ Microbiol 17:1677–1688. doi: 10.1111/1462-2920.12607. [DOI] [PubMed] [Google Scholar]
- 9.Takase R, Ochiai A, Mikami B, Hashimoto W, Murata K. 2010. Molecular identification of unsaturated uronate reductase prerequisite for alginate metabolism in Sphingomonas sp. A1. Biochim Biophys Acta Proteins Proteomics 1804:1925–1936. doi: 10.1016/j.bbapap.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto Y, Nakade K, Konno N. 2011. Endo-β-1,3-glucanase GLU1, from the fruiting body of Lentinula edodes, belongs to a new glycoside hydrolase family. Appl Environ Microbiol 77:8350–8354. doi: 10.1128/AEM.05581-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blattel V, Larisika M, Pfeiffer P, Nowak C, Eich A, Eckelt J, Konig H. 2011. β-1,3-Glucanase from Delftia tsuruhatensis strain MV01 and its potential application in vinification. Appl Environ Microbiol 77:983–990. doi: 10.1128/AEM.01943-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayendra SVN, Kashiwagi Y. 2009. Characterization of a new acid stable exo-β-1,3-glucanase of Rhizoctonia solani and its action on microbial polysaccharides. Int J Biol Macromol 44:92–97. doi: 10.1016/j.ijbiomac.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Peng Y, Liu G-L, Yu X-J, Wang X-H, Jing L, Chi Z-M. 2011. Cloning of exo-β-1,3-glucanase gene from a marine yeast Williopsis saturnus and its overexpression in Yarrowia lipolytica. Mar Biotechnol (NY) 13:193–204. doi: 10.1007/s10126-010-9281-3. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai Y, Ojima T. 2009. Enzymatic properties and the primary structure of a β-1,3-glucanase from the digestive fluid of the Pacific abalone Haliotis discus hannai. Comp Biochem Physiol B Biochem Mol Biol 154:113–120. doi: 10.1016/j.cbpb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Fleet GH, Phaff HJ. 1974. Lysis of yeast cell walls: glucanases from Bacillus circulans WL-12. J Bacteriol 119:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y-M, Hong T-Y, Liu C-C, Meng M. 2009. Cloning and functional characterization of a complex endo-β-1,3-glucanase from Paenibacillus sp. Appl Microbiol Biotechnol 81:1051–1061. doi: 10.1007/s00253-008-1617-9. [DOI] [PubMed] [Google Scholar]
- 17.Jeng W-Y, Wang N-C, Lin C-T, Shyur L-F, Wang AH-J. 2011. Crystal structures of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with inhibitors: essential residues for β-1,3- and β-1,4-glucan selection. J Biol Chem 286:45030–45040. doi: 10.1074/jbc.M111.271213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekborg NA, Gonzalez JM, Howard MB, Taylor LE, Hutcheson SW, Weiner RM. 2005. Saccharophagus degradans gen. nov., sp. nov., a versatile marine degrader of complex polysaccharides. Int J Syst Evol Microbiol 55:1545–1549. doi: 10.1099/ijs.0.63627-0. [DOI] [PubMed] [Google Scholar]
- 19.Taylor LE, Henrissat B, Coutinho PM, Ekborg NA, Hutcheson SW, Weiner RM. 2006. Complete cellulase system in the marine bacterium Saccharophagus degradans strain 2-40T. J Bacteriol 188:3849–3861. doi: 10.1128/JB.01348-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko JK, Jung MW, Kim KH, Choi I-G. 2009. Optimal production of a novel endo-acting β-1,4-xylanase cloned from Saccharophagus degradans 2-40 into Escherichia coli BL21(DE3). N Biotechnol 26:157–164. doi: 10.1016/j.nbt.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Kim HT, Lee S, Lee D, Kim H-S, Bang W-G, Kim KH, Choi I-G. 2010. Overexpression and molecular characterization of Aga50D from Saccharophagus degradans 2-40: an exo-type β-agarase producing neoagarobiose. Appl Microbiol Biotechnol 86:227–234. doi: 10.1007/s00253-009-2256-5. [DOI] [PubMed] [Google Scholar]
- 22.Ha SC, Lee S, Lee J, Kim HT, Ko H-J, Kim KH, Choi I-G. 2011. Crystal structure of a key enzyme in the agarolytic pathway, α-neoagarobiose hydrolase from Saccharophagus degradans 2-40. Biochem Biophys Res Commun 412:238–244. doi: 10.1016/j.bbrc.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 23.Hutcheson SW, Zhang H, Suvorov M. 2011. Carbohydrase systems of Saccharophagus degradans degrading marine complex polysaccharides. Mar Drugs 9:645–665. doi: 10.3390/md9040645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HT, Chung JH, Wang D, Lee J, Woo HC, Choi I-G, Kim KH. 2012. Depolymerization of alginate into a monomeric sugar acid using Alg17C, an exo-oligoalginate lyase cloned from Saccharophagus degradans 2-40. Appl Microbiol Biotechnol 93:2233–2239. doi: 10.1007/s00253-012-3882-x. [DOI] [PubMed] [Google Scholar]
- 25.Honda S, Nishimura Y, Takahashi M, Chiba H, Kakehi K. 1982. A manual method for the spectrophotometric determination of reducing carbohydrates with 2-cyanoacetamide. Anal Biochem 119:194–199. doi: 10.1016/0003-2697(82)90685-6. [DOI] [PubMed] [Google Scholar]
- 26.Farkas V, Maclachlan G. 1988. Stimulation of pea 1,4-β-glucanase activity by oligosaccharides derived from xyloglucan. Carbohydr Res 184:213–219. doi: 10.1016/0008-6215(88)80019-3. [DOI] [Google Scholar]
- 27.Lee CH, Kim HT, Yun EJ, Lee AR, Kim SR, Kim J-H, Choi I-G, Kim KH. 2014. A novel agarolytic β-galactosidase acts on agarooligosaccharides for complete hydrolysis of agarose into monomers. Appl Environ Microbiol 80:5965–5973. doi: 10.1128/AEM.01577-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo J-B, Kang H-N, Woo E-J, Lee S-B. 2014. Molecular cloning and functional characterization of an endo-β-1,3-glucanase from Streptomyces matensis ATCC 23935. Food Chem 148:184–187. doi: 10.1016/j.foodchem.2013.09.137. [DOI] [PubMed] [Google Scholar]
- 29.Lafond M, Navarro D, Haon M, Couturier M, Berrin JG. 2012. Characterization of a broad-specificity β-glucanase acting on β-(1,3)-, β-(1,4)-, and β-(1,6)-glucans that defines a new glycoside hydrolase family. Appl Environ Microbiol 78:8540–8546. doi: 10.1128/AEM.02572-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henrissat B, Claeyssens M, Tomme P, Lemesle L, Mornon JP. 1989. Cellulase families revealed by hydrophobic cluster analysis. Gene 81:83–95. doi: 10.1016/0378-1119(89)90339-9. [DOI] [PubMed] [Google Scholar]
- 31.Sinnott ML. 1990. Catalytic mechanisms of enzymatic glycosyl transfer. Chem Rev 90:1171–1202. doi: 10.1021/cr00105a006. [DOI] [Google Scholar]
- 32.Zechel DL, Withers SG. 2000. Glycosidase mechanisms: anatomy of a finely tuned catalyst. Acc Chem Res 33:11–18. doi: 10.1021/ar970172. [DOI] [PubMed] [Google Scholar]
- 33.van Bueren AL, Morland C, Gilbert HJ, Boraston AB. 2005. Family 6 carbohydrate binding modules recognize the non-reducing end of β-1,3-linked glucans by presenting a unique ligand binding surface. J Biol Chem 280:530–537. doi: 10.1074/jbc.M410113200. [DOI] [PubMed] [Google Scholar]
- 34.Takami H, Nakasone K, Takaki Y, Maeno G, Sasaki R, Masui N, Fuji F, Hirama C, Nakamura Y, Ogasawara N, Kuhara S, Horikoshi K. 2000. Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res 28:4317–4331. doi: 10.1093/nar/28.21.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong T-Y, Cheng C-W, Huang J-W, Meng M. 2002. Isolation and biochemical characterization of an endo-1,3-β-glucanase from Streptomyces sioyaensis containing a C-terminal family 6 carbohydrate-binding module that binds to 1,3-β-glucan. Microbiology 148:1151–1159. doi: 10.1099/00221287-148-4-1151. [DOI] [PubMed] [Google Scholar]
- 36.Aspeborg H, Coutinho PM, Wang Y, Brumer H, Henrissat B. 2012. Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5). BMC Evol Biol 12:186. doi: 10.1186/1471-2148-12-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aires RS, Steindorff AS, Ramada MHS, Siqueira SJL, Ulhoa CJ. 2012. Biochemical characterization of a 27 kDa 1,3-β-d-glucanase from Trichoderma asperellum induced by cell wall of Rhizoctonia solani. Carbohydr Polym 87:1219–1223. doi: 10.1016/j.carbpol.2011.09.001. [DOI] [Google Scholar]
- 38.Shu C-H, Xu C-J, Lin E-S. 2006. Production, purification and partial characterization of a novel endo-β-1,3-glucanase from Agaricus brasiliensis. Process Biochem 41:1229–1233. doi: 10.1016/j.procbio.2005.12.011. [DOI] [Google Scholar]
- 39.Hattori T, Kato Y, Uno S, Usui T. 2013. Mode of action of a β-(1→6)-glucanase from Penicillium multicolor. Carbohydr Res 366:6–16. doi: 10.1016/j.carres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 40.de la Cruz J, Pintor-Toro JA, Benitez T, Llobell A. 1995. Purification and characterization of an endo-β-1,6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J Bacteriol 177:1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bardales RM, Bhavanandan VP. 1989. Transglycosylation and transfer reaction activities of endo-α-N-acetyl-d-galactosaminidase from Diplococcus (Streptococcus) pneumoniae. J Biol Chem 264:19893–19897. [PubMed] [Google Scholar]
- 42.Takahashi M, Yoshioka K, Imai T, Miyoshi Y, Nakano Y, Yoshida K, Yamashita T, Furuta Y, Watanabe T, Sugiyama J, Takeda T. 2013. Degradation and synthesis of β-glucans by a Magnaporthe oryzae endotransglucosylase, a member of the glycoside hydrolase 7 family. J Biol Chem 288:13821–13830. doi: 10.1074/jbc.M112.448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Y, Yan Q, Yang Y, Yang S, Liu Y, Jiang Z. 2015. Expression and characterization of a novel β-glucosidase, with transglycosylation and exo-β-1,3-glucanase activities, from Rhizomucor miehei. Food Chem 175:431–438. doi: 10.1016/j.foodchem.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Malet C, Planas A. 1998. From β-glucanase to β-glucansynthase: glycosyl transfer to α-glycosyl fluorides catalyzed by a mutant endoglucanase lacking its catalytic nucleophile. FEBS Lett 440:208–212. doi: 10.1016/S0014-5793(98)01448-3. [DOI] [PubMed] [Google Scholar]
- 45.Takeda T, Nakano Y, Takahashi M, Konno N, Sakamoto Y, Arashida R, Marukawa Y, Yoshida E, Ishikawa T, Suzuki K. 2015. Identification and enzymatic characterization of an endo-1,3-β-glucanase from Euglena gracilis. Phytochemistry 116:21–27. doi: 10.1016/j.phytochem.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Lafond M, Sulzenbacher G, Freyd T, Henrissat B, Berrin J-G, Garron M-L. 2016. The quaternary structure of a glycoside hydrolase dictates specificity toward β-glucans. J Biol Chem 291:7183–7194. doi: 10.1074/jbc.M115.695999. [DOI] [PMC free article] [PubMed] [Google Scholar]