ABSTRACT
PatzT is an internal promoter of the atzRSTUVW operon that directs the synthesis of AtzT, AtzU, AtzV, and AtzW, components of an ABC-type cyanuric acid transport system. PatzT is σN dependent, activated by the general nitrogen control regulator NtrC with the assistance of protein integration host factor (IHF), and repressed by the LysR-type transcriptional regulator (LTTR) AtzR. We have used a variety of in vivo and in vitro gene expression and protein-DNA interaction assays to assess the mechanisms underlying AtzR-dependent repression of PatzT. Here, we show that repression only occurs when AtzR and NtrC interact simultaneously with the PatzT promoter region, indicating that AtzR acts as an antiactivator to antagonize activation by NtrC. Furthermore, repression requires precise rotational orientation of the AtzR and NtrC binding sites, strongly suggesting protein-protein interaction between the two proteins on the promoter region. Further exploration of the antiactivation mechanism showed that although AtzR-dependent repression occurs prior to open complex formation, AtzR does not alter the oligomerization state of NtrC or inhibit NtrC ATPase activity when bound to the PatzT promoter region. Taken together, these results strongly suggest that PatzT-bound AtzR interacts with NtrC to prevent the coupling of NtrC-mediated ATP hydrolysis with the remodeling of the interactions between E-σN and PatzT that lead to open complex formation.
IMPORTANCE Here, we describe a unique mechanism by which the regulatory protein AtzR prevents the activation of the σN-dependent promoter PatzT. Promoters of this family are always positively regulated, but there are a few examples of overlapping negative regulation. The mechanism described here is highly unconventional and involves an interaction between the repressor and activator proteins to prevent the action of the repressor protein on the RNA polymerase-promoter complex.
INTRODUCTION
Promoters transcribed by RNA polymerase that bear the alternative σ factor σN (E-σN) are obligately subjected to positive control by enhancer-binding proteins (EBPs) that bind DNA at sites that are >100 bp upstream from the E-σN-binding motifs, designated upstream activation sites (UASs). Activation occurs by a unique mechanism involving oligomerization of the EBP at the UAS, interaction with E-σN by looping out the intervening sequences, and stimulation of closed complex to open complex isomerization concomitant to the hydrolysis of ATP by the conserved central domain of the EBP (1–6). In addition, a few σN-dependent promoters are subjected to negative control by proteins other than their EBPs by means of a variety of mechanisms, including (i) interference with DNA loop formation (7–9), (ii) locking an RNA polymerase in a form of closed complex that is insensitive to activation (10), and (iii) competition with the activator for DNA binding (11, 12). Interestingly, all of these mechanisms target the activation process rather than the interaction of the RNA polymerase with the promoter region, which represents a common form of repression for σ70-dependent promoters in bacteria (13, 14). The sole known exception to this rule is AtzR competition with E-σN for DNA binding at the PatzR promoter, a mechanism that was explained by the lack of an UAS in this promoter region, and the concomitant requirement of high promoter occupancy for efficient UAS-independent activation (15).
The Pseudomonas sp. strain ADP 108-kbp plasmid pADP-1 harbors the genes involved in the hydrolytic degradation of the s-triazine atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine) (16). Atrazine degradation requires the products of the constitutively expressed atzA, atzB, and atzC genes, which are involved in atrazine conversion to the central metabolite of s-triazine degradation, cyanuric acid, and the atzDEF operon, encoding the activities required for cyanuric acid conversion to ammonium and carbon dioxide (17, 18). Atrazine and cyanuric acid are primarily used as nitrogen sources, and the atzDEF operon is a target for transcriptional regulation by the general nitrogen control system (19). A regulatory cascade was characterized in which the EBP NtrC activates σN-dependent transcription of atzR, encoding the LysR-type transcriptional regulator (LTTR). AtzR in turn activates the divergent atzDEF operon in response to cyanuric acid and a nitrogen limitation signal transduced by the PII protein GlnK. In addition, AtzR represses its own synthesis (17–21). Recently, we showed that atzR is cotranscribed with five additional genes: atzS, encoding a putative outer membrane protein, and the atzTUVW cluster, encoding an ABC-type transport system that is involved in high-affinity uptake of cyanuric acid (22, 23). However, an additional σN-dependent promoter, PatzT, located within the atzS coding region, is responsible for most of the transcription of the atzTUVW cluster (22). Similarly to PatzR, PatzT is activated by NtrC in response to nitrogen limitation and is repressed by AtzR in a cyanuric acid-independent fashion.
Although PatzR and PatzT share the same regulatory scheme, their architectural features are widely divergent. No UAS for NtrC is present in the PatzR promoter region, and NtrC activates from solution or is nonspecifically bound to DNA, in a UAS-independent fashion (15). UAS-independent activation is known to be fostered by the high occupancy of the promoter by E-σN (24–26). Accordingly, PatzR bears an E-σN binding motif with a high similarity to the consensus that is strongly bound by E-σN (15). A single AtzR binding site overlaps the PatzR E-σN-binding element and is strictly required for repression (15). In contrast with PatzR, our previous results depicted PatzT as an archetypical σN-dependent promoter, featuring an UAS made out of two NtrC binding sites that are required for high-level activation and a binding site for the DNA-bending protein integration host factor (IHF), which is likely involved in looping out the intervening sequences between the NtrC UAS and the E-σN-binding motif (Fig. 1). Nevertheless, PatzT also displays significant levels of UAS-independent activation, suggesting a strong interaction between E-σN and its highly conserved binding site (22). AtzR binds the PatzT promoter region at a single site, containing a strong recognition motif centered at position −112 (Fig. 1). This AtzR binding site was also found to be strictly required for repression (22).
FIG 1.
Comparison of the PatzR and PatzT promoter regions. Cartoon depicting the identified cis-acting elements at the PatzR (top) and PatzT (bottom) promoter regions. Promoters are indicated with black arrows, NtrC binding sites are indicated with black boxes, the symmetrical AtzR-binding sites are indicated with two white boxes, and the IHF binding site is indicated with a gray box. The scale indicates coordinates relative to the transcription start point.
The PatzR and PatzT promoters are two examples of negatively regulated σN-dependent transcription. The mechanism of repression has been solved for PatzR, in which AtzR competes with E-σN for interaction with their overlapping binding sites (15). In contrast, the unusual location of the AtzR binding site in the PatzT promoter region, far upstream from the E-σN recognition motif, and the intriguing observation that AtzR cannot repress PatzT transcription when the NtrC binding sites are absent suggest that an unconventional repression mechanism may operate at this promoter.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this work and their relevant genotypes are summarized in Table 1. A minimal medium containing 25 mM sodium succinate as the sole carbon source was used for in vivo gene expression analysis (27). The nitrogen source was ammonium chloride or l-serine (1 g liter−1). Luria-Bertani (LB) medium was used as a rich medium (28). Liquid cultures were grown in culture tubes or flasks with shaking (180 rpm) at 30 or 37°C (for Pseudomonas putida or Escherichia coli strains, respectively). For the solid medium, Bacto agar (Difco) was added to a final concentration of 18 g liter−1. Antibiotics and other additions were used, when required, at the following concentrations: ampicillin, 100 mg liter−1; kanamycin, 20 mg liter−1; carbenicillin, 500 mg liter−1; rifampin, 10 mg liter−1; chloramphenicol, 15 mg liter−1; tetracycline, 5 mg liter−1; and 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal), (25 mg liter−1). All reagents were purchased from Sigma-Aldrich.
TABLE 1.
Bacterial strains and plasmids used in the present work
| Bacterial strain or plasmid | Genotype/phenotype | Reference/source |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| DH5α | ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rk− mk+) supE 44 thi-1 gyrA relA1 | 48 |
| KT5746 | N5271 [galK ilv his (λ cIts5857 N7N53 ΔBamΔHI)]/pPLhimhimA-5; Apr | 35 |
| NCM631 | hsdS gal λDE3::lacI lacUV5::gen1(T7 RNA polymerase) Δlac linked to Tn10 | 49 |
| P. putida | ||
| KT2440 | mt-2 hsdR1 (r− m+) | 50 |
| KT2440-IHF3 | mt-2 hsdR1 (r− m+) ΔihfA::Tcr | 51 |
| KT2442 | mt-2 hsdR1 (r− m+) Rifr | 50 |
| MPO201 | mt-2 hsdR1 (r− m+) ΔntrC::Tcr | 19 |
| Plasmids | ||
| pBEND2 | Vector for circular permutation analysis; Apr | 52 |
| pIZ227 | pACYC184-derived plasmid containing lacIq and the T7 lysozyme gene; Cmr | 49 |
| pMPO103 | 1.45-kb fragment containing atzR and the 5′ end of atzD, cloned in pBluescript II SK(+); Apr | 19 |
| pMPO109 | atzR coding sequence and promoter region cloned in pKT230; Kmr | 19 |
| pMPO135 | pET23b plasmid derivative overexpressing AtzR-His6; Apr | 21 |
| pMPO234 | Broad-host-range lacZ translational fusion vector, based on pBBR1MCS-4; Apr | 15 |
| pMPO310 | NtrCD55E,S161F expressed from PlacUV5 in a pACYC184-derived plasmid; Cmr | 33 |
| pMPO805 | PatzT-lacZ transcriptional fusion in pMPO234 carrying the wild-type PatzT promoter; Apr | 22 |
| pMPO820 | pBEND2 derivative containing the PatzT promoter region | This work |
| pMPO831 | PatzT wild-type template plasmid for in vitro transcription, based on pTE103; Apr | 22 |
| pMPO835 | PatzT-lacZ transcriptional fusion in pMPO234 bearing a 4-bp insertion between the AtzR and IHF sites; Apr | This work |
| pMPO836 | PatzT-lacZ transcriptional fusion in pMPO234 bearing a 5-bp insertion between the AtzR and IHF sites; Apr | This work |
| pMPO837 | PatzT-lacZ transcriptional fusion in pMPO234 bearing a 10-bp insertion between the AtzR and IHF sites; Apr | This work |
| pMPO849 | PatzT-lacZ transcriptional fusion in pMPO234 bearing a 5-bp insertion between the NtrC2 and AtzR sites; Apr | This work |
| pMPO850 | PatzT-lacZ transcriptional fusion in pMPO234 bearing a 6-bp insertion between the NtrC2 and AtzR sites; Apr | This work |
| pMPO851 | PatzT-lacZ transcriptional fusion in pMPO234 bearing a 10-bp insertion between the NtrC2 and AtzR sites; Apr | This work |
| pMPO853 | PatzT template for in vitro transcription bearing a 5-bp insertion between the NtrC2 and AtzR sites, based on pTE103; Apr | This work |
| pMPO854 | PatzT template for in vitro transcription bearing a 6-bp insertion between the NtrC2 and AtzR sites, based on pTE103; Apr | This work |
| pMPO855 | PatzT template for in vitro transcription bearing a 10-bp insertion between the NtrC2 and AtzR sites, based on pTE103; Apr | This work |
| pMPO863 | PatzT-lacZ transcriptional fusion in pMPO234 bearing a 21-bp insertion between the NtrC2 and AtzR sites; Apr | This work |
| pMPO864 | PatzT-lacZ transcriptional fusion in pMPO234 bearing a 32-bp insertion between the NtrC2 and AtzR sites; Apr | This work |
| pPLhiphimA-5 | IHF overproduction plasmid; Apr | 35 |
| pRK2013 | Helper plasmid used in conjugation; Kmr Tra+ | 53 |
| pTE103 | Vector for in vitro transcription assays; Apr | 54 |
Plasmid construction.
The plasmids used in this work are summarized in Table 1. All DNA manipulations were performed according to standard procedures (28). Restriction enzymes, DNA polymerases, and T4 DNA ligase were purchased from Roche Applied Science. The Klenow fragment or T4 DNA polymerase was routinely used to fill in recessed 3′ ends and trim protruding 3′ ends of incompatible restriction sites. Plasmid DNA preparation and DNA purification kits were purchased from Sigma-Aldrich, General Electric Healthcare, or Macherey-Nagel and were used according to the manufacturers recommendations. Plasmid DNA was transferred to E. coli and P. putida strains by transformation (29) or by triparental mating (30). E. coli DH5α was used as a host in all cloning procedures.
Site-directed mutagenesis of the PatzT promoter region by overlap extension PCR was performed essentially as described previously (31), using mutagenic and external oligonucleotide pairs as primers (sequences available upon request) and pMPO805 as the template. The final PCR products that contained PatzT promoter sequences from −218 to +319 (wild-type PatzT coordinates, relative to the transcriptional start site) were digested with EcoRI and BamHI and were cloned into EcoRI- and BamHI-cleaved pMPO234 to generate the PatzT-lacZ fusion plasmids pMPO835, pMPO836, pMPO837, pMPO849, pMPO850, pMPO851, pMPO863, and pMPO864. The presence of the desired mutations and the absence of unwanted alterations were determined by commercial sequencing (SecuGen). The EcoRI-BamHI fragments that contained the N+5A, N+6A, and N+10A PatzT promoter derivatives were excised from pMPO849, pMPO850, and pMPO851, respectively, and were cloned into EcoRI- and BamHI-digested pTE103 to yield the in vitro transcription template plasmids pMPO853, pMPO854, and pMPO855.
β-Galactosidase assays.
Steady-state β-galactosidase assays were used to examine the expressions of the wild-type and mutant PatzT-lacZ fusions in P. putida KT2442. Preinocula of bacterial strains harboring the relevant plasmids were grown to saturation in a minimal medium under nitrogen-sufficient conditions (ammonium chloride, 1 g liter−1), and cells were then diluted in a minimal medium containing the appropriate nitrogen sources (1 g liter−1 ammonium chloride for nitrogen excess and 1 g liter−1 l-serine for nitrogen limitation). Diluted cultures were shaken for 24 h to mid-exponential phase (A600 = 0.25 to 0.5). Growth was then stopped, and β-galactosidase activity was determined from SDS- and chloroform-permeabilized cells as previously described (32).
Protein purification.
AtzR-His6 was purified from the overproducing strain NCM631, harboring pMPO135 and pIZ227, by nickel affinity chromatography as previously described (21). P. putida NtrCD55E,S161F (33) and σN (34) were kind gifts from A. B. Hervás and V. Shingler. E. coli IHF was purified from the overproducing strain E. coli K5746 by ammonium sulfate fractionation and affinity chromatography on heparin-Sepharose as described previously (35). Core E. coli RNA polymerase was purchased from Epicenter Biotechnologies.
Gel mobility shift and DNase I footprinting assays.
Probes containing the wild-type or mutant PatzT promoter region derivatives were obtained by PCR using the PatzT-lacZ fusion plasmids as the templates (primer sequences available upon request). The PCR products were subsequently digested with EcoRI and BamHI and gel purified. DNA fragments were labeled strand specifically by filling in 5′ overhanging ends using the Klenow fragment in a reaction mixture containing [α-32P]-dCTP. Unincorporated nucleotides were removed using the MSB Spin PCRapace kit (Invitek).
AtzR-DNA complexes were formed at room temperature in 20-μl reaction mixtures that contained 10 ng of the radiolabeled probe and increasing amounts of purified AtzR-His6 (0 to 100 nM) in binding buffer (35 mM Tris acetate [pH 7.9], 70 mM potassium acetate, 20 mM ammonium acetate, 2 mM magnesium acetate, 1 mM calcium chloride, 1 mM dithiothreitol [DTT], 5% glycerol, 100 μg ml−1 salmon sperm DNA, and 250 μg ml−1 bovine serum albumin [BSA]) for 20 min. Reactions were stopped with 4 μl of loading buffer (0.125% [wt/vol] bromophenol blue, 0.125% [wt/vol] xylene cyanol, 10 mM Tris HCl [pH 8], 1 mM EDTA, 30% glycerol), and samples were separated on a 5% polyacrylamide native gel in Tris-borate-EDTA buffer at 4°C. Dried gels were exposed to a phosphoscreen and analyzed using the ImageQuant software (Amersham). For competitive gel mobility shift assays, reaction mixtures were incubated for 20 min in the presence of 400 nM AtzR-His6 and were subsequently challenged with increasing concentrations (0 to 2 μM) of NtrCD55E,S161F for 20 additional minutes or preincubated with 2 μM NtrCD55E,S161F and challenged with 0 to 400 nM AtzR-His6 in the same conditions as above. Reactions were stopped with 4 μl of loading buffer (0.125% [wt/vol] bromophenol blue, 0.125% [wt/vol] xylene cyanol, 10 mM Tris HCl [pH 8], 1 mM EDTA, and 30% glycerol), and samples were separated on 6.5% polyacrylamide native gels in Tris-borate-EDTA buffer at 4°C. Dried gels were exposed to a phosphoscreen and analyzed using the ImageQuant software (Amersham).
Protein-DNA complexes for DNase I footprinting assays were formed as above. Partial digestion of the DNA was initiated by the addition of 1 μl of an empirically determined dilution (typically 10−2 to 10−3) of a DNase I stock solution (10 U ml−1; Roche Diagnostics). Incubation was continued for an additional 30 s, and reactions were stopped by the addition of 5 μl of stop buffer (1.5 M sodium acetate [pH 5.2], 130 mM EDTA, 1 mg ml−1 salmon sperm DNA, and 2.4 mg ml−1 glycogen). DNA was subsequently ethanol precipitated, resuspended in 5 μl loading buffer (0.125% [wt/vol] bromophenol blue, 0.125% [wt/vol] xylene cyanol, 20 mM EDTA, and 95% [vol/vol] formamide), and separated by gel electrophoresis on a 6% polyacrylamide, 6 M urea denaturing sequencing gel. Sequencing reactions were performed with the Sequenase 2.0 kit (USB). Gels were processed and analyzed as above.
In vitro transcription.
Multiround in vitro transcription reactions were performed essentially as described previously (22) in a final volume of 20 μl containing 35 mM Tris-acetate (pH 7.9), 70 mM potassium acetate, 20 mM ammonium acetate, 5 mM magnesium acetate, 1 mM DTT, 10% glycerol, 250 mg liter−1 BSA, 20 nM E-σN, and 0.5 μg of a supercoiled plasmid template containing wild-type PatzT (pMPO831) or its mutant variants (pMPO853, pMPO854, and pMPO855). For PatzT activation assays, E. coli core RNA polymerase (100 nM; Epicentre), P. putida σN factor (200 nM), IHF (75 nM), and 4 mM ATP were added and incubated for 10 min at 30°C. An open complex formation was stimulated by adding NtrCD55E,S161F (0 to 400 nM) and further incubation for 10 min. In repression experiments, AtzR-His6 (0 to 240 nM) was added either before or after the formation of the open complex and was incubated for an additional 10 min at 30°C. A mixture of ATP, GTP, CTP (final concentration, 0.4 mM each), UTP (0.07 mM), and [α-32P]-UTP (0.033 μM; PerkinElmer) was added to initiate transcription. After a 5-min incubation at 30°C, reinitiation was prevented by adding heparin (final concentration, 0.1 mg ml−1). The samples were incubated for an additional 5 min at 30°C, and the reactions were terminated by the addition of 5 μl of stop buffer (150 mM EDTA, 1.05 M NaCl, 14 M urea, 3% glycerol, 0.075% xylene cyanol, and 0.075% bromophenol blue). Samples were run in 6% polyacrylamide-urea gels in Tris-borate-EDTA buffer at room temperature. Gels were processed and analyzed as described above.
ATPase activity assays.
NtrC ATPase activity was assayed by measuring the production of inorganic phosphate (Pi) using the EnzChek phosphate assay kit (Life Technologies). In order to avoid Pi contamination from the AtzR-His6 preparation, the AtzR-His6 buffer was changed to 50 mM Tris HCl (pH 8.0), 0.5 M NaCl, 20% glycerol, and 0.1 mM DTT by discontinuous diafiltration using Nanosep 10K Omega (Pall) centrifugal devices following the manufacturer's recommendations. AtzR-His6 DNA binding activity was essentially unchanged as judged from gel motility shift assays. A 110-bp DNA fragment that contained the PatzT NtrC and AtzR binding sites was obtained by annealing two cDNA oligonucleotides (sequences available upon request).
ATPase assay reactions were carried out as specified by the manufacturer, except that the MgCl concentration was increased to 2 mM, and 10 mM KCl and 1 mM DTT were added. Mixtures containing NtrCD55E,S161F (231 nM dimer), AtzR (240 nM tetramer), and/or the PatzT DNA fragment (770 nM) were preincubated for 20 min at room temperature. Purine nucleotide phosphorylase was added, followed by 10 min of incubation before ATP was added to start the reaction. The kinetics of Pi production was monitored by A360 and used to determine the specific ATPase activity (mol Pi min−1 mol NtrC−1). Phosphate concentrations were derived from standard curves using KH2PO4 as the source of inorganic phosphate. The phosphate release from reactions that did not contain NtrC was negligible.
Blue native gel electrophoresis.
Blue native gel electrophoresis was performed using the NativePAGE bis-Tris gel system (ThermoFisher Scientific). AtzR-His6 and/or NtrCD55E,S161F was diluted in 20 μl of in vitro transcription buffer (35 mM Tris-acetate [pH 7.9], 70 mM potassium acetate, 20 mM ammonium acetate, 5 mM magnesium acetate, 1 mM DTT, and 10% glycerol), incubated for 20 min at room temperature, and loaded on a 4% to 16% bis-Tris blue native gel. Electrophoresis was run, and the gel was further processed according to the manufacturer's recommendations.
RESULTS
AtzR-dependent repression occurs prior to open complex formation.
We have previously shown that PatzT transcription activation can be replicated in vitro in the presence of pure E-σN, the constitutively active NtrC variant NtrCD55E,S161F, and IHF. NtrCD55E,S161F has been shown to activate and repress transcription in vivo and in vitro to levels similar to those obtained with wild-type NtrC in a signal-independent fashion (33, 36). In order to characterize the AtzR-dependent repression of the PatzT promoter in vitro, multiround transcription assays were performed using supercoiled plasmid pMPO831, which bears the PatzT promoter region from position −218 to +319, as the template, Escherichia coli RNA polymerase core and IHF protein, Pseudomonas putida σN and NtrCD55E,S161F, and hexahistidine-tagged AtzR (AtzR-His6). To determine the effect of open complex formation on AtzR-dependent repression, two sets of transcription reactions were prepared. In the first set, the template was incubated with E-σN, IHF, and different concentrations of AtzR-His6 in the presence of ATP. Isomerization to open complex was subsequently triggered by adding NtrCD55E,S161F. In the second set, the DNA template was incubated with NtrCD55E,S161F, E-σN, and IHF in the presence of ATP to allow open complex formation, which was subsequently challenged with different concentrations of AtzR-His6 prior to adding nucleoside triphosphates (NTPs) (see Materials and Methods for details) (Fig. 2A). When added prior to open complex formation, a clear AtzR-His6 concentration-dependent decrease in PatzT activity was observed, to reach a maximum 90% decrease in transcript levels at the highest concentration used. This result indicates that AtzR can efficiently repress PatzT transcription in vitro. In contrast, the addition of AtzR-His6 after open complex formation resulted in low levels of repression, reaching a maximum of 20% at the highest concentration used, which suggests that open complex formation prevents AtzR-dependent repression.
FIG 2.
In vitro repression of the PatzT promoter. In vitro transcription assays using the PatzT promoter region as a template. Isomerization to the open complex was triggered by addition of NtrCD55E,S161F to an ATP-containing mixture (A) or by addition of ATP to an NtrCD55E,S161F-containing reaction mixture (B). Each panel shows an autoradiograph of the representative PAGE gel (top) and a plot of the quantified relative transcript abundance (bottom). AtzR-His6 concentrations were 0 nM (lane 1), 40 nM (lane 2), 80 nM (lane 3), 160 nM (lane 4), or 240 nM (lane 5). Legends denote the order of AtzR-His6 addition before (AtzR-NtrC or AtzR-ATP) or after (NtrC-AtzR or ATP-AtzR) open complex formation. Symbols and error bars represent the averages and standard deviations of at least three independent assays. The significances of the differences between the two data sets for each experiment were assessed by the t test for unpaired samples not assuming equal variances. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Differences were not significant when not indicated.
The data above may also be explained if the presence of NtrC renders the promoter insensitive to repression due to interactions with E-σN or DNA rather than by promoter closed complex isomerization. To distinguish between these two possibilities, two additional sets of in vitro transcription reactions were performed. In the first set, E-σN was preincubated with the PatzT template and NtrCD55E,S161F. The closed complex was challenged with AtzR-His6, and then ATP was added to allow isomerization of the open complex prior to the addition of NTPs. In the second set, ATP was added to the template-NtrCD55E,S161F mixture, thus allowing open complex formation, and the open complex was subsequently challenged with increasing AtzR-His6 concentrations prior to the addition of NTPs (Fig. 2B). The results were equivalent to those obtained above, as efficient PatzT repression was only observed when open complex formation was not allowed prior to the addition of AtzR-His6. These results confirm that isomerization to an open complex protects PatzT from AtzR-dependent repression, strongly suggesting that AtzR acts on the early steps of the initiation pathway to prevent either closed complex formation or isomerization to the open complex.
NtrC and AtzR bind the PatzT promoter region simultaneously.
The location of the AtzR binding motif, centered at position −112, far upstream from the E-σN binding motif and in the vicinity of the NtrC binding site NtrC2 (centered at −130), and the fact that AtzR cannot repress PatzT transcription when it is activated by NtrC in an UAS-independent fashion (22) suggest that rather than tampering with RNA polymerase function, AtzR may antagonize NtrC interaction with the PatzT promoter region (22). To test this possibility, gel mobility shift assays were performed in which PatzT-bound NtrCD55E,S161F was challenged with increasing concentrations of AtzR-His6 and vice versa (Fig. 3). Interaction of NtrCD55E,S161F with the PatzT promoter region resulted in the replacement of the free probe band by a smear spanning much of the corresponding PAGE lane (Fig. 3, lane 2). This was previously observed (22) and suggests that the NtrCD55E,S161F-PatzT complex is unstable during gel electrophoresis. In contrast, AtzR-His6 retarded the PatzT probe to form a single stable complex (Fig. 3, lane 7). Addition of AtzR-His6 to a preformed NtrCD55E,S161F-PatzT complex (Fig. 3, lanes 3 to 6) or NtrCD55E,S161F to a preformed AtzR-His6-PatzT (Fig. 2, lanes 8 to 11) provoked the substitution of the preexisting complexes with a single, stable, slow migrating protein-DNA complex, strongly suggesting that the two proteins can bind the PatzT promoter region simultaneously.
FIG 3.
Gel mobility shift assay of AtzR and NtrC on the PatzT promoter region. Autoradiograph of a representative PAGE gel. Lane 1 shows a free PatzT probe. Lanes 2 to 6 show a preformed complex containing 2 μM NtrCD55E,S161F challenged with 0 nM (lane 2), 50 nM (lane 3), 100 nM (lane 4), 200 nM (lane 5), or 400 nM AtzR-His6. Lanes 7 to 11 show a preformed complex containing 400 nM AtzR-His6, challenged with 0 nM (lane 7), 250 nM (lane 8), 500 nM (lane 9), 1 μM (lane 10), or 2 μM (lane 11) NtrCD55E,S161F. AtzR-DNA, NtrC-DNA, and AtzR-NtrC-DNA complexes are denoted by black, gray, and white arrows, respectively.
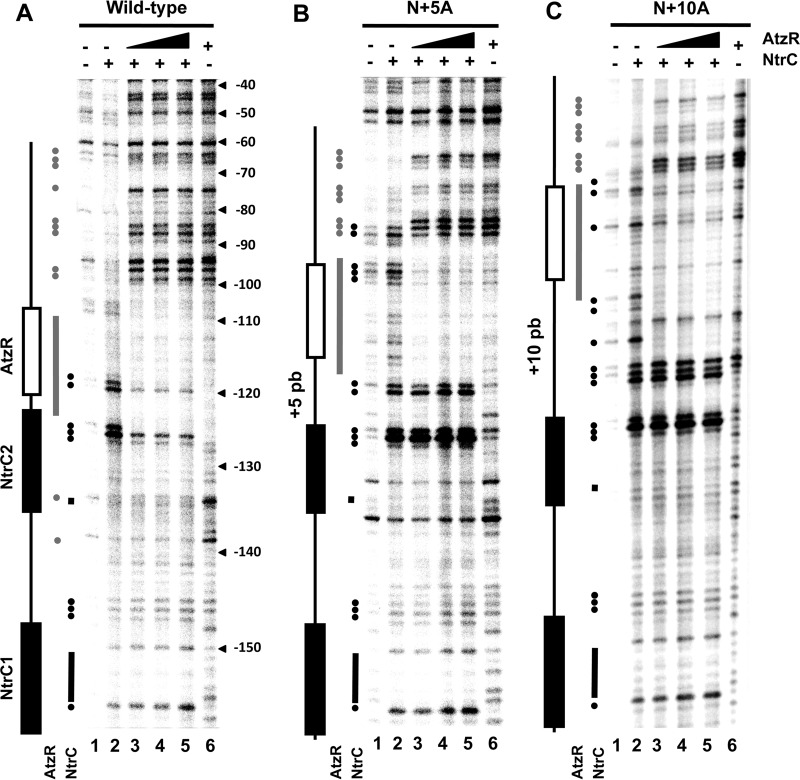
To further characterize the interactions of NtrC and AtzR with the PatzT promoter region, the effect of challenging a preformed protein-DNA complex with the other protein was also assessed by means of DNase I footprinting (Fig. 4). NtrCD55E,S161F binding to the PatzT promoter region (Fig. 4, lane 2) resulted in protection of positions −161, −162, −156 to −150, and −136 and hypersensitivity at positions −157, −149, −148, −127 to −125, −119, −118, and −116, which is consistent with interactions at the NtrC1 and NtrC2 sites (22). AtzR-His6 interaction with its site (Fig. 4, lane 7) resulted in a continuously protected region between −109 and −125 with hypersensitive positions at −139, −136, −128, −99, −98, −86 to −84, and −64 to −62 (22). The addition of increasing concentrations of AtzR-His6 to a preformed NtrCD55E,S161F-PatzT complex (Fig. 4, lanes 3 to 6) did not modify the footprinting pattern at the NtrC1 site and the promoter-distal half of the NtrC2 site. However, the hypersensitivity signatures observed at the promoter-proximal half and downstream from the NtrC2 site (positions −125, −119, −118, and −116) were substituted by the characteristic AtzR footprinting patterns. Challenging a preformed AtzR-His6-PatzT complex with increasing concentrations of NtrCD55E,S161F resulted in the gradual emergence of the NtrC footprinting signatures at NtrC1 and at the promoter-distal half of NtrC2, with no apparent effect on the AtzR footprint. These results are fully consistent with the gel mobility shift results above, indicating that NtrC and AtzR simultaneously bind the PatzT promoter region; however, we cannot rule out that the presence of DNA-bound AtzR may alter the interactions of NtrC with its binding sites or the oligomeric form of NtrC (dimers or hexamers) bound.
FIG 4.
DNase I footprinting assay of AtzR and NtrC on the PatzT promoter region. Autoradiograph of a representative PAGE gel. Lane 1 shows a free PatzT probe. Lanes 2 to 6 show a preformed complex containing 2 μM NtrCD55E,S161F challenged with 0 nM (lane 2), 50 nM (lane 3), 100 nM (lane 4), 200 nM (lane 5), or 400 nM AtzR-His6. Lanes 7 to 11 show a preformed complex containing 400 nM AtzR-His6, challenged with 0 nM (lane 7), 250 nM (lane 8), 500 nM (lane 9), 1 μM (lane 10), or 2 μM (lane 11) NtrCD55E,S161F. Coordinates relative to PatzT transcriptional start are indicated on the right. The approximate locations of the NtrC and AtzR binding sites are denoted by black and white boxes, respectively. Black bars and circles indicate positions rendered protected or hypersensitive, respectively, by NtrC binding. Gray bars and circles indicate positions rendered protected or hypersensitive, respectively, by AtzR binding.
PatzT repression requires precise relative rotational orientation of the NtrC and AtzR sites.
It has long been known that although DNA bending or looping acts as a facilitator of EBP–E-σN interactions in many σN-dependent promoters, a misoriented bend may lead to incorrect alignment of the interacting pair, thus preventing activation (37, 38). This mechanism has been claimed to operate in several examples of σN-dependent promoter repression (7–9). AtzR is a DNA-bending protein (21), and circular permutation analysis has shown that it causes a 74° bend centered at position −92 of the PatzT promoter region (see Fig. S1 in the supplemental material). Thus, interference with DNA loop formation is a feasible mechanism for AtzR repression of the PatzT promoter. To test this hypothesis, we constructed a set of PatzT fusion plasmids in which the rotational orientation and/or the distance between the NtrC and AtzR binding sites or between the two protein binding sites and the downstream elements of the promoter region (IHF and E-σN binding sites) was modified (Fig. 5A). Plasmids pMPO849, pMPO850, pMPO851, pMPO863, and pMPO864 bear 5-, 6-, 10-, 21-, and 32-bp insertions between the NtrC2 and AtzR binding sites (these constructs were designated N+5A, N+6A, N+10A, M+21A, and N+32A, respectively). Plasmids pMPO835, pMPO836, and pMPO837 bear 4-, 6-, and 10-bp insertions between the AtzR and IHF binding sites (constructs A+4I, A+6I, and A+10I). To test the effects of these mutations on in vivo PatzT activation and repression, all fusion plasmids were transferred, along with the wild-type PatzT-lacZ fusion pMPO805, to P. putida KT2442, used here and in our previous work as a surrogate host for in vivo gene expression studies (15, 21–23, 39, 40), or P. putida KT2442 bearing the AtzR-producing plasmid pMPO109. PatzT expression was assessed from cultures grown in minimal medium containing ammonium (nitrogen excess) or serine (nitrogen limitation) by means of β-galactosidase assays (Fig. 5B).
FIG 5.
β-Galactosidase activity of PatzT-lacZ fusions. (A) Schematic of the PatzT promoter derivatives used for gene fusion analysis. Sequences of the NtrC2, AtzR, and IHF binding sites are shown. Binding sites are coded as in Fig. 1. Vertical arrows indicate the location of the insertions. Plasmid name, construct designation, and the number and identity of the nucleotides inserted is shown for each construct. (B) Results of the β-galactosidase assays performed with the PatzT promoter derivatives above fused to lacZ in pMPO234. Columns and error bars represent the averages and standard deviations of at least three independent assays. Significance of AtzR regulation (−AtzR versus +AtzR) was assessed for each promoter variant under nitrogen sufficiency and nitrogen limitation by the t test for unpaired samples not assuming equal variances. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Differences were not significant when not indicated. Significance of nitrogen regulation (ammonium versus serine) was also assessed, and differences were found to be significant (0.05 > P > 0.00002) in all cases (not shown).
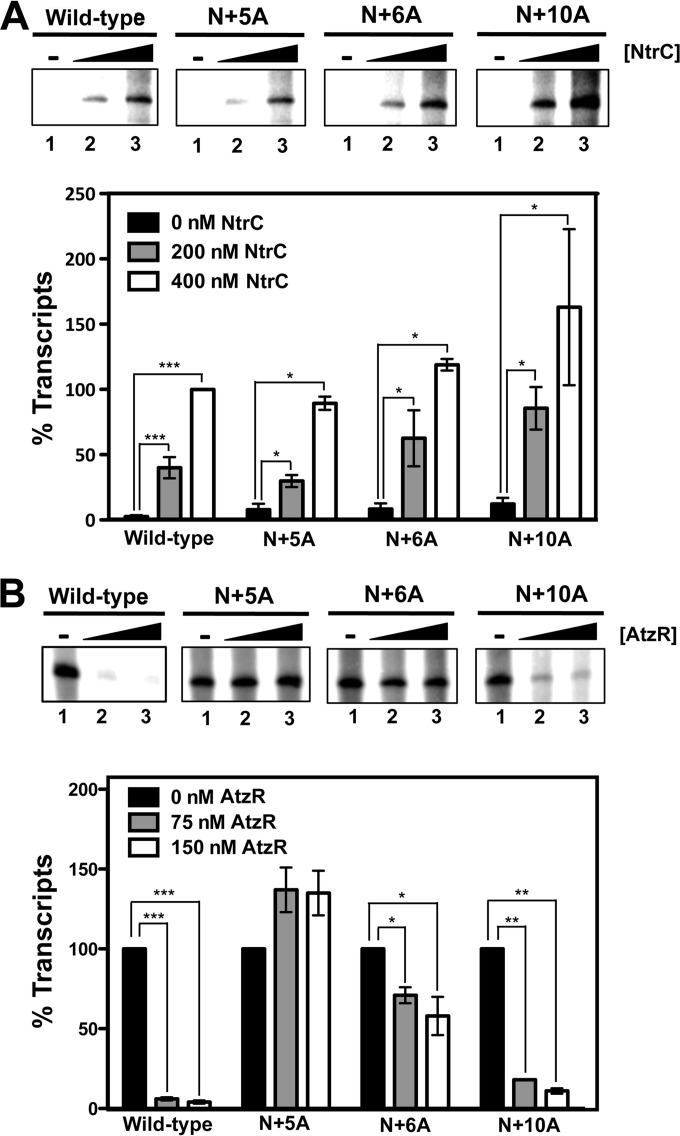
Analysis of in vivo expression from the wild-type PatzT-lacZ fusion in the absence of AtzR revealed a >100-fold increase in response to nitrogen limitation. This is consistent with our previous observations and expected for a promoter regulated by the general nitrogen control system (22). None of the mutant promoter regions displayed a defect in activation, and in fact, all constructs bearing insertions between the NtrC and AtzR sites (N+5A, N+6A, N+10A, M+21A, and N+32A) showed a 1.5- to 2-fold increased induction in nitrogen-limited medium. PatzT expressions from the wild types N+5A and N+10A were also assessed in ihf- and ΔntrC backgrounds (see Fig. S2 in the supplemental material). Nitrogen limitation induction was nearly abolished in the absence of NtrC and was severely decreased in the absence of IHF in all cases, indicating that expression is similarly dependent on the two transcription factors regardless of the construct. The effects of rotational orientation mutations on PatzT promoter expression were further explored by means of multiround in vitro transcription assays using supercoiled pMPO831, pMPO853, pMPO854, or pMPO855, bearing wild-type, N+5A, N+6A, and N+10A PatzT promoter fragments, respectively, as the templates (Fig. 6). In reaction mixtures containing E-σN, IHF, and NtrCD55E,S161F, but lacking AtzR-His6, the mutant constructs exhibited NtrC concentration-dependent PatzT transcript levels that were similar or greater than those obtained by the wild-type template (Fig. 6A), which is consistent with the in vivo observations above. Taken together, our results strongly suggest that NtrC-dependent activation of PatzT is not restricted by the distance or orientation of DNA-bound NtrC relative to promoter-bound E-σN.
FIG 6.
In vitro activation and repression of PatzT promoter derivatives. In vitro transcription assays using PatzT promoter region derivatives as the templates. (A) Activation of PatzT promoter derivatives in the presence of 75 mM IHF and 0 nM (lane 1), 200 nM (lane 2), or 400 nM (lane 3) NtrCD55E,S161F. (B) Repression of PatzT promoter region derivatives in the presence of 400 nM NtrCD55E,S161F, 75 nM IHF, and 0 nM (lane 1), 75 nM (lane 2), or 150 nM (lane 3) AtzR-His6. Each panel shows an autoradiograph of a representative PAGE gel (top) and a plot of the quantified relative transcript abundance (bottom). Bars represent the averages and standard deviations of at least three independent assays. Significances of NtrC-dependent activation (A) and AtzR-dependent repression (B) were assessed for each promoter variant by the t test for unpaired samples not assuming equal variances. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Differences were not significant when not indicated.
Analysis of in vivo expression from the wild-type PatzT-lacZ fusion in the presence of AtzR (Fig. 5) showed a 4-fold decrease in activity under nitrogen limitation, which is consistent with the negative control of the PatzT promoter by AtzR (22). Notably, the mutant promoters bearing insertions of approximately half a helix turn between the NtrC and AtzR sites (N+5A and N+6A) were completely insensitive to AtzR repression, while insertions of approximately one (N+10A), two (N+21A), or three (N+32A) helix turns allowed repression levels similar to those in the wild type. None of the insertions downstream from the AtzR site (A4I, A6I, and A10I) had a significant effect on AtzR-dependent repression of PatzT. In vitro transcription assays performed in the presence of E-σN, IHF, NtrCD55E,S161F, and AtzR-His6 showed a >10-fold AtzR-dependent repression of the wild-type promoter region at the two AtzR-His6 concentrations used (Fig. 6B). A 5-bp insertion (N+5A construct) fully abolished AtzR-dependent repression, while the N+6A template displayed a minor decrease in transcript levels (less than 2-fold). Insertion of a full helix turn (N+10A construct) resulted in repression that was only slightly less efficient than in the wild-type template. Taken together, our results indicate that PatzT repression (i) requires a correct rotational orientation between DNA-bound NtrC and AtzR, (ii) can tolerate an increase in the distance between DNA-bound NtrC and AtzR provided that condition (i) is met, and (iii) does not require correct alignment or a precise distance between the upstream (NtrC and AtzR binding sites) and downstream (IHF and E-σN binding sites) elements of the PatzT promoter region. Considering that NtrC-dependent activation is not sensitive to changes in the distance or orientation of the UAS relative to the E-σN binding site, the evidence obtained does not support the hypothesis that AtzR interference with DNA looping prevents PatzT activation.
The rotational orientation of the binding sites does not significantly alter NtrC and AtzR interactions with the PatzT promoter region.
A trivial explanation for the results above is that the nucleotides inserted in the N+5A and N+6A mutant promoters serendipitously affect AtzR interactions with its binding site, while those inserted in the N+10A, N+21A, and N+32A do not, regardless of their effect on the PatzT promoter architecture. To assess this possibility, AtzR-His6 gel shift assays were performed using wild-type, N+5A, N+6A, and N+10A PatzT promoter fragments as probes (Fig. 7). AtzR-His6 efficiently retarded all four promoter fragments, indicating that the lack of AtzR-dependent repression of the N+5A and N+6A promoter derivatives is not due to a general defect in AtzR binding.
FIG 7.
Gel mobility shift assay of AtzR on PatzT promoter region derivatives. Autoradiograph of a representative PAGE gel, containing the indicated PatzT promoter derivative probes and 0 nM (lane 1), 50 nM (lane 2), or 100 nM (lane 3) AtzR-His6.
Additionally, we questioned whether the architecture of the NtrC-PatzT promoter complexes formed at the opposite side of the helix might somehow hamper AtzR interaction with its binding site at the mutant N+5A and N+6A promoters. To address this question, we performed DNase I footprinting analyses in which NtrCD55E,S161F-PatzT complexes preformed on the wild-type, N+5A, and N+10A promoter variants were challenged with increasing concentrations of AtzR (Fig. 8). The addition of NtrCD55E,S161F to the wild-type and mutant probes resulted in similar footprinting patterns around sites NtrC1 and NtrC2. Additional hypersensitive bands overlapping the AtzR binding site were also observed in a variable pattern that may be attributed to differences in sequence due to the insertions present in the mutant promoters. The addition of AtzR-His6 to preformed NtrCD55E,S161F complexes resulted in the replacement of the NtrC-elicited hypersensitive bands overlapping the AtzR binding site with the characteristic AtzR footprint. AtzR-His6 binding did not alter the NtrCD55E,S161F footprint upstream from position −126 in the wild-type probe, as discussed above, while the NtrCD55E,S161F footprint was discernible down to position −121 in the N+5A mutant and position −116 in the N+10A mutant. This effect is clearly attributable to a decrease in the overlap of the NtrC and AtzR footprinting patterns, as the NtrC2 and AtzR sites are moved apart, and correlates very well with the 5- and 10-bp insertions present in the mutant promoters. No observable feature in the footprinting patterns can be correlated with the repression proficiency of the promoter variants. Taken together, our results suggest that NtrC and AtzR interact with all of the tested PatzT promoter region variants in an independent fashion, and therefore, the ability of AtzR to exert repression cannot be correlated with competition or interference with NtrC for DNA binding.
FIG 8.
DNase I footprinting assay of AtzR and NtrC on PatzT promoter region derivatives. Autoradiograph of representative PAGE gels containing the wild-type (A), N+5A (B), or N+10A (C) probe. Lane 1 shows a free PatzT probe. Lanes 2 to 6 show a preformed complex containing 2 μM NtrCD55E,S161F challenged with 0 nM (lane 2), 50 nM (lane 3), 100 nM (lane 4), 200 nM (lane 5), or 400 nM AtzR-His6. The approximate locations of the NtrC and AtzR binding sites are denoted by black and white boxes, respectively. Black bars and circles indicate positions rendered protected or hypersensitive, respectively, by NtrC binding. Gray bars and circles indicate positions rendered protected or hypersensitive, respectively, by AtzR binding.
AtzR does not alter NtrC ATPase activity or oligomerization state.
The E-σN-dependent promoter activation pathway involves oligomerization of the EBP upon UAS binding to a hexameric conformation that promotes the EBP ATPase activity. Further contacts with the E-σN promoter closed complex couple ATP hydrolysis to the remodeling of the closed complex into a transcriptionally active open complex (41, 42). In order to discern the particular step of this pathway at which AtzR inhibits NtrC activation of PatzT, the possible ability of AtzR to inhibit NtrC oligomerization, AtzR-His6 and NtrCD55E,S161F were incubated separately and simultaneously in an in vitro transcription buffer, and the protein complexes were resolved in blue native gel electrophoresis (see Fig. S3 in the supplemental material). Surprisingly, native AtzR-His6 did not migrate as a discrete band and could not be detected in this electrophoresis system. Also, heat-denatured NtrCD55E,S161F did not migrate to its expected size (∼53 kDa) but as a single band with an apparent molecular mass of ∼90 kDa. The two proteins were previously shown to migrate according to their monomer molecular weights in SDS-PAGE (21, 33). The native form of NtrCD55E,S161F migrated as three bands, with apparent molecular masses of ∼90, ∼180, and ∼540 kDa, suggesting that this constitutive form is a mixture of monomers, dimers, and hexamers in solution. This migration pattern was unchanged in the presence of AtzR-His6, suggesting that the presence of AtzR does not alter the equilibrium between the different oligomeric forms of NtrC.
In addition, ATPase activity assays were performed using pure NtrCD55E,S161F in the absence or in the presence of AtzR-His6 (Fig. 9). Addition of AtzR-His6 did not result in a significant decrease in specific ATPase activity (1.3-fold change; P value = 0.26). This assay was also performed in the presence of an ∼3-fold molar excess of a 110-bp PatzT promoter region fragment spanning the NtrC and AtzR binding sites. In the presence of promoter DNA, a 2-fold increase (P value < 0.05) in ATPase activity was observed in an AtzR-independent fashion, which is consistent with the notion that DNA binding stimulates P. putida NtrC oligomerization and ATPase activity as previously shown for its enterobacterial counterpart and other proteins in this family (41). Significantly, addition of AtzR-His6 did not result in decreased ATP hydrolysis under these conditions.
FIG 9.

Effect of AtzR and PatzT promoter DNA on NtrC ATPase activity. ATPase activity assay of NtrCD55E,S161F (231 nM) in the presence or in the absence of AtzR-His6 (240 nM) and/or PatzT promoter region DNA (770 nM). Data show specific molar activity, assuming that the NtrC active form is a hexamer. Bars represent the averages and standard deviations of at least three independent assays. Significance was assessed by the t test for unpaired samples not assuming equal variances. *, P < 0.05. Differences were not significant when not indicated.
Taken together, these results strongly suggest that AtzR does not inhibit ATP hydrolysis or any of the steps leading to it. As our results above (Fig. 2) indicate that AtzR must operate prior to open complex formation, we propose that PatzT-bound AtzR prevents coupling of ATP hydrolysis to oligomerization of the closed complex into the open complex.
DISCUSSION
Promoters that are dependent on the alternative σ factor σN have been widely studied as unique examples of eukaryotic-like transcriptional activation mechanisms in prokaryotic systems (1, 24, 43). In addition to positive control, a small subset of σN-dependent promoters is subjected to negative regulation. The work presented here demonstrates that a new, unconventional mechanism by which AtzR prevents coupling of NtrC-catalyzed ATP hydrolysis to the isomerization of the E-σN promoter closed complex to the open complex is accountable for the repression of the Pseudomonas sp. ADP PatzT promoter.
Analysis of the few examples of negatively regulated σN-dependent promoters has revealed a fairly diverse array of regulatory mechanisms, generally involving interference of the repressor protein with the activation process (7–12, 15, 22). The location of the AtzR binding site, adjacent to the NtrC UAS and far upstream from the E-σN recognition element, as well as the early observation that AtzR cannot repress the relatively high levels of UAS-independent NtrC-activated PatzT transcription that is observed when the NtrC UAS was inactivated by mutation (22), prompted us to hypothesize that AtzR antagonizes NtrC function when bound to the PatzT promoter region and, therefore, performs the role of an antiactivator. To this end, the simplest mechanism would be activator exclusion, involving competition between repressor and activator for interaction with the promoter region, as previously shown for repression of the XylR-activated P. putida Pu promoter by TurA and PprA (11, 12). However, gel mobility shift assays showed that AtzR and NtrC simultaneously interact with the PatzT promoter region (Fig. 3), and DNase I footprinting revealed the simultaneous occupancy of the NtrC1, NtrC2, and AtzR sites (Fig. 4), strongly suggesting that AtzR does not compete with NtrC for DNA binding. It may be argued that even though AtzR does not exclude NtrC binding of the NtrC2 site, the vicinity of DNA-bound AtzR may alter the mode of interaction with this site, a notion that is supported by the altered NtrC-induced DNase I hypersensitivity pattern around NtrC2 in the presence of AtzR (Fig. 4). However, promoter derivatives that contain insertions of up to 32 bp between the NtrC2 and AtzR sites, in which the sites are moved up to three helix turns apart, still show a normal response to AtzR (Fig. 5), indicating that interference with normal NtrC binding is not likely to be involved in the mechanism of repression. Similarly, the observation that promoter derivatives that bear 4-, 6-, or 10-bp insertions between the AtzR and IHF binding sites still support wild-type levels of activation and repression (Fig. 5) rules out the possibility that AtzR may interfere with IHF binding to the PatzT promoter region.
A second mechanism of antiactivation of σN-dependent promoters exploits the requirement of a DNA loop to facilitate interaction between the DNA-bound EBP and E-σN. Several σN-dependent promoter repressors have been proposed to alter the orientation of the DNA loop and hence prevent activation, including the Klebsiella pneumoniae LTTR Nac at its own promoter (7), CcpA at the Bacillus subtilis levanase operon (8), and CRP at the E. coli glnHp2 promoter (9). As AtzR bends DNA upon binding at the PatzT promoter region (see Fig. S1 in the supplemental material), AtzR-induced bending may alter the orientation of the DNA loop at the PatzT promoter, thus impairing NtrC interaction with E-σN. However, insertions designed to rotate the NtrC UAS to the opposite side of the helix or to separate it up to three full helix turns from the E-σN binding site failed to diminish PatzT activation (Fig. 5 and 6), indicating that the flexibility of the DNA loop is sufficient to accommodate NtrC interaction with RNA polymerase from various orientations and distances. This result is expected for IHF-independent σN-dependent promoters, in which the strong binding of E-σN facilitates interaction with the EBP in a position- and orientation-independent fashion (44, 45). However, for IHF-dependent promoters, strict dependence on the correct rotational orientation for activation has been documented (38). In vivo expressions of the wild-type and mutant PatzT promoters were dependent on NtrC and IHF (see Fig. S2 in the supplemental material). Conversely, the PatzT promoter displays very high similarity to the E-σN consensus (TGGCCC-N5-TTGC versus TGGCAC-N5-TTGC [conserved positions are underlined]). We propose that despite the fact that PatzT activation is aided by IHF, strong interaction of E-σN with its highly conserved binding motif may compensate for the suboptimal orientation of the NtrC UAS, enabling orientation-independent activation. The presence of substantial levels of UAS-independent activation of PatzT (22), a phenomenon that is associated with promoters with high E-σN occupancy (15, 24–26), lends further support to this notion. Strikingly, despite the lack of the rotational orientation dependency of PatzT activation, AtzR repression of PatzT was impaired when a half-helix turn was inserted between the NtrC UAS and the AtzR binding site but not when one, two, or three full helix turns were inserted at this location (Fig. 5 and 6), which is indicative of the rotational orientation-dependent effect of AtzR on PatzT activation. However, this pattern was not reproduced when half or a full helix turn was inserted downstream from the AtzR binding site, indicating that a precise rotational orientation of AtzR relative to the downstream elements of the promoter is not required for repression (Fig. 5). If the role of AtzR was to alter the orientation of the DNA bend, a similar dependence on rotational orientation would be expected on both sides of the AtzR binding site (37, 38). Taken together, the insensitivity of NtrC-dependent activation to changes in position and rotational orientation along with the inconsistent effect of the altered rotational orientation of the AtzR binding site relative to the upstream and downstream promoter elements fail to support an antiactivation model based solely on a distortion exerted by AtzR on the DNA loop formed at the PatzT promoter.
Activation of the σN-dependent promoter is a complex process. NtrC can bind its UAS elements both in its unphosphorylated (inactive) and phosphorylated (active) forms. However, phosphorylation promotes oligomerization to a hexameric conformation that enhances ATPase activity. DNA loop-mediated interaction with E-σN bound to DNA in a closed complex couples ATP hydrolysis with the remodeling of the E-σN promoter closed complex structure, leading to isomerization to the open complex (6, 41, 42). Our experimental results show that (i) AtzR does not interfere with NtrC binding to the PatzT UAS as documented in competitive gel mobility shift and DNase I footprinting assays (Fig. 3, 4, and 8), (ii) AtzR does not alter NtrC oligomerization state as assessed by blue native gel electrophoresis (see Fig. S3 in the supplemental materal), (iii) AtzR does not interfere with NtrC phosphorylation, as repression was observed in vitro with an NtrC variant (NtrCD55E,S161F) that does not require phosphorylation in the absence of a phosphate donor (Fig. 2 and 6), (iv) AtzR does not have a relevant effect on DNA loop formation as evidenced by the in vivo and in vitro phenotypes of our mutants bearing rotationally altered variants of the PatzT promoter region (Fig. 5 and 6), (v) AtzR does not interfere with NtrC ATPase activity or the events (DNA binding and oligomerization) as shown by the fact that AtzR does not decrease the ATPase activity of NtrC even in the presence of PatzT promoter region DNA (Fig. 9), and (vi) our in vitro transcription assays indicate that AtzR repression occurs prior to open complex formation (Fig. 2). Taken together, our experimental evidence leads to the conclusion that AtzR prevents activation of the PatzT promoter by preventing the productive interactions between NtrC and E-σN that result in the isomerization of the stalled transcriptional initiation closed complex to the initiation-proficient closed complex.
The observation that AtzR failed to repress PatzT transcription in vivo when bound at the opposite side of the DNA helix but did so when full helix turn insertions restore the relative orientation of the AtzR and NtrC binding sites (Fig. 5 and 6) indicates that precise alignment between the two DNA-bound proteins is critical for repression. Two different mechanisms of antiactivation are compatible with these observations. First, DNA-bound AtzR may interact specifically with NtrC to form a complex that is not proficient in coupling ATP hydrolysis with closed complex remodeling. Should this be the case, the effects of positive or negative cooperativity between the two proteins may be too subtle to detect with the sensitivity of our DNA-binding assays. Additional experimentation in search for further evidence of specific protein-protein interaction between NtrC and AtzR has unfortunately been inconclusive: in vivo interaction was apparent with the bacterial adenylate cyclase-based two-hybrid (BACTH) assay, but attempts to detect an interaction between the purified proteins in vitro have been so far unsuccessful (data not shown). Alternatively, AtzR may act as a “roadblock” to sterically hinder productive interactions between NtrC and E-σN. This mechanism would not require specific interactions between AtzR and NtrC and may be mediated either by the bulk of the AtzR tetramer bound next to the NtrC binding site or by the loss of flexibility of the intervening DNA between the NtrC and E-σN binding sites due to AtzR-mediated DNA bending. While neither of these mechanisms has been previously documented for the repression of a σN-dependent promoter, they are reminiscent of the repression mechanism at the E. coli CRP-dependent deo promoter, in which CytR binds in the vicinity of the CRP binding site and interacts with CRP to prevent contacts with the carboxyl terminal domain of the RNA polymerase α subunit (46, 47). We hope that our future research will help clarify these specific details of the repression mechanism at this unique bacterial promoter.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ana B. Hervás (CABD, Universidad Pablo de Olavide), Linda U. M. Johansson, Lisandro M. D. Bernardo, Eleonore Skärfstad, and Victoria Shingler (Umeå University) for purified proteins; Guadalupe Martín and Nuria Pérez for technical help; and all members of the Govantes and Santero laboratories at CABD for their insights and helpful suggestions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00906-16.
REFERENCES
- 1.Morett E, Segovia L. 1993. The sigma 54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol 175:6067–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck M, Gallegos MT, Studholme DJ, Guo Y, Gralla JD. 2000. The bacterial enhancer-dependent sigma(54) (sigma(N)) transcription factor. J Bacteriol 182:4129–4136. doi: 10.1128/JB.182.15.4129-4136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Chaney M, Wigneshweraraj SR, Schumacher J, Bordes P, Cannon W, Buck M. 2002. Mechanochemical ATPases and transcriptional activation. Mol Microbiol 45:895–903. doi: 10.1046/j.1365-2958.2002.03065.x. [DOI] [PubMed] [Google Scholar]
- 4.Studholme DJ, Dixon R. 2003. Domain architectures of σ54-dependent transcriptional activators. J Bacteriol 185:1757–1767. doi: 10.1128/JB.185.6.1757-1767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schumacher J, Joly N, Rappas M, Zhang X, Buck M. 2006. Structures and organisation of AAA+ enhancer binding proteins in transcriptional activation. J Struct Biol 156:190–199. doi: 10.1016/j.jsb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Wigneshweraraj S, Bose D, Burrows PC, Joly N, Schumacher J, Rappas M, Pape T, Zhang X, Stockley P, Severinov K, Buck M. 2008. Modus operandi of the bacterial RNA polymerase containing the σ54 promoter-specificity factor. Mol Microbiol 68:538–546. doi: 10.1111/j.1365-2958.2008.06181.x. [DOI] [PubMed] [Google Scholar]
- 7.Feng J, Goss TJ, Bender RA, Ninfa AJ. 1995. Repression of the Klebsiella aerogenes nac promoter. J Bacteriol 177:5535–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Verstraete I, Stulke J, Klier A, Rapoport G. 1995. Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J Bacteriol 177:6919–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao XJ, Huo YX, Buck M, Kolb A, Wang YP. 2007. Interplay between CRP-cAMP and PII-Ntr systems forms novel regulatory network between carbon metabolism and nitrogen assimilation in Escherichia coli. Nucleic Acids Res 35:1432–1440. doi: 10.1093/nar/gkl1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YP, Kolb A, Buck M, Wen J, O'Gara F, Buc H. 1998. CRP interacts with promoter-bound σ54 RNA polymerase and blocks transcriptional activation of the dctA promoter. EMBO J 17:786–796. doi: 10.1093/emboj/17.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rescalli E, Saini S, Bartocci C, Rychlewski L, De Lorenzo V, Bertoni G. 2004. Novel physiological modulation of the Pu promoter of TOL plasmid: negative regulatory role of the TurA protein of Pseudomonas putida in the response to suboptimal growth temperatures. J Biol Chem 279:7777–7784. doi: 10.1074/jbc.M310580200. [DOI] [PubMed] [Google Scholar]
- 12.Vitale E, Milani A, Renzi F, Galli E, Rescalli E, de Lorenzo V, Bertoni G. 2008. Transcriptional wiring of the TOL plasmid regulatory network to its host involves the submission of the σ54-promoter Pu to the response regulator PprA. Mol Microbiol 69:698–713. doi: 10.1111/j.1365-2958.2008.06321.x. [DOI] [PubMed] [Google Scholar]
- 13.Rojo F. 1999. Repression of transcription initiation in bacteria. J Bacteriol 181:2987–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojo F. 2001. Mechanisms of transcriptional repression. Curr Opin Microbiol 4:145–151. doi: 10.1016/S1369-5274(00)00180-6. [DOI] [PubMed] [Google Scholar]
- 15.Porrúa O, García-González V, Santero E, Shingler V, Govantes F. 2009. Activation and repression of a σN-dependent promoter naturally lacking upstream activation sequences. Mol Microbiol 73:419–433. doi: 10.1111/j.1365-2958.2009.06779.x. [DOI] [PubMed] [Google Scholar]
- 16.Martinez B, Tomkins J, Wackett LP, Wing R, Sadowsky MJ. 2001. Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J Bacteriol 183:5684–5697. doi: 10.1128/JB.183.19.5684-5697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govantes F, García-González V, Porrúa O, Platero AI, Jiménez-Fernández A, Santero E. 2010. Regulation of the atrazine-degradative genes in Pseudomonas sp. strain ADP. FEMS Microbiol Lett 310:1–8. doi: 10.1111/j.1574-6968.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 18.Govantes F, Porrúa O, García-González V, Santero E. 2009. Atrazine biodegradation in the lab and in the field: enzymatic activities and gene regulation. Microbial Biotechnol 2:178–185. doi: 10.1111/j.1751-7915.2008.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-González V, Govantes F, Porrúa O, Santero E. 2005. Regulation of the Pseudomonas sp. strain ADP cyanuric acid degradation operon. J Bacteriol 187:155–167. doi: 10.1128/JB.187.1.155-167.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-González V, Jiménez-Fernández A, Hervás AB, Canosa I, Santero E, Govantes F. 2009. Distinct roles for NtrC and GlnK in nitrogen regulation of the Pseudomonas sp. strain ADP cyanuric acid utilization operon. FEMS Microbiol Lett 300:222–229. doi: 10.1111/j.1574-6968.2009.01784.x. [DOI] [PubMed] [Google Scholar]
- 21.Porrúa O, García-Jaramillo M, Santero E, Govantes F. 2007. The LysR-type regulator AtzR binding site: DNA sequences involved in activation, repression and cyanuric acid-dependent repositioning. Mol Microbiol 66:410–427. doi: 10.1111/j.1365-2958.2007.05927.x. [DOI] [PubMed] [Google Scholar]
- 22.Platero AI, García-Jaramillo M, Santero E, Govantes F. 2012. Transcriptional organization and regulatory elements of a Pseudomonas sp. strain ADP operon encoding a LysR-type regulator and a putative solute transport system. J Bacteriol 194:6560–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platero AI, Santero E, Govantes F. 2014. Genetic evidence of a high-affinity cyanuric acid transport system in Pseudomonas sp. ADP. FEMS Microbiol Lett 352:150–156. doi: 10.1111/1574-6968.12392. [DOI] [PubMed] [Google Scholar]
- 24.Buck M, Cannon W. 1989. Mutations in the RNA polymerase recognition sequence of the Klebsiella pneumoniae nifH promoter permitting transcriptional activation in the absence of NifA binding to upstream activator sequences. Nucleic Acids Res 17:2597–2612. doi: 10.1093/nar/17.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morett E, Buck M. 1989. In vivo studies on the interaction of RNA polymerase-σ54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters. The role of NifA in the formation of an open promoter complex. J Mol Biol 210:65–77. [DOI] [PubMed] [Google Scholar]
- 26.Hoover TR, Santero E, Porter S, Kustu S. 1990. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell 63:11–22. doi: 10.1016/0092-8674(90)90284-L. [DOI] [PubMed] [Google Scholar]
- 27.Mandelbaum RT, Wackett LP, Allan DL. 1993. Mineralization of the s-triazine ring of atrazine by stable bacterial mixed cultures. Appl Environ Microbiol 59:1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Russell DW. 2001. Molecular cloning, a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 29.Inoue H, Nojima H, Okayama H. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23–28. doi: 10.1016/0378-1119(90)90336-P. [DOI] [PubMed] [Google Scholar]
- 30.Espinosa-Urgel M, Salido A, Ramos JL. 2000. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J Bacteriol 182:2363–2369. doi: 10.1128/JB.182.9.2363-2369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiyar A, Xiang Y, Leis J. 1996. Site-directed mutagenesis using overlap extension PCR. Methods Mol Biol 57:177–191. [DOI] [PubMed] [Google Scholar]
- 32.Miller JH. 1992. A short course in bacterial genetics: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 33.Hervás AB, Canosa I, Little R, Dixon R, Santero E. 2009. NtrC-dependent regulatory network for nitrogen assimilation in Pseudomonas putida. J Bacteriol 191:6123–6135. doi: 10.1128/JB.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson LU, Solera D, Bernardo LM, Moscoso JA, Shingle V. 2008. Sigma54-RNA polymerase controls sigma70-dependent transcription from a non-overlapping divergent promoter. Mol Microbiol 70:709–723. doi: 10.1111/j.1365-2958.2008.06440.x. [DOI] [PubMed] [Google Scholar]
- 35.Nash HA, Robertson CA, Flamm E, Weisberg RA, Miller HI. 1987. Overproduction of Escherichia coli integration host factor, a protein with nonidentical subunits. J Bacteriol 169:4124–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hervás AB, Canosa I, Santero E. 2010. Regulation of glutamate dehydrogenase expression in Pseudomonas putida results from its direct repression by NtrC under nitrogen-limiting conditions. Mol Microbiol 78:305–319. doi: 10.1111/j.1365-2958.2010.07329.x. [DOI] [PubMed] [Google Scholar]
- 37.Claverie-Martin F, Magasanik B. 1992. Positive and negative effects of DNA bending on activation of transcription from a distant site. J Mol Biol 227:996–1008. doi: 10.1016/0022-2836(92)90516-M. [DOI] [PubMed] [Google Scholar]
- 38.Molina-Lopez JA, Govantes F, Santero E. 1994. Geometry of the process of transcription activation at the σ54-dependent nifH promoter of Kebsiella pneumoniae. J Biol Chem 269:25419–25425. [PubMed] [Google Scholar]
- 39.Porrúa O, Platero AI, Santero E, Del Solar G, Govantes F. 2010. Complex interplay between the LysR-type regulator AtzR and its binding site mediates atzDEF activation in response to two distinct signals. Mol Microbiol 76:331–347. doi: 10.1111/j.1365-2958.2010.07100.x. [DOI] [PubMed] [Google Scholar]
- 40.Porrúa O, López-Sánchez A, Platero AI, Santero E, Shingler V, Govantes F. 2013. An A-tract at the AtzR binding site assists DNA binding, inducer-dependent repositioning and transcriptional activation of the PatzDEF promoter. Mol Microbiol 90:72–87. [DOI] [PubMed] [Google Scholar]
- 41.Bush M, Dixon R. 2012. The role of bacterial enhancer binding proteins as specialized activators of σ54-dependent transcription. Microbiol Mol Biol Rev 76:497–529. doi: 10.1128/MMBR.00006-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joly N, Zhang N, Buck M, Zhang Z. 2012. Coupling AAA protein function to regulated gene expression. Biochim Biophys Acta 1823:108–116. doi: 10.1016/j.bbamcr.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Kustu S, Santero E, Keener J, Popham D, Weiss D. 1989. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev 53:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ninfa A, Reitzer L, Magasanik B. 1987. Initiation of transcription at the bacterial glnAp2 promoter by purified E. coli components is facilitated by enhancers. Cell 50:1039–1046. doi: 10.1016/0092-8674(87)90170-X. [DOI] [PubMed] [Google Scholar]
- 45.Reitzer L, Magasanik B. 1986. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell 45:785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- 46.Valentin-Hansen P, Sogaard-Andersen L, Pedersen H. 1996. A flexible partnership: the CytR anti-activator and the cAMP-CRP activator protein, comrades in transcription control. Mol Microbiol 20:461–466. doi: 10.1046/j.1365-2958.1996.5341056.x. [DOI] [PubMed] [Google Scholar]
- 47.Kristensen HH, Valentin-Hansen P, Sogaard-Andersen L. 1997. Design of CytR-regulated, cAMP-CRP dependent class II promoters in Escherichia coli: RNA polymerase-promoter interactions modulate the efficiency of CytR repression. J Mol Biol 266:866–876. doi: 10.1006/jmbi.1996.0852. [DOI] [PubMed] [Google Scholar]
- 48.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 49.Govantes F, Molina-Lopez JA, Santero E. 1996. Mechanism of coordinated synthesis of the antagonistic regulatory proteins NifL and NifA of Klebsiella pneumoniae. J Bacteriol 178:6817–6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franklin FC, Bagdasarian M, Bagdasarian MM, Timmis KN. 1981. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci U S A 78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marqués S, Gallegos MT, Manzanera M, Holtel A, Timmis KN, Ramos JL. 1998. Activation and repression of transcription at the double tandem divergent promoters for the xylR and xylS genes of the TOL plasmid of Pseudomonas putida. J Bacteriol 180:2889–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Zwieb C, Wu C, Adhya S. 1989. Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene 85:15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- 53.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elliott T, Geiduschek EP. 1984. Defining a bacteriophage T4 late promoter: absence of a “-35” region. Cell 36:211–219. doi: 10.1016/0092-8674(84)90091-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.