ABSTRACT
The heavy metal cadmium (Cd) is an environmental pollutant that causes adverse health effects in humans and animals. Our previous work demonstrated that oral administration of probiotics can significantly inhibit Cd absorption in the intestines of mice, but further evidence is needed to gain insights into the related protection mode. The goal of this study was to evaluate whether probiotics can inhibit Cd absorption through routes other than the Cd binding, with a focus on gut barrier protection. In the in vitro assay, both the intervention and therapy treatments of Lactobacillus plantarum CCFM8610 alleviated Cd-induced cytotoxicity in the human intestinal cell line HT-29 and protected the disruption of tight junctions in the cell monolayers. In a mouse model, probiotics with either good Cd-binding or antioxidative ability increased fecal Cd levels and decreased Cd accumulation in the tissue of Cd-exposed mice. Compared with the Cd-only group, cotreatment with probiotics also reversed the disruption of tight junctions, alleviated inflammation, and decreased the intestinal permeability of mice. L. plantarum CCFM8610, a strain with both good Cd binding and antioxidative abilities, exhibited significantly better protection than the other two strains. These results suggest that along with initial intestinal Cd sequestration, probiotics can inhibit Cd absorption by protecting the intestinal barrier, and the protection is related to the alleviation of Cd-induced oxidative stress. A probiotic with both good Cd-binding and antioxidative capacities can be used as a daily supplement for the prevention of oral Cd exposure.
IMPORTANCE The heavy metal cadmium (Cd) is an environmental pollutant that causes adverse health effects in humans and animals. For the general population, food and drinking water are the main sources of Cd exposure due to the biomagnification of Cd within the food chain; therefore, the intestinal tract is the first organ that is susceptible to Cd contamination. Moreover, Cd exposure causes the disruption of the intestinal barrier and further induces the amplification of Cd absorption. The present study confirms that, along with initial intestinal Cd sequestration, oral administration of probiotics can inhibit Cd absorption by protecting the intestinal barrier. A probiotic with both good Cd-binding and antioxidative capacities can be used as a daily supplement for the prevention of oral Cd exposure.
INTRODUCTION
Cadmium (Cd) is one of the most toxic and widely distributed heavy metals in the environment. Cd exposure causes a broad range of adverse health effects in humans, including hepatic, renal, skeletal, reproductive, and cardiovascular dysfunction (1–3). For the general population, food and drinking water are the main sources of Cd exposure due to the biomagnification of Cd within the food chain (4); therefore, the intestinal tract is the first organ that is susceptible to Cd contamination. Early in vitro and animal studies have shown that the intestinal barrier plays a crucial role in limiting Cd absorption (5, 6), and Cd exposure causes an inflammatory response, death of epithelial cells, and damage to tight junctions in the intestines, leading to the disruption of the intestinal barrier and the amplification of Cd absorption (6–10). A recent research study also revealed that subchronic oral Cd exposure significantly affects the gut ecology and susceptibility to colitis in mice (11).
Recently, several studies have revealed that specific Lactobacillus strains have the potential to be developed as probiotics for the alleviation and treatment of heavy metal toxicity (12, 13). Our previous work reported the protective effects of a probiotic against Cd toxicity, demonstrating that Lactobacillus plantarum CCFM8610, a selected probiotic with good Cd-binding and antioxidative capabilities (14), can significantly inhibit Cd absorption in the intestines of mice, which in turn decreases Cd accumulation in tissues and alleviates Cd-induced tissue histopathology (15, 16). The Cd-binding ability of CCFM8610 appears to be important for the inhibition of intestinal Cd absorption, because it enables CCFM8610 to sequester Cd before intestinal absorption and increase Cd excretion as Lactobacillus strains are excreted through feces (17). However, as a considerable number of studies have shown that oral administration of probiotics can prevent intestinal barrier dysfunction by modulating immune responses, inducing tight-junction maturation and inhibiting abnormal necrosis of epithelial cells (18–21), further evidence is needed to establish whether probiotics, such as CCFM8610, can inhibit Cd absorption by protecting the intestinal barrier.
In this work, we sought to evaluate the protective effects of probiotics with different physiological characteristics against Cd absorption in the intestines of mice and to test the hypothesis that in addition to Cd-binding capacity, the protection of the intestinal barrier by probiotics is also vital for the inhibition of Cd absorption.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Three L. plantarum strains (CCFM8610, CCFM11, and CCFM8614) were obtained from the in-house Culture Collections of Food Microbiology (CCFM), Jiangnan University (Wuxi, China). The strains were cultured in de Man, Rogosa, and Sharpe (MRS) broth (Hope Bio-technology Company, Qingdao, China) at 37°C for 18 h. All of the bacteria were subcultured twice before the experiment. For application of the strains in the animal experiments, the cells were cultured and lyophilized with skim milk as a protectant (15).
Cell culture and treatment.
HT-29 cells (a human colon adenocarcinoma cell line) were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI 1640 medium (HyClone, USA) supplemented with 10% (vol/vol) fetal bovine serum (Gibco, USA) and 100 U/ml penicillin-streptomycin (Gibco) at 37°C with 5% CO2.
The HT-29 cells were seeded into 96-well plates, 6-well plates, Millicell CM culture plate inserts, and 2-cm-diameter cover glass-bottom dishes (1 × 105 cells/well), respectively, for the assays shown in Table 1. The cells were treated as shown in Table 1, with a bacterium-to-cell ratio of 1,000. For the therapy assay, the cells were washed three times with a phosphate-buffered saline solution (pH 7.2) after 24 h to remove residual Cd or antibiotics. The dose of Cd exposure was 50 μM CdCl2, selected on the basis of data from our preliminary experiment (see Fig. S1 in the supplemental material) and from the literature (22, 23) to correspond to a moderate toxic effect of Cd on intestinal epithelial cells.
TABLE 1.
Cell experimental protocol
| Group | Treatment on the indicated period bya: |
||
|---|---|---|---|
| Intervention assay (0–24 h) | Therapy assay |
||
| 0–24 h | 24–48 h | ||
| Control | Medium | Medium | Medium |
| Cd only | Medium + Cd | Medium + Cd | Medium |
| 8610 only | Medium + CCFM8610 | Medium | Medium + CCFM8610 |
| Cd + 8610 | Medium + Cd + CCFM8610 | Medium + Cd | Medium + CCFM8610 |
Medium, RPMI 1640 medium supplemented with fetal bovine serum and penicillin-streptomycin; medium + Cd, 50 μM CdCl2 in medium; medium + CCFM8610, 1 × 108 CFU/well L. plantarum CCFM8610 in medium (without antibiotics); medium + Cd + CCFM8610, 50 μM CdCl2 and 1 × 108 CFU/well L. plantarum CCFM8610 in medium (without antibiotics). For the therapy assay, the cells were washed three times with phosphate-buffered saline after 24 h.
Cytotoxicity measurements.
A 3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyl-tetrazolium bromide (MTT) assay was performed as previously described to determine cell viability (24), using an assay kit purchased from the Institute of Beyotime Biotechnology (Jiangsu, China).
Apoptotic and necrotic cells were quantified using an annexin V-fluorescein isothiocyanate (FITC)-propidium iodide (PI) kit (Institute of Beyotime Biotechnology) and detected using a flow cytometric assay (FACSCalibur; Becton Dickinson) (25).
Measurement of intracellular reactive oxygen species level and lipid peroxidation.
The accumulation of intracellular reactive oxygen species (ROS) in the HT-29 cells was determined using an assay kit from the Institute of Beyotime Biotechnology (Jiangsu, China), and the analysis was based on the incorporation of dichlorodihydrofluorescein diacetate (DCFH-DA) into the cells (26). The ROS level was detected by measuring the fluorescence intensity using a fluorescence plate reader (F-7000; Hitachi) and a laser confocal scanning microscope (LSM710; Zeiss).
Malondialdehyde (MDA), a terminal product and indicator of the lipid peroxidation process, was measured to estimate the level of lipid peroxidation in the HT-29 cells (27). The MDA level in cell homogenates was determined using an assay kit from the Jiancheng Bioengineering Institute (Jiangsu, China).
Measurement of cytokine profile of cell cultures.
The levels of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), IL-6, IL-8, and IL-10 were determined from the cell supernatant using an enzyme-linked immunosorbent assay (ELISA) (Abcam, United Kingdom), according to the manufacturer's instructions.
Evaluation of tight-junction morphology in cell monolayers.
The morphology of the tight junction was evaluated by immunofluorescence staining (28, 29). Briefly, cell monolayers were fixed, permeabilized, blocked, and incubated with the primary antibodies anti-claudin-1 and anti-ZO-1 (1:50 dilution; Life Technologies, Rockford, IL, USA) overnight at 4°C. The samples were then incubated with a secondary antibody (Alexa Fluor 488-conjugated goat anti-rabbit antibody, 1:50 dilution; Life Technologies) for 1 h at room temperature. Nuclear staining was finally performed using 4′,6-diamidino-2-phenylindole (DAPI) (Institute of Beyotime Biotechnology, Jiangsu, China) for 10 min at room temperature. Images of the stained cells were acquired with confocal laser scanning microscopy (TCS SP8; Leica).
Determination of Cd-binding ability and antioxidative activity of Lactobacillus strains.
The Cd-binding ability and antioxidative activity of Lactobacillus strains were analyzed as described in our previous study (14). Three L. plantarum strains with different Cd-binding and antioxidative characteristics were selected for the animal study.
Animals and experimental design.
Adult male C57BL/6 mice obtained from the Shanghai Laboratory Animal Center (Shanghai, China) were used in all of the experiments. The mice were kept in stainless steel cages in a temperature- and humidity-controlled room that was equipped to maintain a 12-h light-dark cycle. The mice were fed standard commercial mouse food, and water was provided ad libitum. All of the protocols for this study were approved by the Ethics Committee of Jiangnan University, China (JN no. 20141120-0124[48]). The procedures of this study were carried out in accordance with the European Community guidelines (30) for the care and use of experimental animals.
As shown in Table 2, the mice were randomly divided into five groups (control, Cd only, Cd plus L. plantarum CCFM8610, Cd plus L. plantarum CCFM11, and Cd plus L. plantarum CCFM8614), with 10 animals in each group. The L. plantarum strains were provided at a dose of 1 × 109 CFU in 0.5 ml of skim milk that was administered once daily via gavage. Mice in the control group received the same volume of skim milk (without lactobacilli) once daily via gavage. An oral dose of CdCl2 at 100 mg/liter of drinking water was used for modeling of an environmentally relevant low-concentration Cd exposure (31–33). During the 8-week treatment period, each mouse was moved into a clean and empty cage each week for 1 h, and fecal samples were collected. At the end of the 8th week, all of the mice were sacrificed under light ether anesthesia.
TABLE 2.
Animal experimental protocol
| Group (no. of mice) | Treatmenta |
|---|---|
| Control (10) | SM + PW |
| Cd only (10) | SM + Cd |
| Cd + 8610 (10) | SM + CCFM8610 + Cd |
| Cd + 11 (10) | SM + CCFM11 + Cd |
| Cd + 8614 (10) | SM + CCFM8614 + Cd |
SM, 0.5 ml of skim milk once daily via gavage; PW, plain water for drinking; Cd, CdCl2 at 100 mg/liter in drinking water; SM + CCFM8610 (or CCFM8611 or CCFM8614), 1 × 109 CFU L. plantarum CCFM8610 (or CCFM8611 or CCFM8614) in 0.5 ml of skim milk once daily via gavage. The treatments lasted 8 weeks.
Determination of Cd levels in tissues and feces.
Liver, kidney, and fecal samples were transferred to metal-free digestion vessels (Omni; CEM) and digested in concentrated HNO3 with a microwave digestion system (MARS; CEM). Cd concentrations in the samples were determined with a flame or graphite furnace atomic absorption spectrophotometer (Spectr AAS or AA; Varian).
Determination of tight-junction mRNA expression in intestinal tissues.
The small bowel (jejunum section) and colon segments (approximately 0.1 g) were isolated and immediately stored in liquid nitrogen. Total RNA was extracted with TRIzol reagent (Ambion, USA), as previously described (34), and RNA purity and integrity were determined by NanoDrop and gel electrophoresis. RNA was converted to cDNA using a reverse transcription kit (TaKaRa, Japan). Gene expression was determined with the respective primers (Table 3) using iTaq Universal SYBR green Supermix (Bio-Rad, USA) on a real-time quantitative PCR system (CFX Connect; Bio-Rad). Relative quantification of the target gene was analyzed using a comparison with the β-actin gene and calculated according to the 2−ΔΔCT method (35).
TABLE 3.
Primer sequences used for real-time quantitative PCR
| Primera | Sequence (5′–3′) |
|---|---|
| β-actin-F | CCTAAGAGGAGGATGGTCGC |
| β-actin-R | CTCAACACCTCAACCCCCTC |
| ZO-1-F | TCTTCCATCATTTCGCTGTGT |
| ZO-1-R | TCTGAAACCATCAAGTCCACA |
| ZO-2-F | TCTCCGGACAATAGGACAACC |
| ZO-2-R | GTGGTTCCAGACAACGGACA |
| Occludin-F | TCACTTTTCCTGCGGTGACT |
| Occludin-R | GGGAACGTGGCCGATATAATG |
| Claudin-1-F | ATGCAAAGATGTTTTGCCACAG |
| Claudin-1-R | TACAAATTCCCATTGCAGCCC |
F, forward primer; R, reverse primer.
Determination of cytokines and secretory immunoglobulin in intestinal tissues.
The remaining segments of the small bowel (jejunum and ileum sections) and colon tissues were homogenized in 1 ml of radioimmunoprecipitation assay (RIPA) buffer (Beyotime Biotechnology, Jiangsu, China), and the lysate was centrifuged at 15,000 × g and 4°C for 15 min (36, 37). The levels of TNF-α, IL-1β, IL-6, IL-8, IL-10, and secretory immunoglobulin (sIgA) in the supernatant were measured using ELISA, according to the manufacturer's instructions (Abcam, United Kingdom).
Determination of intestinal permeability.
The intestinal permeability of the mice was measured using 4,000-Da fluorescent dextran-FITC (DX-4000-FITC) and endotoxin assays, as previously described (28, 38). For the DX-4000-FITC assay, the mice were fasted for 6 h at 3 days before the end of the experiment and then given DX-4000-FITC (Sigma-Aldrich, USA) at a dose of 500 mg/kg of body weight. After 1 h, 100 μl of blood from the tip of the tail vein was collected, and the plasma was analyzed for DX-4000-FITC concentration with a fluorescence spectrophotometer (SpectraMax; Molecular Devices). At the end of the experiment, the endotoxin levels in the serum were measured with ELISA, according to the manufacturer's instructions (Abcam, United Kingdom).
Statistical analysis.
The data were expressed as the mean ± standard error of the mean (SEM) values. A minimum of three independent experiments were carried out for each in vitro assay. Differences between the means of the test were analyzed using one-way analysis of variance, followed by Tukey's post hoc test. A P value of <0.05 was considered to be statistically significant. Statistical analyses of the obtained data were performed using the SPSS 16.0 software for Windows (SPSS Inc., Chicago, IL).
RESULTS
Effects of L. plantarum CCFM8610 on Cd-induced cytotoxicity.
Cd exposure significantly decreased the viability of HT-29 cells (Fig. 1A and B). Both intervention (simultaneously treated with Cd exposure) and therapy (treated after Cd exposure) with L. plantarum CCFM8610 alleviated Cd-induced cytotoxicity (P < 0.05). It was also noted that the treatment of the strain itself (CCFM8610-only group) did not damage cell viability. Flow cytometry (Fig. 1C to E) showed that apoptotic cells drastically increased from approximately 6% in the control group to 36.07% in the intervention assay and 36.66% in the therapy assay after Cd exposure. Compared to the cells treated with Cd alone, treatment of L. plantarum CCFM8610 exhibited significant protection against Cd-induced cell apoptosis (P < 0.05).
FIG 1.
Effects of L. plantarum CCFM8610 on Cd-induced cytotoxicity in HT-29 cells. (A and B) Cell viability in intervention (A) and therapy (B) assays, measured with the MTT method. (C to E) Cell apoptosis measured using flow cytometry with annexin V-FITC-PI staining. (C) Representative histogram of flow cytometric analysis. Q4, normal cells; Q3, early apoptotic cells; Q2, late apoptotic cells; Q1, necrotic cells. I, intervention assay; T, therapy assay. (D and E) Percentage of apoptotic cells in intervention (D) and therapy (E) assays. The results are expressed as the percentage of apoptotic cells that include the cells in early (Q3 in panel C) and late apoptosis (Q2 in panel C). The data are the mean ± standard error of the mean (SEM) values of the results from six replicates. (A, B, D, and E) Significant differences (P < 0.05) between the groups are indicated with different letters above the bars.
Effects of L. plantarum CCFM8610 on Cd-induced oxidative stress in HT-29 cells.
Confocal microscopy observation confirmed that Cd exposure significantly enhanced ROS levels in cells (Fig. 2A). Compared with the control, the dichlorofluorescein (DCF) fluorescence increased by 270.53% in the intervention assay and 358.58% in the therapy assay after Cd exposure (Fig. 2B and C). Moreover, an abnormal level of lipid peroxidation was observed in cells treated with Cd alone (Fig. 2D). It was noted that both intervention and therapy treatment of L. plantarum CCFM8610 significantly inhibited Cd-induced ROS generation and decreased MDA levels in HT-29 cells (P < 0.05).
FIG 2.
Effects of L. plantarum CCFM8610 on Cd-induced oxidative stress in HT-29 cells. (A) Confocal microscopy observations of the intracellular ROS levels. The ROS was stained using DCFH-DA (green), and the red arrows show the accumulation of ROS. I, intervention assay; T, therapy assay. (B and C) Intracellular ROS levels in intervention (B) and therapy (C) assays detected with a fluorescence plate reader. (D) Intracellular MDA levels in intervention and therapy assays. The data are the mean ± SEM values of the results from three replicates for ROS detection and five replicates for MDA detection. Significant differences (P < 0.05) between groups are indicated with different letters above the bars.
Effects of L. plantarum CCFM8610 on Cd-induced alterations of cytokine production in HT-29 cells.
As shown in Fig. 3, Cd exposure significantly increased the levels of chemokine IL-8 and proinflammatory cytokines, including TNF-α, IL-1β, and IL-6 (P < 0.05). Both intervention and therapy treatments of L. plantarum CCFM8610 recovered the levels of IL-8 and IL-6, and the intervention treatment also decreased the levels of TNF-α and IL-1β. Neither Cd nor probiotic treatment caused significant differences in the level of IL-10, a cytokine with anti-inflammatory effects (39), compared with the control groups (see Fig. S2 in the supplemental material).
FIG 3.
Effects of L. plantarum CCFM8610 on Cd-induced alterations of cytokine production in HT-29 cells. The data are the mean ± SEM values of the results from six replicates. Significant differences (P < 0.05) between groups are indicated with different letters above the bars.
Effects of L. plantarum CCFM8610 on Cd-induced disruption of tight junctions in HT-29 cell monolayers.
Tight-junction proteins, including ZO-1 and claudin-1, in the therapy were observed with immunofluorescence staining (Fig. 4A and B). For the untreated cells, the tight-junction proteins were present as an uninterrupted belt along the cell borders. In the Cd-exposed group, the fluorescence associated with the tight-junction proteins was more dispersed, with discontinuous and irregular distribution at sites of cell-to-cell contact. Cotreatment with Cd and L. plantarum CCFM8610 significantly reversed the Cd-induced disruption of tight junctions. Similar protective effects of the strain on tight junctions were also observed in the intervention assay (data not shown).
FIG 4.
Effects of L. plantarum CCFM8610 on Cd-induced disruption of tight junctions ZO-1 (A) and claudin-1 (B) in HT-29 cell monolayers in the therapy assay. Tight-junction proteins were stained by Alexa Fluor 488 (green) and imaged by confocal microscopy. The red arrows show the discontinuous and irregular distribution of tight-junction proteins at sites of cell-to-cell contact.
Selection of L. plantarum strains for animal experiments.
To test the hypothesis that, in addition to the intestinal Cd sequestration ability of probiotics due to their Cd-binding capacity, probiotics can also inhibit Cd absorption by protecting the intestinal barrier, L. plantarum strains with different Cd-binding capacities were selected for animal experiments. Moreover, the antioxidative activity of the strains was considered to investigate whether the protection against Cd-induced oxidative stress in the intestines is a mechanism for intestinal barrier protection. Therefore, based on our previous in vitro study (14), L. plantarum CCFM8610 (with good Cd-binding and antioxidative abilities), L. plantarum CCFM11 (with good Cd-binding ability but relatively poor antioxidative activity), and L. plantarum CCFM8614 (with relatively poor Cd-binding ability but good antioxidative activity) were selected for the animal experiments. The data for the Cd-binding and antioxidative abilities of three L. plantarum strains are presented in Fig. S3 and Tables S1 and S2 in the supplemental material.
Effects of L. plantarum strains on Cd levels in the feces, liver, and kidneys of mice.
Alterations in the Cd levels in feces during the 8-week treatment period are represented in Fig. 5A. The fecal Cd concentrations in the control groups were much lower than those of the other groups (<0.15 μg/g of wet feces over the 8 weeks), so these data were not included in Fig. 5A. Compared with the Cd-only group, oral administration of three L. plantarum strains markedly increased fecal Cd levels at each time point (P < 0.05), with the exception of L. plantarum CCFM8614 treatment during the first week. L. plantarum CCFM8610 treatment exhibited a more significant effect on the promotion of fecal Cd levels than that with the other strains. During the first 5 weeks, fecal Cd levels in the Cd-plus-CCFM11 group were higher than those in the Cd-plus-CCFM8614 group (with the exception of the 2nd week). From the 6th week to the end of the experiment, the Cd levels remained stable in the Cd-plus-CCFM11 group but continued to rise in the Cd-plus-CCFM8614 group, and the levels in the Cd-plus-CCFM8614 group surpassed those in the Cd-plus-CCFM11 group during the 8th week.
FIG 5.
Effects of L. plantarum strains on Cd levels in the feces, liver, and kidneys of mice. (A) Cd levels in feces of mice during the 8-week treatment. The data are the mean ± SEM values of the results from 10 mice per group. Groups with different letters at each time point differ significantly (P < 0.05). (B) Cd levels in the livers and kidneys of mice. For each tissue, groups with different letters differ significantly (P < 0.05).
The Cd levels in the liver and kidneys of Cd-treated mice are shown in Fig. 5B. The Cd concentrations in tissues of the control group were very low (<0.0001 μg/g of tissue in the liver and 0.05 μg/g of tissue in the kidney), so these data are not included in Fig. 5B. Compared with the control group, oral exposure of Cd significantly increased tissue Cd levels (P < 0.05). Cotreatment with Cd and L. plantarum strains significantly decreased the hepatic and renal Cd levels, and the administration of L. plantarum CCFM8610 resulted in more-significant protection effects than those with the other strains (P < 0.05).
Effects of L. plantarum strains on mRNA expression levels of tight-junction proteins in intestines of Cd-exposed mice.
Cd exposure caused marked reductions in mRNA expression of ZO-1, ZO-2, occludin, and claudin-1 proteins in the small bowel (jejunum section) and colon of mice (Fig. 6A and B). Compared with the Cd-only group, all of these reductions were reversed by oral administration of L. plantarum CCFM8610, whereas treatment with CCFM11 only increased the expression of claudin-1 in the jejunum, and treatment with CCFM8614 increased the expression of ZO-1, occludin, and claudin-1 proteins in the jejunum and occludin and claudin-1 proteins in the colon (P < 0.05). Therefore, compared with the other two L. plantarum strains, CCFM8610 exhibited greater protection against Cd-induced disruption of tight junctions in the intestines.
FIG 6.
Effects of L. plantarum strains on mRNA expression levels of tight-junction proteins in the jejunum (A) and colon (B) of Cd-exposed mice. The data are expressed as the fold change versus control group (set to 1) and are the mean ± SEM values of the results from of five mice per group. Significant differences (P < 0.05) between groups are indicated with different letters above the bars.
Effects of L. plantarum strains on immune response in the intestines of Cd-exposed mice.
Cd exposure significantly decreased the level of sIgA in the small bowel (jejunum and ileum sections) and colon (Fig. 7A). Compared with the Cd-only group, oral administration of three L. plantarum strains markedly increased sIgA levels in the intestines of mice (P < 0.05).
FIG 7.
Effects of L. plantarum strains on immune response in the intestines of Cd-exposed mice. (A to F) Levels of sIgA (A), TNF-α (B), IL-1β (C), IL-6 (D), IL-8 (E), and IL-10 (F) in the small bowel (jejunum and ileum sections) and colon of mice. The data are the mean ± SEM values of the results from six mice per group for the sIgA assay and five mice per group for the cytokine assays. For each tissue, groups with different letters differ significantly (P < 0.05).
The levels of TNF-α, IL-1β, IL-6, and IL-8 in the intestines significantly increased after Cd exposure, with the exception of the IL-6 level in the colon (Fig. 7B to F). Secretion of the anti-inflammatory cytokine IL-10 was inhibited in the colon of Cd-exposed mice. Compared with the Cd-only group, oral administration of L. plantarum CCFM8610 mitigated all of the Cd-induced alterations of the production of cytokines and chemokines in the intestines of mice, whereas treatment with the other two strains only partly reversed those alterations.
Effects of L. plantarum strains on gut permeability of Cd-exposed mice.
As shown in Fig. 8A and B, the intensity of DX-4000-FITC fluorescence and the level of endotoxin were significantly increased in the serum of Cd-exposed mice (P < 0.05), indicating that Cd exposure was associated with an increase in gut permeability. Oral administration of three L. plantarum strains markedly decreased gut permeability, and CCFM8610 provided the best protection. Strain CCFM8614 showed a stronger resistance to the Cd-induced increase of gut permeability than CCFM11 (P < 0.05).
FIG 8.
Effects of L. plantarum strains on gut permeability of Cd-exposed mice. (A) DX-4000-FITC levels in the serum of mice. (B) Endotoxin levels in the serum of mice. The data are the mean ± SEM values of the results from five mice per group. Significant differences (P < 0.05) between groups are indicated with different letters above the bars. EU, endotoxin units.
DISCUSSION
Our previous study confirmed the ability of a probiotic, L. plantarum CCFM8610, to inhibit Cd absorption in the intestines of mice, and the protective mechanism is partly due to the intestinal sequestration by the Cd-binding ability of the strain (15, 16). This study was performed to evaluate whether probiotics can inhibit Cd absorption through other routes besides Cd binding, with a focus on protection of the gut barrier.
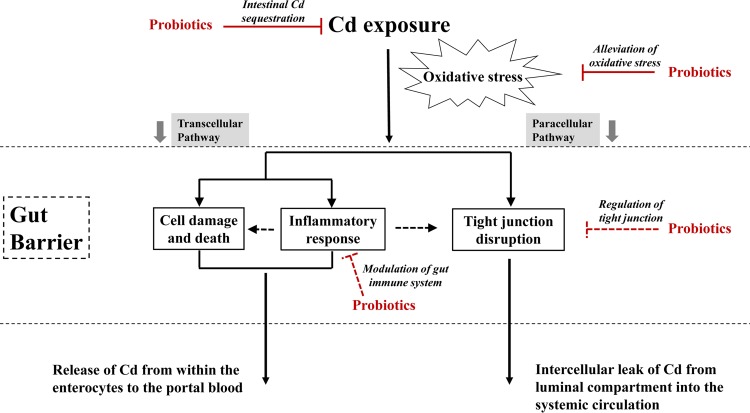
The toxic effects of Cd on vital organs, such as the liver, kidneys, bones, and reproductive system, are well documented, and oxidative stress is an important mechanism of the toxicity (31, 40). Recently, the adverse effects of Cd exposure on the intestinal tract have attracted increasing attention, because this organ is the first target of Cd exposure for the general population. This study provides biological evidence that Cd exposure causes significant damage to the gut barrier, including the toxicity of enterocytes, induction of inflammatory response, disruption of tight junctions, and increased intestinal permeability. It was previously reported that similar to calcium absorption, Cd can be absorbed by both transcellular and paracellular routes in the intestines, and an intact gut barrier is crucial to limiting absorption (5, 6, 41, 42). As shown in Fig. 9, absorption could be amplified after chronic Cd exposure, because Cd-induced cell death may cause a leak in the epithelial layer, resulting in a larger amount of Cd permeating from within the enterocytes to the portal blood (8, 41). Moreover, Cd-induced disruption of tight junctions may lead to an intercellular leak, providing an abnormal path for Cd to pass through the intestinal barrier (6, 7, 41). On the basis of these analyses, protection of the gut barrier against Cd toxicity is important for the inhibition of intestinal Cd absorption.
FIG 9.
Proposed mechanism for Cd-induced gut barrier disruption and potential pathways of protection by probiotics against intestinal Cd absorption.
Using the HT-29 intestinal epithelial cell model, we observed obvious protection against cell damage when L. plantarum CCFM8610 was introduced simultaneously with Cd exposure (intervention assay). This was partly due to the intestinal Cd sequestration by CCFM8610, which attenuated the Cd exposure by Cd binding. To evaluate the possibility of other protective routes, we bypassed the Cd-binding ability of the strain by setting up a therapy assay (CCFM8610 treated after Cd exposure), thus avoiding direct contact of the metal with the strain. Interestingly, cotreatment with Cd and CCFM8610 in the therapy assay significantly alleviated Cd-induced cytotoxicity, inhibited the secretion of inflammation-related chemokines and cytokines, and reversed the disruption of tight junctions. These results indicate that CCFM8610 is able to protect the barrier function when it is unable to sequester Cd. The CCFM8610 treatment also significantly inhibited Cd-induced ROS generation and decreased MDA levels in the therapy assay, indicating direct protection against Cd-induced oxidative stress by the strain.
It has been reported that oxidative stress induced by xenobiotics, including Cd, can cause necrosis or apoptosis of the enterocytes, trigger an inflammatory response, and disrupt the tight junctions in the intestines (22, 43–45), and these adverse effects could be reversed by antioxidants (46–48). On the other hand, other studies have demonstrated that probiotics can protect against intestinal barrier disruption and decrease intestinal permeability by alleviating oxidative stress in animals (49, 50). It has been confirmed that L. plantarum CCFM8610 exhibits good antioxidative ability in vitro and in vivo (14, 15). Such an ability may protect it from the chain effects triggered by Cd-induced oxidative stress in the intestines.
To further confirm that, along with the initial intestinal Cd sequestration, probiotics can inhibit Cd absorption by protecting the intestinal barrier, three L. plantarum strains with different physiological characteristics were individually introduced to Cd-exposed mice via oral administration. CCFM8614, which has a relatively poor Cd-binding ability compared with CCFM8610 and CCFM11, was found to significantly increase fecal Cd levels and decrease Cd accumulation in the tissues of Cd-exposed mice. Fecal Cd excretion in the Cd-plus-CCFM8614 group was higher than that in the Cd-plus-CCFM11 group at the end of the animal experiment. Compared with the Cd-only group, cotreatment with Cd and CCFM8614 also increased the mRNA expression of tight-junction proteins, alleviated inflammation, and decreased the intestinal permeability of mice. These results indicate that probiotics can inhibit Cd absorption by protecting the intestinal barrier, and such protection is not simply an indirect effect due to the initial intestinal Cd sequestration by the Cd-binding ability of the strains. According to the analyses of our cell assay and considering the good antioxidative activity of CCFM8614, this strain may alleviate Cd-induced oxidative stress in the intestines, thus protecting the barrier function, decreasing Cd permeation, and inhibiting Cd absorption. We also found that although both CCFM8610 and CCFM11 have a good Cd-binding capacity, CCFM8610 exhibits significantly better protective effects by the inhibition of Cd absorption than CCFM11. This can also be explained by the superior antioxidative activity of CCFM8610, which can further protect gut barrier function against Cd-induced oxidative stress, whereas CCFM11 simply sequesters Cd through binding and decreases the opportunity for exposure in the intestines. A further study to test the underlying role of antioxidative activity of strains against Cd-induced dysfunction of the gut barrier is now in progress, in which the intestinal Cd sequestration of strains is bypassed via genetic modification.
In addition to the protective effect due to the initial intestinal Cd sequestration and the oxidative stress alleviation by probiotics, direct effects on the modulation of the gut immune system (19, 51) and regulation of the tight junction (52, 53) cannot be ruled out (Fig. 9). The tolerance of the strains to Cd and gastrointestinal tract conditions should also be taken into consideration to ensure the viability of bacteria after oral administration.
The absorption of Cd mainly occurs in the small intestine, while only a small number of lactobacilli stay in this gut section. The small intestine is an important immune organ containing a relatively small population size of the endogenous microbiota, allowing transient dominance of foodborne microorganisms, including probiotics (19). L. plantarum strains have been proven to be one of the predominant transient lactobacilli in the jejunum and ileum (54). Besides their Cd sequestration and antioxidative stress activities in the small intestine, L. plantarum strains may interact with dendritic cells in the small intestine lamina propria to influence T cell responses and influence STAT1 and NF-κB p65 translocation in the epithelial cells and macrophages of small intestine (55, 56). Additionally, a previous human study showed that L. plantarum strains can enhance barrier function by rearrangement of the tight-junction protein conformation in response to Toll-like receptor 2 (TLR2) signaling in the small intestine (52). It is possible that the strains used in the present study also modulate the immune system and protect tight junctions in the small intestine through these routes.
In conclusion, this study has demonstrated that treatment with probiotics can relieve Cd-induced cytotoxicity, alleviate oxidative stress and the inflammatory response, reverse tight-junction disruption, and decrease the gut permeability in both intestinal epithelial cells and animals. Such protection of the gut barrier resulted in an increase in fecal Cd levels and a decrease in Cd accumulation in mouse tissues, indicating the inhibition of intestinal Cd absorption by probiotics. We also confirmed that along with the initial intestinal Cd sequestration, the antioxidative ability of L. plantarum strains is important for protection against Cd-induced disruption of the gut barrier. Lactobacillus strains with both good Cd-binding and antioxidative capacities, such as L. plantarum CCFM8610, can be considered for testing as a daily supplement for the prevention of oral Cd exposure.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ruipeng Yu for providing advice on AAS analysis.
This work was supported by the National Natural Science Foundation of China Key Program (grant 31530056), the National Natural Science Foundation of China (grants 31470161 and 31371721), the BBSRC Newton Fund Joint Centre Award, the 111 Project B07019, the Program for ChangJiang Scholars, and the Self-Determined Research Program of Jiangnan University (grant JUSRP 115A23).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00695-16.
REFERENCES
- 1.Hong F, Jin T, Zhang A. 2004. Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population. Biometals 17:573–580. doi: 10.1023/B:BIOM.0000045741.22924.d8. [DOI] [PubMed] [Google Scholar]
- 2.Thompson J, Bannigan J. 2008. Cadmium: toxic effects on the reproductive system and the embryo. Reprod Toxicol 25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. 2008. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Environ Health Perspect 116:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordberg GF, Nogawa K, Nordberg M, Friberg L. 2011. Cadmium, p 446–451. In Nordberg GF, Fowler BA, Nordberg M, Friberg L (ed), Handbook on the toxicology of metals, 3rd ed Academic Press, Burlington, MA. [Google Scholar]
- 5.Jumarie C, Campbell P, Houde M, Denizeau F. 1999. Evidence for an intracellular barrier to cadmium transport through Caco-2 cell monolayers. J Cell Physiol 180:285–297. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Blais A, Lecoeur S, Milhaud G, Tomé D, Kolf-Clauw M. 1999. Cadmium uptake and transepithelial transport in control and long-term exposed Caco-2 cells: the role of metallothionein. Toxicol Appl Pharmacol 160:76–85. doi: 10.1006/taap.1999.8735. [DOI] [PubMed] [Google Scholar]
- 7.Prozialeck WC. 2000. Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol 164:231–249. doi: 10.1006/taap.2000.8905. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z, Hyun JS, Satsu H, Kakuta S, Shimizu M. 2006. Oral exposure to cadmium chloride triggers an acute inflammatory response in the intestines of mice, initiated by the over-expression of tissue macrophage inflammatory protein-2 mRNA. Toxicol Lett 164:144–154. doi: 10.1016/j.toxlet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Ninkov M, Popov Aleksandrov A, Demenesku J, Mirkov I, Mileusnic D, Petrovic A, Grigorov I, Zolotarevski L, Tolinacki M, Kataranovski D, Brceski I, Kataranovski M. 2015. Toxicity of oral cadmium intake: impact on gut immunity. Toxicol Lett 237:89–99. doi: 10.1016/j.toxlet.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Breton J, Le Clere K, Daniel C, Sauty M, Nakab L, Chassat T, Dewulf J, Penet S, Carnoy C, Thomas P, Pot B, Nesslany F, Foligne B. 2013. Chronic ingestion of cadmium and lead alters the bioavailability of essential and heavy metals, gene expression pathways and genotoxicity in mouse intestine. Arch Toxicol 87:1787–1795. doi: 10.1007/s00204-013-1032-6. [DOI] [PubMed] [Google Scholar]
- 11.Breton J, Daniel C, Vignal C, Body-Malapel M, Garat A, Plé C, Foligné B. 2016. Does oral exposure to cadmium and lead mediate susceptibility to colitis? The dark-and-bright sides of heavy metals in gut ecology. Sci Rep 6:19200. doi: 10.1038/srep19200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, Reid G. 2014. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 5:e01580-14. doi: 10.1128/mBio.01580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monachese M, Burton JP, Reid G. 2012. Bioremediation and tolerance of humans to heavy metals through microbial processes: a potential role for probiotics? Appl Environ Microbiol 78:6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai Q, Yin R, Yu L, Wang G, Tian F, Yu R, Zhao J, Liu X, Chen YQ, Zhang H, Chen W. 2015. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control 54:23–30. doi: 10.1016/j.foodcont.2015.01.037. [DOI] [Google Scholar]
- 15.Zhai Q, Wang G, Zhao J, Liu X, Narbad A, Chen Y, Zhang H, Tian F, Chen W. 2014. Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl Environ Microbiol 80:4063–4071. doi: 10.1128/AEM.00762-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhai Q, Wang G, Zhao J, Liu X, Tian F, Zhang H, Chen W. 2013. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl Environ Microbiol 79:1508–1515. doi: 10.1128/AEM.03417-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul D, Hoskins LC. 1972. Effect of oral lactobacillus feedings on fecal Lactobacillus counts. Am J Clin Nutr 25:763–765. [DOI] [PubMed] [Google Scholar]
- 18.Lutgendorff F, Nijmeijer RM, Sandström PA, Trulsson LM, Magnusson K-E, Timmerman HM, van Minnen LP, Rijkers GT, Gooszen HG, Akkermans LMA, Soderholm JD. 2009. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLoS One 4:e4512. doi: 10.1371/journal.pone.0004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bron PA, van Baarlen P, Kleerebezem M. 2012. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10:66–78. [DOI] [PubMed] [Google Scholar]
- 20.Zakostelska Z, Kverka M, Klimesova K, Rossmann P, Mrazek J, Kopecny J, Hornova M, Srutkova D, Hudcovic T, Ridl J., Tlaskalova-Hogenova H. 2011. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS One 6:e27961. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. 2012. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol 180:626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyun JS, Satsu H, Shimizu M. 2007. Cadmium induces Interleukin-8 production via NF-κB activation in the human intestinal epithelial cell, Caco-2. Cytokine 37:26–34. doi: 10.1016/j.cyto.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Rossi A, Poverini R, Di Lullo G, Modesti A, Modica A, Scarino M. 1996. Heavy metal toxicity following apical and basolateral exposure in the human intestinal cell line Caco-2. Toxicol In Vitro 10:27–36. doi: 10.1016/0887-2333(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 24.Guo C, He Z, Wen L, Zhu L, Lu Y, Deng S, Yang Y, Wei Q, Yuan H. 2012. Cytoprotective effect of trolox against oxidative damage and apoptosis in the NRK-52e cells induced by melamine. Cell Biol Int 36:183–188. doi: 10.1042/CBI20110036. [DOI] [PubMed] [Google Scholar]
- 25.Nie F, Zhang X, Qi Q, Yang L, Yang Y, Liu W, Lu N, Wu Z, You Q, Guo Q. 2009. Reactive oxygen species accumulation contributes to gambogic acid-induced apoptosis in human hepatoma SMMC-7721 cells. Toxicology 260:60–67. doi: 10.1016/j.tox.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Halliwell B, Whiteman M. 2004. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L. 2010. l-Carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept 161:58–66. doi: 10.1016/j.regpep.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, Chiarella SE, Radigan KA, Gonzalez A, Jakate S, Keshavarzian A, Budinger GR, Mutlu GM. 2011. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol 8:19. doi: 10.1186/1743-8977-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. 2007. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 30.European Parliament, Council of the European Union. 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Union 276:337–339. [Google Scholar]
- 31.Thijssen S, Cuypers A, Maringwa J, Smeets K, Horemans N, Lambrichts I, Van Kerkhove E. 2007. Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology 236:29–41. doi: 10.1016/j.tox.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Satarug S, Moore MR. 2004. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect 112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damek-Poprawa M, Sawicka-Kapusta K. 2003. Damage to the liver, kidney, and testis with reference to burden of heavy metals in yellow-necked mice from areas around steelworks and zinc smelters in Poland. Toxicology 186:1–10. doi: 10.1016/S0300-483X(02)00595-4. [DOI] [PubMed] [Google Scholar]
- 34.Simms D, Cizdziel PE, Chomczynski P. 1993. TRIzol: a new reagent for optimal single-step isolation of RNA. Focus 15:532–535. [Google Scholar]
- 35.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 36.Lee H-S, Han S-Y, Bae E-A, Huh C-S, Ahn Y-T, Lee J-H, Kim D-H. 2008. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int Immunopharmacol 8:574–580. doi: 10.1016/j.intimp.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Schiavi E, Barletta B, Butteroni C, Corinti S, Boirivant M, Di Felice G. 2011. Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy 66:499–508. doi: 10.1111/j.1398-9995.2010.02501.x. [DOI] [PubMed] [Google Scholar]
- 38.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee T-S, Chau L-Y. 2002. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Qu W, Kadiiska MB. 2009. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalups RK, Ahmad S. 2003. Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol 186:163–188. doi: 10.1016/S0041-008X(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 42.Vesey DA. 2010. Transport pathways for cadmium in the intestine and kidney proximal tubule: focus on the interaction with essential metals. Toxicol Lett 198:13–19. doi: 10.1016/j.toxlet.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. 2003. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 44.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. 2003. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem 278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 45.Banan A, Choudhary S, Zhang Y, Fields J, Keshavarzian A. 1999. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther 291:1075–1085. [PubMed] [Google Scholar]
- 46.Meyer TN, Schwesinger C, Ye J, Denker BM, Nigam SK. 2001. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem 276:22048–22055. doi: 10.1074/jbc.M011477200. [DOI] [PubMed] [Google Scholar]
- 47.Martín AR, Villegas I, La Casa C, de la Lastra CA. 2004. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol 67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Srigiridhar K, Nair KM, Subramanian R, Singotamu L. 2001. Oral repletion of iron induces free radical mediated alterations in the gastrointestinal tract of rat. Mol Cell Biochem 219:91–98. doi: 10.1023/A:1011023111048. [DOI] [PubMed] [Google Scholar]
- 49.Ueno N, Fujiya M, Segawa S, Nata T, Moriichi K, Tanabe H, Mizukami Y, Kobayashi N, Ito K, Kohgo Y. 2011. Heat-killed body of Lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm Bowel Dis 17:2235–2250. doi: 10.1002/ibd.21597. [DOI] [PubMed] [Google Scholar]
- 50.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. 2009. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Hara AM, O'Regan P, Fanning A, O'Mahony C, MacSharry J, Lyons A, Bienenstock J, O'Mahony L, Shanahan F. 2006. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology 118:202–215. doi: 10.1111/j.1365-2567.2006.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer R-JM, Wells JM. 2010. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 298:G851–G859. [DOI] [PubMed] [Google Scholar]
- 53.Seth A, Yan F, Polk DB, Rao RK. 2008. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC-and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 294:G1060–G1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reuter G. 2001. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol 2:43–53. [PubMed] [Google Scholar]
- 55.Smelt MJ, de Haan BJ, Bron PA, van Swam I, Meijerink M, Wells JM, Faas MM, de Vos P. 2013. Probiotics can generate FoxP3 T-cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS One 8:e68952. doi: 10.1371/journal.pone.0068952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trapecar M, Goropevsek A, Gorenjak M, Gradisnik L, Slak Rupnik M. 2014. A co-culture model of the developing small intestine offers new insight in the early immunomodulation of enterocytes and macrophages by Lactobacillus spp. through STAT1 and NF-κB p65 translocation. PLoS One 9:e86297. doi: 10.1371/journal.pone.0086297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.