ABSTRACT
We investigated communities of arbuscular mycorrhizal fungi (AMF) in the roots and the rhizosphere soil of Brachypodium retusum in six different natural soils under field conditions. We explored phylogenetic patterns of AMF composition using indicator species analyses to find AMF associated with a given habitat (root versus rhizosphere) or soil type. We tested whether the AMF characteristics of different habitats or contrasting soils were more closely related than expected by chance. Then we used principal-component analysis and multivariate analysis of variance to test for the relative contribution of each factor in explaining the variation in fungal community composition. Finally, we used redundancy analysis to identify the soil properties that significantly explained the differences in AMF communities across soil types. The results pointed out a tendency of AMF communities in roots to be closely related and different from those in the rhizosphere soil. The indicator species analyses revealed AMF associated with rhizosphere soil and the root habitat. Soil type also determined the distribution of AMF communities in soils, and this effect could not be attributed to a single soil characteristic, as at least three soil properties related to microbial activity, i.e., pH and levels of two micronutrients (Mn and Zn), played significant roles in triggering AMF populations.
IMPORTANCE Communities of arbuscular mycorrhizal fungi (AMF) are main components of soil biota that can determine the productivity of ecosystems. These fungal assemblages vary across host plants and ecosystems, but the main ecological processes that shape the structures of these communities are still largely unknown. A field study in six different soil types from semiarid areas revealed that AMF communities are significantly influenced by habitat (soil versus roots) and soil type. In addition, three soil properties related to microbiological activity (i.e., pH and manganese and zinc levels) were the main factors triggering the distribution of AMF. These results contribute to a better understanding of the ecological factors that can shape AMF communities, an important soil microbial group that affects multiple ecosystem functions.
INTRODUCTION
Arbuscular mycorrhizal fungi (AMF) represent an important soil microbial group that affects multiple ecosystem functions and processes, including nutrient cycling, plant productivity and competition, and plant diversity. As a consequence, the number of ecological studies concerning AMF has increased considerably in recent years (1–9). Those studies considered AMF communities associated with different host plants in many different ecosystems. However, studies comparing the occurrence of specific AMF species and communities in different soil types are scarce and have focused mainly on cultivated soils and different land uses (10–13). Differences in soil types have been reported to be key factors determining AMF community composition (10), and this is particularly relevant in stressed environments such as serpentine soils (14–16), thermal soils (17), heavy metal soils, and saline soils (18–23).
Traditionally, studies on AMF abundance and distribution have been made by spore extraction from soil and identification based on the morphology and ontogeny of the spores. Thus, identification of spores has also been widely used to characterize AMF communities in soil (10, 24, 25).
The introduction of molecular methods to the study of AMF has revealed a previously unexpected degree of complexity in the ecology of fungi and their relationships with the host plants. PCR-based methods have been used to detect AMF in plant roots and in soil in numerous studies in natural and seminatural ecosystems, including grasslands (26), wetlands (27), agricultural ecosystems (12), urban soils (28), semiarid shrubland (29, 30), and a temperate forest (31). Recently, some investigations have incorporated fungal DNA extraction from soil in addition to root extractions (9, 32–35) as tools to describe the total AMF soil diversity, including actively functioning fungal taxa as well as dormant spores.
In semiarid ecosystems, AMF play key roles in improving the function and adaptation of plant communities to these stressed environments. Despite their importance in semiarid regions, few studies have investigated AMF diversity and community composition, e.g., in plants from gypsum soils (6, 29, 36), in semiarid prairies (30), in degraded areas (37), and in a shrub community (38).
There is clear evidence that AMF community composition and distribution at different sites or in different habitats are affected mainly by host plant species and environmental factors such as soil type (6, 7, 10, 29, 31, 33, 39–41). If soil type determines the composition and species richness of AMF communities, what are the key soil parameters defining such communities? Is a single parameter or a set of physical, chemical, and/or biological properties involved? In recent studies, Gosling et al. (42) found that different AMF communities in agricultural fields colonized the same host plants, depending on phosphorus concentrations in the soil, and Hazard et al. (40) established that soil pH has a stronger effect than land use itself on AMF communities in agroecosystems and crops. However, those studies considered only a limited set of soil properties.
Here we investigated AMF communities in the roots and the rhizosphere soil of Brachypodium retusum (Pers.) P. Beauv., a common plant species of broad distribution that grows in different types of soil in semiarid Mediterranean areas. Fungal DNA extracted from both root and soil samples represented the total AMF assemblages, including actively colonizing fungal taxa as well as those present in soil. Natural soils investigated had different chemical, physical, and biological characteristics but virtually the same environmental conditions (mean annual temperatures and precipitation levels). Under these conditions, we hypothesized that physical, chemical, and biological soil characteristics could shape AMF communities. We used indicator species analyses to assess whether certain AMF operational taxonomic units (OTUs) tended to occur in different habitats (root versus rhizosphere) and sites with contrasting physicochemical properties, and then we tested whether OTUs associated with each habitat or site tended to be closely related (i.e., phylogenetic signals). Finally, inasmuch as host plant species and climatic conditions do not vary between sites, the differences in AMF communities in the rhizosphere of Brachypodium retusum could be attributable to differences in soil characteristics. Hence, we studied the relationships between physical, chemical, and biological soil characteristics and the compositions of AMF communities found in the roots and in the rhizosphere soil.
MATERIALS AND METHODS
Experimental sites and root and soil sampling.
This study was carried out in Campo de Cartagena, Province of Murcia, in southeastern Spain. The area is a coastal plain, where geological complexity yields diverse soil types that differ in edaphic characteristics but are subject to very similar environmental factors and demonstrate the same species compositions in their plant communities. The climate is semiarid, with a pronounced dry season from June to September, an average temperature of 19.1 ± 0.25°C, an average rainfall of 271 ± 4 mm, and annual potential evapotranspiration of 1,000 ± 14 mm (data are averages of records from 6 weather stations located in the zone; for more detailed information, see Table S1 in the supplemental material). The soils surveyed were Lithic Xerorthent (XER), Xeric Torriorthent (TOR), Typic Haplargid (THA), Typic Haplosalid (THS), Lithic Haploxeroll (LHP), and Typic Haploxeroll (THP), according to the Soil Survey Staff (SSS) (43).
In order to reduce the biotic factors affecting the AMF distribution, this study focused in one target plant, namely, Brachypodium retusum (Pers.) P. Beauv., a perennial herbaceous species belonging to the family Poaceae that is widely distributed in semiarid soils of southeastern Spain and was the most abundant in all of the locations sampled. The plant community was the same in all locations and belonged to the association Teucrio pseudochamaepitys-Brachypodietum retusi O. Bolòs. The community was grassland composed mainly of annual and perennial grasses, including Brachypodium retusum (Pers.) P. Beauv., Dactylis glomerata L., Lygeum spartum L., Stipa tenacissima L., and Brachypodium distachyon (L.) Beauv., as well as small shrub species such as Rosmarinus officinalis L., Asparagus horridus L., Thymus hyemalis Lange, Rhamnus lycioides L., and Anthyllis terniflora (Lag.) Pau.
All samples were collected in May 2014 (late spring). Three individual plants were sampled in each of 18 sites across soil types (three replication sites per soil type) in different locations (see Table S2 in the supplemental material). Plants, including root systems, were collected and placed in polyethylene bags for transport to the laboratory, where fine roots were separated from rhizosphere soil. Roots were briefly rinsed, quickly dried on paper, and used for molecular analysis. Rhizosphere soil was used partly for characterization of soil properties and partly for molecular analysis.
Soil analysis.
Soil pH and electrical conductivity were measured in a 1:5 (wt/vol) aqueous solution. The percentage of stable aggregates was determined according to the method of Lax et al. (44).
Dehydrogenase activity was determined according to the methods of García et al. (45) and Trevors (46). Urease and N-α-benzoyl-l-arginine amide (BAA)-hydrolyzing protease activities were determined in 0.1 M phosphate buffer (pH 7); 1 M urea and 0.03 M BAA, respectively, were used as the substrates. Two milliliters of buffer and 0.5 ml of substrate were added to 0.5 g of soil sieved to <2 mm, and the mixture was incubated for 90 min at 30°C (urease) or 39°C (protease). Both activities were determined as the NH4+ released in the hydrolysis reaction (47).
Alkaline phosphatase activity was determined using p-nitrophenyl phosphate disodium (PNPP) (0.115 M) as the substrate. The p-nitrophenol (PNP) formed was determined by spectrophotometry at 398 nm (48). β-Glucosidase was determined using p-nitrophenyl-β-d-glucopyranoside (PNG) (0.05 M) as the substrate. The assay is based on the release and detection of PNP. The amount of PNP was determined at 398 nm (49).
Glomalin-related soil protein (GRSP) was measured in the easily extractable glomalin form, according to the method of Wright and Anderson (50). Total organic C and total N levels were determined by dry combustion using a Leco Tru-Spec CN analyzer (Leco Corp., St. Joseph, MI, USA). Levels of assimilable P extracted with 0.5 M NaHCO3, assimilable K, Ca, Na, and Mg extracted with ammonium acetate, and B, Fe, Mn, Cu, S, and Zn extracted with water were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES) (Iris Intrepid II XDL; Thermo Elemental Co.). Levels of water-soluble carbohydrates and total carbohydrates were determined by the method of Brink et al. (51).
Root and soil DNA extraction and PCR.
DNA extractions from 36 samples (1 root sample and 1 soil sample per replicate for each soil type) were carried out. For root samples, 0.1 g fresh root material was placed in a 2-ml screw-cap propylene tube together with two tungsten carbide balls (3 mm) and was ground (for 3 min at 13,000 rpm) using a mixer mill (MM 400; Retsch, Haan, Germany). Total DNA was extracted using a DNeasy plant minikit, following the manufacturer's recommendations (Qiagen). Two extractions per root sample were performed (0.2 g), and the extracted DNA was resuspended in 20 μl of water and stored at −20°C.
For each soil sample, DNA was extracted from 0.5 g of soil using a FastDNA Spin kit for soil, according to the recommendations of the manufacturer (Q-BIOgene, Heidelberg, Germany). The extracted DNA was resuspended in 20 μl of water and stored at −20°C. Several dilutions of extracted DNA (1:10, 1:50, and 1:100) were prepared, and 2 μl was used as the template. Partial small subunit (SSU) rRNA gene fragments were amplified using nested PCR with the universal eukaryotic primers NS1 and NS4 (52). PCR was carried out in a final volume of 25 μl, using PureTaq Ready-To-Go PCR beads (Amersham Pharmacia Biotech), 0.2 μM deoxynucleoside triphosphates (dNTPs), and 0.5 μM each primer; the PCR conditions were as follows: 94°C for 3 min, 30 cycles of 94°C for 30 s, 40°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 10 min.
Two microliters of several dilutions (1:10, 1:20, 1:50, and 1:100) from the first PCR were used as the template DNA in a second PCR, which was performed using the specific primers AML1 and AML2 (53). PCRs were carried out in a final volume of 25 μl using PureTaq Ready-To-Go PCR beads (Amersham Pharmacia Biotech), 0.2 μM dNTPs, and 0.5 μM each primer; the PCR conditions were as follows: 94°C for 3 min, 30 cycles of 1 min of denaturation at 94°C, 1 min of primer annealing at 50°C, and 1 min of extension at 72°C, and a final extension at 72°C for 10 min. Positive and negative controls using PCR-positive products and sterile water, respectively, were included in all amplifications. All PCRs were run on a PerkinElmer Cetus DNA thermal cycler. Reaction yields were estimated by using a 1.2% agarose gel containing GelRed (Biotium).
Cloning and sequencing.
The PCR products were purified using a gel extraction kit (Qiagen), cloned into pGEM-T Easy (Promega), and transformed into Escherichia coli XL1-Blue. Thirty-two positive transformants were screened in each resulting SSU rRNA gene library, using 0.7 units of RedTaq DNA polymerase (Sigma) and reamplification with the AML1 and AML2 primers under the same conditions as described above. Product quality and size were checked in agarose gels as described above. All clones with inserts of the correct size (795 bp) in each library were sequenced. Clones were grown in liquid culture, and the plasmid was extracted using a QIAprep Spin miniprep kit (Qiagen). Sequencing was performed by the Laboratory of Sistemas Genómicos (Valencia, Spain), using the universal primers SP6 and T7.
AMF richness.
Sequence editing was performed using the program FinchTV 1.4.0 (Geospiza, Inc., Seattle, WA, USA). Sequence similarities were determined using BLASTn (54), provided by the National Center for Biotechnology Information (NCBI). Phylogenetic analysis was carried out on the sequences obtained in this study and those corresponding to the closest matches from GenBank, as well as sequences from cultured AMF taxa, including representatives of the major taxonomical groups described by Redecker et al. (55). All of the sequences were aligned using the multiple sequence comparison program MAFFT (version 7.0) (http://mafft.cbrc.jp/alignment/software), and the alignment was adjusted manually with BioEdit software (version 7.0.4.1) (56). The program CHIMERA_CHECK 2.7 (Ribosomal Database Project II) (http://rdp.cme.msu.edu) was used to check for chimeric artifacts among the 18S rDNA sequences.
Maximum likelihood (ML) phylogenetic tree inference was performed with MEGA software (version 5.05) (57). Nucleotide data files were first tested to find the best DNA evolution model. The general time reversible model with a discrete gamma distribution showed the lowest Bayesian information criterion (BIC) scores and was deemed to best describe the nucleotide substitution pattern. Initial trees for the heuristic search were obtained by applying the neighbor-joining method to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach. The robustness of all trees obtained was evaluated with 1,000 bootstrap replications. Endogone pisiformis Link and Mortierella polycephala Coem. were used as the outgroups. Different AMF sequence types or operational taxonomic units (OTUs) were defined as groups of closely related sequences, with a high level of bootstrap support in the phylogenetic analysis (>85%) and/or sequence similarity of ≥97% with every other sequence.
Statistical analysis.
The number of clones for each AMF OTU in each soil sample (combining root and rhizosphere soil AMF communities) was used to calculate the rarefaction curves. The rarefaction curves were produced by plotting the number of OTUs observed against the number of sequences obtained, using the freely available Analytic Rarefaction software (version 1.3) (http://www.uga.edu/∼strata/software/anRareReadme.html).
We used indicator species analyses to generate a numerical classification of OTUs (58). This method uses a reciprocal averaging ordination to classify the OTUs with respect to apparently important environmental properties (59). It calculates two different probabilities, i.e., (i) the probability that the surveyed site belongs to a given environment, given the fact that the species has been found there (i.e., specificity of the species as an indicator of an environment type), and (ii) the probability of finding the species in sites belonging to a given environment (i.e., fidelity or sensitivity of the species as an indicator of an environment type). Two independent analyses were performed to test whether there were specific OTUs associated with a certain type of habitat (rhizosphere soil versus roots) or soil type (six soil types). The indicator value (IndVal) index (60) was used to measure the associations. Finally, the statistical significance of the relationships was tested using a permutation test with 999 permutations. These analyses were performed using the indcspecies package implemented in R (version 3.2.1) (61).
It was then determined whether the OTUs identified as being characteristic of different habitats (rhizosphere and roots) or contrasting soils were more closely related than expected by chance, using the method proposed by Maddison and Slatkin (62). This test estimates whether the minimum number of evolutionary steps in a character on a phylogenetic tree is smaller than expected by chance, as determined by comparing the observed minimum number of steps with a null model in which data were reshuffled 1,000 times across the tips of the phylogeny. The character used was the OTU's association or not (i.e., significant or nonsignificant IndVal value) with a given habitat or soil, based on the indicator species analyses. These analyses were performed in R (version 3.2.1), using the function phylo.signal.disc developed by Enrico Rezende.
The relationship between the habitat (roots or rhizosphere soil) and the soil type (explanatory variables) regarding the distribution of AMF sequence types was studied using a combination of principal-component analysis (PCA) and multivariate analysis of variance (MANOVA). We used a constrained ordination process, PCA, to seek the combination of environmental variables that best explained the variations in fungal community composition. To avoid biases mediated by the PCR methods, only the absence or presence (0 or 1) of the different OTUs was considered in the analysis. We performed different principal-component analyses to quantify the amounts of variation explained by different sets of environmental variables. First we calculated the amount of variation in fungal community composition explained by habitat (i.e., soils [n = 18 samples] versus roots [18 samples]). These analyses included 3 replicates per soil type for each level. In order to eliminate effects of pseudoreplication, the same analysis was repeated 3 times with one replicate per soil type at a time (n = 6 for both soil and root samples), and the results were consistent; therefore, only the results of the first analysis are reported. Then we quantified the variation explained by soil type (6 soil types, with 3 replicates of the combined AMF communities in roots and rhizosphere soil). This analysis was also performed by considering rhizosphere soil and root AMF communities independently.
In order to provide variance partitioning considering both factors (habitat and soil type) at the same time, we performed a principal-component analysis with the community matrix (49 OTUs across 36 samples) and selected a sufficient number of axes to account for 90% of the variance explained. This matrix with the selected axes was used as a proxy of the community composition. Then we performed a multivariate analysis of variance (MANOVA) with this matrix as the dependent variable and habitat (root versus rhizosphere), site, and habitat-site interaction as explanatory variables. Finally, we calculated the proportion of the variance associated with each factor as eta-square. These analyses were performed in R (version 3.2.1), using the heplots package.
Finally, we quantified the amount of fungal community variation in the soil-plant system (root plus rhizosphere soil communities) for each soil type that was explained by specific soil properties. Redundancy analysis (RDA) was then applied. As a forward procedure, Monte Carlo permutation tests were conducted using 999 permutations, and the variables were ranked according to their importance and significance for the distribution of the AMF communities. Only soil variables with significant effects (P < 0.05) are shown in the bi-plot diagram. This analysis was conducted with CANOCO for Windows (version 4.5) (63).
Nucleotide sequence accession numbers.
A total of 144 representative sequences of OTUs from root and soil samples from different soil types generated in this study have been deposited in GenBank under accession numbers HG380100 to HG380243.
RESULTS
PCR and sequence analysis.
All of the root and rhizosphere soil samples extracted were amplified successfully by nested PCR and generated PCR products of the expected band size of approximately 795 bp, which were used for cloning and creation of the clone libraries. We screened 1,152 clones in total from soil and roots (32 clones were analyzed per library); of those, 1,092 clones contained an SSU rDNA fragment and subsequently were sequenced. The BLAST search revealed that 981 sequences had a high degree (≥95%) of similarity to sequences from taxa belonging to the phylum Glomeromycota, while the remaining 111 sequences showed BLAST similarity to plants and fungi belonging to Ascomycotina.
AMF richness.
Forty-nine OTUs (see Table S4 in the supplemental material) could be distinguished on the basis of bootstrap values of more than 85% (see Fig. S2 in the supplemental material). Sequences from the families Glomeraceae (29 OTUs), Paraglomeraceae (7 OTUs), Claroideoglomeraceae (4 OTUs), Diversisporaceae (3 OTUs), Archaeosporaceae (2 OTUs), Ambisporaceae (2 OTUs), Acaulosporaceae (1 OTU), and Gigasporaceae (1 OTU) were obtained. Ten OTUs clustered with previously identified sequences, i.e., Glomus macrocarpum (G3), Sclerocystis sinuosa (Sc1), Rhizophagus clarus (Rh1), Rhizophagus intraradices-irregularis-fasciculatus (Rh2), Diversispora spurca-aurantia (D1), Redeckera fulvum (Red1), Acaulospora laevis-lacunosa-spinosa (Ac1), Claroideoglomus luteum-claroideum-lamellosum (Cl1), Archaeospora schenckii-trappei (Ar2), and Ambispora leptoticha (Amb2). Twenty OTUs clustered with uncultured Glomeromycota sequences recorded in the database. The remaining 19 OTUs were Glomeromycota not clustering with any sequences in the database.
Effects of soil type and habitat (roots or rhizosphere soil) on AMF community composition.
In order to determine whether the number of clones sequenced was sufficient to represent the AMF diversity in the roots and in the rhizosphere soil, rarefaction curves were constructed (see Fig. S1A and B in the supplemental material). For the rhizosphere soil samples, the clones sequenced were sufficient to allow the detection of the majority of OTUs. For the root samples, there was a well-defined plateauing of the curves, and it is highly unlikely that the sequencing of more clones would have revealed more OTUs, except in XER soil.
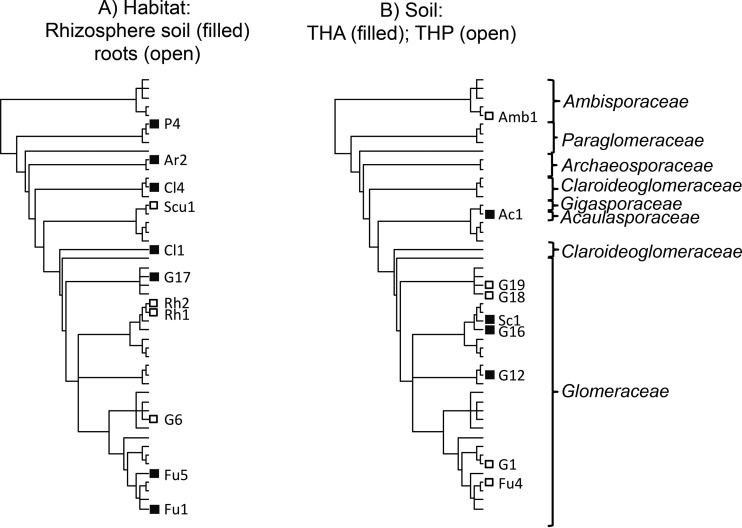
Indicator species analyses were conducted to find specific OTUs associated with root or rhizosphere soil samples and specific OTUs associated with soil types. Seven OTUs were more prone to be found in rhizosphere soil samples; five of them were specific for rhizosphere soil samples (G17, Fu5, P4, Cl4, and Ar2), and the other two both were specific for and showed fidelity for rhizosphere soil samples (Fu1 and Cl1) (Table 1). On the other hand, 4 OTUs were associated with the root habitat; the OTU Rh2 presented more fidelity to the root habitat, and Rh1, G6, and Scu1 showed both specificity and fidelity for roots (Table 1). Although there was a wide range of phylogenetic diversity in both habitats, OTUs significantly associated with roots were more closely related than expected by chance (observed transitions [OTs], 3; null transitions [NTs] [i.e., transitions in the null model], 4; P = 0.05), while this was not the case for OTUs associated with rhizosphere soil (OTs, 7; NTs, 7; P > 0.05) (Fig. 1).
TABLE 1.
Indicator species analyses
| OTU and associationa | Probabilityb |
Indicator value index | P | |
|---|---|---|---|---|
| A | B | |||
| OTUs associated with habitat | ||||
| Rhizosphere soil | ||||
| Fu1 | 1.000 | 0.778 | 0.882 | 0.001 |
| Cl1 | 0.708 | 0.945 | 0.818 | 0.003 |
| G17 | 1.000 | 0.556 | 0.745 | 0.001 |
| Fu5 | 1.000 | 0.500 | 0.707 | 0.002 |
| P4 | 1.000 | 0.389 | 0.624 | 0.006 |
| Cl4 | 1.000 | 0.333 | 0.577 | 0.022 |
| Ar2 | 1.000 | 0.278 | 0.527 | 0.045 |
| Roots | ||||
| Rh1 | 0.833 | 0.833 | 0.833 | 0.001 |
| G6 | 0.929 | 0.722 | 0.819 | 0.001 |
| Rh2 | 0.621 | 1.000 | 0.788 | 0.004 |
| Scu1 | 0.733 | 0.611 | 0.669 | 0.047 |
| OTUs associated with soil types | ||||
| THP | ||||
| G18 | 1.000 | 1.000 | 1.000 | 0.001 |
| G19 | 1.000 | 1.000 | 1.000 | 0.001 |
| G1 | 1.000 | 0.667 | 0.816 | 0.003 |
| Fu4 | 1.000 | 0.500 | 0.707 | 0.017 |
| Amb1 | 1.000 | 0.500 | 0.707 | 0.008 |
| THA | ||||
| G16 | 1.000 | 0.500 | 0.707 | 0.016 |
| Sc1 | 1.000 | 0.500 | 0.707 | 0.016 |
| Ac1 | 1.000 | 0.500 | 0.707 | 0.016 |
| G12 | 0.429 | 1.000 | 0.655 | 0.009 |
| LHP | ||||
| D2 | 1.000 | 0.500 | 0.707 | 0.018 |
| TOR | ||||
| Fu2 | 1.000 | 0.500 | 0.707 | 0.016 |
| XER | ||||
| G11 | 1.000 | 0.667 | 0.816 | 0.001 |
| G20 | 0.3529 | 1.000 | 0.594 | 0.038 |
| THS | ||||
| G14 | 1.000 | 0.500 | 0.707 | 0.012 |
Soil types were as follows: THS, Typic Haplosalid; XER, Lithic Xerorthent; TOR, Xeric Torriorthent; THA, Typic Haplargid; LHP, Lithic Haploxeroll; THP, Typic Haploxeroll.
Probability A, the probability that the surveyed site belongs to a given environment, given the fact that the species has been found; probability B, the probability of finding the species in sites belonging to a given environment.
FIG 1.
Phylogenetic distribution of the AMF OTUs characteristic of habitat, i.e., rhizosphere soil (■) and roots (□) (A), and the two soils with the most contrasting microbiological activities, i.e., THP (low activity) (□) and THA (high activity) (■) (B). The abbreviations correspond to the codes of the operational taxonomic units. The phylogenetic relationships between the selected OTUs are extracted from Fig. S2 in the supplemental material.
There were also OTUs associated with specific soil types. Five OTUs tended to occur in Typic Haploxeroll (THP) (G18, G19, G1, Fu4, and Amb1), four in Typic Haplargid (THA) (G16, Sc1, Ac1, and G12), two in Lithic Xerorthent (XER) (G11 and G20), and one each in Lithic Haploxeroll (LHP) (D2), Xeric Torriorthent (TOR) (Fu2), and Typic Haplosalid (THS) (G14) (Table 1). Therefore, only in THP and THA were there enough OTUs to test for a phylogenetic signal in the association with a given soil. In both soils, there was not a phylogenetic signal (THP: OTs, 5; NTs, 5; P > 0.05; THA: OTs, 4; NTs, 4; P > 0.05) (Fig. 1).
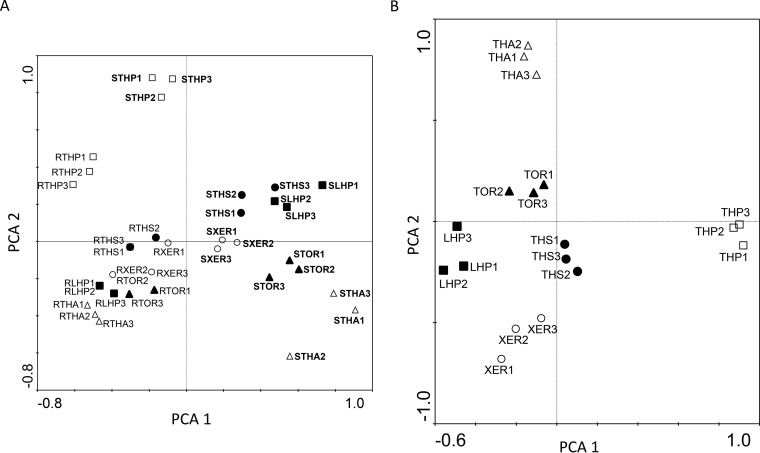
We used principal-component analysis (PCA) to examine the influence of habitat (root or rhizosphere soil) on AMF community variation. Habitat explained 54.3% of the variation in AMF community composition, showing a clear effect of habitat on AMF distribution (Fig. 2A). Considering the complete AMF community associated with Brachypodium retusum as the sum of the AMF communities of rhizosphere soil and roots, soil type explained 69.7% of the AMF variation (Fig. 2B). When the two factors and their interaction were considered, there was also a significant difference in AMF community composition across habitats (F = 91.83, df = 1, partial eta-square = 0.96, P < 0.001), sites (F = 36.19, df = 5, partial eta-square = 0.69, P < 0.001), and habitat-site interaction (F = 23.41, df = 5, partial eta-square = 0.65, P < 0.001), confirming the significant influence of both habitat and soil type on AMF distribution.
FIG 2.
(A) Principal-component analysis (PCA) of the AMF community composition in the roots and in the rhizosphere soil of Brachypodium retusum in six different soil types. The amounts of variation explained by the first two PCA axes were as follows: PCA1, 0.20; PCA2, 0.13. The model explained 54.3% of the whole variance. THS, Typic Haplosalid; XER, Lithic Xerorthent; TOR, Xeric Torriorthent; THA, Typic Haplargid; LHP, Lithic Haploxeroll; THP, Typic Haploxeroll; S, rhizosphere soil; R, roots. (B) PCA of the global AMF community composition under Brachypodium retusum in six different soil types. The amounts of variation explained by the first two PCA axes were as follows: PCA1, 0.19; PCA2, 0.18. The model explained 69.7% of the whole variance.
Soil characteristics.
The evaluation of soil physicochemical and biological parameters showed significant differences among soil types for most of the soil properties considered (see Table S3 in the supplemental material). THS and THA soils presented the highest pH values, although all of the soils had similar pH ranges. Electrical conductivity values were significantly greater for TOR soil. The highest values for aggregate stability were recorded in THA and LHP soils, whereas the lowest values were found in TOR and THS soils.
Regarding the chemical properties of the soils sampled, XER soil showed significantly lower values for total carbon and calcium levels, whereas TOR soil presented the highest values for those properties. The latter soil, TOR, showed the lowest values for most chemical properties, such as nitrogen, organic carbon, available phosphorus, sodium, magnesium, iron, manganese, and zinc levels.
In relation to biological properties (enzyme activities and glomalin-related soil protein levels), the lowest dehydrogenase, urease, protease, and alkaline phosphatase activities and glomalin-related soil protein levels were observed in TOR and THP soils. In contrast, the highest levels were found in THA soil. The highest levels of water-soluble carbohydrates and total carbohydrates were recorded in LHP soil, whereas the lowest levels were found in TOR soil.
Soil properties triggering AMF community structure.
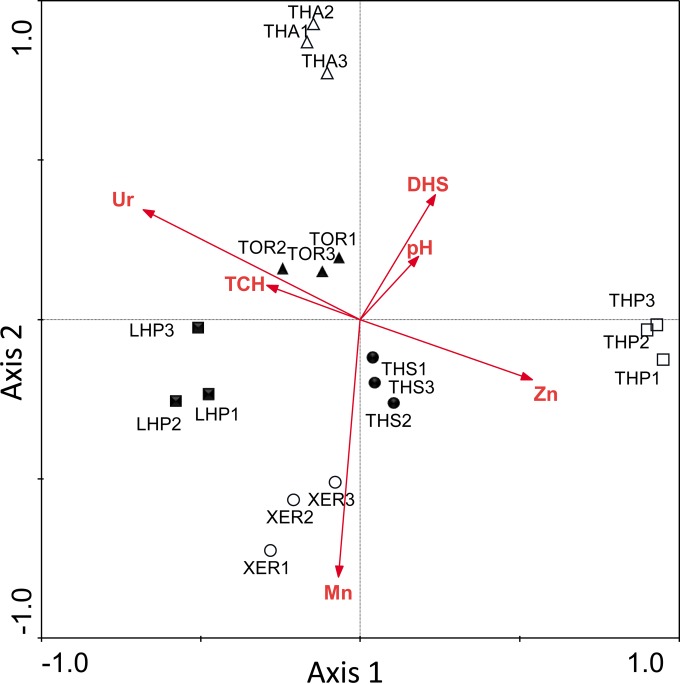
The different soils tested differed significantly in most of the properties evaluated, which allowed us to try to establish relationships between the edaphic factors and AMF communities. Multivariate analysis based on constrained ordination, RDA, was used to investigate the influence of soil properties (used as explanatory variables) on the AMF community composition in B. retusum rhizospheres (Fig. 3). The first two axes explained 37.1% of the total variance (69.7% for the model). RDA and subsequent forward procedures selected 6 soil properties as significantly triggering AMF community composition, i.e., three related to microbiological activity (namely, urease, dehydrogenase, and total carbohydrate levels; P < 0.01), soil pH (P < 0.01), and levels of two essential micronutrients (i.e., Mn and Zn; P < 0.05 and P < 0.01, respectively).
FIG 3.
Redundancy analysis (RDA) showing the influence of soil properties on the AMF community composition in B. retusum rhizospheres. The first two axes explained 37.1% of the total variance (69.7% for the model). Only soil variables with significant effects in Monte Carlo tests (P < 0.05) are shown in the bi-plot diagram. THS, Typic Haplosalid; XER, Lithic Xerorthent; TOR, Xeric Torriorthent; THA, Typic Haplargid; LHP, Lithic Haploxeroll; THP, Typic Haploxeroll; DHS, dehydrogenase; TCH, total carbohydrates; Ur, urease.
DISCUSSION
Our results pointed out a tendency of AMF communities in roots to be more similar to each other and different from those in the rhizosphere soil. The indicator species analyses revealed 7 OTUs associated with rhizosphere soil and 4 OTUs with the root habitat. In general, AMF in soil are considered to represent a pool of species from which plants recruit only a fraction at any time (64, 65). Previous morphologically based studies documented differences in AMF present in the roots or rhizosphere of the same plants, probably due to differential sporulation dynamics (66), to seasonal changes in the AMF community (67), or to different life history strategies of AMF (68). Our results coincide with those of Saks et al. (69), showing that root-colonizing AMF represent a phylogenetically clustered subset of AMF available in soil.
In this sense, Varela-Cervero et al. (70) observed that AMF communities detected in root samples from different plant species in a semiarid Mediterranean area were more similar to each other than those in extraradical mycelium and spore fractions, which were more variable. Glomeraceae were described previously as abundant in roots but scarce in soil (37, 70, 71). Although our results showed that Glomeraceae were also common in rhizosphere soil, three of the four OTUs identified by the indicator species analyses as characteristic of roots are Glomeraceae (Fig. 1A), supporting the previous evidence to some extent. This phylogenetic pattern suggests that, among AMF, there are niche preferences and this is probably one important factor regulating AMF community composition (70).
From the findings described above, it seems that a given OTU of an AMF in an individual plant species can be detected in the roots, the rhizosphere soil, or both. In this sense, we considered the AMF communities as the sum of both soil and root populations, to establish their relationships with the edaphic characteristics tested. There is evidence of several biotic factors with strong influences in regulating AMF community composition, with the best studied being the host plant (29, 30, 72–74) and host functional traits (6, 37). Among the abiotic factors that can have relevant roles in driving AMF communities are soil moisture (75), rainfall patterns, and geographical distance (40, 76). There is no doubt that soil type has a role in the AMF distribution (10, 34, 35, 40, 77–80), and our work also demonstrated that soil type is a major factor driving AMF assemblages, after elimination of the host factor and other environmental variables not related to soil characteristics. Although individual soil characteristics have been reported to play important roles in AMF community structure and composition (7, 11, 13, 40, 42, 78, 81–83), very limited data sets regarding soil characteristics were used in these studies, and the relative incidences of each soil property determined after complete soil characterization have not been reported previously.
Multivariate analysis based on constrained ordination (RDA) identified urease, dehydrogenase, total carbohydrates, pH, Zn, and Mn as the soil properties significantly influencing the AMF community distribution in Brachypodium retusum (roots plus rhizosphere). Some of these soil properties have been reported previously to affect the growth and distribution of AMF.
Soil enzyme activities and microbial processes are particularly important because they usually are good indicators of system sustainability (45, 84). Enzyme activities can play an important role, since some authors have reported direct effects of these on AMF colonization development (85). Enzyme activities are involved in nutrient cycling and decomposition of organic matter and respond quickly to any form of change occurring in the system. The importance of urease, dehydrogenase, and total carbohydrates in shaping AMF communities suggests that, in the semiarid soils studied, where biological activity is generally very low (86), the structure of AMF assemblages is partly determined by the soil parameters directly related to microbial activity (13). Our results also showed that the phylogenetic compositions of AMF communities in soils with high (THA) or low (THP) microbiological activity were not phylogenetically clustered. The OTUs of these two contrasting soils tended to belong to different clades in the phylogeny (Fig. 2B), suggesting that there may be some niche segregation between species characteristic of soils with contrasting microbiological activities.
In this survey, pH was found to be a significant factor shaping AMF community composition. Soil acidity is one of the most important drivers (environmental filters) of microbial communities and particularly of AMF communities (7, 87). Recently, Bainard et al. (9) concluded that soil pH is the only environmental variable that appears to be a key factor in the assembly of AMF communities in the Canadian prairie landscape.
According to our results, besides pH, consistent chemical drivers of AMF communities were Zn and Mn soil contents. These micronutrients are important for metabolic processes in plants (88), and their uptake can be positively influenced by AMF (89, 90). Several studies showed strong negative effects of Zn soil contents on AMF abundance and diversity in polluted soils (20–23). The Zn contents in our surveyed soils were far from those found in heavy metal-polluted soils; however, it seems that Zn can be a determinant in shaping the structure of AMF communities also in nonpolluted soils. Little is known about the influence of Mn on the abundance and diversity of AMF, as well as on the composition of their communities. Wei et al. (91) found that AMF root colonization and diversity were negatively correlated with total extractable Mn concentrations in contaminated soils, and they concluded that Mn contamination affected AMF diversity and shaped AMF community structure. As in the case of Zn, our results point to a relevant role of Mn also in noncontaminated soils from semiarid areas.
It can be concluded that both soil type and habitat (root versus rhizosphere) determine the distribution of AMF communities in semiarid Mediterranean soils. In addition, the driving effect of soil type could not be attributed to a single soil characteristic, and the use of extensive soil characterization revealed that up to three soil properties related to microbial activity, i.e., pH and the levels of two micronutrients (Mn and Zn), play significant roles in triggering AMF populations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Plan Nacional-FEDER (grant AGL2012-39057-CO2-01). A.M.-N. was supported by a postdoctoral contract from the Ministerio de Economía y Competitividad (grant FPDI-2013-16266) and an Early Career Project Grant from the BES (grant 3975-4849).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03982-15.
REFERENCES
- 1.Hausmann NT, Hawkes CV. 2010. Order of plant host establishment alters the composition of arbuscular mycorrhizal communities. Ecology 91:2333–2343. doi: 10.1890/09-0924.1. [DOI] [PubMed] [Google Scholar]
- 2.Hu Y, Rillig MC, Xiang D, Hao Z, Chen B. 2013. Changes of AM fungal abundance along environmental gradients in the arid and semi-arid grasslands of Northern China. PLoS One 8:e57593. doi: 10.1371/journal.pone.0057593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V. 2010. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ Microbiol 12:2165–2179. [DOI] [PubMed] [Google Scholar]
- 4.Öpik M, Metsis M, Daniell TJ, Zobel M, Moora M. 2009. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol 184:424–437. doi: 10.1111/j.1469-8137.2009.02920.x. [DOI] [PubMed] [Google Scholar]
- 5.van der Heijden MGA, Bardgett RD, Van Straalen NM. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 6.Torrecillas E, Alguacil MM, Roldán A, Díaz G, Montesinos-Navarro A, Torres P. 2014. Modularity reveals the tendency of arbuscular mycorrhizal fungi to interact differently with generalist and specialist plant species in gypsum soils. Appl Environ Microbiol 80:5457–5760. doi: 10.1128/AEM.01358-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansa J, Erb A, Oberholzer HR, Smilauer P, Egli S. 2014. Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol Ecol 23:2118–2135. doi: 10.1111/mec.12706. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary VB, O'Dell TE, Rillig MC, Johnson NC. 2014. Multiscale patterns of arbuscular mycorrhizal fungal abundance and diversity in semiarid shrublands. Fungal Ecol 12:32–43. doi: 10.1016/j.funeco.2014.06.003. [DOI] [Google Scholar]
- 9.Bainard LD, Dai M, Furrazola Gómez E, Torres-Arias Y, Bainard JD, Sheng M, Eilers W, Hamel C. 2015. Arbuscular mycorrhizal fungal communities are influenced by agricultural land use and not soil type among the Chernozem great groups of the Canadian Prairies. Plant Soil 387:351–362. doi: 10.1007/s11104-014-2288-1. [DOI] [Google Scholar]
- 10.Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, van der Heijden M, Sieverding E. 2010. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738. doi: 10.1016/j.soilbio.2010.01.006. [DOI] [Google Scholar]
- 11.Santos-González JC, Nallanchakravarthula S, Alström S, Finlay RD. 2011. Soil, but not cultivar, shapes the structure of arbuscular mycorrhizal fungal assemblages associated with strawberry. Microb Ecol 62:25–35. doi: 10.1007/s00248-011-9834-7. [DOI] [PubMed] [Google Scholar]
- 12.Verbruggen E, van der Heijden MGA, Weedon JT, Kowalchuk GA, Röling WFM. 2012. Community assembly, species richness and nestedness of arbuscular mycorrhizal fungi in agricultural soils. Mol Ecol 21:2341–2353. doi: 10.1111/j.1365-294X.2012.05534.x. [DOI] [PubMed] [Google Scholar]
- 13.Alguacil MM, Torrecillas E, García-Orenes F, Roldán A. 2014. Changes in the composition and diversity of AMF communities mediated by management practices in a Mediterranean soil are related with increases in soil biological activity. Soil Biol Biochem 76:34–44. doi: 10.1016/j.soilbio.2014.05.002. [DOI] [Google Scholar]
- 14.Fitzsimons MS, Miller RM. 2010. Serpentine soil has little influence on the root-associated microbial community composition of the serpentine tolerant grass species Avenula sulcata. Plant Soil 330:393–405. doi: 10.1007/s11104-009-0213-9. [DOI] [Google Scholar]
- 15.Schechter SP, Bruns TD. 2008. Serpentine and non-serpentine ecotypes of Collinsia sparsiflora associate with distinct arbuscular mycorrhizal fungal assemblages. Mol Ecol 17:3198–3210. doi: 10.1111/j.1365-294X.2008.03828.x. [DOI] [PubMed] [Google Scholar]
- 16.Schechter SP, Bruns TD. 2013. A common garden test of host-symbiont specificity supports a dominant role for soil type in determining AMF assemblage structure in Collinsia sparsiflora. PLoS One 8:e55507. doi: 10.1371/journal.pone.0055507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lekberg Y, Meadow J, Rohr JR, Redecker D, Zabinski CA. 2011. Importance of dispersal and thermal environment for mycorrhizal communities: lessons from Yellowstone National Park. Ecology 92:1292–1302. doi: 10.1890/10-1516.1. [DOI] [PubMed] [Google Scholar]
- 18.Sonjak S, Beguiristain T, Leyval C, Regvar M. 2009. Temporal temperature gradient gel electrophoresis (TTGE) analysis of arbuscular mycorrhizal fungi associated with selected plants from saline and metal polluted environments. Plant Soil 314:25–34. doi: 10.1007/s11104-008-9702-5. [DOI] [Google Scholar]
- 19.Sonjak S, Udovič M, Wraber T, Likar M, Regvar M. 2009. Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Sečovlje salterns. Soil Biol Biochem 41:1847–1856. doi: 10.1016/j.soilbio.2009.06.006. [DOI] [Google Scholar]
- 20.Ban Y, Zhouying X, Zhang H, Chen H, Tang M. 2015. Soil chemistry, translocation of heavy metals, and mycorrhizal fungi associated with six plant species growing on lead-zinc mine tailings. Ann Microbiol 65:503–515. doi: 10.1007/s13213-014-0886-z. [DOI] [Google Scholar]
- 21.Zarei M, Konig S, Hempel S, Nekouei MK, Savaghebi G, Buscot F. 2008. Community structure of arbuscular mycorrhizal fungi associated to Veronica rechingeri at the Anguran zinc and lead mining region. Environ Pollut 156:1277–1283. doi: 10.1016/j.envpol.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Zarei M, Saleh-Rastin N, Jouzani GS, Savaghebi G, Buscot F. 2008. Arbuscular mycorrhizal abundance in contaminated soils around a zinc and lead deposit. Eur J Soil Biol 44:381–391. doi: 10.1016/j.ejsobi.2008.06.004. [DOI] [Google Scholar]
- 23.Zarei M, Hempel S, Wubet T, Schafer T, Savaghebi G, Jouzani GS, Nekouei MK, Buscot F. 2010. Molecular diversity of arbuscular mycorrhizal fungi in relation to soil chemical properties and heavy metal contamination. Environ Pollut 158:2757–2765. doi: 10.1016/j.envpol.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Robinson-Boyer L, Grzyb I, Jeffries P. 2009. Shifting the balance from qualitative to quantitative analysis of arbuscular mycorrhizal communities in field soils. Fungal Ecol 2:1–9. doi: 10.1016/j.funeco.2008.11.001. [DOI] [Google Scholar]
- 25.Tchabi A, Coyne D, Hountondji F, Lawouin L, Wiemken A, Oehl F. 2008. Arbuscular mycorrhizal fungal communities in sub-Saharan savannas of Benin, West Africa, as affected by agricultural land use intensity and ecological zone. Mycorrhiza 18:181–195. doi: 10.1007/s00572-008-0171-8. [DOI] [PubMed] [Google Scholar]
- 26.Mummey DL, Rillig MC. 2006. The invasive plant species Centaurea maculosa alters arbuscular mycorrhizal fungal communities in the field. Plant Soil 288:81–90. doi: 10.1007/s11104-006-9091-6. [DOI] [Google Scholar]
- 27.Wolfe BE, Mummey DL, Rillig MC, Klironomos JN. 2007. Small-scale spatial heterogeneity of arbuscular mycorrhizal fungal abundance and community composition in a wetland plant community. Mycorrhiza 17:175–183. doi: 10.1007/s00572-006-0089-y. [DOI] [PubMed] [Google Scholar]
- 28.Hassan SED, Boon E, St-Arnaud M, Hijri M. 2011. Molecular biodiversity of arbuscular mycorrhizal fungi in trace metal-polluted soils. Mol Ecol 20:3469–3483. doi: 10.1111/j.1365-294X.2011.05142.x. [DOI] [PubMed] [Google Scholar]
- 29.Alguacil MM, Roldán A, Torres MP. 2009. Assessing the diversity of AM fungi in arid gypsophilous plant communities. Environ Microbiol 11:2649–2659. doi: 10.1111/j.1462-2920.2009.01990.x. [DOI] [PubMed] [Google Scholar]
- 30.Torrecillas E, Alguacil MM, Roldán A. 2012. Host preferences of arbuscular mycorrhizal fungi colonizing annual herbaceous plant species in semiarid Mediterranean prairies. Appl Environ Microbiol 78:6180–6186. doi: 10.1128/AEM.01287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helgason T, Merryweather JW, Denison J, Wilson P, Young JPW, Fitter AH. 2002. Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J Ecol 90:371–384. doi: 10.1046/j.1365-2745.2001.00674.x. [DOI] [Google Scholar]
- 32.Alguacil MM, Torrecillas E, Torres MP, García-Orenes F, Roldán A. 2012. Long-term effects of irrigation with waste water on soil AM fungi diversity and microbial activities: the implications for agro-ecosystem resilience. PLoS One 7:e47680. doi: 10.1371/journal.pone.0047680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davison J, Öpik M, Zobel M, Vasar M, Metsis M, Moora M. 2012. Communities of arbuscular mycorrhizal fungi detected in forest soil are spatially heterogeneous but do not vary throughout the growing season. PLoS One 7:e41938. doi: 10.1371/journal.pone.0041938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciccolini V, Bonari E, Pellegrino E. 2015. Land-use intensity and soil properties shape the composition of fungal communities in Mediterranean peaty soils drained for agricultural purposes. Biol Fertil Soils 51:719–731. doi: 10.1007/s00374-015-1013-4. [DOI] [Google Scholar]
- 35.Borriello R, Berruti A, Lumini E, Della Beffa MT, Scariot V, Bianciotto V. 2015. Edaphic factors trigger diverse AM fungal communities associated to exotic camellias in closely located Lake Maggiore (Italy) sites. Mycorrhiza 25:253–265. doi: 10.1007/s00572-014-0605-4. [DOI] [PubMed] [Google Scholar]
- 36.Alguacil MM, Torrecillas E, Roldán A, Díaz G, Torres MP. 2012. Perennial plant species from semiarid gypsum soils support higher AMF diversity in roots than the annual Bromus rubens. Soil Biol Biochem 49:132–138. doi: 10.1016/j.soilbio.2012.02.024. [DOI] [Google Scholar]
- 37.Torrecillas E, Alguacil MM, Roldán A. 2012. Differences in the AMF diversity in soil and roots between two annual and perennial gramineous plants co-occurring in a Mediterranean, semiarid degraded area. Plant Soil 354:97–106. doi: 10.1007/s11104-011-1047-9. [DOI] [Google Scholar]
- 38.Martínez-García LB, Armas C, Miranda JDD, Padilla FM, Pugnaire FI. 2011. Shrubs influence arbuscular mycorrhizal fungi communities in a semi-arid environment. Soil Biol Biochem 43:682–689. doi: 10.1016/j.soilbio.2010.12.006. [DOI] [Google Scholar]
- 39.Dumbrell AJ, Ashton PD, Aziz N, Feng G, Nelson M, Dytham C, Fitter AH, Helgason T. 2011. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol 190:794–804. doi: 10.1111/j.1469-8137.2010.03636.x. [DOI] [PubMed] [Google Scholar]
- 40.Hazard C, Gosling P, Van Der Gast CJ, Mitchell DT, Doohan FM, Bending GD. 2013. The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J 7:498–508. doi: 10.1038/ismej.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davison J, Moora M, Öpik M, Adholeya A, Ainsaar L, Bâ A, Burla S, Diedhiou AG, Hiiesalu I, Jairus T, Johnson NC, Kane A, Koorem K, Kochar M, Ndiaye C, Pärtel M, Reier Ü, Saks Ü, Singh R, Vasar M, Zobel M. 2015. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 394:970–973. doi: 10.1126/science.aab1161. [DOI] [PubMed] [Google Scholar]
- 42.Gosling P, Mead A, Proctor M, Hammond JP, Bending GD. 2013. Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol 198:546–556. doi: 10.1111/nph.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soil Survey Staff. 2010. Keys to soil taxonomy. Natural Resources Conservation Service, Washington, DC. [Google Scholar]
- 44.Lax A, Díaz E, Castillo V, Albaladejo J. 1994. Reclamation of physical and chemical properties of a salinized soil by organic amendment. Arid Soil Res Rehabil 8:9–17. [Google Scholar]
- 45.García C, Hernandez T, Costa F. 1997. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun Soil Sci Plan 28:123–134. doi: 10.1080/00103629709369777. [DOI] [Google Scholar]
- 46.Trevors JT. 1984. Dehydrogenase activity in soil: a comparison between the INT and TTC assay. Soil Biol Biochem 16:673–674. doi: 10.1016/0038-0717(84)90090-7. [DOI] [Google Scholar]
- 47.Nannipieri P, Ceccanti B, Cervelli S, Matarese E. 1980. Extraction of phosphatase, urease, protease, organic carbon and nitrogen from soil. Soil Sci Soc Am J 44:1011–1016. doi: 10.2136/sssaj1980.03615995004400050028x. [DOI] [Google Scholar]
- 48.Tabatabai MA, Bremner JM. 1969. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307. doi: 10.1016/0038-0717(69)90012-1. [DOI] [Google Scholar]
- 49.Tabatabai MA. 1982. Soil enzymes, p 903–947. In Page AL, Miller RH, Keeney DR (ed), Methods of soil analysis, part 2. American Society of Agronomy, Madison, WI. [Google Scholar]
- 50.Wright SF, Anderson RL. 2000. Aggregate stability and glomalin in alternative crop rotations for the central Great Plains. Biol Fertil Soils 31:249–253. doi: 10.1007/s003740050653. [DOI] [Google Scholar]
- 51.Brink RH, Dubar P, Linch DL. 1960. Measurements of carbohydrates in soil hydrolysates with anthrone. Soil Sci 89:157–166. doi: 10.1097/00010694-196003000-00006. [DOI] [Google Scholar]
- 52.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, New York, NY. [Google Scholar]
- 53.Lee J, Lee S, Young JPW. 2008. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 65:339–349. doi: 10.1111/j.1574-6941.2008.00531.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- 55.Redecker D, Schüßler A, Stockinger H, Stürmer SL, Morton JB, Walker C. 2013. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531. doi: 10.1007/s00572-013-0486-y. [DOI] [PubMed] [Google Scholar]
- 56.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. [Google Scholar]
- 57.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill MO, Bunce RG, Shaw MW. 1975. Indicator species analysis, a divisive polythetic method of classification, and its application to a survey of native pinewoods in Scotland. J Ecol 63:597–613. doi: 10.2307/2258738. [DOI] [Google Scholar]
- 59.Hill MO. 1973. Reciprocal averaging: an eigenvector method of ordination. J Ecol 61:237–249. doi: 10.2307/2258931. [DOI] [Google Scholar]
- 60.Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monograph 67:345–366. doi: 10.2307/2963459. [DOI] [Google Scholar]
- 61.De Cáceres M, Legendre P. 2009. Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574. doi: 10.1890/08-1823.1. [DOI] [PubMed] [Google Scholar]
- 62.Maddison WP, Slatkin M. 1991. Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution 45:1184–1197. doi: 10.2307/2409726. [DOI] [PubMed] [Google Scholar]
- 63.ter Braak CFJ, Smilauer P. 2004. CANOCO reference manual and CanoDraw for Windows. Biometris, Wageningen, Netherlands. [Google Scholar]
- 64.Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB. 2003. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908. doi: 10.1890/0012-9658(2003)084[1895:NEAMAA]2.0.CO;2. [DOI] [Google Scholar]
- 65.Davison J, Öpik M, Daniell TJ, Moora M, Zobel M. 2011. Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microb Ecol 78:103–115. doi: 10.1111/j.1574-6941.2011.01103.x. [DOI] [PubMed] [Google Scholar]
- 66.Oehl F, Sieverding E, Ineichen K, Mäder P, Wiemken A, Boller T. 2009. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agr Ecosyst Environ 134:257–268. doi: 10.1016/j.agee.2009.07.008. [DOI] [Google Scholar]
- 67.López-García Á, Azcón-Aguilar C, Barea JM. 2014. The interactions between plant life form and fungal traits of arbuscular mycorrhizal fungi determine the symbiotic community. Oecologia 176:1075–1086. doi: 10.1007/s00442-014-3091-7. [DOI] [PubMed] [Google Scholar]
- 68.Denison RF, Kiers ET. 2011. Life histories of symbiotic rhizobia and mycorrhizal fungi. Curr Biol 21:775–785. doi: 10.1016/j.sbi.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Saks Ü, Davison J, Öpik M, Vasar M, Moora M, Zobel M. 2014. Root-colonizing and soil-borne communities of arbuscular mycorrhizal fungi in a temperate forest understory. Botany 92:277–285. doi: 10.1139/cjb-2013-0058. [DOI] [Google Scholar]
- 70.Varela-Cervero S, Vasar M, Davison J, Barea JM, Öpik M, Azcón-Aguilar C. 2015. The composition of arbuscular mycorrhizal fungal communities differs among the roots, spores and extraradical mycelia associated with five Mediterranean plant species. Environ Microbiol 17:2882–2895. doi: 10.1111/1462-2920.12810. [DOI] [PubMed] [Google Scholar]
- 71.Hempel S, Renker C, Buscot F. 2007. Differences in the species composition of arbuscular mycorrhizal fungi in spore, root and soil communities in a grassland ecosystem. Environ Microbiol 9:1930–1938. doi: 10.1111/j.1462-2920.2007.01309.x. [DOI] [PubMed] [Google Scholar]
- 72.Alguacil MM, Torres MP, Torrecillas E, Díaz G, Roldán A. 2011. Plant type differently promote the arbuscular mycorrhizal fungi biodiversity in the rhizosphere after revegetation of a degraded, semiarid land. Soil Biol Biochem 43:167–173. doi: 10.1016/j.soilbio.2010.09.029. [DOI] [Google Scholar]
- 73.Li LF, Li T, Zhang Y, Zhao ZW. 2010. Molecular diversity of arbuscular mycorrhizal fungi and their distribution patterns related to host-plants and habitats in a hot and arid ecosystem, southwest China. FEMS Microbiol Ecol 71:418–427. doi: 10.1111/j.1574-6941.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 74.Sýkorová Z, Wiemken A, Redecker D. 2007. Cooccurring Gentiana verna and Gentiana acaulis and their neighboring plants in two Swiss upper montane meadows harbor distinct arbuscular mycorrhizal fungal communities. Appl Environ Microbiol 73:5426–5434. doi: 10.1128/AEM.00987-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deepika S, Kothamasi D. 2015. Soil moisture: a regulator of arbuscular mycorrhizal fungal community assembly and symbiotic phosphorous uptake. Mycorrhiza 25:67–75. doi: 10.1007/s00572-014-0596-1. [DOI] [PubMed] [Google Scholar]
- 76.Alguacil MM, Torrecillas E, Lozano Z, Roldán A. 2015. Arbuscular mycorrhizal fungi communities in a coral cay system (Morrocoy, Venezuela) and their relationships with environmental variables. Sci Total Environ 505:805–813. doi: 10.1016/j.scitotenv.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 77.Lekberg Y, Koide RT, Rohr JR, Aldrich-Wolfe L, Morton JB. 2007. Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J Ecol 95:95–105. doi: 10.1111/j.1365-2745.2006.01193.x. [DOI] [Google Scholar]
- 78.Helgason T, Fitter AH. 2009. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J Exp Bot 60:2465–2480. doi: 10.1093/jxb/erp144. [DOI] [PubMed] [Google Scholar]
- 79.Balestrini R, Magurno F, Walker C, Lumini E, Bianciotto V. 2010. Cohorts of arbuscular mycorrhizal fungi (AMF) in Vitis vinifera, a typical Mediterranean fruit crop. Environ Microbiol Rep 2:594–604. doi: 10.1111/j.1758-2229.2010.00160.x. [DOI] [PubMed] [Google Scholar]
- 80.Haug I, Setaro S, Suárez JP. 2013. Reforestation sites show similar and nested AMF communities to an adjacent pristine forest in a tropical mountain area of South Ecuador. PLoS One 8:e63524. doi: 10.1371/journal.pone.0063524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schreiner RP, Mihara KL. 2009. The diversity of arbuscular mycorrhizal fungi amplified from grapevine roots (Vitis vinifera L.) in Oregon vineyards is seasonally stable and influenced by soil and vine age. Mycologia 101:599–611. doi: 10.3852/08-169. [DOI] [PubMed] [Google Scholar]
- 82.Alguacil MM, Roldán A, Torres MP. 2009. Complexity of semiarid gypsophilous shrub communities mediates the AMF biodiversity at the plant species level. Microb Ecol 57:718–727. doi: 10.1007/s00248-008-9438-z. [DOI] [PubMed] [Google Scholar]
- 83.Alguacil MM, Lozano Z, Campoy M, Roldán A. 2010. Phosphorus fertilisation management modifies the biodiversity of AM fungi in a tropical savanna forage system. Soil Biol Biochem 42:1114–1122. doi: 10.1016/j.soilbio.2010.03.012. [DOI] [Google Scholar]
- 84.DeLuca TH, Keeney DR. 1993. Soluble anthrone-reactive carbon in soils: effect of carbon and nitrogen amendments. Soil Sci Soc Am J 57:1296–1300. doi: 10.2136/sssaj1993.03615995005700050022x. [DOI] [Google Scholar]
- 85.Huijuan G, Xueli H, Yingpeng L. 2012. Spatial distribution of arbuscular mycorrhiza and glomalin in the rhizosphere of Caragana korshinskii Kom. in the Otindag sandy land, China. Afr J Microbiol Res 6:5745–5753. [Google Scholar]
- 86.Caravaca F, Alguacil MM, Torres P, Roldán A. 2005. Plant type mediates rhizospheric microbial activities and soil aggregation in a semiarid Mediterranean salt marsh. Geoderma 124:375–382. doi: 10.1016/j.geoderma.2004.05.010. [DOI] [Google Scholar]
- 87.Da Silva IR, de Mello CMA, Neto RAF, da Silva DKA, de Melo AL, Oehl F, Maia LC. 2014. Diversity of arbuscular mycorrhizal fungi along an environmental gradient in the Brazilian semiarid. Appl Soil Ecol 84:166–175. doi: 10.1016/j.apsoil.2014.07.008. [DOI] [Google Scholar]
- 88.Marschner H. 1995. Mineral nutrition of higher plants, 2nd ed Academic Press, London, United Kingdom. [Google Scholar]
- 89.Lehmann A, Rillig MC. 2015. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops: a meta-analysis. Soil Biol Biochem 81:147–158. doi: 10.1016/j.soilbio.2014.11.013. [DOI] [Google Scholar]
- 90.Cavagnaro TR. 2014. Arbuscular mycorrhizas and their role in plant zinc nutrition, p 189–200. In Solaiman Z, Abbott LK, Varma A (ed), Mycorrhizal fungi: use in sustainable agriculture and land restoration. Springer Verlag, Berlin, Germany. [Google Scholar]
- 91.Wei Y, Hou H, Li J, ShangGuan Y, Xu Y, Zhang J, Zhao L, Wang W. 2014. Molecular diversity of arbuscular mycorrhizal fungi associated with an Mn hyperaccumulator—Phytolacca americana, in Mn mining area. Appl Soil Ecol 82:11–17. doi: 10.1016/j.apsoil.2014.05.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.