ABSTRACT
In the summer of 2010, Vibrio parahaemolyticus caused an outbreak in Maryland linked to the consumption of oysters. Strains isolated from both stool and oyster samples were indistinguishable by pulsed-field gel electrophoresis (PFGE). However, the oysters contained other potentially pathogenic V. parahaemolyticus strains exhibiting different PFGE patterns. In order to assess the identity, genetic makeup, relatedness, and potential pathogenicity of the V. parahaemolyticus strains, we sequenced 11 such strains (2 clinical strains and 9 oyster strains). We analyzed these genomes by in silico multilocus sequence typing (MLST) and determined their phylogeny using a whole-genome MLST (wgMLST) analysis. Our in silico MLST analysis identified six different sequence types (STs) (ST8, ST676, ST810, ST811, ST34, and ST768), with both of the clinical and four of the oyster strains being identified as belonging to ST8. Using wgMLST, we showed that the ST8 strains from clinical and oyster samples were nearly indistinguishable and belonged to the same outbreak, confirming that local oysters were the source of the infections. The remaining oyster strains were genetically diverse, differing in >3,000 loci from the Maryland ST8 strains. eBURST analysis comparing these strains with strains of other STs available at the V. parahaemolyticus MLST website showed that the Maryland ST8 strains belonged to a clonal complex endemic to Asia. This indicates that the ST8 isolates from clinical and oyster sources were likely not endemic to Maryland. Finally, this study demonstrates the utility of whole-genome sequencing (WGS) and associated analyses for source-tracking investigations.
IMPORTANCE Vibrio parahaemolyticus is an important foodborne pathogen and the leading cause of bacterial infections in the United States associated with the consumption of seafood. In the summer of 2010, Vibrio parahaemolyticus caused an outbreak in Maryland linked to oyster consumption. Strains isolated from stool and oyster samples were indistinguishable by pulsed-field gel electrophoresis (PFGE). The oysters also contained other potentially pathogenic V. parahaemolyticus strains with different PFGE patterns. Since their identity, genetic makeup, relatedness, and potential pathogenicity were unknown, their genomes were determined by using next-generation sequencing. Whole-genome sequencing (WGS) analysis by whole-genome multilocus sequence typing (wgMLST) allowed (i) identification of clinical and oyster strains with matching PFGE profiles as belonging to ST8, (ii) determination of oyster strain diversity, and (iii) identification of the clinical strains as belonging to a clonal complex (CC) described only in Asia. Finally, WGS and associated analyses demonstrated their utility for trace-back investigations.
INTRODUCTION
Vibrio parahaemolyticus is an important foodborne pathogen and the leading cause of bacterial infections in United States associated with the consumption of seafood (1). Vibrio parahaemolyticus strains are considered pathogenic when they carry genes encoding thermostable direct hemolysin (tdh) and/or thermostable direct hemolysin-related hemolysin (trh) (2), although these potentially pathogenic strains usually represent a small fraction of all environmental strains (3). In addition to the tdh and trh genes, pathogenic V. parahaemolyticus strains carry other pathogenicity-related genes, such as type III secretion effectors, which are needed for producing infections at the intestinal level and are usually located in pathogenicity islands (4–7).
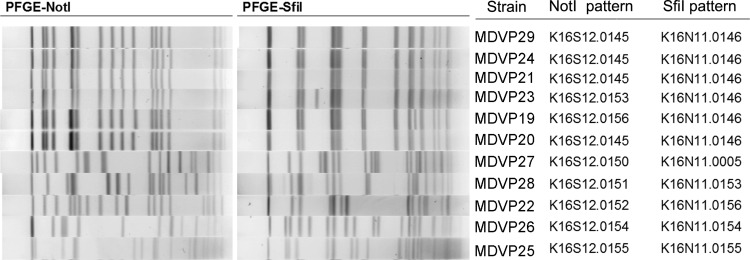
In the summer of 2010, two individuals became ill after eating raw oysters in two different restaurants in Baltimore, MD. In both cases, V. parahaemolyticus strains were isolated from their stools, and preliminary analyses by pulsed-field gel electrophoresis (PFGE) using two enzymes (SfiI and NotI) found that the two strains differed by only two bands (Table 1). On this basis, they were considered part of the same outbreak. Neither patient had traveled outside his/her home state 7 days prior to illness onset, nor did they have preexisting high-risk conditions for Vibrio infection. The two cases were initially linked based on the similar PFGE patterns produced by the strains isolated from these cases and later by the oyster tags, which are collected for every positive Vibrio exposure.
TABLE 1.
Characteristics of the V. parahaemolyticus strains used in this studyb
| Isolate | CFSAN identification no. | ST | Source | Virulence gene |
PFGE profilea |
||
|---|---|---|---|---|---|---|---|
| tdh | trh | NotI | SfiI | ||||
| MDVP19 | CFSAN007452 | 8 | Stool | None | 2 | K16S12.0156 | K16N11.0146 |
| MDVP20 | CFSAN007453 | 8 | Stool | None | 2 | K16S12.0145 | K16N11.0146 |
| MDVP21 | CFSAN012491 | 8 | Oyster | None | 2 | K16S12.0145 | K16N11.0146 |
| MDVP23 | CFSAN012492 | 8 | Oyster | None | 2 | K16S12.0153 | K16N11.0146 |
| MDVP24 | CFSAN012493 | 8 | Oyster | None | 2 | K16S12.0145 | K16N11.0146 |
| MDVP29 | CFSAN012494 | 8 | Oyster | None | 2 | K16S12.0145 | K16N11.0146 |
| MDVP22 | CFSAN007454 | 676 | Oyster | 2 | 1 | K16S12.0152 | K16N11.0156 |
| MDVP25 | CFSAN007456 | 810 | Oyster | None | 2 | K16S12.0155 | K16N11.0155 |
| MDVP26 | CFSAN007457 | 811 | Oyster | None | 2 | K16S12.0154 | K16N11.0154 |
| MDVP27 | CFSAN007458 | 34 | Oyster | None | 2 | K16S12.0150 | K16S11.0005 |
| MDVP28 | CFSAN007459 | 768 | Oyster | 2 | 1 | K16S12.0151 | K16N11.0153 |
PFGE profiles assigned by the CDC. Boldface indicates identical PFGE profiles in the same column.
All strains were isolated in Maryland in 2010.
The V. parahaemolyticus outbreak in Maryland in 2010 provided both an unusual opportunity and several puzzles, as causative strains are rarely isolated from food sources (5, 8–10). V. parahaemolyticus outbreak strains are typically available only from clinical samples. In this case, oysters containing the outbreak strain were identified. However, these oysters also contained V. parahaemolyticus strains that were not related to the outbreak. Furthermore, the PFGE pattern of the 2010 outbreak strains has not been detected in any subsequent V. parahaemolyticus cases in Maryland. Key questions include the following. What happened to the strain causing the 2010 outbreak? How related are these outbreak strains to previously archived strains? How genetically related are these outbreak strains to the other potentially pathogenic V. parahaemolyticus strains isolated from the same oysters? Can the genetic identity and phylogenetic relationship among these and other V. parahaemolyticus strains help us identify the possible origin of the outbreak strains?
Recently, scientists have been using next-generation sequencing techniques to reanalyze historical collections of pathogens and outbreak strains, in efforts to provide new insights for outbreak investigations. Whole-genome sequencing (WGS) together with single nucleotide polymorphism (SNP) (11–16) or whole-genome multilocus sequence typing (MLST) (wgMLST) (17–20) data analyses allow us to better understand both population dynamics and the mechanisms that contribute to increased virulence among foodborne bacterial pathogens.
To address the key questions about the identity, genetic makeup, and phylogenetic relationships among the V. parahaemolyticus strains collected during the 2010 Maryland outbreak, we sequenced the genomes of 11 of these strains: 2 from clinical samples and 9 from outbreak-implicated oysters. By comparing these genomes to each other and to the other V. parahaemolyticus genomes archived in GenBank, we are able to propose a possible origin for these outbreak strains as well as demonstrate the utility of WGS and associated analyses for such investigations.
MATERIALS AND METHODS
Identification of oyster isolates.
A trace-back investigation was conducted, and tags from suspect oysters were used to identify the implicated growing area. Additional oysters were collected from the growing area and analyzed for V. parahaemolyticus by using a most-probable-number (MPN)–real-time PCR method described previously (21). Thiosulfate-citrate-bile salts-sucrose (TCBS) agar plates were streaked from PCR-positive MPN tubes for isolation of V. parahaemolyticus. Typical isolated colonies were confirmed by using the same real-time PCR as the one used for screening. All tdh+ and/or trh+ isolates were analyzed by PFGE using the standard PulseNet protocol.
Bacterial strains and media.
The V. parahaemolyticus strains sequenced in this study, along with their assigned CFSAN identification numbers, are listed in Table 1. Isolates sequenced in our CFSAN/FDA facility are given a unique CFSAN identification number for future tracking. All isolates were retrieved from storage (−80°C freezer), transferred to Luria-Bertani (LB) medium with 3% NaCl, and incubated at 37°C with shaking at 250 rpm.
DNA extraction and quantification.
Genomic DNA from each strain was isolated from cultures grown overnight by using the DNeasy blood and tissue kit (Qiagen, Valencia, CA). The quality of the DNA was checked by using a NanoDrop 1000 instrument (Thermo Scientific, Rockford, IL), and the concentration was determined by using a Qubit double-stranded DNA (dsDNA) high-sensitivity (HS) assay kit and a Qubit 2.0 fluorometer (Thermo Scientific), according to each manufacturer's instructions.
Whole-genome sequencing, contig assembly, and annotation.
The genomes of the strains were sequenced by using 250-bp paired-end libraries, with a MiSeq reagent kit (v2), using a MiSeq sequencer (Illumina, San Diego, CA), according to the manufacturer's instructions, at ∼80× average coverage. The genome libraries were constructed by using a Nextera XT DNA sample prep kit (Illumina). Genomic sequence contigs were de novo assembled by using default settings within CLC Genomics Workbench v7.6.1 (Qiagen), with a minimum contig size threshold of 500 bp. The draft genomes were annotated by using the NCBI Prokaryotic Genome Automatic Annotation Pipeline (PGAAP) (http://www.ncbi.nlm.nih.gov/genomes/static/Pipeline.html) (22).
In silico MLST phylogenetic analysis.
The initial analysis and identification of the strains were performed by using an in silico V. parahaemolyticus MLST approach, based on information available at the V. parahaemolyticus MLST Database (http://pubmlst.org/vparahaemolyticus/), and by using Ridom SeqSphere+ software v2.3 (Ridom, Münster, Germany). Seven loci (dnaE, gyrB, recA, dtdS, pntA, pyrC, and tnaA) described previously for V. parahaemolyticus (23) were used for MLST analysis. The same V. parahaemolyticus MLST database was also used to assign numbers for alleles and sequence types (STs).
Assignment to clonal complexes.
eBURST v3 (http://eburst.mlst.net/) was used to assign STs to clonal complexes (CCs) using 1,000 bootstrap resamplings (23). In order to be included in a particular CC, isolates needed to share at least 5 of the 7 alleles with members of that CC. Single-locus variants (SLVs) shared at least 6 of the 7 alleles. Double-locus variants (DLVs) were defined as those STs which shared 5 of 7 alleles.
Phylogenomic analysis and targeted SNP analysis.
The phylogenetic relationship among these isolates was assessed by wgMLST using Ridom SeqSphere+ software v2.4.0 (20, 24, 25). The genome of strain RIMD2210633 was used as a reference. Core genes were defined as those that were shared among this strain and V. parahaemolyticus strain 10329 (GenBank accession number AFBW01000000), and accessory genes were defined as those that were present only in RIMD2210633. Ridom SeqSphere+ identified 2,526 loci as core genes and 587 loci as accessory genes for chromosome I. For chromosome II, 1,370 and 365 loci were identified as core and accessory genes, respectively.
The DNA distance method described previously by Nei et al. (26) was used for calculating the matrix of genetic distance, taking only the number of same/different alleles in the core genes into consideration. After eliminating any loci that were missing from the genomes of any strains used in our analyses, we performed wgMLST analysis. The total number of core genes employed for each analysis varied depending on which strains were being analyzed. In some cases, values are missing for certain loci because that gene either was missing or became truncated due to its position at either end of the de novo-assembled contigs. Therefore, the total number of relevant loci in each wgMLST figure was clarified, and a neighbor-joining (NJ) tree using the appropriate genetic distances for each analysis was then constructed.
Nucleotide sequence accession numbers.
The draft genome sequences for all 11 V. parahaemolyticus strains used in our analyses are available in GenBank under the accession numbers listed in Table 2.
TABLE 2.
Summary report of the de novo assembly of the strains from this study
| Isolate | GenBank accession no. | No. of contigs | Size (bp) | GC content (%) | N50 | Minimum contig size (bp) | Maximum contig size (bp) | Avg contig size (bp) | Avg coverage (×) |
|---|---|---|---|---|---|---|---|---|---|
| MDVP19 | JNTJ00000000 | 37 | 5,127,281 | 45.1 | 527,441 | 504 | 1,521,871 | 138,575 | 99 |
| MDVP20 | JNTK00000000 | 39 | 5,126,628 | 45.2 | 567,671 | 511 | 1,521,355 | 131,452 | 105 |
| MDVP21 | JNUG00000000 | 35 | 5,126,325 | 45.1 | 526,001 | 511 | 995,851 | 146,466 | 97 |
| MDVP23 | JNUH00000000 | 34 | 5,128,956 | 45.2 | 485,136 | 510 | 1,192,043 | 150,852 | 113 |
| MDVP24 | JNUI00000000 | 47 | 5,123,442 | 45.2 | 329,201 | 511 | 635,564 | 109,009 | 79 |
| MDVP29 | JNUJ00000000 | 34 | 5,127,159 | 45.2 | 527,658 | 511 | 1,521,578 | 150,799 | 95 |
| MDVP22 | JNUO00000000 | 32 | 5,017,786 | 45.3 | 445,027 | 704 | 799,796 | 139,383 | 77 |
| MDVP25 | JNUK00000000 | 56 | 5,206,921 | 45.2 | 480,822 | 501 | 878,464 | 92,981 | 95 |
| MDVP26 | JNUL00000000 | 33 | 5,188,815 | 45.1 | 540,584 | 691 | 1,242,084 | 157,237 | 113 |
| MDVP27 | JNUM00000000 | 52 | 5,061,948 | 45.2 | 321,455 | 520 | 729,103 | 97,345 | 66 |
| MDVP28 | JNUN00000000 | 45 | 5,205,568 | 45.1 | 425,650 | 554 | 1,317,831 | 115,679 | 87 |
RESULTS
Identification of oyster isolates.
Oysters from the growing area implicated in the outbreak were screened for total and pathogenic V. parahaemolyticus bacteria. Of the 479 V. parahaemolyticus strains isolated, 11 of these isolates were determined to be potentially pathogenic. PFGE two-enzyme analysis found four of the nine tdh-negative trh+ isolates to be indistinguishable from one of the human isolates (Table 1).
Draft genome assemblies.
The draft genomes of the 11 V. parahaemolyticus strains isolated during the 2010 Maryland outbreak were generated by whole-genome sequencing using MiSeq. The estimated average coverage for these strains was between 60× and 110×. Genome assembly statistics for each strain are summarized in Table 2. The estimated genome sizes varied between 5.02 and 5.2 Mb. The average G+C content was between 45.1 and 45.3%; these values are within the range reported previously for other V. parahaemolyticus strains (27–29).
In silico MLST.
In silico MLST identified 7 different STs among the 11 sequenced strains. The two clinical strains were of ST8, as were the four oyster strains that matched by PFGE (Table 1 and Fig. 1). The other oyster strains appear to belong to previously undetected STs (ST810, ST811, and ST676), and one was of ST34, which is an ST commonly found in coastal areas of the United States (5, 23), mainly in the Gulf of Mexico (http://pubmlst.org/vparahaemolyticus).
FIG 1.
PFGE profiles of the V. parahaemolyticus strains used in this study, using two restriction enzymes (NotI and SfiI).
Phylogenetic analysis.
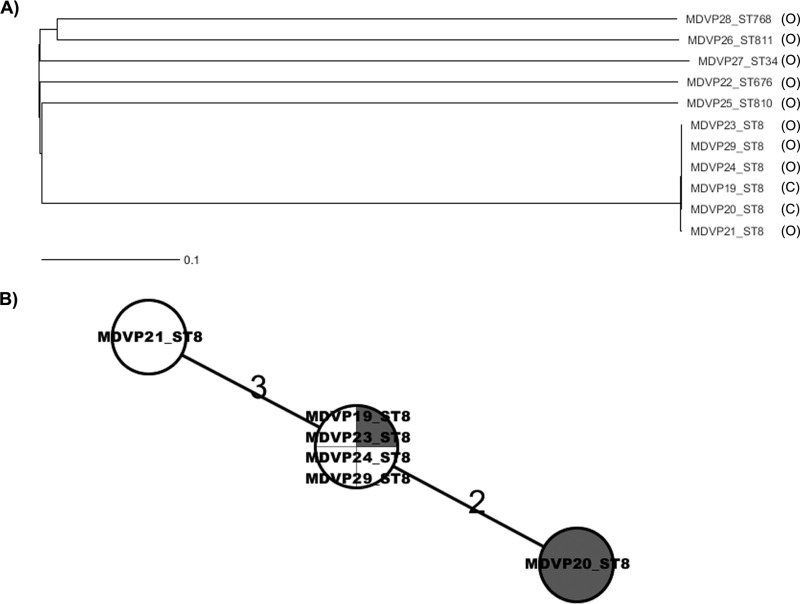
The phylogenetic relationships among the V. parahaemolyticus 2010 Maryland strains analyzed in this study were determined by using wgMLST analysis (Fig. 2). The V. parahaemolyticus strains isolated from outbreak-associated oysters were genetically diverse and belonged to different populations, as documented by the high number of locus differences that defined each branch (minimum spanning tree [MST]) (see Fig. S1 in the supplemental material). Interestingly, the Maryland ST8 strains differed from the other oyster strains by >3,000 loci. Nonetheless, strains with the same ST clustered together. Further wgMLST analysis using only Maryland ST8 strains showed that among the 4,349 loci used for the comparison, 4,344 of them were identical across all these strains, and two strains, MDVP20 and MDVP21, differed by only 2 and 3 loci, respectively (Fig. 2B). The differences in those loci were caused by SNPs (Table 3).
FIG 2.
Phylogeny of the V. parahaemolyticus strains isolated during the outbreak of 2010 in Maryland assessed by wgMLST analysis. Ridom SeqSphere+ identified 3,896 and 952 loci as core and accessory genes, respectively, for both chromosomes in V. parahaemolyticus. (A) NJ tree showing the high level of diversity of V. parahaemolyticus strains isolated from oysters and their relationship to the clinical samples (C, clinical; O, oysters) (3,955 loci were shared among the strains analyzed). (B) Minimum spanning tree showing the locus differences among ST8 strains from oysters (no shading) and from clinical samples (shaded). Of 4,349 loci shared by all ST8 strains, there were overall 5 loci differing among the strains, showing clonality of the strains. Also evident is that the ST8 oyster strains were indistinguishable from ST8 clinical strains. This result, combined with the epidemiological data, confirmed that the tested oysters were the source of the outbreak cases. The numbers above the connected lines are locus differences. The lines are not drawn to scale.
TABLE 3.
SNP differences among the Maryland ST8 strains, their positions, and locus locations
| Target | Position | Abs. positiona | Product | Nucleotide |
|||||
|---|---|---|---|---|---|---|---|---|---|
| MDVP20 | MDVP23 | MDVP21 | MDVP29 | MDVP24 | MDVP19 | ||||
| VP1807 | 373 | 1915623 | Hypothetical protein | G | A | A | A | A | A |
| VPA0532 | 60 | 536660 | Hypothetical protein | G | G | T | G | G | G |
| VPA1445 | 461 | 1538990 | Secreted calcium-binding protein | T | T | C | T | T | T |
| VPA1674 | 2 | 1795177 | Ribulokinase | A | T | T | T | T | T |
| VP1647 | 649 | 1766093 | Methylcitrate synthase | G | G | A | G | G | G |
Base position in the genome of RIMD2210633.
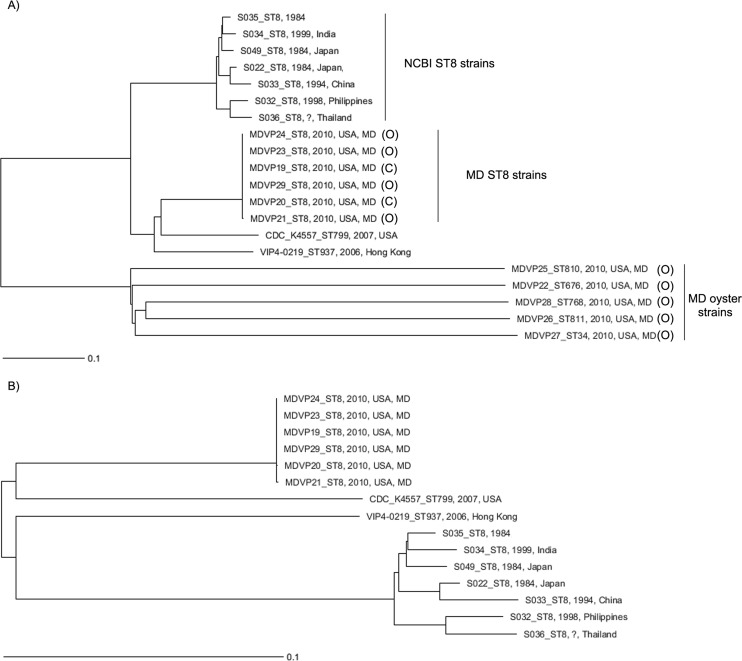
Comparison of ST8 Maryland outbreak strains with closely related V. parahaemolyticus genomes in GenBank.
Additional wgMLST comparisons of Maryland ST8 strains with their closest relatives available in GenBank (Fig. 3 and Table 4) confirmed that they were highly related and different from the other Maryland oyster strains (Fig. 3A). However, Maryland ST8 strains had important differences from ST8 strains collected from Asia between 1984 and 1999 (Fig. 2A). wgMLST of only Maryland ST8 strains and their genome group members showed that they were genetically more closely related to strains of other STs belonging to the same genome group that were isolated more recently (VIP4-0219 [ST937], isolated in 2006 in Hong Kong from salmon sashimi, and CDC_K4557 [ST799], isolated in 2006 from stool samples) than to other ST8 strains isolated >10 years ago (Fig. 3B).
FIG 3.
Genome comparison of the 2010 V. parahaemolyticus ST8 strains from Maryland with other members of the genome group in the NCBI database by wgMLST analysis. (A) Comparison of all Maryland 2010 strains (clinical and oyster sources) with strains belonging to the ST8 genome group in the NCBI database (using 3,376 total loci). (B) Comparison of genomes of only ST8 Maryland 2010 strains with genomes of the strains belonging to the ST8 genome group in the NCBI database (http://www.ncbi.nlm.nih.gov/genome/genomes/691?genome_assembly_id=group167998).
TABLE 4.
List of genomes in GenBank that belong to the same genome group according to genomic BLAST analysis, excluding the Maryland ST8 isolatesb
| Strain | Region | Yr of isolation | Sourcea | Serotype | GenBank accession no. | ST |
|---|---|---|---|---|---|---|
| CDC_K4557 | USA | 2007 | C | O1:K33 | NC_021848.1, NC_021822.1 | 799 |
| S049 | Japan | 1984 | C | O4:K68 | AWLM01000000 | 8 |
| S036 | Thailand | ? | C | ? | AWLZ01000000 | 8 |
| S035 | Japan | 1984 | C | O4:K53 | AWMA01000000 | 8 |
| S034 | India | 1999 | C | ? | AWMB01000000 | 8 |
| S033 | China | 1994 | C | O5:K60 | AWMC01000000 | 8 |
| S032 | Philippines | 1998 | C | O1:K56 | AWMD01000000 | 8 |
| S022 | Japan | 1984 | C | O5:K15 | AWMN01000000 | 8 |
| VIP4-0219 | Hong Kong | 2006 | E | ? | NZ_AXNQ01000000 | 937 |
C, clinical; E, environmental.
?, unknown.
Origin of the Maryland ST8 strains.
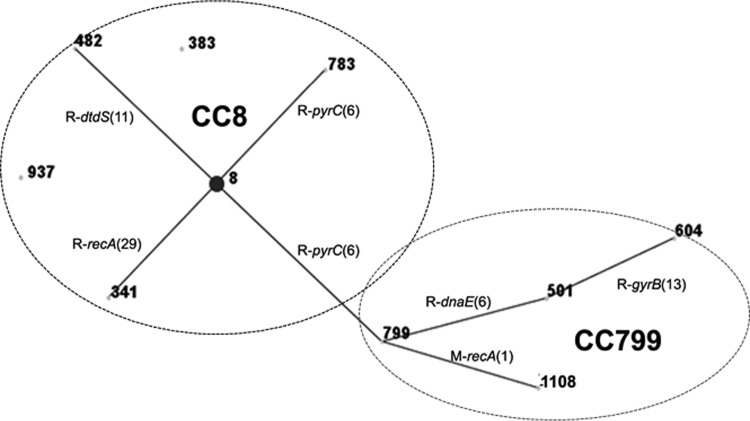
eBURST analysis using other strains from the V. parahaemolyticus MLST database showed that the strains within ST8 belong to a CC that encompasses both CC8 and CC799 (Fig. 4 and Tables 4 and 5). ST8 and ST799 are SLVs, and both STs are the predicted ancestral ST of their own respective clonal complexes (Table 4). As observed by whole-genome analysis, the main sources of changes identified by MLST analysis (seven housekeeping genes) are recombination events (Fig. 4; see also Fig. S3 in the supplemental material). As shown in Table 4, 20 strains (representing 11 different STs) already found in the MLST database are members of these two CCs. Interestingly, most strains (95%) belonging to CC8 and CC799 were isolated from Asian sources. The only exception is CDC_K4557 (ST799), which was isolated from a clinical sample in the United States in 2006 (30).
FIG 4.
V. parahaemolyticus population “snapshot” of CC8 and SLV CC799 obtained by using eBURST v3, using data available in the MLST database. ST8 and ST799 were identified as the predicted clonal ancestors of CC8 and CC799, respectively. STs that are SLVs of each other are shown connected by black lines. Recombination events (R), loci, and the number of SNPs between the connecting STs are shown. Numbers of SNP differences are in parentheses.
TABLE 5.
Sequence types in each of the two CCs (V. parahaemolyticus MLST database) identified as being related to the Maryland strains causing the outbreak in 2010 (ST8) by eBURST analysisa
| CC | ST | Frequency (no. of strains)b | Variant | Country(ies) (no. of strains) | Yr of isolation | Source(s) (no. of strains) |
|---|---|---|---|---|---|---|
| 8 | 8 | 10c | Ancestral type | China (5), Japan (2), Philippines (1), India (1), Thailand (1) | 1984–2008 | C (9), E(1) |
| 341 | 1 | SLV | China | 2010 | C | |
| 783 | 1 | SLV | China | 2008 | C | |
| 482 | 1 | SLV | China | 2010 | C | |
| 1016 | 1 | SLV | NA | NA | NA | |
| 383 | 1 | DLV | China | 2005 | E | |
| 937 | 1 | DLV | China | 2006 | E | |
| 799 | 799 | 1 | Ancestral type | USA | 2006 | C |
| 1108 | 1 | SLV | China | 2006 | E | |
| 501 | 1 | SLV | China | 2008 | E | |
| 604 | 1 | DLV | China | NA | NA |
C, clinical; E, environmental; NA, information not available.
Number of strains in the V. parahaemolyticus MLST database.
Excluding the six ST8 strains from the Maryland 2010 outbreak.
Distribution of the type III secretion system and other genomic regions in V. parahaemolyticus strains from this study.
While analyzing the genetic makeup of the studied strains, we found that potentially pathogenic V. parahaemolyticus strains lacked the full set of pathogenicity islands (PIs) found in pandemic strain RIMD2210633 (VPaI-1 to -7) (7) (Table 6). However, they all carried type III secretion system 2 beta (T3SS-2 beta) (region containing the trh gene) (6), hypothetical proteins of unknown function (NK), T3SS-1, osmotolerance (chromosome I), gametolysin and osmotolerance (chromosome II), capsule polysaccharide (CPS), type I secretion, type I pilus, multidrug efflux, and ferric uptake genes. Also, most of these strains carried a T6SS that differed from that of the pandemic strain.
TABLE 6.
In silico screen for pathogenicity markers tested for V. parahaemolyticus strains sequenced in this study
| Location and pathogenicity marker | Region or source | Presence or type of markera |
|||||
|---|---|---|---|---|---|---|---|
| MDVP20 | MDVP22 | MDVP25 | MDVP26 | MDVP27 | MDVP28 | ||
| Chromosome I | |||||||
| NK | VP0081–VP0092 | + | + | + | + | + | + |
| LPS | VP0218–VP0234 | − | − | − | − | − | − |
| VPaI-1 | VP0380–VP0403 | − | − | − | − | − | − |
| VPaI-2 | VP0634–VP0643 | − | + (dif) | − | − | − | − |
| VPaI-3 | VP1071–VP1095 | − | − | − | − | − | − |
| T6SS | VP1386–VP1420 | + | + | − | + | + | + |
| Phage f237 | VP1549–VP1590 | + (dif) | + (dif) | + (dif) | + (dif) | − | + (dif) |
| T3SS-1 | VP1658–VP1702 | + | + | + | + | + | + |
| Osmotolerance | VP1719–VP1728 | + | + | + | + | + | + |
| Integron class 1 | VP1787–VP1865 | + (dif) | + (dif) | + (dif) | + (dif) | + (dif) | + (dif) |
| VPaI-4 | VP2131–VP2144 | − | − | − | − | − | − |
| VPaI-5 | VP2900–VP2910 | − | − | − | − | − | − |
| Chromosome II | |||||||
| Degradative | VPA0434–VPA0458 | − | + | − | − | − | − |
| Phage f237-like | VPA0887–VPA0914 | + (dif) | + (dif) | + (dif) | + (dif) | + (dif) | − |
| Biofilm | VPA0950–VPA0962 | − | − | − | − | − | − |
| Gametolysin | VPA0989–VPA0999 | + | + | + | + | + | + |
| Osmotolerance | VPA1102–VPA1115 | + | + | + | + | + | + |
| VPaI-6 | VPA1253–VPA1270 | − | − | − | − | − | − |
| VPaI-7 (T3SS-2 alpha) | VPA1312–VPA1395 | − | − | − | − | − | − |
| CPS | VPA1403–VPA1412 | + | + | + | + | + | + |
| Type I secretion | VPA1440–VPA1444 | + | + | + | + | + | + |
| Type I pilus | VPA1503–VPA1521 | + | + | + | + | + | + |
| Multidrug efflux | VPA1559–VPA1583 | + | + | + | + | + | + |
| Ferric uptake | VPA1652–VPA1679 | + | + | + | + | + | + |
| T3SS-2 beta | TH3996 trh1 regionb | + | + | + | + | + | + |
| tdh type | − | 2 | − | − | − | − | |
| trh type | 2 | 1 | 2 | 2 | 2 | 2 | |
| ST | MLST V. parahaemolyticus website | 8 | 676 | 810 | 811 | 34 | 768 |
+, present; −, absent; + (dif), present but with a different sequence or element.
See GenBank accession number AB455531.
DISCUSSION
In this study, we found that V. parahaemolyticus strains causing an outbreak in Maryland in 2010 and isolated from stool samples belonged to ST8. Vibrio parahaemolyticus strains isolated from oysters implicated in this outbreak had a PFGE pattern indistinguishable from that of the clinical strains, belonged to ST8, and were nearly indistinguishable by wgMLST (differing in 2 or 3 loci of 4,349 loci tested), with these differences being caused by SNPs. These results confirm that Chesapeake Bay oysters were the source of the vibriosis cases in 2010, since the genomic identity at the nucleotide level between oyster and clinical strains was >99.999%, which is the level of similarity that can be found between colonies of the same strain (11).
However, our research during the 2010 outbreak investigations revealed that the oysters analyzed also carried other potentially pathogenic V. parahaemolyticus strains that belong to different populations and differed in >3,000 loci from the outbreak strains (see Fig. S3 and Table S1 in the supplemental material). There were 115,875 SNPs within these 3,000 loci (see Table S2 in the supplemental material). One oyster strain, of ST34 and carrying trh2, was linked previously to clinical cases (31) (http://pubmlst.org/vparahaemolyticus). Such strains, regardless of their pathogenic potential, were not observed among any other clinical cases from 2010. This could have been because their concentration was lower in oysters and not high enough to cause illnesses but high enough to be detectable by the MPN method. Sporadic illnesses and/or small outbreaks often go unnoticed, and therefore, the amount of illness caused by any strain of V. parahaemolyticus is probably underreported (1).
Genomic analysis of all potentially pathogenic V. parahaemolyticus strains isolated from the oysters implicated in the outbreak showed that they carried several pathogenicity-related genes (i.e., type III secretion effectors) besides the tdh and/or trh genes. However, they lacked all pathogenicity islands described for pandemic strain RIMD2210633 (7). We believe that these strains probably have their own PIs that have not yet been described, but this speculation must be interpreted with caution since a more detailed investigation using closed genomes could reveal additional elements that cannot currently be assessed.
When we compared Maryland ST8 strains with their most closely related genomic sequences available in GenBank (Table 3; see also Fig. S1 in the supplemental material), we found that the Maryland ST8 strains were more closely related to strains of other STs in their genome group than to the other ST8 strains (Fig. 3A). The significant genomic differences between both groups of ST8 strains could be explained by the time lapse between both samplings (>20 years separate the two ST8 groups), and habitat conditions/selective pressures in Asia could be different from those in the Chesapeake Bay, all of which could result in significant genomic differences.
In V. parahaemolyticus, the typical mechanism of evolution is believed to be recombination instead of mutation, with recombination/mutation ratios estimated to be 2.5:1 and 8.8:1 by allele and site, respectively (23). An example from the current set of isolates is MDVP23, which differs from S035 by 806 loci, but analysis at the SNP level shows that these strains differ by 7,562 SNPs within these loci, with most of the loci containing possible recombination signatures of between 5 and 6 SNPs per locus, for an estimated recombination ratio of ∼9:1 by locus (see Fig. S3 in the supplemental material). Our empirical calculations suggest that strains of V. parahaemolyticus of ST8 are experiencing strong evolutionary pressures favoring multiple recombination events.
How nonautochthonous V. parahaemolyticus strains from Asia can be present in oysters harvested from the eastern coast of the United States, specifically in the Chesapeake Bay, remains a matter of speculation. This bay has a lot of maritime traffic, and ballast water from ships coming from Asia, ocean currents, or other events such as the introduction of nonnative oysters or exotic fish may have introduced the nonautochthonous strains into this area. Since 2010, ST8 strains have not been linked to any additional illnesses, and most of the outbreaks in Maryland (2012 to 2013) have been linked to autochthonous East Coast U.S. strains (e.g., ST631) (5) or pandemic strains (10). The disappearance of the ST8 strains from Maryland could have happened due to natural replacement by new or autochthonous V. parahaemolyticus strains and/or by the action of bacteriophages (usually present in higher levels in seawater [32]) after the probable unknown source was removed from the Chesapeake environment. The reduction or elimination of V. parahaemolyticus by phages has been described for pandemic strains in the south of Chile (33), and other authors have employed bacteriophage therapy to reduce Vibrio numbers in oysters (34–36).
Taken together, our findings demonstrate that whole-genome sequencing allowed a detailed retrospective study of outbreak and nonoutbreak strains of V. parahaemolyticus, revealing their phylogenetic relationships and confirming their local vector and their likely path from Asia to the Chesapeake Bay. The wgMLST method employed was easy, robust, and scalable to multiple strains to be used in future V. parahaemolyticus outbreak investigations. Furthermore, we demonstrate the potential consequences of nonautochthonous V. parahaemolyticus strains introduced into a new habitat.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by FDA Foods Program intramural funds.
We thank Lili Fox Vélez for her scientific writing assistance on the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00096-16.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner JW, Paranjpye RN, Landis ED, Biryukov SV, Gonzalez-Escalona N, Nilsson WB, Strom MS. 2013. Population structure of clinical and environmental Vibrio parahaemolyticus from the Pacific Northwest coast of the United States. PLoS One 8:e55726. doi: 10.1371/journal.pone.0055726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DePaola A, Kaysner CA, Bowers J, Cook DW. 2000. Environmental investigations of Vibrio parahaemolyticus in oysters after outbreaks in Washington, Texas, and New York (1997 and 1998). Appl Environ Microbiol 66:4649–4654. doi: 10.1128/AEM.66.11.4649-4654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noriea NF III, Johnson CN, Griffitt KJ, Grimes DJ. 2010. Distribution of type III secretion systems in Vibrio parahaemolyticus from the northern Gulf of Mexico. J Appl Microbiol 109:953–962. doi: 10.1111/j.1365-2672.2010.04722.x. [DOI] [PubMed] [Google Scholar]
- 5.Haendiges J, Timme R, Allard MW, Myers RA, Brown EW, Gonzalez-Escalona N. 2015. Characterization of Vibrio parahaemolyticus clinical strains from Maryland (2012-2013) and comparisons to a locally and globally diverse V. parahaemolyticus strains by whole-genome sequence analysis. Front Microbiol 6:125. doi: 10.3389/fmicb.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park KS, Iida T, Yamaichi Y, Oyagi T, Yamamoto K, Honda T. 2000. Genetic characterization of DNA region containing the trh and ure genes of Vibrio parahaemolyticus. Infect Immun 68:5742–5748. doi: 10.1128/IAI.68.10.5742-5748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd EF, Cohen AL, Naughton LM, Ussery DW, Binnewies TT, Stine OC, Parent MA. 2008. Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus. BMC Microbiol 8:110. doi: 10.1186/1471-2180-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Urtaza J, Baker-Austin C, Jones JL, Newton AE, Gonzalez-Aviles GD, DePaola A. 2013. Spread of Pacific Northwest Vibrio parahaemolyticus strain. N Engl J Med 369:1573–1574. doi: 10.1056/NEJMc1305535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton AE, Garrett N, Stroika SG, Halpin JL, Turnsek M, Mody RK. 2014. Notes from the field: increase in Vibrio parahaemolyticus infections associated with consumption of Atlantic Coast shellfish—2013. MMWR Morb Mortal Wkly Rep 63:335–336. [PMC free article] [PubMed] [Google Scholar]
- 10.Haendiges J, Rock M, Myers RA, Brown EW, Evans P, Gonzalez-Escalona N. 2014. Pandemic Vibrio parahaemolyticus, Maryland, USA, 2012. Emerg Infect Dis 20:718–720. doi: 10.3201/eid2004.130818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allard MW, Luo Y, Strain E, Li C, Keys CE, Son I, Stones R, Musser SM, Brown EW. 2012. High resolution clustering of Salmonella enterica serovar Montevideo strains using a next-generation sequencing approach. BMC Genomics 13:32. doi: 10.1186/1471-2164-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakker HC, Switt AI, Cummings CA, Hoelzer K, Degoricija L, Rodriguez-Rivera LD, Wright EM, Fang R, Davis M, Root T, Schoonmaker-Bopp D, Musser KA, Villamil E, Waechter H, Kornstein L, Furtado MR, Wiedmann M. 2011. A whole-genome single nucleotide polymorphism-based approach to trace and identify outbreaks linked to a common Salmonella enterica subsp. enterica serovar Montevideo pulsed-field gel electrophoresis type. Appl Environ Microbiol 77:8648–8655. doi: 10.1128/AEM.06538-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin C-S, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, Bullard J, Webster DR, Kasarskis A, Peluso P, Paxinos EE, Yamaichi Y, Calderwood SB, Mekalanos JJ, Schadt EE, Waldor MK. 2011. The origin of the Haitian cholera outbreak strain. N Engl J Med 364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, Paxinos EE, Sebra R, Chin CS, Iliopoulos D, Klammer A, Peluso P, Lee L, Kislyuk AO, Bullard J, Kasarskis A, Wang S, Eid J, Rank D, Redman JC, Steyert SR, Frimodt-Moller J, Struve C, Petersen AM, Krogfelt KA, Nataro JP, Schadt EE, Waldor MK. 2011. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med 365:709–717. doi: 10.1056/NEJMoa1106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allard MW, Luo Y, Strain E, Pettengill J, Timme R, Wang C, Li C, Keys CE, Zheng J, Stones R, Wilson MR, Musser SM, Brown EW. 2013. On the evolutionary history, population genetics and diversity among isolates of Salmonella Enteritidis PFGE pattern JEGX01.0004. PLoS One 8:e55254. doi: 10.1371/journal.pone.0055254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Escalona N, Timme R, Raphael BH, Zink D, Sharma SK. 2014. Whole-genome single-nucleotide-polymorphism analysis for discrimination of Clostridium botulinum group I strains. Appl Environ Microbiol 80:2125–2132. doi: 10.1128/AEM.03934-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovanen SM, Kivisto RI, Rossi M, Schott T, Karkkainen UM, Tuuminen T, Uksila J, Rautelin H, Hanninen ML. 2014. Multilocus sequence typing (MLST) and whole-genome MLST of Campylobacter jejuni isolates from human infections in three districts during a seasonal peak in Finland. J Clin Microbiol 52:4147–4154. doi: 10.1128/JCM.01959-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolley KA, Maiden MC. 2014. Using MLST to study bacterial variation: prospects in the genomic era. Future Microbiol 9:623–630. doi: 10.2217/fmb.14.24. [DOI] [PubMed] [Google Scholar]
- 19.Schmid D, Allerberger F, Huhulescu S, Pietzka A, Amar C, Kleta S, Prager R, Preussel K, Aichinger E, Mellmann A. 2014. Whole genome sequencing as a tool to investigate a cluster of seven cases of listeriosis in Austria and Germany, 2011–2013. Clin Microbiol Infect 20:431–436. doi: 10.1111/1469-0691.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohl TA, Diel R, Harmsen D, Rothganger J, Walter KM, Merker M, Weniger T, Niemann S. 2014. Whole-genome-based Mycobacterium tuberculosis surveillance: a standardized, portable, and expandable approach. J Clin Microbiol 52:2479–2486. doi: 10.1128/JCM.00567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordstrom JL, Vickery MC, Blackstone GM, Murray SL, DePaola A. 2007. Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl Environ Microbiol 73:5840–5847. doi: 10.1128/AEM.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klimke W, Agarwala R, Badretdin A, Chetvernin S, Ciufo S, Fedorov B, Kiryutin B, O'Neill K, Resch W, Resenchuk S, Schafer S, Tolstoy I, Tatusova T. 2009. The National Center for Biotechnology Information's Protein Clusters Database. Nucleic Acids Res 37:D216–D223. doi: 10.1093/nar/gkn734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Escalona N, Martinez-Urtaza J, Romero J, Espejo RT, Jaykus LA, DePaola A. 2008. Determination of molecular phylogenetics of Vibrio parahaemolyticus strains by multilocus sequence typing. J Bacteriol 190:2831–2840. doi: 10.1128/JB.01808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bletz S, Bielaszewska M, Leopold SR, Kock R, Witten A, Schuldes J, Zhang W, Karch H, Mellmann A. 2013. Evolution of enterohemorrhagic Escherichia coli O26 based on single-nucleotide polymorphisms. Genome Biol Evol 5:1807–1816. doi: 10.1093/gbe/evt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruppitsch W, Pietzka A, Prior K, Bletz S, Fernandez HL, Allerberger F, Harmsen D, Mellmann A. 2015. Defining and evaluating a core genome multilocus sequence typing scheme for whole-genome sequence-based typing of Listeria monocytogenes. J Clin Microbiol 53:2869–2876. doi: 10.1128/JCM.01193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nei M, Tajima F, Tateno Y. 1983. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. J Mol Evol 19:153–170. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Escalona N, Gavilan RG, Brown EW, Martinez-Urtaza J. 2015. Transoceanic spreading of pathogenic strains of Vibrio parahaemolyticus with distinctive genetic signatures in the recA gene. PLoS One 10:e0117485. doi: 10.1371/journal.pone.0117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee S, Petronella N, Chew LC, Farber J. 2015. Draft genome sequences of four Vibrio parahaemolyticus isolates from clinical cases in Canada. Genome Announc 3(1):e01482-14. doi: 10.1128/genomeA.01482-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalburge SS, Polson SW, Boyd Crotty K, Katz L, Turnsek M, Tarr CL, Martinez-Urtaza J, Boyd EF. 2014. Complete genome sequence of Vibrio parahaemolyticus environmental strain UCM-V493. Genome Announc 2(2):e00159-14. doi: 10.1128/genomeA.00159-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludeke CH, Kong N, Weimer BC, Fischer M, Jones JL. 2015. Complete genome sequences of a clinical isolate and an environmental isolate of Vibrio parahaemolyticus. Genome Announc 3(2):e00216-15. doi: 10.1128/genomeA.00216-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellingsen AB, Olsen JS, Granum PE, Rorvik LM, Gonzalez-Escalona N. 2013. Genetic characterization of trh positive Vibrio spp. isolated from Norway. Front Cell Infect Microbiol 3:107. doi: 10.3389/fcimb.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang SC, Paul JH. 1998. Gene transfer by transduction in the marine environment. Appl Environ Microbiol 64:2780–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia K, Bastias R, Higuera G, Torres R, Mellado A, Uribe P, Espejo RT. 2013. Rise and fall of pandemic Vibrio parahaemolyticus serotype O3:K6 in southern Chile. Environ Microbiol 15:527–534. doi: 10.1111/j.1462-2920.2012.02883.x. [DOI] [PubMed] [Google Scholar]
- 34.Jun JW, Kim HJ, Yun SK, Chai JY, Park SC. 2014. Eating oysters without risk of vibriosis: application of a bacteriophage against Vibrio parahaemolyticus in oysters. Int J Food Microbiol 188:31–35. doi: 10.1016/j.ijfoodmicro.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Tskhvediani A, Khukhunashvili T, Eliashvili T, Tsertsvadze G, Gachechiladze N, Tediashvili M. 2014. The possible use of V. parahaemolyticus-specific bacteriophages for prevention and therapy of infections caused by V. parahaemolyticus. Georgian Med News 2014(231):82–88. [PubMed] [Google Scholar]
- 36.Pereira C, Silva YJ, Santos AL, Cunha A, Gomes NC, Almeida A. 2011. Bacteriophages with potential for inactivation of fish pathogenic bacteria: survival, host specificity and effect on bacterial community structure. Mar Drugs 9:2236–2255. doi: 10.3390/md9112236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.