ABSTRACT
Nitrosomonas europaea is a chemolithoautotrophic bacterium that oxidizes ammonia (NH3) to obtain energy for growth on carbon dioxide (CO2) and can also produce nitrous oxide (N2O), a greenhouse gas. We interrogated the growth, physiological, and transcriptome responses of N. europaea to conditions of replete (>5.2 mM) and limited inorganic carbon (IC) provided by either 1.0 mM or 0.2 mM sodium carbonate (Na2CO3) supplemented with atmospheric CO2. IC-limited cultures oxidized 25 to 58% of available NH3 to nitrite, depending on the dilution rate and Na2CO3 concentration. IC limitation resulted in a 2.3-fold increase in cellular maintenance energy requirements compared to those for NH3-limited cultures. Rates of N2O production increased 2.5- and 6.3-fold under the two IC-limited conditions, increasing the percentage of oxidized NH3-N that was transformed to N2O-N from 0.5% (replete) up to 4.4% (0.2 mM Na2CO3). Transcriptome analysis showed differential expression (P ≤ 0.05) of 488 genes (20% of inventory) between replete and IC-limited conditions, but few differences were detected between the two IC-limiting treatments. IC-limited conditions resulted in a decreased expression of ammonium/ammonia transporter and ammonia monooxygenase subunits and increased the expression of genes involved in C1 metabolism, including the genes for RuBisCO (cbb gene cluster), carbonic anhydrase, folate-linked metabolism of C1 moieties, and putative C salvage due to oxygenase activity of RuBisCO. Increased expression of nitrite reductase (gene cluster NE0924 to NE0927) correlated with increased production of N2O. Together, these data suggest that N. europaea adapts physiologically during IC-limited steady-state growth, which leads to the uncoupling of NH3 oxidation from growth and increased N2O production.
IMPORTANCE Nitrification, the aerobic oxidation of ammonia to nitrate via nitrite, is an important process in the global nitrogen cycle. This process is generally dependent on ammonia-oxidizing microorganisms and nitrite-oxidizing bacteria. Most nitrifiers are chemolithoautotrophs that fix inorganic carbon (CO2) for growth. Here, we investigate how inorganic carbon limitation modifies the physiology and transcriptome of Nitrosomonas europaea, a model ammonia-oxidizing bacterium, and report on increased production of N2O, a potent greenhouse gas. This study, along with previous work, suggests that inorganic carbon limitation may be an important factor in controlling N2O emissions from nitrification in soils and wastewater treatment.
INTRODUCTION
During nitrification in aerobic environments, ammonia (NH3) oxidation to nitrite (NO2−) is initiated by ammonia-oxidizing bacteria (AOB) and archaea (1). These microorganisms are predominantly chemolithoautotrophs that derive energy for growth from NH3 and meet their carbon (C) needs by fixing carbon dioxide (CO2) (1). All known AOB fix CO2 via the Calvin-Benson-Bassham (CBB) cycle for growth, utilizing ribulose bisphosphate carboxylase/oxygenase (RuBisCO) (1).
Extensive previous work and recent studies have delved into how the model AOB Nitrosomonas europaea oxidizes NH3 to hydroxylamine (NH2OH) via ammonia monooxygenase (AMO) and subsequently NH2OH to NO2− via hydroxylamine dehydrogenase (HAO) (1–3). Oxidation of NH3 is closely linked with anabolism of CO2 since carbon assimilation consumes reductant and serves to regenerate key metabolic intermediates in electron transport.
The uptake and assimilation of inorganic carbon (IC) in AOB are understudied phenomena. N. europaea lacks carboxysomes and responds to IC limitation by upregulating RuBisCO (4–6). Regardless of the generally assumed advantages offered by carboxysomes, a previous study found that N. europaea dominated over other AOB in low-IC continuous-flow bioreactors (7). Investigation of the cbb operon in N. europaea has shown that it encodes a green-like type I RuBisCO and that the cbb operon expression increases in response to low CO2 concentrations (4). The RuBisCO form in N. europaea is type IAq, a form associated with intermediate affinity for CO2 and an intermediate catalytic rate of CO2 fixation in the presence of O2 (8). Interestingly, N. europaea and the nitrite-oxidizing bacterium Nitrobacter winogradskyi grown in coculture responded by decreasing expression of RuBisCO under replete Na2CO3 conditions compared to their responses when grown singly, which perhaps suggests an increased efficiency of RuBisCO during coculture (9).
The form of IC supplied (bicarbonate/carbonate or CO2 gas) has been shown to change carbonic anhydrase activity, growth rate, and nitrification efficiency (6, 7, 10–13). The work of Jiang et al. (6) demonstrated that changes in IC supply, particularly when IC was supplied as a gas, substantially affected the production of nitric oxide (NO) and nitrous oxide (N2O); the latter is a potent greenhouse gas (14). This study used continuous culturing, sophisticated gas detection instruments, quantitative PCR (qPCR), and proteomic techniques to make a compelling case that deprivation of IC from the medium increases nitrogen oxide (NOx) gas production (6). However, Jiang et al. were unable to establish steady-state IC-limiting conditions in their chemostats; the cells washed out when aerated with CO2-free air to provide limiting concentrations of IC (6). As a consequence, gene expression data reported by Jiang et al. represented transitional and not steady-state conditions (6). Other studies have suggested that N2O is produced via reduction of nitric oxide (NO) formed either by reduction of NO2− or by incomplete oxidation of NH2OH (15–17). N2O production by AOB has traditionally been associated with high NH4+/NH3 and NO2− concentrations and with low oxygen conditions (18–20), and further studies are needed to confirm the mechanism of N2O production under IC-limiting conditions.
In our study, we sought to expand on the work of Jiang et al. (6) to better understand the physiological adaptation of N. europaea to IC limitation and its relationship with N2O production. By manipulating the IC provided in the medium combined with reducing aeration with air, we established steady-state continuous cultures under IC limitation. Using these growth conditions combined with comprehensive high-throughput mRNA sequencing (mRNA-Seq) analyses, we show that limiting Na2CO3 in solution and controlling aeration of the culture vessel result in incomplete NH3 oxidation, increased production of N2O, and increased energy requirements for cellular maintenance. Furthermore, changes in growth rate and in NH3 oxidation rate under low Na2CO3 conditions changed N2O production. Finally, our work demonstrates that IC limitation affects the expression of a significant percentage of the genetic inventory (20%) in N. europaea, particularly those genes involved in C fixation and metabolism, energy transformation and electron transport, reductant consumption, and stress responses.
MATERIALS AND METHODS
Bacterial strains and routine culture conditions.
Nitrosomonas europaea (ATCC 19718) was routinely cultivated in 30 mM (NH4)2SO4 minimal medium in batch culture as described previously (9).
Chemostat experiments.
Chemostat culture experiments were carried out at 30°C in 2-liter bioreactors (ADI 1025 and ADI 1010; Applikon Biotechnology) with stirring at 600 rpm, constant aeration (filtered [0.2-μm pore size] atmospheric air), and a pH maintained at 7.5. Bioreactors were treated with Sigmacote (Sigma-Aldrich, St. Louis, MO) to inhibit biofilm formation. The medium consisted of 30 mM (NH4)2SO4 as described previously (9), with the exception that K2HPO4 and NaH2PO4 (25 and 2.5 mM, respectively) were added to buffer the medium to pH 8. Chemostat cultures were inoculated with 2%-volume exponential-phase batch cultures and grown in batch mode until ≥20 mM ammonium (NH4+) was consumed. At this point, medium feed commenced and continuous culturing began. Continuous cultures were allowed at least three population doublings to reach steady state, which was defined as one population doubling with a ≤5% change in cell density (21). Approximately 200-μl aliquots of chemostat cultures were plated periodically on Luria-Bertani agar plates to confirm axenic culture conditions.
Continuous cultures were maintained at steady state under three different inorganic carbon (IC) conditions: one IC-replete condition (2 mM Na2CO3 with 100 ml min−1 filtered [0.2-μm pore size] atmospheric air) and two IC-limiting conditions (1.0 mM Na2CO3 and 0.2 mM Na2CO3; both of these IC-limiting conditions used 50 ml min−1 filtered [0.2-μm pore size] atmospheric air). The rationale for providing IC via medium addition and aeration was that aeration with CO2-free air resulted in the removal of all IC from the system. This effect is similar to that obtained by sparging medium with inert gas to remove oxygen. The pH under the replete condition was controlled with the addition of 10% (wt/vol) Na2CO3 as necessary. In IC-limiting treatments, pH was maintained with the addition of 0.4 M NaOH as necessary. The same growth medium was used under the three IC conditions, with the exception of Na2CO3 levels among treatments. The three IC treatments were compared at steady state by measuring transcriptome responses, optical density (OD), NH4+, NO2−, total IC as CO2, N2O, and protein, all at a dilution rate (D) of 0.016 h−1 (doubling time [Td] = 44.7 h). Higher D values of 0.020, 0.024, and 0.026 h−1 (Td values of 34.7, 28.9, and 26.7 h, respectively) were implemented to determine how growth rate influenced rates of NH3 oxidation and N2O production during the 0.2 mM Na2CO3 treatment. Further chemostat experiments used to calculate maintenance energy are outlined in the Materials and Methods section in the supplemental material.
Analytical methods.
NH4+ and NO2− concentrations were determined by chemical assays as described previously (22). Cellular maintenance energy was calculated using Pirt's equation based on D, NH3 consumption, and growth yield as described in the Materials and Methods section in the supplemental material (23–25). CO2/HCO3−/CO32− levels in air and the growth medium were determined by gas chromatography using a thermal conductivity detector and a 2-m HayeSep Q (80/100) column (1/8-in. outside diameter [o.d.], 0.085-in. inside diameter [i.d.], stainless steel tubing). Briefly, 10 ml of medium was placed in a 30-ml serum vial capped with a gray butyl stopper and crimped, and 2 ml of 1 M HCl was added. Serum vials were incubated for 60 min at room temperature to allow full equilibration, and CO2 in the headspace (100-μl injection) was measured by comparison to a standard Na2CO3 aqueous solution. N2O was measured using a Varian 3700 gas chromatograph equipped with an electron capture detector and a 1.8-m Porapak Q column (0.32-cm o.d., stainless steel). The carrier gas was composed of 95% argon and 5% methane. Cell density was determined by measuring OD at 600 nm (OD600), and protein content was measured with a Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Rockford, IL).
RNA preparation and sequencing.
Total RNA was extracted as described previously with minor modification (9). Briefly, bacterial cells were collected from three biological replicates, concentrated by centrifugation, and lysed via sonication, and RNA was purified with an RNeasy minikit (Qiagen, Germantown, MD) as recommended by the manufacturer. The MICROBExpress RNA isolation kit was used to deplete rRNA from total RNA by following the instructions of the manufacturer (Ambion/Life Technologies, NY). The quality of the depleted RNA was assayed using a 6000 Nano LabChip kit and an Agilent Bioanalyzer (Agilent Technologies Inc., Palo Alto, CA). An Illumina-targeted RNA expression kit and at least 200 ng of RNA containing less than 5% rRNA were used to prepare mRNA-Seq libraries. The libraries were sequenced (100-mer, paired end) on a HiSeq 2000 sequencer (Illumina, San Diego, CA) at the Center for Genome Research and Biocomputing Core Laboratories at Oregon State University.
Transcriptome data analysis.
The mRNA-Seq reads were analyzed using the CLC Main Workbench (CLC bio, Aarhus, Denmark). The module for empirical analysis of differential gene expression (DGE) was used as described previously (26, 27) with default values of the software and normalized to the number of reads per kilobase of transcript per million mapped reads (RPKM). Significant DGE for two-group comparisons was determined for all three possible but nonreciprocal pairwise comparisons (P value threshold of ≤0.05) (26, 27). The fold change in mRNA abundance indicates the difference between the number of mRNA transcripts in the control and treatment groups. If the relative abundance of a transcript in the control was smaller than in the treatment, the number was given a positive value; conversely, if the transcript count was larger in the control than in the treatment group, the number was given a negative value.
Corroboration of gene expression was performed for selected genes by quantitative reverse transcription PCR (qRT-PCR), with total RNA from biological replicates as the template and with corresponding primers (see Tables S2 and S3 in the supplemental material). The qRT-PCRs were carried out in a MyiQ real-time PCR system with SYBR green I-based detection kits by following the directions of the manufacturer (Bio-Rad Laboratories, Hercules, CA) and normalized to cDNA concentrations measured with a NanoDrop ND UV–visible-light (UV-Vis) spectrophotometer (Thermo Scientific, Waltham, MA). Synthesis of cDNAs was carried out as previously described (28). Cycling parameters were 5 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Dissociation curves were drawn to ensure the absence of nonspecific amplification. Reaction efficiency and starting concentrations of the template were calculated with LinRegPCR version 2015.1 as described previously (29).
Three DGE comparisons were analyzed: replete versus 1.0 mM Na2CO3, replete versus 0.2 mM Na2CO3, and 1.0 mM Na2CO3 versus 0.2 mM Na2CO3. Similar changes in gene expression trends were observed when replete-Na2CO3 conditions were compared to the IC-limiting conditions, and there were few differences between 1.0 mM Na2CO3 and 0.2 mM Na2CO3. Therefore, Results and Discussion are focused on differences between the replete and both IC-limiting conditions. Overall observations were considered significant when P was ≤0.05, unless otherwise specified.
Nucleotide sequence accession number.
Unprocessed and processed data sets are available in the Gene Expression Omnibus (GEO) database at the National Center for Biotechnology Information (NCBI) under accession no. GSE70988.
RESULTS
Inorganic carbon limitation in N. europaea reduces ammonia oxidation and cell density.
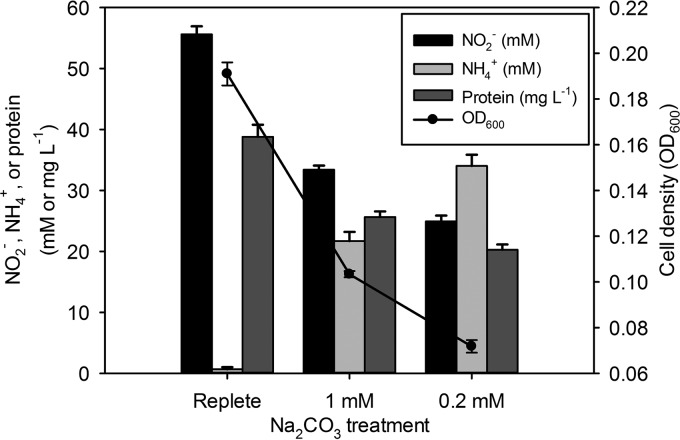
To determine the effect of IC limitation on N. europaea, we maintained continuous chemostat cultures at a steady-state dilution rate of 0.016 h−1 under replete- and reduced-IC conditions consisting of Na2CO3 and a known input of CO2 from the atmospheric air supply. Nonlimiting-IC conditions resulted in efficient consumption of ammonium (less than 1.0 mM NH4+ remaining) and accumulation of approximately 55.6 mM NO2−, similar to previous results (Fig. 1) (9). IC-limited (1.0 mM and 0.2 mM Na2CO3) steady-state chemostat cultures showed incomplete NH4+ consumption (approximately 64% and 43% of the NH4+ was consumed in the 1.0 and 0.2 mM Na2CO3 treatments, respectively) (Fig. 1). Furthermore, the cell yield (based on the OD600 and protein concentration) of the 1.0 mM Na2CO3 culture was reduced to between 54 and 66% of that of the replete-IC culture, and that of the 0.2 mM Na2CO3 culture was reduced to between 37 and 52% of that of the replete-IC culture (Fig. 1). The IC-limiting treatments also resulted in much less NO2− than the replete treatment (1.0 mM Na2CO3 accumulated approximately 33.4 mM NO2− and 0.2 mM Na2CO3 accumulated approximately 24.7 mM NO2−) (Fig. 1). However, based on the previously reported IC uptake-to-NH3 oxidation ratio (0.086 mol C per mol N), we hypothesized that there was a significant contribution of IC from atmospheric CO2 (30).
FIG 1.
Chemostat culture during replete- and limiting-IC conditions. Treatment is indicated on the x axis. Bars represent concentrations of NO2− (millimolar, black), NH4+ (millimolar, light gray), and protein (milligrams per liter, dark gray) measured during a steady-state dilution rate of 0.016 h−1 (left y axis). Circles (line graph) indicate cell density measured as optical density at 600 nm (OD600) (right y axis). Values are the means of results from three independent biological replicates. Error bars indicate the standard deviations of the means.
N. europaea can increase its capacity to utilize atmospheric carbon dioxide.
To estimate the amount of atmospheric CO2 consumed during continuous chemostat culture, we measured total IC in steady-state culture medium and the change in CO2 concentration between influent and effluent air (Table 1). Steady-state culture samples contained more residual IC as the initial Na2CO3 concentration increased (Table 1). Measurements of bioreactor effluent air compared to influent atmospheric air showed that atmospheric CO2 was reduced by approximately 32.8% in the 0.2 mM Na2CO3 treatment and by approximately 25.5% in the 1.0 mM Na2CO3 treatment (Table 1). In the replete-Na2CO3 treatment, effluent air contained more than twice the CO2 content of atmospheric air (Table 1). Based on the approximately 49.6 μmol CO2 h−1 supplied by aeration and IC in the medium feed, the two IC-limited treatments consumed similar amounts of total IC (Table 1). However, the 0.2 mM Na2CO3 treatment consumed more atmospheric CO2 (Table 1). The detection of increased CO2 from the replete treatment air effluent suggests that significant amounts of IC can be purged by aeration. In addition, despite the increased contribution from atmospheric CO2, IC consumption in the IC-limited treatment samples was less than that predicted based on the previously reported IC uptake-to-NH3 oxidation ratio (30). These data suggested that some NH3 oxidation was uncoupled from IC assimilation under these circumstances.
TABLE 1.
IC consumption during continuous chemostat culturea
| Na2CO3 treatment | IC in culture (mM) | CO2 in effluent air (μM)b | IC contributed by pH control (μmol liter−1 h−1)c | IC consumed from medium (μmol liter−1 h−1)d | CO2 consumed from air (μmol liter−1 h−1)d | Total IC consumed (μmol liter−1 h−1)d |
|---|---|---|---|---|---|---|
| Replete | 3.49 (1.06) | 29.1 (2.42) | —e | — | — | 74.6e |
| 1.0 mM | 0.57 (0.11) | 8.15 (0.97) | 3.14 (0.06) | 10.6 | 12.6 | 23.2 |
| 0.2 mM | 0.13 (0.02) | 7.35 (0.98) | 2.38 (0.005) | 4.80 | 16.3 | 21.1 |
Values are the means of results from three biological replicates. Values in parentheses are the standard deviations of the means.
Values are based on 400 ppm or approximately 16.5 μM CO2 in the atmosphere under standard conditions.
IC contributions from the background IC in the NaOH addition to control pH are based on the flow rate and on an IC concentration of 0.60 mM.
The average rate of consumption is based on the weighted average of the IC in the culture and the IC contributed by an added pH control to the total reduction in atmospheric CO2 (estimated to be 400 ppm in atmosphere; 49.6 μmol h−1 was supplied in air).
—, the IC contribution from a pH control and after consumption from the medium and air in the IC-replete treatment was not calculated. A value of 74.6 μmol liter−1 h−1 should theoretically be consumed based on the NH3 oxidation rate (30).
Inorganic carbon limitation increases nitrous oxide production and cellular maintenance energy.
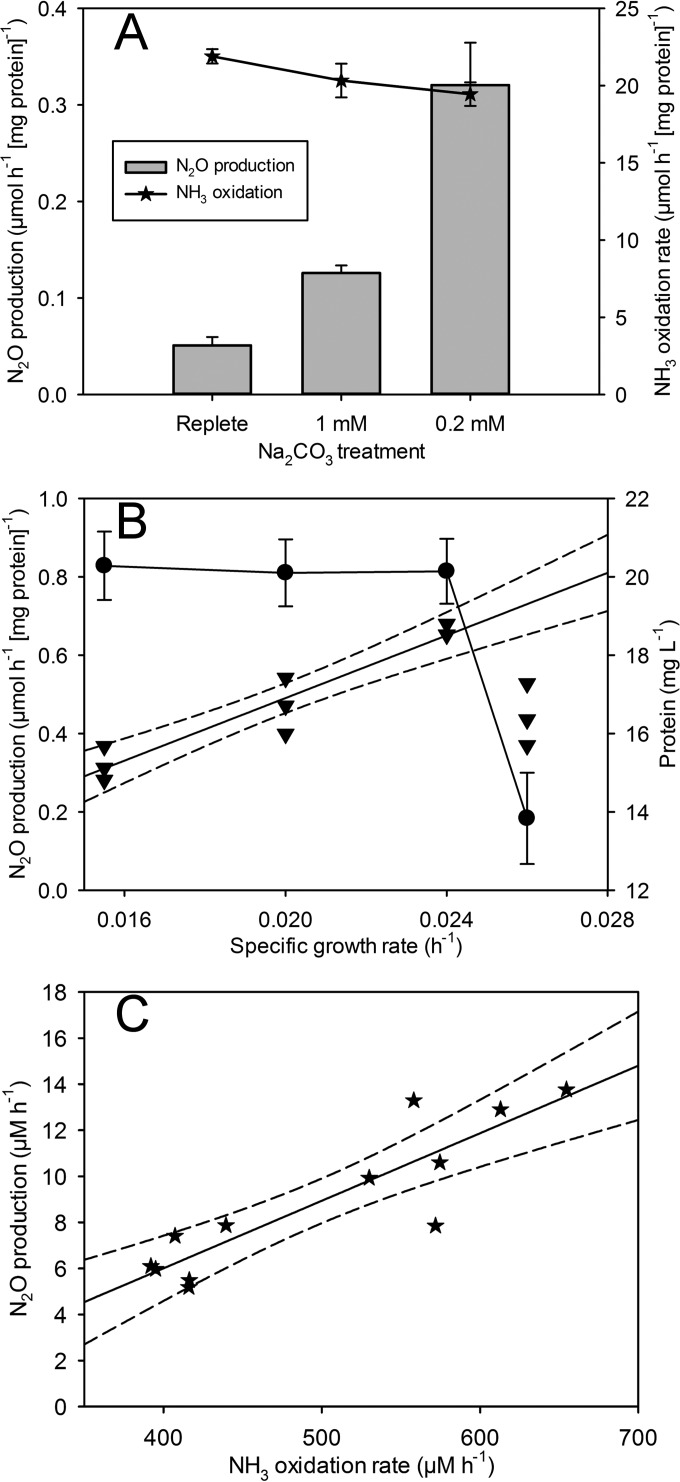
By following previous work by Jiang et al. (6) that showed increased N2O production during IC limitation, we quantified N2O production by gas chromatography. We found that N2O production normalized to culture protein content increased 2.5-fold and 6.3-fold when Na2CO3 was reduced to 1.0 mM and 0.2 mM, respectively (Fig. 2A). In addition, the NH3 oxidation rate, normalized to protein, showed a slight decrease during IC limitation from 21.9 to 19.4 μmol (mg protein)−1 h−1 (Fig. 2A).
FIG 2.
IC, growth rate, and NH3 oxidation-dependent production of N2O during chemostat culture. (A) Bars represent N2O production normalized to protein concentration (micromoles per hour per milligram of protein) (left y axis). Stars (line graph) indicate the NH3 oxidation rate normalized to protein (micromoles per hour per milligram of protein) (right y axis). Treatment is indicated on the x axis. All values were measured during steady state (dilution rate = 0.016 h−1). (B) Triangles represent individual experimental replicate N2O production levels normalized to protein (micromoles per hour per milligram of protein) (left y axis). The straight black line is the regression of N2O production from a specific growth rate of 0.016 to 0.024 h−1 (R2 = 0.91), and dotted lines indicate the 95% confidence band. Circles (line graph) indicate protein content (milligram per liter) (right y axis). (C) Stars represent individual experimental replicate measurements of N2O production (micromolar per hour) (y axis) plotted against the NH3 oxidation rate (micromolar per hour) (x axis) at the different dilution rates shown in panel B. The straight black line is the regression of N2O production (R2 = 0.79), and dotted lines indicate the 95% confidence band.
Previous work has shown that IC limitation during batch culture substantially decreases the growth rate of N. europaea (10). We hypothesized that IC limitation coupled with energy wasted on N2O production would increase cellular maintenance energy and decrease the optimal growth rate, leading to a washout at a slower doubling time (Td) than previously reported (31, 32). Continuous-culture theory predicts that the dilution rate is equal to μ at steady state and that cells will begin to wash out when the culture approaches the maximum specific growth rate (μmax) (21, 33). As predicted, IC-limited cultures began to wash out at a μ of 0.026 h−1 (Td, 26.7 h), and cellular maintenance energy during C-limited growth was estimated to be 14.0 mmol NH3 consumed per gram of cells (dry weight) (gDCW) h−1 (Fig. 2B; see also Fig. S1A in the supplemental material). Based on previously reported continuous-culture data, N. europaea maintenance energy under NH3-limited growth was calculated to be 4.4 mmol NH3 gDCW−1 h−1, and we estimated it to be 6.12 mmol NH3 gDCW−1 h−1 (Fig. S1B) (34). The maximum growth rate under IC limitation appears to be significantly lower than the previously published NH3-limited Td values of 5.5 to 11 h (μmax = 0.126 to 0.063 h−1) (31, 32).
In our experiments, there was a positive linear correlation between growth rate and N2O production (R2 = 0.92) (Fig. 2B). N2O production increased as μ increased, and production peaked at approximately 0.34 μmol N2O (mg protein)−1 h−1 (Fig. 2B). At peak N2O production, approximately 4.4% of the NH3-N oxidized was transformed to N2O-N (Fig. 2B). When the chemostat culture approached the maximum growth rate and washout occurred, N2O production decreased with decreasing cell density (Fig. 2B). In addition, the NH3 oxidation rate showed a positive linear correlation (R2 = 0.79) with N2O production (Fig. 2C). These experiments suggest that IC limitation and growth rate affect N2O production in N. europaea, in part by changing the NH3 oxidation rate. To further explore the response of N. europaea to IC limitation, we extracted RNA for mRNA-Seq and transcriptome analyses.
Transcriptome responses to IC limitation.
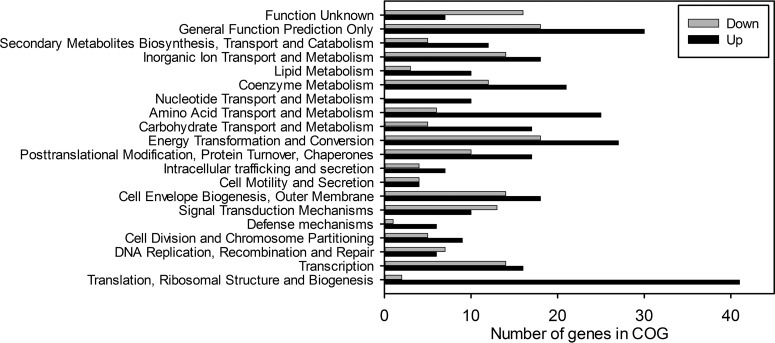
We compared the transcriptomes of N. europaea under replete-C and the two IC-limiting conditions. The transcriptome analysis showed that 488 genes were differentially expressed between the replete-IC and the IC-limited conditions (see Table S1 and Data Set S1 in the supplemental material). Except with a few genes, the same expression trend for gene clusters occurred in the two IC-limited conditions, with the 0.2 mM Na2CO3 treatment generally showing a lower fold change (Table S1 and Data Set S1). When expression changes were grouped into clusters of orthologous group (COG) functional categories, COGs associated with energy transformation, translation, transcription, signal transduction, lipid metabolism, and secondary metabolites substantially (≥20% of category COGs) changed in expression (Fig. 3). We found many transcriptome changes in genes associated with carbon fixation and metabolism, energy transformation and electron transport, reductant consumption, and stress response-related genes (see below and Table 2).
FIG 3.
Clusters of orthologous group (COG) assignments of differentially expressed genes during IC limitation. Bars in black indicate the number of genes with increased expression and bars in gray indicate the number of genes with decreased expression during IC limitation for each functional group.
TABLE 2.
Statistically significant changes in expression of C fixation and metabolism, electron transport and transformation, reductant consumption, and stress response-related genes
| Category and gene(s) | Gene(s) | Role(s) | Fold changea |
|---|---|---|---|
| C fixation and metabolism | |||
| NE0325–NE0328 | pykA, pgk, cbbG, cbbT | Gluconeogenesis | 1.39–1.82 |
| NE1917–NE1922 | cbbOQSR, rbcL | RuBisCO gene cluster | 1.81–19.88 |
| NE1926 | cynT | Carbonic anhydrase | 1.42–1.45 |
| Carbon metabolism linked to photorespiration | |||
| NE0221–NE0223, NE0362 | folD | Folate metabolism | 1.43–1.89 |
| NE0606 | cah | Carbonic anhydrase | −1.25 to −1.56 |
| NE0607–NE0611 | gcv gene loci | Glycine cleavage system | 1.46 to 2.66 |
| Energy transformation and electron transport | |||
| NE0199–NE0206 | atp gene loci | ATP synthase | 1.00 to 2.30 |
| NE0448 | rh50 | NH3 transport | −2.04 to −3.58 |
| NE0941–NE0945, NE2057–NE2064 | amo gene loci 1 and 2 | Ammonia oxidation | −1.33 to −2.57 |
| NE0278, NE0508–NE0510, NE0764, NE0765 | SCO1 gene or senC, ccmA, ccmB | Heme and cytochrome biosynthesis/assembly | 1.26–2.51b |
| NE0681–NE0684, NE1313–NE1315 | SCO1 gene or senC, coxA2, coxB, ccp gene loci | Cytochrome c oxidase | −1.47 to −2.13 |
| NE1541, NE1542 | Putative hemin transport | 2.04–5.60b | |
| NE1764–NE1777 | nuo gene loci | NADH dehydrogenase | 1.00–1.97 |
| NE1838, NE1868, NE1869 | ubiA, ubiB, putative gene ubiJ | Ubiquinone biosynthesis | 1.56–2.07 |
| Reductant consumption | |||
| NE0924–NE0927 | aniA (nirK) gene loci | Nitrite reductase and electron transport | 1.57–3.31 |
| NE2003, NE2004 | norC, norB | NO reductase | 1.00 to −1.59c |
| Stress response | |||
| NE0027–NE0031, NE0035, NE0584–NE0586, NE1312, NE1948, NE1950 | groES, groEL, tig, clpP, rpoH, cspD2_1, dnaJ, grpE | Temperature stress response of chaperonins, etc. | 1.28–5.39 |
“Fold change” is the difference in mRNA transcript levels between the control (replete IC) and the treatment (1.0 mM or 0.2 mM Na2CO3) (P ≤ 0.05). A value of 1.00 indicates no statistically significant change in at least one of the listed genes.
A significant fold change was observed only in the 0.2 mM Na2CO3 treatment.
A significant fold change was observed only in the 1.0 mM Na2CO3 treatment.
Carbon fixation and metabolism gene expression.
As an autotrophic bacterium, N. europaea fixes carbon via the CBB cycle using a type I RuBisCO to catalyze the reaction between CO2 and d-ribulose-1,5-bisphosphate to form 2 molecules of 3-phospho-d-glycerate (3). Under IC limitation, expression of the entire cbb gene cluster increased, with 7.4- and 10.8-fold increases in numbers of genes encoding the small and large subunits of RuBisCO, cbbS (NE1920) and rbcL (NE1921) (Table 2). In addition, levels of expression of genes involved in gluconeogenesis and the Calvin cycle, including pykA, pgk, cbbG, and cbbT (NE0325 to NE0328), were also increased (Table 2).
Another response to IC-limited conditions was the increase in expression of carbonic anhydrase used to enhance the intracellular supply of CO2. N. europaea has two putative carbonic anhydrases encoded by NE1926 and NE0606. The gene NE1926 was expressed 1.4- to 1.5-fold more than NE0606 (−1.3- to −1.6-fold) (Table 2). NE1926 is located in close proximity to the cbb gene cluster and is likely coregulated.
Interestingly, other genes involved in C1 metabolism were upregulated, including the folate biosynthesis gene and genes involved in transfer of C1 compounds to folate (NE0221 to NE0223 and NE0362, 1.4- to 1.9-fold) (Table 2). In addition, genes in the glycine synthase/cleavage system (NE0607 to NE0612, gcv) were increased in expression 1.4- to 2.7-fold (Table 2). This system can either consume or generate CO2, NH4+, NADH, and tetrahydrofolate, and it is an important step during salvage of C after the oxygenase activity of RuBisCO that commonly occurs under CO2 limitation (known as photorespiration in cyanobacteria and C3 plants) (35).
Expression of genes associated with amino acid, pyrimidine, phospholipid, and fatty acid biosynthesis changed during IC limitation. There was a 1.3- to 2.3-fold increase in the expression of various genes that participate in leucine (NE1320, leuA1), valine/isoleucine (NE1323, ilvC), and arginine and pyrimidine (NE1661 and NE1662, carAB) biosynthesis (see Table S1 in the supplemental material). Five genes that take part in phosphatidylethanolamine biosynthesis (NE1645, NE0612, NE1713, NE1321, NE1322; plsX, plsC, cdsA, pssA, psd) were upregulated 1.4- to 2.4-fold, while the expression of two gene clusters associated with fatty acid degradation decreased (NE0741 and NE2348 to NE2353, −1.5- to −2.5-fold) (Table S1).
Energy transformation and electron transport.
Expression of the putative ammonium/ammonia transporter gene rh50 (NE0448) was downregulated −2.0- to −3.6-fold as was that of all amo-related genes (NE0941 to NE0945 and NE2057 to NE2064, −1.3- to −2.6-fold) except amoC3 (NE1411), which showed no change in expression (Table 2; see also Table S1 in the supplemental material).
Changes in the levels of expression of genes involved in electron transport, particularly cytochrome-associated genes, were evident in IC-limited transcriptomes (Fig. 3 and Table 2). Expression of some cytochrome and heme biosynthesis and heme transporter genes increased (NE0278, NE0508 to NE0510, NE0764, NE0765, NE1541, and NE1542, 1.3- to 5.6-fold), while expression of electron transport-associated genes decreased, including that of the cytochrome c oxidase genes coxAB (NE0681 to NE0684, −1.4- to −2.1-fold), cytochrome c554-associated cycX3 and cycX1 (NE0959 and NE 2336, −1.4-fold; only 1 mM Na2CO3 treatment), and the putative cytochrome c551 genes (NE1313 to NE1315, −1.4- to −2.0-fold) (Table 2; see also Table S1 in the supplemental material). The increase in transcription of heme biosynthesis genes is supported by a decrease in expression of the tricarboxylic acid (TCA) cycle reaction that consumes succinyl-coenzyme A (CoA) (NE0050 and NE0051, sucCD, −1.4- to −1.8-fold) since heme biosynthesis requires succinyl-CoA as a reactant. In addition, expression of NADH dehydrogenase (NE1764 to NE1777), ubiquinone biosynthesis (NE1838, NE1868, and NE1869), and ATP synthase biosynthesis (NE0199 to NE0206) genes increased up to 2.3-fold (Table 2).
Reductant consumption.
Under IC limitation, expression of NO2− reductase and associated cytochromes (NE0924 to NE0927) increased 1.6- to 3.3-fold, suggesting a possible role in the generation of NO from the reduction of NO2− (Table 2). However, expression of norCD (NE2003 and NE2004), N. europaea's only known NO reductase for nitrifier denitrification, was either unchanged or decreased up to −1.6-fold (Table 2). It is possible that basal expression of norCD is sufficient to account for the increased generation of N2O. However, as suggested by others, this result may also point to alternative methods of NO reduction, such as NO binding by cytochrome c′-beta (17, 36, 37). However, no significant change in expression was observed for this gene.
Stress responses.
Another finding in the IC-limited transcriptome analysis of N. europaea was induction of stress response regulators, protein chaperones, and ribosomal components. Translation-related ribosomal proteins and protein-processing genes were overrepresented and generally increased in expression (Fig. 3; see also Table S1 and Data Set S1 in the supplemental material). Both increases and decreases in gene expression were observed for numerous extracytoplasmic function (ECF) sigma factors and other transcriptional regulators (NE0533 to NE0536, NE0557, NE1079, NE2138, NE2435), but a recurring theme was greater expression of temperature stress response genes (Table 2; Table S1). Under IC-limiting conditions, Lon protease and several chaperonins associated with thermal stress (NE0027 to NE0031, NE0035, NE0585, NE0586, NE1312, NE1948, NE1950) were upregulated 1.3- to 5.4-fold (Table 2; Table S1). In addition, expression of rpoH (NE0584, annotated as heat shock sigma factor 32) increased 1.4- to 2.6-fold (Table 2; Table S1).
DISCUSSION
IC limitation during continuous culture of N. europaea: overview.
We hypothesized that adaptation to IC limitation by N. europaea was focused on two goals: maintenance of its CO2-fixing ability and removal of reductant generated in excess of biosynthetic needs. These goals were reflected in the physiological changes observed during IC-limited culturing experiments, including N. europaea's inability to fully oxidize available NH3, reduction in cell density, and an increase in maintenance energy. The IC-limited culture's increased production of N2O further supports this hypothesis. Furthermore, gene expression changes revealed by mRNA-Seq analysis provided insight into this phenomenon. It is interesting to note that while physiological differences between the two IC-limited conditions were observed, there were few transcriptome differences, suggesting potential limitations to mRNA-Seq analyses. These results, along with the positive correlations observed between growth rate, NH3 oxidation rate, and N2O production, suggest that IC limitation uncouples NH3 oxidation from growth, diverting excess reductant toward the production of N2O.
IC utilization by N. europaea.
The increase in abundance of mRNA for RuBisCO and other CBB pathway genes during low-IC conditions is in agreement with previous reports which suggest overexpression of this pathway is an attempt to assimilate low concentrations of CO2 (4, 6). The higher expression of the gene for the carbonic anhydrase encoded by NE1926, which catalyzes the reaction of bicarbonate (HCO3−) to CO2 and H2O, might increase periplasmic or intracellular CO2 (38). Indeed, previous work has shown that carbonic anhydrase activity increases during IC limitation, whether supplied as CO2 or HCO3− (10). By pulling more IC out of solution for C fixation, the cells may shift the CO2/HCO3−/CO32− equilibrium, forcing further gaseous CO2 into solution to diffuse across the cell membrane. However, expression of a putative carbonic anhydrase (NE0606) decreased during C limitation and disagrees with our logic unless NE0606 has an alternative function.
Interestingly, NE0606 is clustered with the upregulated gcv gene cluster (NE0607 to NE0611), which might be implicated in salvaging CO2 through cleavage of glycine. It is well recognized that under conditions of low CO2 and high O2, RuBisCO functions as an oxygenase cleaving ribulose-1,5-bisphosphate to form phosphoglycolate. In cyanobacteria and C3 plants, phosphoglycolate is reclaimed through a series of reactions, including the glycine cleavage system (35). The oxygenase activity of RuBisCO is energetically wasteful and may partially explain the increased maintenance energy required during IC limitation (35). Future experiments are needed to investigate RuBisCO oxygenase activity and its consequences in N. europaea.
IC-limiting conditions may uncouple ammonia oxidation from growth due to accumulation of reduced metabolic intermediates.
IC-limited cells are expected to accumulate high-energy reductant due to reduced demand for NAD(P)H (39). The first evidence for this hypothesis may be the decreased expression of genes involved in NH3 uptake and oxidation during IC limitation. A previous study by Belser suggested that 8% (0.086 mol C per mol N) of reductant generated from NH3 oxidation is directed to CO2 fixation (30). When CO2 becomes limiting, we speculate that N. europaea attempts to deal with this discrepancy by redirecting some of this reductant to NOx gas production. Indeed, N2O measurements show that up to approximately 4.4% of NH3-N may be directed to N2O-N production under IC limitation, particularly at higher growth rates. However, the proportion of NH3-N that accumulates as NO-N remains unknown in this study. Previous work showed that NH3-N can be directed to NO production, especially during IC limitation, which further supports our hypothesis (6, 40–42). Our gene expression data are in contrast with those in the previous study by Jiang et al. (6), who showed increased expression of rh50, amo, and hao by qPCR, but these differences may be reflections of a different medium and different growth conditions in their study (6).
Another potential effect of the accumulation of reduced metabolic intermediates is electron radical stress. Transcriptome analysis under IC limitation suggests that N. europaea cells might need to remove damaged proteins, as suggested by increased expression of ribosomal components and heat shock chaperonins (Fig. 3 and Table 2; see also Table S1 and Data Set S1 in the supplemental material) (43). For example, rpoH activity in Escherichia coli is thought to be induced when the cell senses misfolded proteins (44).
Implications of IC limitation on N2O production by ammonia-oxidizing bacteria.
Transcriptome analysis of N. europaea under IC limitation adds to a growing body of evidence linking IC limitation to production of NOx gases (6, 45). Previous studies have suggested that alkalinity is likely the chief determinant of IC availability in soil and wastewater treatment systems (45, 46). Alkalinity varies widely in the two systems, and pH control may be a possible method to control N2O production. In addition, there is mounting evidence that N2O production is dependent on growth rate due to the increased rate of ammonia oxidation, based on NH3 oxidation and growth rate data generated in our work and other studies (42, 47). Our data suggest that IC limitation exacerbates these effects on N2O production by uncoupling NH3 oxidation from growth (Fig. 2).
There is controversy about how electron flow around NH3 oxidation occurs and how dynamic electron channeling may lead to production of N2O. A metabolic network modeling study of NOx gas production during the anoxic-oxic transition by N. europaea suggests that N2O production is associated with cytochrome-mediated electron flow imbalances (20). Clearly, more experiments are needed to study the effects of IC limitation on nitrifiers and on N2O evolution. Comparison of the biochemistry of N. europaea cells grown under IC and their biochemistry under energy limitation may be a useful tool to address how electron flow leads to the formation of N2O. Future studies on electron flow, RuBisCO oxygenase activity, and N2O production during IC limitation in N. europaea may help researchers to better understand and control sources of this potent greenhouse gas.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brent Kronmiller for bioinformatics consulting, David Myrold for gas chromatography instrument use, and Anne Taylor, Michael Dobie, Neeraja Vajrala, and Aiden Maxwell for helpful discussions and laboratory assistance.
Funding Statement
The DOE provided funding to the co-principal investigators L.A.S.-S. and P.J.B. under award ER65192, the USDA provided funding to P.J.B. under USDA-NIFA award 2012-67019-3028, and the NSF provided funding to principal investigator F.C. and co-principal investigator L.A.S.-S. under EAGER award CBET 1239870. During the duration of this project, B.L.M. was partially supported by a USDA-NIFA postdoctoral fellowship, award 2016-67012-24691. The funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00294-16.
REFERENCES
- 1.Sayavedra-Soto LA, Arp DJ. 2011. Ammonia-oxidizing bacteria: their biochemistry and molecular biology, p 11–37. In Ward BB, Arp DJ, Klotz MG (ed), Nitrification. ASM Press, Washington, DC. [Google Scholar]
- 2.Arp DJ, Sayavedra-Soto LA, Hommes NG. 2002. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch Microbiol 178:250–255. doi: 10.1007/s00203-002-0452-0. [DOI] [PubMed] [Google Scholar]
- 3.Chain P, Lamerdin J, Larimer F, Regala W, Lao V, Land M, Hauser L, Hooper A, Klotz M, Norton J, Sayavedra-Soto L, Arciero D, Hommes N, Whittaker M, Arp D. 2003. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J Bacteriol 185:2759–2773. doi: 10.1128/JB.185.9.2759-2773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei X, Sayavedra-Soto LA, Arp DJ. 2004. The transcription of the cbb operon in Nitrosomonas europaea. Microbiology 150:1869–1879. doi: 10.1099/mic.0.26785-0. [DOI] [PubMed] [Google Scholar]
- 5.Yeates TO, Kerfeld CA, Heinhorst S, Cannon GC, Shively JM. 2008. Protein-based organelles in bacteria: carboxysomes and related microcompartments. Nat Rev Microbiol 6:681–691. doi: 10.1038/nrmicro1913. [DOI] [PubMed] [Google Scholar]
- 6.Jiang D, Khunjar WO, Wett B, Murthy SN, Chandran K. 2015. Characterizing the metabolic trade-off in Nitrosomonas europaea in response to changes in inorganic carbon supply. Environ Sci Technol 49:2523–2531. doi: 10.1021/es5043222. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima T, Whang LM, Chiang TY, Lin YH, Chevalier LR, Chen MC, Wu YJ. 2013. Nitrifying bacterial community structures and their nitrification performance under sufficient and limited inorganic carbon conditions. Appl Microbiol Biotechnol 97:6513–6523. doi: 10.1007/s00253-012-4436-y. [DOI] [PubMed] [Google Scholar]
- 8.Badger MR, Bek EJ. 2008. Multiple RubisCO forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle. J Exp Bot 59:1525–1541. [DOI] [PubMed] [Google Scholar]
- 9.Pérez J, Buchanan A, Mellbye B, Ferrell R, Chang JH, Chaplen F, Bottomley PJ, Arp DJ, Sayavedra-Soto LA. 2015. Interactions of Nitrosomonas europaea and Nitrobacter winogradskyi grown in co-culture. Arch Microbiol 197:79–89. doi: 10.1007/s00203-014-1056-1. [DOI] [PubMed] [Google Scholar]
- 10.Jahnke LS, Lyman C, Hooper AB. 1984. Carbonic anhydrase, carbon-dioxide levels and growth of Nitrosomonas. Arch Microbiol 140:291–293. doi: 10.1007/BF00454945. [DOI] [Google Scholar]
- 11.Wett B, Rauch W. 2003. The role of inorganic carbon limitation in biological nitrogen removal of extremely ammonia concentrated wastewater. Water Res 37:1100–1110. doi: 10.1016/S0043-1354(02)00440-2. [DOI] [PubMed] [Google Scholar]
- 12.Guisasola A, Petzet S, Baeza JA, Carrera J, Lafuente J. 2007. Inorganic carbon limitations on nitrification: experimental assessment and modelling. Water Res 41:277–286. doi: 10.1016/j.watres.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Tan C, Ma F, Qiu S. 2013. Impact of carbon to nitrogen ratio on nitrogen removal at a low oxygen concentration in a sequencing batch biofilm reactor. Water Sci Technol 67:612–618. [DOI] [PubMed] [Google Scholar]
- 14.Thomson AJ, Giannopoulos G, Pretty J, Baggs EM, Richardson DJ. 2012. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos Trans R Soc Lond B Biol Sci 367:1157–1168. doi: 10.1098/rstb.2011.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper AB. 1968. A nitrite-reducing enzyme from Nitrosomonas europaea. Preliminary characterization with hydroxylamine as electron donor. Biochim Biophys Acta 162:49–65. [DOI] [PubMed] [Google Scholar]
- 16.Whittaker M, Bergmann D, Arciero D, Hooper AB. 2000. Electron transfer during the oxidation of ammonia by the chemolithotrophic bacterium Nitrosomonas europaea. Biochim Biophys Acta 1459:346–355. doi: 10.1016/S0005-2728(00)00171-7. [DOI] [PubMed] [Google Scholar]
- 17.Kozlowski JA, Price J, Stein LY. 2014. Revision of N2O-producing pathways in the ammonia-oxidizing bacterium Nitrosomonas europaea ATCC 19718. Appl Environ Microbiol 80:4930–4935. doi: 10.1128/AEM.01061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goreau TJ, Kaplan WA, Wofsy SC, McElroy MB, Valois FW, Watson SW. 1980. Production of NO2 and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl Environ Microbiol 40:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandran K, Stein LY, Klotz MG, van Loosdrecht MC. 2011. Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem Soc Trans 39:1832–1837. doi: 10.1042/BST20110717. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Garcia O, Villas-Boas SG, Swift S, Chandran K, Singhal N. 2014. Clarifying the regulation of NO/N2O production in Nitrosomonas europaea during anoxic-oxic transition via flux balance analysis of a metabolic network model. Water Res 60:267–277. doi: 10.1016/j.watres.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Ziv N, Brandt NJ, Gresham D. 2013. The use of chemostats in microbial systems biology. J Vis Exp 80:50168. doi: 10.3791/50168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood-Nowotny R, Hinko-Najera Umana N, Inselbacher E, Oswald-Lachouani P, Wanek W. 2010. Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Sci Soc Am J 74:1018–1027. doi: 10.2136/sssaj2009.0389. [DOI] [Google Scholar]
- 23.Pirt SJ. 1965. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci 163:224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- 24.Russell JB, Baldwin RL. 1979. Comparison of maintenance energy expenditures and growth yields among several rumen bacteria grown on continuous culture. Appl Environ Microbiol 37:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Bodegom P. 2007. Microbial maintenance: a critical review on its quantification. Microb Ecol 53:513–523. doi: 10.1007/s00248-006-9049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson MD, Smyth GK. 2008. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 9:321–332. [DOI] [PubMed] [Google Scholar]
- 27.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. 2009. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belser LW. 1984. Bicarbonate uptake by nitrifiers: effects of growth rate, pH, substrate concentration, and metabolic inhibitors. Appl Environ Microbiol 48:1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engel MS, Alexander M. 1958. Growth and autotrophic metabolism of Nitrosomonas europaea. J Bacteriol 76:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato C, Schnoor JL, McDonald DB, Huey J. 1985. Test medium for the growth of Nitrosomonas europaea. Appl Environ Microbiol 49:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellbye B, Schuster M. 2014. Physiological framework for the regulation of quorum sensing-dependent public goods in Pseudomonas aeruginosa. J Bacteriol 196:1155–1164. doi: 10.1128/JB.01223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keen GA, Prosser JI. 1987. Steady-state and transient growth of autotrophic nitrifying bacteria. Arch Microbiol 147:73–79. doi: 10.1007/BF00492908. [DOI] [Google Scholar]
- 35.Berg IA. 2011. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol 77:1925–1936. doi: 10.1128/AEM.02473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaumont HJ, van Schooten B, Lens SI, Westerhoff HV, van Spanning RJ. 2004. Nitrosomonas europaea expresses a nitric oxide reductase during nitrification. J Bacteriol 186:4417–4421. doi: 10.1128/JB.186.13.4417-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klotz MG, Stein LY. 2011. Genomics of ammonia-oxidizing bacteria and insights into their evolution, p 57–94. In Ward BB, Arp DJ, Klotz MG (ed), Nitrification. ASM Press, Washington, DC. [Google Scholar]
- 38.Smith KS, Ferry JG. 2000. Prokaryotic carbonic anhydrases. FEMS Microbiol Rev 24:335–366. doi: 10.1111/j.1574-6976.2000.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 39.Bar-Even A, Noor E, Milo R. 2012. A survey of carbon fixation pathways through a quantitative lens. J Exp Bot 63:2325–2342. doi: 10.1093/jxb/err417. [DOI] [PubMed] [Google Scholar]
- 40.Hooper AB, Terry KR. 1979. Hydroxylamine oxidoreductase of Nitrosomonas. Production of nitric oxide from hydroxylamine. Biochim Biophys Acta 571:12–20. [DOI] [PubMed] [Google Scholar]
- 41.Kampschreur MJ, Tan NC, Picioreanu C, Jetten MS, Schmidt I, van Loosdrecht MC. 2006. Role of nitrogen oxides in the metabolism of ammonia-oxidizing bacteria. Biochem Soc Trans 34:179–181. doi: 10.1042/BST0340179. [DOI] [PubMed] [Google Scholar]
- 42.Yu R, Kampschreur MJ, van Loosdrecht MC, Chandran K. 2010. Mechanisms and specific directionality of autotrophic nitrous oxide and nitric oxide generation during transient anoxia. Environ Sci Technol 44:1313–1319. doi: 10.1021/es902794a. [DOI] [PubMed] [Google Scholar]
- 43.Cabiscol E, Tamarit J, Ros J. 2000. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8. [PubMed] [Google Scholar]
- 44.Arsene F, Tomoyasu T, Bukau B. 2000. The heat shock response of Escherichia coli. Int J Food Microbiol 55:3–9. doi: 10.1016/S0168-1605(00)00206-3. [DOI] [PubMed] [Google Scholar]
- 45.Peng L, Ni BJ, Ye L, Yuan Z. 2015. N2O production by ammonia oxidizing bacteria in an enriched nitrifying sludge linearly depends on inorganic carbon concentration. Water Res 74:58–66. doi: 10.1016/j.watres.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Shaaban M, Peng QA, Hu R, Wu Y, Lin S, Zhao J. 2015. Dolomite application to acidic soils: a promising option for mitigating N2O emissions. Environ Sci Pollut Res Int 22:19961–19970. doi: 10.1007/s11356-015-5238-4. [DOI] [PubMed] [Google Scholar]
- 47.Law Y, Ni BJ, Lant P, Yuan Z. 2012. N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res 46:3409–3419. doi: 10.1016/j.watres.2012.03.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.