ABSTRACT
Microbes can produce molecular hydrogen (H2) via fermentation, dinitrogen fixation, or direct photolysis, yet the H2 dynamics in cyanobacterial communities has only been explored in a few natural systems and mostly in the laboratory. In this study, we investigated the diel in situ H2 dynamics in a hot spring microbial mat, where various ecotypes of unicellular cyanobacteria (Synechococcus sp.) are the only oxygenic phototrophs. In the evening, H2 accumulated rapidly after the onset of darkness, reaching peak values of up to 30 μmol H2 liter−1 at about 1-mm depth below the mat surface, slowly decreasing to about 11 μmol H2 liter−1 just before sunrise. Another pulse of H2 production, reaching a peak concentration of 46 μmol H2 liter−1, was found in the early morning under dim light conditions too low to induce accumulation of O2 in the mat. The light stimulation of H2 accumulation indicated that nitrogenase activity was an important source of H2 during the morning. This is in accordance with earlier findings of a distinct early morning peak in N2 fixation and expression of Synechococcus nitrogenase genes in mat samples from the same location. Fermentation might have contributed to the formation of H2 during the night, where accumulation of other fermentation products lowered the pH in the mat to less than pH 6 compared to a spring source pH of 8.3.
IMPORTANCE Hydrogen is a key intermediate in anaerobic metabolism, and with the development of a sulfide-insensitive microsensor for H2, it is now possible to study the microdistribution of H2 in stratified microbial communities such as the photosynthetic microbial mat investigated here. The ability to measure H2 profiles within the mat compared to previous measurements of H2 emission gives much more detailed information about the sources and sinks of H2 in such communities, and it was demonstrated that the high rates of H2 formation in the early morning when the mat was exposed to low light intensities might be explained by nitrogen fixation, where H2 is formed as a by-product.
INTRODUCTION
Cyanobacterial mats are found in various limnic, marine, and hypersaline environments. The thickest and most homogeneous mats are found in extreme environments such as hypersaline habitats and geothermal springs with a scarcity or total absence of grazers. Such microbial mats are important natural model systems for studying microbial interactions and the fundamental links between structure, diversity, and function in microbial communities (1, 2). Another reason for the extensive interest in these mats is their strong apparent similarity to ancient microbial mats inhabiting the early Earth before grazers evolved and now preserved as stromatolites (3). Knowledge about the functioning of modern microbial mats may thus give insights into the functioning of early microbial ecosystems on planet Earth. Hydrogen (H2) presumably played a major role in the metabolism of primitive microbial communities (4), and outgassing of H2 originating or escaping from these microbial communities might have played a major role in the gradual oxidation of our planet (5).
Coastal and hypersaline cyanobacterial mats are known to be rather vigorous producers of H2 during darkness (5–10), and some H2 production may also be observed in the beginning of a light period due to photolysis (10). Microcoleus sp., which was a dominating cyanobacterium in the mat H2 evolution studies listed above, is known to be a vigorous H2 producer during fermentation (11). Burow et al. (7) showed that the H2 production in the dark in a hypersaline microbial mat might be ascribed to cyanobacterial fermentation, whereas significant H2 production from N2 fixation as a by-product of nitrogenase activity (N2 + 8H+ + 8e− → 2NH3 + H2) was not verified. A detailed analysis of an intertidal cyanobacterial mat dominated by Microcoleus sp. using sulfide-insensitive H2 microsensors showed that the maximum H2 concentrations reached 40 μmol H2 liter−1 (depending on the previous light exposure history of the mat) within the first hour after darkening (10), whereafter the H2 concentration decreased to near zero within about 7 h. A main reason for this decrease was consumption by sulfate-reducing bacteria (SRB), as addition of the sulfate analogue and SRB inhibitor molybdate led to even higher H2 concentrations that persisted for a much longer time period. In another coastal microbial mat, SRB were also identified as the major hydrogenotrophic prokaryotes (12).
The hot spring microbial mats in the siliceous alkaline Mushroom Spring and nearby Octopus Spring have been extensively studied for more than 40 years as natural model systems for investigating in situ interactions of metabolic processes and microbial diversity (1, 2). In these hot springs, microbial mats growing at water temperatures of >54°C show a restricted diversity, with various ecotypes of the cyanobacterium Synechococcus (13) being the only oxygenic phototrophs. Metagenomic and metatranscriptomic analyses have demonstrated a relatively low diversity of taxonomic groups, including several photoheterotrophic or photomixotrophic Chloroflexi (Roseiflexus-, Chloroflexus-, and Anaerolineae-like taxa), photoheterotrophic acidobacteria (Chloracidobacterium), Chlorobi (“Candidatus Thermochlorobacter”), and two novel aerobic heterotrophic taxa (14–17). Such relatively limited diversity has made these hot spring mats attractive for the study of metabolic interactions among taxa under in situ conditions and under a natural diel cycle. In the slightly alkaline 50 to 70°C Octopus Spring mat, for example, it was shown that the two most abundant taxa, Synechococcus and Roseiflexus, produce glycogen (18) (and in Roseiflexus, polyhydroxyalkanoic acid as well [17, 19]), which they ferment at nighttime to produce large amounts of acetate and propionate that are photoassimilated by filamentous, Chloroflexus-like organisms (probably related to Roseiflexus [20]) the next morning (19, 21, 22). Both Anderson et al. (22) and van der Meer (23) reported that H2 was formed during darkness in the mats, but the accumulation was restricted by diffusion into the overlying water (19) and by H2 consumption by SRB (24, 25) and methanogenic bacteria (21, 26) in the 50 to 60°C mat. The rates of H2 accumulation were much higher in the 65°C mat, which did not exhibit any methanogenesis.
Genomic and metagenomic analyses of microbial mats in Mushroom Spring and Octopus Spring have demonstrated that Roseiflexus (15, 27), but not Synechococcus (28), possesses hydrogenases and might thus produce H2 by fermentation. The lack of hydrogenases in Synechococcus means that any H2 produced by Synechococcus nitrogen fixation is lost to the environment where other mat organisms may use it as an electron donor in anoxygenic photosynthesis or respiration. Metatranscriptomic studies conducted on a 60°C mat in Mushroom Spring demonstrated that the expression of Synechococcus nitrogenase genes occurred in darkness and that N2 fixation peaked in the early morning under dim light conditions below the compensation irradiance, keeping the mat largely anoxic (16, 29, 30). Based on these previous observations, we hypothesized that H2 formation in Mushroom Spring and Octopus Spring cyanobacterial mats would exhibit a peak in the early morning due to N2 fixation and a peak after sunset that might be caused by fermentation and N2 fixation supplied with ATP from fermentation.
In this study, we tested this hypothesis by quantifying the diel H2 dynamics in a Mushroom Spring microbial mat at a hitherto unreached spatiotemporal resolution using a newly developed sulfide-insensitive H2 microsensor (31). Earlier studies showed the potential for H2 production in dark-incubated mats (22), including a few, albeit uncalibrated, H2 microsensor measurements (23). The sensitivity of the previous H2 microsensors to hydrogen sulfide strongly limited their applicability for studying the zonation and dynamics of H2 metabolism in microbial mats. By use of the improved microsensor for H2 in combination with microsensors for O2, H2S, and pH, it was possible to describe the chemical microenvironment in the mat in great detail, enabling new insights into the regulation of H2 dynamics.
MATERIALS AND METHODS
Mushroom Spring is a siliceous, slightly alkaline hot spring (2) located in the Lower Geyser Basin of Yellowstone National Park (Wyoming). The spring has a large source pool that drains 68 to 70°C water through a single effluent channel that gradually widens, while the spring water is cooling down. Our study site had a water temperature throughout the diel cycle of 58 ± 3°C and harbored a microbial mat with an ∼1-mm-thick green cyanobacterial layer on top of an orange undermat. Field measurements were conducted on 12 to 13 August 2014, and the associated laboratory experiments were done a few days earlier.
Most measurements were done in situ, but a few cores (8-mm diameter, about 1-cm deep) were sampled and brought back to the laboratory for preliminary analysis. Samples were transported to the laboratory in a 1-liter stainless steel thermos filled with in situ water and were then immediately placed in a 58°C water bath filled with stirred in situ water. A fiberoptic halogen lamp equipped with a collimating lens (KL-2500; Schott, Germany) illuminated the mat samples with a downwelling photon irradiance of 1000 μmol photons m−2 s−1, as determined with a photosynthetically active radiation (PAR) quantum irradiance sensor (MQS-B; Heinz Walz GmbH, Effeltrich, Germany).
Concentrations of dissolved H2 and O2 and pH were measured in the mat and overlying spring water using microsensors. The H2 microsensors (Unisense A/S) had tip diameters of 70 μm and were made H2S insensitive by placing a ZnCl2-propylene carbonate-based H2S trap in front of the H2 microsensor tip. The addition of the sulfide trap, furthermore, lowered the baseline current by lowering the H2O influx to the anode so that the sensors could be used at temperatures up to about 60°C (31). The sensors optimally have a detection limit of about 20 nmol H2 liter−1 (31), but at the high temperature of the spring and lack of continuous signal recording, the detection limit was 0.2 μmol H2 liter−1, corresponding to a 1-pA change in signal. The sensors were four-point calibrated (0 to 69 μmol H2 liter−1) in the laboratory at 58°C, verifying the linearity of the signal, and two-point calibrated (0 to 34 μmol H2 liter−1) in the field. The standards were prepared using dilutions of water saturated with H2 at 20°C (805 μmol H2 liter−1 at sea level pressure, but 693 μmol H2 liter−1 at the barometric pressure of Bozeman, MT, where the bottle was prepared) (32). This procedure may induce some inaccuracy in the calibration, as the temperature of our supposedly 693 μmol H2 liter−1 solution containing a H2 headspace varied under field conditions, causing changes in the equilibrium between gas-phase and dissolved H2, and the temperature of the 34 μmol H2 liter−1 standard was also affected by the dilution. We estimate that the potential error in the calibration might be as high as 15%. The sensors gave a linear response of about 4 pA per μM H2 at the in situ temperature of 58°C. The sensors needed about 30 s of equilibration at each depth and concentration profile; we therefore only measured with 0.5-mm depth resolution.
The O2 microsensors (33) had a tip diameter of 10 μm and were made in our laboratory at Aarhus University (AU). They were linearly calibrated in the field based on sensor readings in anoxic parts of the mat (zero oxygen) and in spring water (58°C) equilibrated with air by vigorous shaking of a 50-ml centrifuge tube that was about 70% filled with spring water and with intermittent placement in the spring to keep the temperature near 58°C. At the atmospheric pressure for the 2,227-m elevation of Mushroom Spring, it is necessary to multiply the saturations from standard tables (http://www.unisense.com/files/PDF/Diverse/Seawater%20&%20Gases%20table.pdf) by a factor of 0.77, resulting in an O2 concentration of 118 μmol liter−1. Due to nonperfect temperature control during air equilibration, we estimate a potential error in the calibration of up to 15%.
The pH microelectrode was made in our laboratory at AU and had a 250-μm-long pH-sensitive glass tip and a tip diameter of 40 μm (34). It was calibrated in pH 6.96 and pH 9.76 buffers at 58°C using a commercial reference electrode (REF201; Radiometer, Denmark) and yielding a sensitivity of 58 mV per pH unit.
Laboratory profiles of H2S were measured with an amperometric H2S microsensor (35) having a tip diameter of 30 μm. The sensor was made in our laboratory at AU and was painted black to avoid light interference on the signal (35). The sensor was linearly calibrated from signal readings in H2S-free water and in a standard prepared by dissolving a washed Na2S · 9H2O crystal in 10 μmol liter−1 anoxic HCl (10 mmol liter−1) to a final concentration of 200 μmol H2S liter−1. The calibration was done within a few minutes of preparation of the standard.
The signals from the O2, H2, and H2S microsensors were read by a custom-built, battery-operated pA meter with a resolution of 10−13 A. The signal from the pH sensor was read with a custom-built, battery-operated high-impedance voltmeter with an internal resistance of 1014 ohms. Both meters were enclosed in plastic bags with silica gel during field measurements. In the laboratory and during daytime field measurements, the instruments were connected to a battery-operated strip-chart recorder (SE-110; Gossen-Metrawatt, Germany), while signals were read directly from the meter display during nighttime as steam from the hot spring water caused excessive condensation of water on all surfaces.
The microsensors were mounted and advanced vertically into the microbial mat with manually operated micromanipulators that were read with better than 10-μm accuracy. The micromanipulators were attached to heavy metal stands.
The field measurements were initiated in the late afternoon, but in the afternoon and evening only single profiles for all three parameters were measured. In the morning, this was changed to triplicate measurements for H2, but still only single profiles for O2 and pH. The three locations for measurement of H2 were chosen at random within the 2.5- by 2.5-cm square covered by the x-y positioning of the micromanipulator.
Simultaneous with the diel microsensor measurements, 1-liter bottles were filled to capacity with 58°C spring water, and an 8-ml headspace of atmospheric air was introduced into the bottles through a butyl rubber septum. After equilibration of the water with the gas phase via 30 s of vigorous shaking, the H2 gas concentration in the headspace was immediately analyzed on-site using a portable gas chromatograph (GC) (490 micro GC; Agilent Technologies, Santa Clara, CA, USA) using a thermal conductivity detector connected to a 10-m unheated MolSieve 5-Å column, running with a flow of 99.9999% pure argon as the carrier gas. A standard curve for the analysis was obtained by adding various volumes of H2-saturated water to spring water sampled during daytime when there was no H2 in the spring water. The detection limit of the GC was 4 nmol H2 liter−1.
Temperature loggers (iButton DS1922L; Maxim Integrated Products, Inc., San Jose, CA, USA) were placed in the spring on the biofilm surface, logging the spring water temperature at 5-min intervals. Downward photon irradiance of PAR (400 to 700 nm) was monitored continuously in air throughout the field measurements with a PAR quantum irradiance sensor (MQS-B; Heinz Walz GmbH, Effeltrich, Germany) placed next to the spring and connected to a light meter (ULM-500; Heinz Walz GmbH).
Based on the in situ concentration profiles measured over a diel cycle, isopleths of pH, O2, and H2 concentrations were generated using the software package Ocean Data View (http://odv.awi.de) based on weighted-average gridding.
RESULTS
The temperature of the spring water flowing above the mat was relatively constant over time, but lower air temperatures at night reduced the water temperature to ∼55°C and solar exposure during daytime caused maximum temperatures of up to 60°C at noon (Fig. 1). The incident photon irradiance was fluctuating in the afternoon prior to 19:00 due to shifting cloud cover but exhibited a very smooth curve in the interesting regions around sunset and sunrise. A photon irradiance of ∼1% of maximum noontime irradiance (1,500 μmol photons m−2 s−1) was reached at 20:20 in the evening and at 6:28 in the morning.
FIG 1.
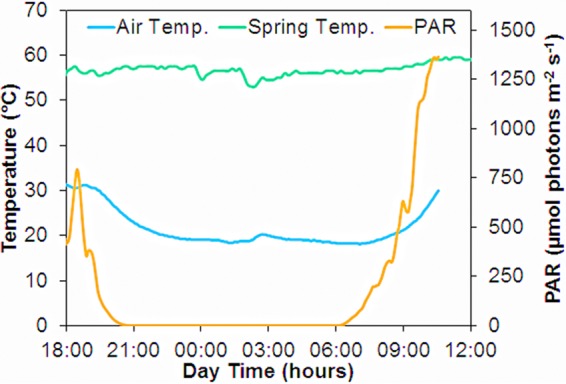
Photon irradiance (400 to 700 nm), air temperature 10 cm above the water, and water temperature at the mat surface measured throughout the diel experimental period. The dip in irradiance at 18:00 was due to cloud cover.
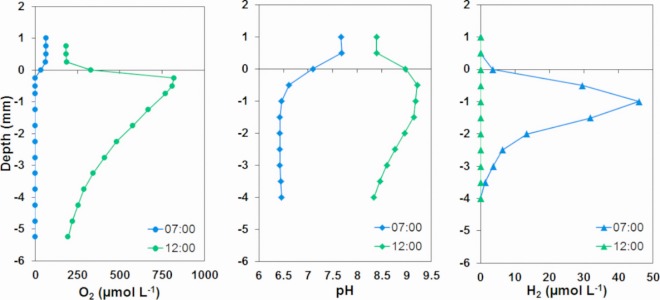
The chemistry of the Mushroom Spring mat changed dramatically throughout the diel cycle. To illustrate this, pH, O2, and H2 concentration profiles measured at noon and at sunrise (7:00) down to 4- to 6-mm depth in the mat are shown in Fig. 2. At sunrise, the irradiance was too low (60 μmol photons m−2 s−1) (Fig. 1) to cause a net production of O2, and O2 only penetrated to about 0.3-mm depth. At noon, oxygenic photosynthesis caused an oxygen peak in the surface layer of >800 μmol O2 liter−1, and O2 was present in high concentrations down to the deepest analyzed layer at 5.25 mm. The pH values showed similar large changes with the water pH increasing from 7.4 at 7:00 to 8.5 at noon and pH at 1-mm depth increasing from 6.1 to 9.4 in the same period. Hydrogen was present down to 3-mm depth at 7:00, and a maximum concentration of 46 μmol H2 liter−1 was found at 1.5-mm depth. At noontime, no H2 was detected in the mat.
FIG 2.
In situ vertical profiles of O2, pH, and H2 levels in the Mushroom Spring mat measured during the period with maximum H2 concentrations (7:00) and at noontime (12:00). The conversion factor from micromolar H2 to parts per million by volume (ppmv) is 1 μmol H2 liter−1 = 1,613 ppmv (at the elevation of Mushroom Spring).
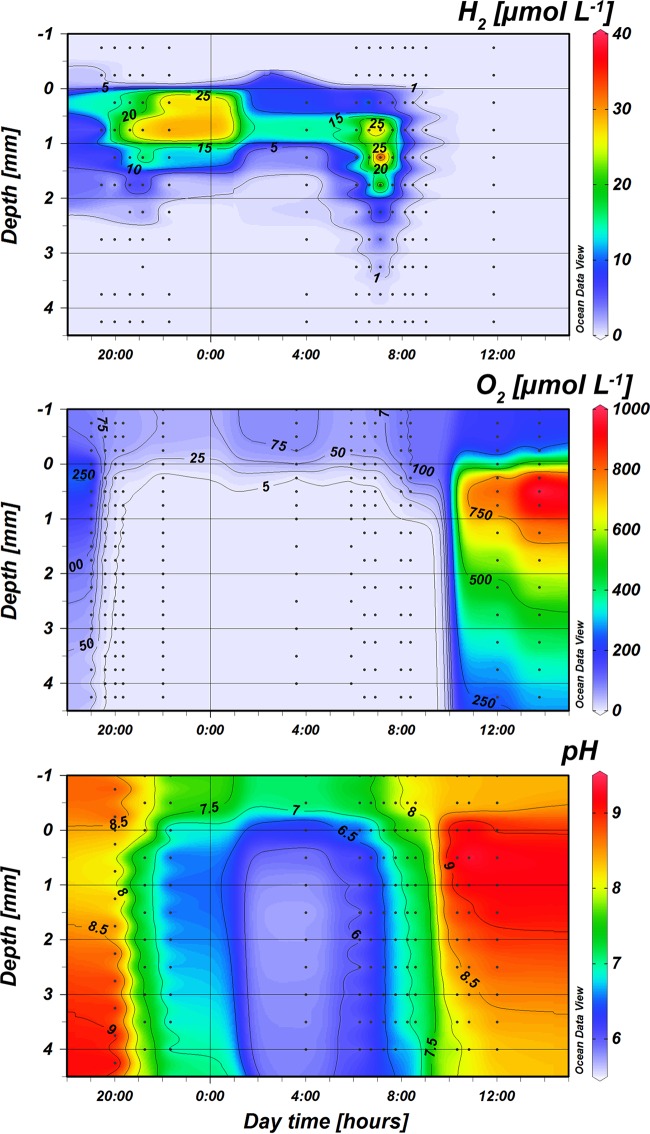
Isopleths of pH, O2, and H2 concentrations in the mat through the diel cycle (18:00 to 12:00) are shown in Fig. 3. The data points used for construction of the H2 isopleths are the average values for three measurements in the period from 4:00 to 10:00.
FIG 3.
Isopleths of O2, pH, and H2 depth distribution over a diel cycle in the 58°C Mushroom Spring mat. The measured profiles on which the interpolations were based are shown as dots. The last O2 profile in the morning was recorded at 8:22 when the first peak of O2 was recorded, the upper 1 mm of the mat became oxic, and H2 became nondetectable. Notice that the interpolation for H2 between about 23:00 and 3:00 is uncertain due to a lack of measuring points during this time interval.
The diel variation in the H2 concentrations within the mat exhibited two peaks (Fig. 4): a broader peak with maximum concentrations of about 30 μmol H2 liter−1 after sunset (20:30 to 22:15) that faded away to reach about 11 μmol H2 liter−1 just before sunrise; and a sharp peak in the early morning at 7:00 under a low diffuse photon irradiance of ∼60 μmol photons m−2 s−1. The average maximum H2 concentration from the three profiles measured at 7:00 was 46 μmol H2 liter−1, but the profiles were very different with maximum concentrations at 1- to 1.5-mm depth of 49, 27, and 64 μmol H2 liter−1, respectively. The depth penetration of H2 was also variable, with two profiles extending down to 2 mm, whereas one profile had a H2 penetration of 4 mm. The deep H2 penetration seen in Fig. 3 at 7:00 is thus due to one atypical profile and should not be overinterpreted. Similar variations among sites were also found in the other morning H2 concentration profiles as evident from the large standard deviations in Fig. 4.
FIG 4.
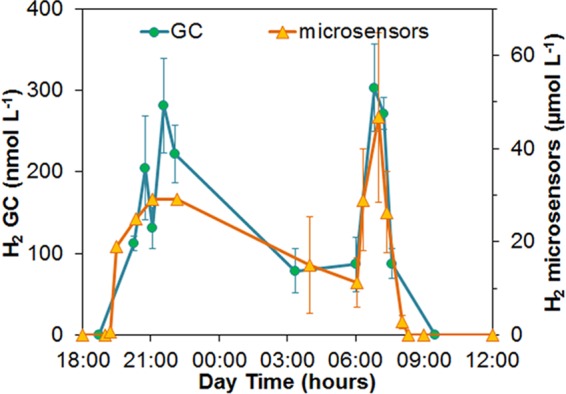
Maximum in situ H2 concentrations in the 58°C mat as measured with microsensors, and H2 concentrations in the overlying spring water as measured by GC during the diel cycle. Only one profile was measured for each time point in the evening, whereas three profiles at random positions were measured in the morning. Standard deviations are thus shown for the morning values.
The first trace of H2 (0.7 μmol H2 liter−1) in the evening was found at 19:25 when there was still an irradiance of 170 μmol photons m−2 s−1. An O2 profile was recorded 10 min later (19:35; at an irradiance of 100 μmol photons m−2 s−1), and at that time the O2 peak had disappeared and the mat was anoxic below 0.5-mm depth. A similar high photon irradiance was needed to cause a significant buildup of O2 in the mat in the morning, where a photon irradiance of 250 μmol photons m−2 s−1 at 7:58 did not cause significant change. However, the following O2 concentration profile measured at 8:22 (350 μmol photons m−2 s−1) exhibited an O2 peak and a 0.75-mm increase in O2 penetration depth compared to that for the dark profiles. The H2 concentration decreased to one-third from 7:00 to 7:36, although we did not detect a change in the O2 concentration profile until 7.58, where no H2 was detected in the mat. The depth resolution of our measurements was, however, rather crude with O2 being measured only every 0.25 mm and pH and H2 every 0.5 mm. While a slight increase in O2 penetration between 7:00 and 7:58 that went undetected cannot be ruled out, the O2 concentration in the overlying water remained constant at 61 to 63 μmol O2 liter−1 during the measurements at 5:53, 6:26, and 6:53, before showing a slight increase to 91 μmol O2 liter−1 at 7:58. Consequently, there cannot have been any change in the O2 profile until after 6:53 (and possibly considerably later), while the morning peak in H2 concentrations in the mat was measured from 6:32 to 7:40.
The standard deviations on the measured H2 concentrations are substantial, and based on the data from the H2 microprofiles alone, we cannot conclude that the two peaks in H2 accumulation are representative. However, the efflux of H2 from the mat was investigated by GC analysis, and the data from these measurements also showed a two-peak pattern (Fig. 4). However, the H2 content in the spring water was probably strongly affected by the H2 emission from mats further upstream and thus presents some kind of integration of the H2 efflux from mats growing at 58 to 70°C.
Concentration profiles of O2 and H2S were measured in mat cores that were brought back to the laboratory. We also attempted H2S measurements in the field, but insufficient optical insulation of the H2S microsensors (35) destroyed these before H2S might have accumulated during the night. The laboratory measurements showed a maximal concentration of 70 μmol H2S liter−1 (nondissociated H2S; total sulfide is higher) at ∼1.5 mm below the mat surface after 2 h of dark incubation, decreasing to 40 μmol H2S liter−1 at 6-mm depth (data not shown). An earlier study of the Mushroom Spring mat found even lower free H2S concentrations (23), while Dillon et al. (25) reported maximum concentrations of 250 μmol H2S liter−1 and substantial sulfate reduction rates in the upper mat layers. The H2 sensor applied in the field was only tested for H2S insensitivity up to ∼200 μmol H2S liter−1, but H2S interference on the H2 signal would show up as a high signal increasing with depth (9, 10), while all H2 profiles showed zero concentration in deeper layers.
DISCUSSION
Earlier studies have also reported H2 formation in hot spring microbial mats, where H2 production may be due to fermentation (18, 19, 22, 36) or to formation as a by-product of nitrogenase activity during N2 fixation (30). The relative importance of these processes for H2 dynamics in hot spring cyanobacterial communities has remained unclear, while studies of hypersaline and coastal cyanobacterial mats have identified fermentation as the major H2 source (7–9). In this study, we show that the H2 production/accumulations in the Mushroom Spring mat correlate well with the patterns of nitrogenase expression and activity described in previous studies.
Most nonheterocystous cyanobacteria are prone to O2 inhibition of the nitrogenase enzyme catalyzing N2 fixation (37), although some can perform the process under fully oxic conditions (38–40). In cyanobacterial mats found at ∼55 to 65°C in Mushroom Spring and nearby Octopus Spring, N2 fixation is limited to conditions of hypoxia or anoxia in the mat during low irradiance and nighttime (29, 30). Dinitrogen fixation is a very energy-demanding process, and the finding of the highest levels of nitrogenase activity at sunrise was explained by the presence of light for ATP production, while the mat was still largely anoxic due to the consumption of O2 within the mat being higher than the production (30).
We observed a peak in H2 production exactly in the period where the cyanobacterial layer of the mat was still largely anoxic under a low incident photon irradiance of about 60 μmol photons m−2 s−1. However, it cannot be ruled out that the H2 production continued to be high later in the morning also, but that autotrophic or mixotrophic anoxygenic phototrophy by Chloroflexus-like organisms (17, 23) and oxic metabolism based on cryptic oxygenic photosynthesis consumed this H2. Prokaryotes having high-affinity terminal oxidases are not restricted in their O2 consumption until they experience concentrations of a few nanomoles of O2 per liter (41), and an environment with the simultaneous presence of H2 and cryptic O2 production by oxygenic photosynthesis would select for knallgas bacteria (i.e., bacteria oxidizing H2 with O2) having such high-affinity terminal oxidases.
High levels of nitrogenase gene expression (16, 29, 30) and N2 fixation activity (30) have been observed at sunset in the Octopus Spring and Mushroom Spring mats. While we cannot rule out the possibility that all H2 production in the afternoon-evening transmission was due to nitrogenase activity, the massive accumulation of fermentation products shown in dark-incubated mats from Mushroom Spring (19, 22) and the very low nighttime pH values measured in this study provide evidence that fermentation might be another source of H2 accumulation in the mat. Previously measured accumulations of acetate plus propionate in dark-incubated vials containing a 1-cm-deep core of the mat (22) thus indicate that volatile fatty acids may build up to tens of millimolar concentrations in the upper millimeters of the mat during the night. Furthermore, metagenomic analysis of the mat has demonstrated the presence of many taxa that possess hydrogenase genes and should be capable of producing H2 during fermentation (42).
The balance between nitrogenase- and fermentation-mediated H2 production in a hypersaline mat dominated by Microcoleus sp. was investigated by Burow et al. (7), and they only found evidence of fermentation as a source of H2, which was further supported in a recent study of H2 dynamics in intertidal cyanobacterial mats (9). Diel changes in N2 fixation in Mushroom Spring were investigated by Steunou et al. (30), who found early morning peak rates of nitrogenase activity (i.e., acetylene reduction) in the illuminated but largely anoxic mat that were >10 times higher than the nitrogenase activity measured just before sunset, indicating a pronounced potential stimulation of H2 formation by low light. There are no known mechanisms whereby light stimulates fermentation, but low irradiance might provide energy for cyanobacterial N2 fixation (and associated H2 production) in three different ways: (i) by cryptic oxygenic photosynthesis producing both ATP and NADH, but where O2 does not build up as the consumption in the mat is larger than the production, (ii) by respiratory use of the O2 produced by cryptic oxygenic photosynthesis, and (iii) by photosystem I (PSI)-driven ATP formation without O2 evolution by use of the Mehler reaction (43). We conclude that the high H2 concentration measured in the Mushroom Spring mat during the morning was most likely due to light-driven alleviation of energy limitation fueling intense N2 fixation. Diazotrophs other than Synechococcus might in principle produce H2, but the light stimulation of H2 formation indicates that the dominating N2-fixing organisms should be phototrophs, and the abundant anoxygenic phototrophs in the mat (Roseiflexus and Chloroflexus) have not been shown to express genes for N2 fixation (30). Instead of producing H2, these organisms may use H2 as an electron donor (30). The quantitative contribution of fermentation to H2 production in the Mushroom Spring mat remains unclear. Steunou et al. (30) found that N2 fixation (nitrogenase activity) in the evening just after sunset was only 20% of the peak morning values at low light intensities, whereas we found only 1 1/2 times higher (H2 profiles, Fig. 4) or similar (overlying water H2, Fig. 4) H2 concentrations in the morning peak compared to those in the evening. Just before sunrise, the nitrogenase activity was <5% of the peak rates found just 2 h later. The discrepancy between the nitrogenase activity pattern and H2 accumulation indicates that fermentation might be a major factor responsible for the H2 accumulation in the evening and night.
High morning rates of N2 fixation and associated H2 production might also be significant in other types of cyanobacterial mats. Diel variations in N2 fixation in marine cyanobacterial mats have been subjected to investigation (44), but the expression of nitrogenase genes and rates of N2 fixation were not found to correlate, and no morning peak in N2 fixation was detected. However, if high morning rates were present, it is unlikely that they would result in massive H2 accumulation as H2 produced by nitrogenase activity in the early morning may be consumed rapidly by SRB that are much more abundant in marine and hypersaline mats and that may be very active after several hours of anoxia. It was thus shown that high concentrations of H2 were found in a marine cyanobacterial mat throughout an 8-h dark period when molybdate was added as an inhibitor of sulfate reduction, while a mat without inhibitor addition was depleted of H2 within a few hours (10). We note that SRB might potentially also contribute significantly to N2 fixation in such mats, but a recent study only showed a minor contribution of SRB to N2 fixation in a hypersaline mat (45).
We only found measurable H2 levels down to 4.5 mm below the mat surface, and H2 never accumulated in the deeper parts of the Mushroom Spring mat. High rates of sulfate reduction have been measured in the Mushroom Spring mat (25), and sulfate reduction might thus cause depletion of H2 in deeper layers. In nearby Octopus Spring, methanogenesis was shown to be the major sink for H2 in dark-incubated 55°C mats (22), where addition of the methanogenesis inhibitor bromoethane sulfonic acid (BES) led to massive efflux of H2 from mat samples. An efflux of 0.2 μmol H2 cm−2 h−1 can be calculated from the profile in Fig. 2 using Fick's first law of diffusion and the diffusion coefficient for H2 at 58°C in water (9.7 × 10−5 cm2 s−1) (tables at www.unisense.com), and that is essentially identical to the H2 formation rate after BES addition found earlier. Similar rates of methanogenesis (0.04 μmol CH4 cm−2 h−1 corresponding to a consumption of 0.16 μmol H2 cm−2 h−1) were measured in the 60°C Mushroom Spring mat (46), i.e., close to the site analyzed in this study (58°C). Both sulfate reducers and methanogens might thus have contributed to keeping the H2 concentrations below the detection limit in deeper mat layers (>4.5-mm depth). High-sensitivity gas chromatography on anoxic mat material from deeper layers would probably have shown H2 concentrations of 1 to 10 nmol liter−1 as typically found in methanogenic and sulfate-reducing environments (47), but such concentrations are more than 1 order of magnitude lower than the detection limit of our sensors, which at the high temperature of the mat was 0.2 μmol liter−1. The mat was oxic far below 4.5-mm depth during the daytime (Fig. 3), but analysis of mat samples (from Octopus Spring) that were oxic during the daytime showed abundant presence of methanogenic archaea (107 to 108 cells g−1 wet weight), and pure cultures isolated from the samples survived hours of oxic conditions (24; N. P. Revsbech and D. M. Ward, unpublished results).
We found early morning pH levels (<pH 6) in the mats that were lower than the pH minimum of 7 measured earlier in Octopus Spring (48) and Mushroom Spring (49) mats, and we speculate whether a change in sensor calibration during the measuring period might be the explanation. A change in the reference potential might cause such effects, while it is not possible to obtain an increase in pH microsensor sensitivity significantly above the 58 mV per pH unit determined in the calibration. The measured pH at 2- to 3-mm depth was 1.4 pH unit below the water reading at 4:00 and as other investigations (48–50) found early morning pH values in the overlying water from 7.7 to 7.9 in similar settings, it can be concluded that the pH in the mat must have been below 6.5. Our site was far outside the main flow channel, and the overlying water pH might have been further lowered as seen in our data (pH 7.1 at 4:00) by a long contact time with the mat, resulting in a pH minimum in the mat considerably below 6.5. The extensive effects of mat-water exchange on the overlying water chemistry are also evident from the O2 measurements showing minimum O2 concentrations of 61 μmol O2 liter−1 from 6:00 to 7:00 and up to 185 μmol O2 liter−1 at 12:00.
Although we did not observe any change in O2 profile in the mat until after 8:00 in the morning, with the first significant change observed at 8:22, a substantial change in the pH profile was observed. At 4:00, the overlying water was at pH 7.1, and the minimum pH at 1.5-mm depth was ∼5.7. At 7:12, coinciding with the maximum H2 accumulation, the pH in the water had increased to pH 7.7, and the pH at 1.5-mm depth was pH 6.4. At 7:43, the pH in the water was 8.0, and the pH at 1.5-mm depth was 6.8. These rather large deviations in overlying water pH compared to that for the source water (pH 8.3) (23) indicate that there was high photosynthetic activity in the mat prior to 8:22, although this did not result in an accumulation of O2 as the incident photon irradiance was at or below the compensation irradiance, where O2 production and consumption processes balanced each other. It is not possible to estimate how much of this photosynthetic activity was due to the oxygenic cyanobacteria or the anoxygenic phototrophs in the mat, but photomixotrophic anoxygenic metabolism has been documented for the filamentous anoxygenic phototrophic bacteria, Chloroflexus spp. and Roseiflexus spp., that are abundant in the Mushroom Spring mat (19), and these organisms may thus both fix CO2 and photoassimilate volatile fatty acids (VFAs) (22).
Both the N2 fixation by Synechococcus and the photoassimilation of fermentation products are facilitated by the diel changes in chemistry and the light regime of the mat. While Chloroflexus aurantiacus and Roseiflexus castenholzii can be cultured in laboratory media supplied with, for example, acetate (27, 51), high concentrations of acetate are usually not found in static environments. Microbial specialization to utilize the metabolic opportunities created by cyclic changes in environmental conditions (e.g., 19) may be more common than generally realized. Other well-known examples are the accumulation of polyphosphate by, e.g., “Candidatus Accumulibacter phosphatis” during the oxic part of oxic/anoxic cycles (52) and the accumulation of elemental sulfur in the beginning of a light period by purple sulfur bacteria (53).
In conclusion, our in situ measurements of the diel H2, O2, and pH dynamics in the Mushroom Spring microbial mat showed strong evidence for nitrogenase activity being a major source of H2 accumulating in the mat during the evening and especially in the early morning, when dim irradiance alleviated energy limitation in the upper mat layers. Photosynthetic activity at such times with low irradiance may have profound effects on microbial communities by supplying energy to anoxic types of metabolism and possibly also oxic metabolism occurring at nondetectable O2 concentrations.
ACKNOWLEDGMENTS
We thank Preben Sørensen and Lars Borregaard Pedersen for the construction of the microsensors, Emilio Garcia-Robledo for help with illustrations, and the US National Park Service and personnel from Yellowstone National Park for their permission to conduct this work and their helpful assistance.
This study was supported by the European Research Council (“Oxygen,” grant 267233; N.P.R.), the Grundfos Foundation (N.P.R.), an Elite Researcher Travel Stipend from the Danish Council for Independent Research (E.T.), an instrument grant from the Carlsberg Foundation (M.K.), and a Sapere-Aude Advanced Grant from the Danish Council for Independent Research/Natural Sciences (M.K.). D.M.W. appreciates the support from the Montana Agricultural Experiment Station (project 352).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Ward DM, Bateson MM, Ferris MJ, Nold SC. 1998. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev 62:1353–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward DM, Bateson MM, Ferris MJ, Kühl M, Wieland A, Koeppel A, Cohan FM. 2006. Cyanobacterial ecotypes in the microbial mat community of Mushroom Spring (Yellowstone National Park, Wyoming) as species-like units linking microbial community composition, structure and function. Philos Trans R Soc Lond B Biol Sci 361:1997–2008. doi: 10.1098/rstb.2006.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awramik SM. 1984. Ancient stromatolites and microbial mats, p 1–22. In Cohen Y, Castenholz RW, Halvorson HO (ed), Microbial mats: stromatolites. Liss, New York, NY. [Google Scholar]
- 4.Rees DC, Howard JB. 2003. The interface between the biological and inorganic worlds: iron-sulfur metalloclusters. Science 300:929–931. doi: 10.1126/science.1083075. [DOI] [PubMed] [Google Scholar]
- 5.Hoehler T, Bebout BM, Des Marais DJ. 2001. The role of microbial mats in the production of reduced gases on the early Earth. Nature 412:324–327. doi: 10.1038/35085554. [DOI] [PubMed] [Google Scholar]
- 6.Skyring GW, Lynch RM, Smith GD. 1989. Quantitative relationships between carbon, hydrogen, and sulfur metabolism in cyanobacterial mats, p 170–179. In Cohen Y, Rosenberg E (ed), Microbial mats, physiological ecology of benthic microbial communities. American Society for Microbiology, Washington, DC. [Google Scholar]
- 7.Burow LC, Woebken D, Bebout BM, McMurdie PJ, Singer SW, Pett-Ridge J, Prufert-Bebout L, Spormann AM, Weber PK, Hoehler TM. 2012. Hydrogen production in photosynthetic microbial mats in the Elkhorn Slough estuary, Monterey Bay. ISME J 6:863–874. doi: 10.1038/ismej.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JZ, Burow LC, Woebken D, Everroad RC, Kubo MD, Spormann AM, Weber PK, Pett-Ridge J, Bebout BM, Hoehler TM. 2014. Fermentation couples Chloroflexi and sulfate-reducing bacteria to cyanobacteria in hypersaline microbial mats. Front Microbiol 5:61. doi: 10.3389/fmicb.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann D, Maldonado J, Wojciechowski MF, Garcia-Pichel F. 2015. Hydrogen export from intertidal cyanobacterial mats: sources, fluxes, and the influence of community composition. Environ Microbiol 17:3738–3753. doi: 10.1111/1462-2920.12769. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen M, Revsbech NP, Kühl M. 2015. Microsensor measurements of hydrogen gas dynamics in cyanobacterial microbial mats. Front Microbiol 6:726. doi: 10.3389/fmicb.2015.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kothari A, Potrofka R, Garcia-Pichel F. 2012. Diversity in hydrogen evolution from bidirectional hydrogenases in cyanobacteria from terrestrial, freshwater and marine intertidal environments. J Biotechnol 162:105–114. doi: 10.1016/j.jbiotec.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Burow LC, Woebken D, Marshall IPG, Singer SW, Pett-Ridge J, Prufert-Bebout L, Spormann AM, Bebout BM, Weber PK, Hoehler TM. 2014. Identification of Desulfobacterales as primary hydrogenotrophs in a complex microbial mat community. Geobiology 12:221–230. doi: 10.1111/gbi.12080. [DOI] [PubMed] [Google Scholar]
- 13.Ward DM, Castenholz RW, Miller SR. 2012. Cyanobacteria in geothermal habitats, p 39–63. In Whitton BA. (ed), Ecology of Cyanobacteria, 2nd ed Springer Science+Business Media, Heidleberg, Germany. [Google Scholar]
- 14.Bryant DA, Costas AMG, Maresca JA, Chew AGM, Klatt CG, Bateson MM, Tallon LJ, Hostetler J, Nelson WC, Heidelberg JF, Ward DM. 2007. Candidatus Chloracidobacterium thermophilum: an aerobic phototrophic acidobacterium. Science 317:523–526. doi: 10.1126/science.1143236. [DOI] [PubMed] [Google Scholar]
- 15.Klatt CG, Wood JM, Rusch DB, Bateson MM, Hamamura N, Heidelberg JF, Grossman AR, Bhaya D, Cohan FM, Kühl M, Bryant DA, Ward DM. 2011. Community ecology of hot spring cyanobacterial mats: predominant populations and their functional potential. ISME J 5:1262–1278. doi: 10.1038/ismej.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Klatt CG, Ludwig M, Rusch DB, Jensen SI, Kühl M, Ward DM, Bryant DA. 2012. “Candidatus Thermochlorobacter aerophilum”: an aerobic chlorophotoheterotrophic member of the phylum Chlorobi defined by metagenomics and metatranscriptomics. ISME J 6:1869–1882. doi: 10.1038/ismej.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klatt CG, Liu Z, Ludwig M, Kühl M, Jensen SI, Bryant DA, Ward DM. 2013. Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a mat in a geothermal spring. ISME J 7:1775–1789. doi: 10.1038/ismej.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Meer MTJ, Schouten S, Sinninghe Damsté JS, Ward DM. 2007. Impact of carbon metabolisms on 13C signatures of cyanobacteria and green nonsulfur-like bacteria inhabiting a microbial mat from an alkaline siliceous hot spring in Yellowstone National Park (USA). Environ Microbiol 9:482–491. doi: 10.1111/j.1462-2920.2006.01165.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y-M, Nowack S, Olsen MT, Becraft ED, Wood JM, Thiel V, Klapper I, Kühl M, Fredrickson JK, Bryant DA, Ward DM, Metz TO. 2015. Diel metabolomics analysis of a hot spring chlorophototrophic microbial mat leads to new hypotheses of community member metabolisms. Front Microbiol 6:209. doi: 10.3389/fmicb.2015.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nübel U, Bateson MM, Vandieken V, Kühl M, Ward DM. 2002. Microscopic examination of distribution and phenotypic properties of phylogenetically diverse Chloroflexaceae-related bacteria in hot spring microbial mats. Appl Environ Microbiol 68:4593–4603. doi: 10.1128/AEM.68.9.4593-4603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandbeck KA, Ward DM. 1981. Fate of immediate methane precursors in low sulfate hot spring algal-bacterial mats. Appl Environ Microbiol 41:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson KL, Tayne TA, Ward DM. 1987. Formation and fate of fermentation products in hot spring cyanobacterial mats. Appl Environ Microbiol 53:2343–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Meer MT, Schouten S, Bateson MM, Nübel U, Wieland A, Kühl M, de Leeuw JW, Sinninghe Damste JS, Ward DM. 2005. Diel variations in carbon metabolism by green nonsulfur-like bacteria in alkaline siliceous hot spring microbial mats from Yellowstone National Park. Appl Environ Microbiol 71:3978–3986. doi: 10.1128/AEM.71.7.3978-3986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward D, Beck E, Revsbech NP, Sandbeck KA, Winfrey MR. 1984. Decomposition of hot spring microbial mats, p 191–214. In Cohen Y, Castenholz RW, Halvorson HO (ed), Microbial mats: stromatolites. Liss, New York, NY. [Google Scholar]
- 25.Dillon JG, Fishbain S, Miller SR, Bebout BM, Habicht KS, Webb SM, Stahl DA. 2007. High rates of sulfate reduction in a low-sulfate hot spring microbial mat are driven by a low level of diversity of sulfate-respiring microorganisms. Appl Environ Microbiol 73:5218–5226. doi: 10.1128/AEM.00357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward DM. 1978. Thermophilic methanogenesis in a hot-spring algal-bacterial mat (71 to 30 degrees C). Appl Environ Microbiol 35:1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Meer MTJ, Klatt CG, Wood J, Bryant DA, Bateson MM, Lammerts L, Schouten S, Sinninghe Damsté JS, Madigan MT, Ward DM. 2010. Cultivation and genomic, nutritional and lipid biomarker characterization of Roseiflexus strains closely related to predominant in situ populations inhabiting Yellowstone hot spring microbial mats. J Bacteriol 192:3033–3042. doi: 10.1128/JB.01610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhaya D, Grossman AR, Steunou AS, Khuri N, Cohan FM, Hamamura N, Melendrez MC, Bateson MM, Ward DM, Heidelberg JF. 2007. Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J 1:703–713. doi: 10.1038/ismej.2007.46. [DOI] [PubMed] [Google Scholar]
- 29.Steunou AS, Bhaya D, Bateson M, Melendrez M, Ward DM, Brecht E, Peters JW, Kühl M, Grossman A. 2006. In situ analysis of nitrogen fixation and metabolic switching in unicellular thermophilic cyanobacteria inhabiting hot spring microbial mats. Proc Natl Acad Sci U S A 103:2398–2403. doi: 10.1073/pnas.0507513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steunou AS, Jensen SI, Brecht E, Becraft ED, Bateson MM, Kilian O, Bhaya D, Ward DM, Peters JW, Grossman AR, Kühl M. 2008. Regulation of nif gene expression and the energetics of N2 fixation over the diel cycle in a hot spring microbial mat. ISME J 2:364–378. doi: 10.1038/ismej.2007.117. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen M, Larsen LH, Ottosen LDM, Revsbech NP. 2015. Hydrogen microsensors with hydrogen sulfide traps. Sens Actuators B Chem 215:1–8. doi: 10.1016/j.snb.2015.03.035. [DOI] [Google Scholar]
- 32.Wiesenburg DA, Guinasso NL. 1979. Equilibrium solubilities of methane, carbon monoxide and hydrogen in water and sea water. J Chem Eng Data 24:359–360. [Google Scholar]
- 33.Revsbech NP. 1989. An oxygen microelectrode with a guard cathode. Limnol Oceanogr 34:472–476. [Google Scholar]
- 34.Revsbech NP, Jørgensen BB. 1986. Microelectrodes: Their use in microbial ecology. Adv Microb Ecol 9:293–352. doi: 10.1007/978-1-4757-0611-6_7. [DOI] [Google Scholar]
- 35.Kühl M, Steuckart C, Eickert G, Jeroschewski P. 1998. A H2S microsensor for profiling sediments and biofilms: application in acidic sediment. Aquat Microb Ecol 15:201–209. doi: 10.3354/ame015201. [DOI] [Google Scholar]
- 36.Otaki H, Everroad RC, Matsuura K, Haruta S. 2012. Production and consumption of hydrogen in hot spring microbial mats dominated by filamentous anoxygenic photosynthetic bacterium. Microbes Environ 27:293–299. doi: 10.1264/jsme2.ME11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fay P. 1992. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev 56:340–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpenter EJ, Price CC. 1976. Marine Oscillatoria (Trichodesmium)—explanation for aerobic nitrogen-fixation without heterocysts. Science 191:1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- 39.Reddy KJ, Haskell JB, Sherman DM, Sherman LA. 1993. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J Bacteriol 175:1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson ST, Foster RA, Zehr JP, Karl DM. 2010. Hydrogen production by Trichodesmium erythraeum, Cyanothece sp and Crocosphaera watsonii. Aquat Microb Ecol 59:197–206. doi: 10.3354/ame01407. [DOI] [Google Scholar]
- 41.Gong X, Garcia-Robledo E, Schramm A, Revsbech NP. 2016. Respiratory kinetics of marine bacteria exposed to decreasing oxygen concentrations. Appl Environ Microbiol 82:1412–1422. doi: 10.1128/AEM.03669-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klatt CG, Inskeep WP, Herrgard M, Jay ZJ, Rusch DB, Tringe SG, Parenteau MN, Ward DM, Boomer SM, Bryant DA, Miller SR. 2013. Community structure and function of high-temperature chlorophototrophic microbial mats inhabiting diverse geothermal environments. Front Microbiol 4:106. doi: 10.3389/fmicb.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milligan AJ, Berman-Frank I, Gerchman Y, Dismukes GC, Falkowski PG. 2007. Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection. J Phycol 43:845–852. doi: 10.1111/j.1529-8817.2007.00395.x. [DOI] [Google Scholar]
- 44.Severin I, Stal LJ. 2010. Temporal and spatial variability of nifH expression in three filamentous cyanobacteria in coastal microbial mats. Aquat Microb Ecol 60:59–70. doi: 10.3354/ame01405. [DOI] [Google Scholar]
- 45.Woebken D, Burow LC, Behnam F, Mayali X, Schintlmeister A, Fleming ED, Prufert-Bebout L, Singer SW, Cortes AL, Hoehler TM, Pett-Ridge J, Spormann AM, Wagner M, Weber PK, Bebout BM. 2015. Revisiting N2 fixation in Guerrero Negro intertidal microbial mats with a functional single-cell approach. ISME J 9:485–496. doi: 10.1038/ismej.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandbeck KA, Ward DM. 1982. Temperature adaptations in the terminal processes of anaerobic decomposition of Yellowstone National Park and Icelandic hot spring microbial mats. Appl Environ Microbiol 44:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodwin S, Lovley DR. 1988. Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim Cosmochim Acta 52:2993–3003. doi: 10.1016/0016-7037(88)90163-9. [DOI] [Google Scholar]
- 48.Revsbech NP, Ward DM. 1984. Microelectrode studies of interstitial water chemistry and photosynthetic activity in a hot spring microbial mat. Appl Environ Microbiol 48:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen SI, Stenou AS, Bhaya D, Kühl M, Grossman AR. 2011. In situ dynamics of O2, pH and cyanobacterial transcripts associated with CCM, photosynthesis and detoxification of ROS. ISME J 5:317–328. doi: 10.1038/ismej.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsing NB, Ferris MJ, Ward DM. 2000. Highly ordered vertical structure of Synechococcus populations within the one-millimeter-thick photic zone of a hot spring cyanobacterial mat. Appl Environ Microbiol 66:1038–1049. doi: 10.1128/AEM.66.3.1038-1049.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madigan MT, Petersen SR, Brock TD. 1974. Nutritional studies of Chloroflexus, a filamentous photosynthetic, gliding bacterium. Arch Microbiol 100:97–103. doi: 10.1007/BF00446309. [DOI] [Google Scholar]
- 52.Nguyen HTT, Nielsen JL, Nielsen PH. 2012. “Candidatus Halomonas phosphatis,” a novel polyphosphate-accumulating organism in full-scale enhanced biological phosphorus removal plants. Environ Microbiol 14:2826–2837. doi: 10.1111/j.1462-2920.2012.02826.x. [DOI] [PubMed] [Google Scholar]
- 53.van Gemerden H. 1974. Coexistence of organisms competing for the same substrate: an example among the purple sulfur bacteria. Microb Ecol 1:104–119. doi: 10.1007/BF02512382. [DOI] [PubMed] [Google Scholar]