Abstract
Objective
To evaluate the occurrence, clinical presentations and diagnostic methods for tuberculosis in a cohort of HIV-infected infants, children and adolescents from Latin America.
Methods
A retrospective analysis of children with tuberculosis and HIV was performed within a prospective observational cohort study conducted at multiple clinical sites in Latin America.
Results
Of 1114 HIV-infected infants, children, and adolescents followed from 2002 to 2011, 69 that could be classified as having confirmed or presumed tuberculosis were included in this case series; 52.2% (95% CI: 39.8–64.4%) had laboratory-confirmed tuberculosis, 15.9% (95% CI: 8.2–26.7%) had clinically confirmed disease and 31.9% (95% CI: 21.2–44.2%) had presumed tuberculosis. Sixty-six were perinatally HIV-infected. Thirty-two (61.5%) children had a history of contact with an adult tuberculosis case; however information on exposure to active tuberculosis was missing for 17 participants. At the time of tuberculosis diagnosis, 39 were receiving antiretroviral therapy. Sixteen of these cases may have represented immune reconstitution inflammatory syndrome.
Conclusions
Our study emphasizes the need for adequate contact tracing of adult tuberculosis cases and screening for HIV or tuberculosis in Latin American children diagnosed with either condition. Preventive strategies in tuberculosis-exposed, HIV-infected children should be optimized.
Keywords: Tuberculosis, HIV, Children, Latin America
Introduction
HIV/AIDS is responsible for exacerbating the tuberculosis (TB) epidemics in both adults and children in many low and middle income countries.1 Unfortunately, diagnosing TB in children is a challenging task because symptoms are non-specific and sputum specimens are usually not accessible, making bacteriologic confirmation difficult.
Diagnosis in children often requires reliance on clinical, epidemiological, and radiographic criteria.1 The diagnosis of TB is further complicated in HIV-infected children2 presenting with advanced immune-suppression when Pneumocystis jiroveci, disseminated bacterial, fungal and viral infections, or other mycobacterial agents mimic or coexist with TB disease, and treatment is started empirically for more than one etiological agent in severely ill patients.3 Interpretation of tuberculin skin tests is difficult, with false negative results occurring in HIV-infected children who are immunocompromised and false positive results occurring in Latin-American regions with moderate to high endemicity like Brazil, where BCG is routinely given at birth.4, 5
Many investigators have reported on pediatric HIV-TB co-infection in Africa,6, 7, 8, 9 but few such studies have been conducted in Latin America.10, 11, 12 The largest Latin American study reported 29 cases of TB in a cohort of 360 HIV-infected children using a retrospective, chart abstraction of hospitalization records between 1989 and 1999.10 Most cases in this study had pulmonary disease; bacteriological confirmation was obtained in half of the cases, and no data on antiretroviral therapy (ART) were reported.
The main purpose of our study was to describe cases of TB occurring in HIV-infected children and youth who were participating in a large, multicenter observational cohort study conducted in Latin American countries. Our aims included describing HIV-TB co-infection, methods used to diagnose TB cases, clinical presentation, the timing of the TB diagnosis in relation to timing of initiating treatment for HIV, therapeutic response to TB treatment, and the association between TB clinical presentation and patient immunological status (when appropriate data were available).
Methods
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International Site Development Initiative (NISDI) Pediatric protocol was a prospective cohort study that enrolled and followed HIV-infected children at multiple clinical sites in Latin America between 2002 and 2011. The primary purpose of the study was to describe the demographic, clinical, immunological and virologic characteristics of HIV-infected children and youth at participating clinical sites, as well as characterize outcomes related to HIV disease, including exposure to ARV treatment. A detailed description of this study has been published elsewhere.13 Briefly, HIV-infected children and youth <21 years of age were enrolled at outpatient clinics and treated with ART according to each country's standard protocol. Historical laboratory and clinical data were systematically collected at enrollment; protocol evaluations, including medical and treatment history and clinical and laboratory assessments, were conducted every six months. The protocol was approved by the ethical review boards at each clinical site, by the sponsoring institution (NICHD), the data management and statistical center (Westat), and the Brazilian National Ethics Committee (CONEP). Informed consent was obtained from parents and/or guardians.
This case series was obtained from a retrospective medical record review of all TB cases reported among HIV infected subjects participating in the NISDI Pediatric protocol (2002–2007) and prospective case ascertainment after 2007. Participating clinical sites in Brazil (eleven sites), Argentina (two sites), Peru (two sites), and Mexico (two sites) were asked to: (1) review the medical records for all subjects with a clinical diagnosis of TB, or any diagnosis where Mycobacterium tuberculosis was cultured; and (2) complete a TB case report form for each case. Data collected from available medical records at the time of diagnosis included the basis for the TB diagnosis (laboratory, epidemiological, radiological, clinical signs and symptoms); the clinical presentation of TB; receipt of ART prior to the TB diagnosis; receipt of anti-TB drug therapy; and CD4 and viral load nearest (within six months before and one month after) the time of TB diagnosis. HAART was defined as at least three anti-retroviral medications from at least two classes.
To be included in this case series, each subject had to have documentation of having been prescribed anti-TB therapy and some evidence of TB obtained from the medical records at the time of the TB diagnosis. Cases were classified in one of three ways: laboratory-confirmed, clinically confirmed, or presumed TB.
The diagnosis of TB was considered laboratory-confirmed if one or more of the following conditions were met:
-
•
Isolation of M. tuberculosis from a clinical specimen.
-
•
Demonstration of M. tuberculosis complex from a clinical specimen by nucleic acid amplification test.
-
•
Demonstration of acid-fast bacilli in a clinical specimen when a culture had not been or could not be obtained.
-
•
Histopathology consistent with TB.
In the absence of any laboratory evidence, TB was classified as clinically confirmed if all of the following conditions were met:
-
•
A positive tuberculin skin test (≥5 mm induration) for M. tuberculosis.
-
•
Clinical signs and symptoms (e.g., fever, cough, weight loss), radiologic evidence (e.g., abnormal chest radiograph [CXR], abnormal chest computerized tomography [CT] scan or ultrasound evidence of hepatomegaly, splenomegaly, and/or lymphadenopathy) compatible with TB.
-
•
Treatment with two or more anti-TB medications.
If a case could not be classified as laboratory- or clinically confirmed, the case was considered presumed TB if the subject was treated with three or more anti-TB medications and there was evidence in at least two of three broad categories (epidemiologic, radiologic, clinical signs or symptoms).
-
•
Report of close contact with an active TB case was considered epidemiologic evidence.
-
•
Radiological evidence included (1) CXR and/or CT demonstrating cavitation, hilar lymphadenopathy, an infiltrate, or findings consistent with miliary TB and/or (2) ultrasound evidence of the following: hepatomegaly, splenomegaly and/or lymphadenopathy.
-
•
Clinical signs/symptoms included a report of symptoms compatible with TB (cough, fever, weight loss, lymphadenopathy, or liver and/or spleen enlargement).
Those cases with insufficient evidence for classification as outlined above were not included in the final case series.
A clinical response to treatment with anti-tuberculosis medication was defined as a return to normal temperature after reported fever and/or gaining 10% of body weight among those who had previously lost 10% of their body weight one month prior to the diagnosis of TB.
Results
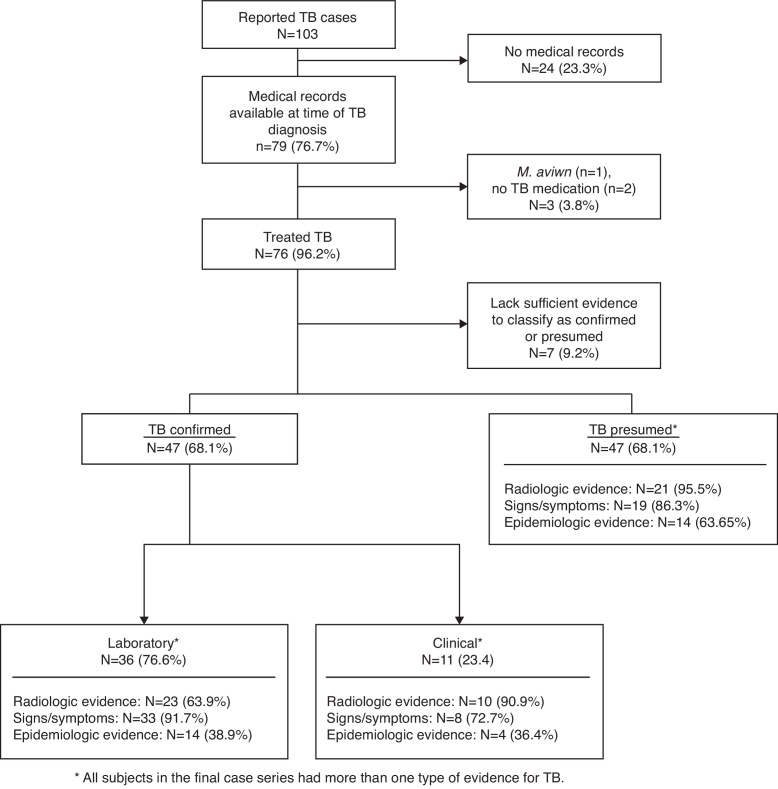
Of the 1114 HIV-infected infants, children, and adolescents enrolled in NISDI, 103 (9.2%) had some mention of TB in their case report forms and 79 (76.7%) of these had medical records available at the time of the TB diagnosis (Fig. 1). Of these 79 cases, one was excluded for a positive culture for Mycobacterium avium and two were excluded as there was no documentation of treatment for TB. Of the remaining 76 subjects, seven lacked sufficient evidence for classification and were excluded, resulting in 69 TB cases to be described, of which 36 (52.2%; 95% CI: 39.8–64.4) were classified as laboratory-confirmed TB, 11 (15.9%; 95% CI: 8.2–26.7) as clinically confirmed TB, and 22 (31.9%; 95% CI: 21.2–44.2) as presumed TB. Thus, 87.3% (69/79) of reported TB could be classified by our chart review.
Fig. 1.
Different sources of evidence for classification of reported TB cases.
As shown in Table 1, among these 69 TB cases, 49 (71.0%) were from Brazil, reflecting the overall distribution of enrollments in the cohort; 66 (95.7%) had perinatally acquired HIV infection, and the majority (84.1%) had received BCG vaccination. Of the 52 subjects with information available on contact history, 32 (61.5%) had a history of contact with an adult TB case, with 94% of these reporting daily contact with this person. The median age at TB diagnosis was 2.5 years, ranging from birth to 22 years of age. No deaths from TB occurred among these 69 cases during study follow-up.
Table 1.
Demographic and clinical features of the 69 tuberculosis (TB) cases.
| Variable | n (%) |
|---|---|
| Country of origin | |
| Argentina | 3 (4.3) |
| Brazil | 49 (71.0) |
| Mexico | 7 (10.1) |
| Peru | 10 (14.5) |
| Gender | |
| Male | 37 (53.6) |
| Female | 32 (46.4) |
| Mode of HIV acquisition | |
| Perinatal | 66 (95.7) |
| Horizontal | 3 (4.3) |
| History of contact with adult TB case | |
| Yes | 32 (61.5) |
| No | 20 (38.5) |
| Missing | 17 |
| BCG vaccination | |
| Yes | 58 (84.1) |
| Not confirmed | 11 (15.9) |
| If yes, age at BCG vaccination (months) | |
| <12 | 51 (94.4) |
| ≥12 | 3 (5.6) |
| Missing | 4 |
| If yes, months since BCG at TB diagnosis | |
| <12 | 16 (30.2) |
| 12 < 24 | 8 (15.1) |
| ≥24 | 29 (54.7) |
| Missing | 5a |
| Age at TB diagnosis (years) | |
| <1 | 20 (29.0) |
| 1–4 | 27 (39.1) |
| 5–14 | 19 (27.5) |
| >14 | 3 (4.4) |
| Median | 2.5 |
| Range | Birth to 22 |
| Relationship of TB diagnosis (dx) to ART | |
| TB dx prior to any ART | 25 (36.2) |
| TB dx within 3 days of starting any ART | 3 (4.3) |
| TB dx after being on ART >3 days | 39 (56.5) |
| Missing ART dates | 2 (2.9) |
| Clinical presentation | |
| Pulmonary | 33 (47.8) |
| Miliary | 24 (34.8) |
| Lymphadenitis | 9 (13.0) |
| Other | 3 (4.4) |
| Clinical features | |
| Any symptoms consistent with TB | 60 (87.0) |
| Unknown/missing | 9 (13.0) |
| Fever | |
| Yes | 42 (70.0) |
| No | 18 (30.0) |
| Cough | |
| Yes | 39 (65.0) |
| No | 21 (35.0) |
| Weight loss within 1 month | |
| Yes | 22 (36.7) |
| No | 38 (63.3) |
| Lymphadenopathy | |
| Yes | 7 (11.7) |
| No | 53 (88.3) |
| Hospitalization for ≥28 days | |
| Yes | 42 (60.9) |
| No or unknown | 27 (39.1) |
One case of TB occurred prior to BCG administration.
Clinical features/presentation and diagnostic methods
Sixty cases (87.0%) had symptoms consistent with TB recorded with the remaining nine (13%) subjects missing information regarding their symptoms (Table 1). Fever was the most frequent symptom recorded (70%), with most fevers lasting ≥14 days [24/42 (57.1%)], followed by cough (65%), recent (within 1 month) weight loss (36.7%), and lymphadenopathy (11.7%).
Pulmonary TB was the most frequent diagnosis (47.8%), followed by miliary TB (34.8%), and TB lymphadenitis (13.0%) (Table 1). Table 2 shows the clinical presentation of the TB cases and how they were diagnosed and distributed by age category.
Table 2.
Tuberculosis (TB) cases by age at time of TB diagnosis, clinical presentation, and type of evidence used for diagnosis.
| Age at time of TB diagnosis | Clinical presentation | Type of evidence used for diagnosis |
Total | ||
|---|---|---|---|---|---|
| Laboratory confirmed TB | Clinically confirmed TB | Presumed TB | |||
| 0 ≤ 12 mo | Lymphadenitis | 2 | 1 | 0 | 3 |
| Miliary TB | 5 | 2 | 1 | 8 | |
| Pulmonary TB | 6 | 1 | 1 | 8 | |
| Congenital TB | 1 | 0 | 0 | 1 | |
| 1–4 yr | Lymphadenitis | 3 | 0 | 0 | 3 |
| Miliary TB | 3 | 2 | 6 | 11 | |
| Pulmonary TB | 4 | 2 | 7 | 13 | |
| 5–14 yr | Lymphadenitis | 2 | 0 | 1 | 3 |
| Miliary TB | 2 | 0 | 2 | 4 | |
| Pulmonary TB | 7 | 2 | 2 | 11 | |
| Renal TB (6 yr) | 0 | 1 | 0 | 1 | |
| >14 yr | Lymphadenitis | 0 | 0 | 0 | 0 |
| Miliary TB | 0 | 0 | 1 | 1 | |
| Pulmonary TB | 0 | 0 | 1 | 1 | |
| TB meningitis (22 yr) | 1 | 0 | 0 | 1 | |
| Total | 36 | 11 | 22 | 69 | |
Among the 36 laboratory-confirmed cases, 18 (50.0%) had a positive culture and another 18 (50.0%) had at least one specimen positive for acid fast bacilli (AFB) and/or histopathology suggestive of TB (one by histopathology alone) (data not shown).
Among 61 records reporting CXR and/or CT exams, 54 (88.5%) were abnormal; 18 (33.3%) of the 54 were read as miliary TB, 15 (27.8%) with local or diffuse infiltrates, 14 (25.9%) with hilar/lymph node enlargement, three (5.6%) with cavitation and four (7.4%) other. Eleven (84.6%) of the 13 abdominal ultrasounds performed demonstrated hepatomegaly, splenomegaly, and/or lymphadenopathy.
Tuberculin skin tests were recorded for 37 (53.6%) of the 69 TB cases, of which 16 (43.2%) were positive. Five out of 16 (31.3%) tests among the 36 bacteriologically confirmed cases were positive.
Clinical response to treatment
Among 61 cases with available weight and fever information, 72.5% (n = 44) demonstrated a positive response after TB treatment; 17 (27.5%) charts documented both an increase in body weight of ≥10% and disappearance of fever, and 27 (44.9%) documented either an increase of ≥10% in body weight or disappearance of fever (data not shown).
Timing of TB and ART
The vast majority (87.0%) of TB cases were diagnosed prior to enrollment into NISDI; only nine cases of TB (13.0%) were diagnosed after study enrollment. Most ART start dates also occurred prior to study enrollment. Two (2.9%) cases did not have ART start dates available, 25 (36.2%) cases of TB were diagnosed prior to the start of ART, three (4.3%) had the diagnosis of TB and ART initiation occur within three days of each other, and 39 (56.5%) cases were on ART [26 were on HAART-PI, three on HAART-NNRTI, and ten on dual therapy] prior to TB diagnosis (Table 1). Eleven of the 39 cases on ART prior to TB diagnosis had been on HAART for less than three months when TB manifested itself and another five cases were diagnosed between three and six months following initiation of HIV treatment (data not shown).
Immunological markers
In the majority of cases no CD4 counts were available at the time of TB diagnosis. Twenty eight cases had CD4 results available within six months before or one month after TB diagnosis: 11 (39.3%) had CD4% <15%, 12 (42.9%) had CD4% between 15% and 25%, and five (17.9%) had CD4% >25%. There was no apparent association between CD4 percentages and clinical presentation (data not shown).
Discussion
Of the 1114 HIV-infected subjects enrolled in the NISDI study, 103 (9.2%) had some mention of TB in their case report forms. This crude prevalence is similar to the 7.4% (51/689) prevalence reported among Kenyan children enrolled in a prospective study from six weeks to 14 years of age.14
Of the 79 available medical records, 69 (87.3%) contained sufficient evidence to classify these cases as follows: 52.2% (95% CI: 39.8–64.4) were classified as laboratory-confirmed TB, 15.9% (95% CI: 8.2–26.7) as clinically confirmed TB, and 31.9% (95% CI: 21.2–44.2) as presumed TB. As previously noted, the diagnosis of TB in children is a challenging task, often requiring reliance on clinical, epidemiological, and radiographic criteria.
Among 218 children with symptoms suggestive of pulmonary tuberculosis (cases) that enrolled from August, 2002, to January, 2007 at two hospitals in Lima, Peru, only 22 (10%) had at least one positive M. tuberculosis culture.15 In contrast, in our case series, 26% (18/69) of cases were culture confirmed, perhaps due, in part, to the strict criteria utilized to classify cases.
Thirty-nine (56.5%) subjects were receiving ART at least three or more days prior to TB diagnosis, with eleven cases receiving ART for less than three months when TB was diagnosed and five cases diagnosed between three and six months following initiation of ART. Given the timing ART initiation in relationship to the timing of TB diagnosis, these 16 (23%) cases may represent immune reconstitution inflammatory syndrome (IRIS). A recent systematic literature review of TB IRIS found only thirteen pertinent studies.16 They found the median time from start of ART to TB IRIS diagnosis (reported by eight studies) ranged from eight days to 16 weeks. This review included only one study from Latin America, that reported on two cases of disseminated BCG in HIV infected children less than one year of age.17 Thus, our report of 16 potential TB IRIS cases from Latin America is the largest we are aware of.
There are some potential limitations of this study that are worth noting when interpreting the results. In Latin American countries, BCG immunization is recommended in the first month of life because of its proven efficacy in avoiding severe forms of the disease.18 It is however not recommended in children with symptomatic HIV disease because of its potential to cause severe local or even disseminated disease.19, 20 Most, if not all, children in this cohort were not known to be HIV-infected at the time they received BCG, so it is not possible to exclude the Mycobacterium bovis strain as the cause of one or more cases in the present cohort.
In this observational cohort, most cases of TB (87%) occurred prior to enrollment into NISDI, and therefore, were based on chart review which may have missed important clinical and epidemiological details. Patients were enrolled at 15 sites in four countries over nine years, thus standard of care for diagnosis and treatment of TB and HIV could have varied considerably over time and across sites. Despite these limitations we could classify the majority of cases reported.
A recent report by WHO21 shows that this region of the Americas is second to Africa with respect to prevalence of HIV among TB patients; 44% of TB patients in Africa found to be HIV-positive compared to 17% in the region of the Americas. In fact, the diagnosis of TB may have led to the HIV diagnosis in our study, since over one-third of the TB cases were not on ART until months to years later. Therefore, there is an urgent need to find, prevent and treat TB in people living with HIV, and to screen for HIV and TB in children upon diagnosis of either condition.
Our finding that nearly half of all cases (32) had a history of contact with an adult TB case emphases the need for more aggressive contact tracing among adult TB cases and provision of preventive therapy for exposed HIV infected children.
Research on optimizing preventive strategies in TB-exposed, HIV-infected children is needed.
Funding
Funded by Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland, NICHD Contract # HHSN267200800001C (NICHD Control # N01-HD-8-0001).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the children and families who participated in the NISDI pediatric project and the site staff involved in the conduct of the study.
Principal investigators, co-principal investigators, study coordinators, data management center representatives and NICHD staff include: Brazil: Belo Horizonte: Jorge Pinto, Flávia Faleiro (Universidade Federal de Minas Gerais); Caxias do Sul: Rosa Dea Sperhacke, Nicole Golin, Sílvia Mariani Costamilan (Universidade de Caxias do Sul/Serviço Municipal de Infectologia); Nova Iguacu: Jose Pilotto, Beatriz Grinsztejn, Valdilea Veloso, Luis Felipe Moreira, Ivete Gomes (Hospital Geral Nova de Iguacu–HIV Family Care Clinic); Porto Alegre: Rosa Dea Sperhacke, Breno Riegel Santos, Rita de Cassia Alves Lira (Universidade de Caxias do Sul/Hospital Conceição); Rosa Dea Sperhacke, Mario Ferreira Peixoto, Elizabete Teles (Universidade de Caxias do Sul/Hospital Fêmina); Rosa Dea Sperhacke, Marcelo Goldani, Carmem Lúcia Oliveira da Silva, Margery Bohrer Zanetello (Universidade de Caxias do Sul/Hospital de Clínicas de Porto Alegre); Regis Kreitchmann, Marcelo Comerlato Scotta, Debora Fernandes Coelho (Irmandade da Santa Casa de Misericordia de Porto Alegre); Ribeirão Preto: Marisa M. Mussi-Pinhata, Maria Célia Cervi, Márcia L. Isaac, Fernanda Tomé Sturzbecher, Bento V. Moura Negrini (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo); Rio de Janeiro: Ricardo Hugo S. Oliveira, Maria C. Chermont Sapia (Instituto de Puericultura e Pediatria Martagão Gesteira); Esau Custodio Joao, Maria Leticia Cruz, Ana Paula Antunes, Jacqueline Anita de Menezes (Hospital dos Servidores do Estado); São Paulo: Regina Celia de Menezes Succi, Daisy Maria Machado (Escola Paulista de Medicina - Universidade Federal de São Paulo); Marinella Della Negra, Wladimir Queiroz, Yu Ching Lian (Instituto de Infectologia Emilio Ribas); Mexico: Mexico City: Noris Pavía-Ruz, Dulce Morales-Pérez, Jorge Gamboa-Cardeña (Hospital Infantil de México Federico Gómez); Peru: Lima: Jorge Alarcón Villaverde (Instituto de Medicina Tropical “Daniel Alcides Carrión”- Sección de Epidemiologia, UNMSM), María Castillo Díaz (Instituto Nacional de Salud del Niño), Mary Felissa Reyes Vega (Instituto de Medicina Tropical “Daniel Alcides Carrión” - Sección de Epidemiologia, UNMSM); Data Management and Statistical Center: Yolanda Bertucci, Laura Freimanis Hance, René Gonin, D. Robert Harris, Roslyn Hennessey, Margot Krauss, James Korelitz, Kathryn Miller, Sharon Sothern de Sanchez, Sonia K. Stoszek (Westat, Rockville, MD, USA); NICHD: Rohan Hazra, Lynne M. Mofenson, George K. Siberry (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the Department of Health and Human Services.
References
- 1.Marais B.J., Schaaf H.S. Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin North Am. 2010;24:727–749. doi: 10.1016/j.idc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Verhagen L.M., Warris A., van Soolingen D., et al. Human Immunodeficiency Virus and tuberculosis co-infection in children. Pediatr Infect Dis. 2010;29:e63–e70. doi: 10.1097/INF.0b013e3181ee23ae. [DOI] [PubMed] [Google Scholar]
- 3.Jeena P.M., Pillay P., Pillay T., et al. Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa. Int J Tuberc Lung Dis. 2002;6:672–678. [PubMed] [Google Scholar]
- 4.World Health Organization Country profiles 2011, Brazil. http://www.who.int/tb/publications/global_report/2011/gtbr11_a2.pdf [accessed 16.12.13].
- 5.Feja K., Saiman L. Tuberculosis in children. Clin Chest Med. 2005;26:295–312. doi: 10.1016/j.ccm.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Elenga N., Kouakoussui K.A., Bonard D., et al. Diagnosed tuberculosis during the follow-up of a cohort of human immunodeficiency virus-infected children in Abidjan, Cote d’Ivoire ANRS 1278 study. Pediatr Infect Dis J. 2005;24:1077–1082. doi: 10.1097/01.inf.0000190008.91534.b7. [DOI] [PubMed] [Google Scholar]
- 7.Hesseling A.C., Westra A.E., Werschkull H., et al. Outcome of HIV-infected children with culture confirmed tuberculosis. Arch Dis Child. 2005;90:1171–1174. doi: 10.1136/adc.2004.070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braitstein P., Nyandiko W., Vreeman R., et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J. 2009;28:626–632. doi: 10.1097/INF.0b013e31819665c5. [DOI] [PubMed] [Google Scholar]
- 9.Chisti M.J., Ahmed T., Pietroni M.A., et al. Pulmonary tuberculosis in severely-malnourished or HIV-infected children with pneumonia: a review. J Health Popul Nutr. 2013;31:308–313. doi: 10.3329/jhpn.v31i3.16516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertolini D.V., Sato H.K., Marques H.H.S. Tuberculose em crianças com Aids. J Bras AIDS. 2002;3:17–22. [Google Scholar]
- 11.Ramirez-Cardich M.E., Kawai V., Oberhelman R.A., et al. Clinical correlates of tuberculosis co-infection in HIV-infected children hospitalized in Peru. Int J Infect Dis. 2006;10:278–281. doi: 10.1016/j.ijid.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Viani R.M., Lopez G., Chacon-Cruz E., et al. Poor outcome is associated with delayed tuberculosis diagnosis in HIV-infected children in Baja California, Mexico. Int J Tuberc Lung Dis. 2008;12:411–416. [PubMed] [Google Scholar]
- 13.Hazra R., Stoszek S.K., Freimanis-Hance L., et al. Cohort Profile: NICHD International Site Development Initiative (NISDI): a prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and Caribbean countries. Int J Epidemiol. 2009;38:1207–1214. doi: 10.1093/ije/dyn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abuogi L.L., Mwachari C., Leslie H.H., et al. Impact of expanded antiretroviral use on incidence and prevalence of tuberculosis in children with HIV in Kenya. Int J Tuberc Lung Dis. 2013;17:1291–1297. doi: 10.5588/ijtld.12.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberhelman R.A., Soto-Castellares G., Gilman R.H., et al. Diagnostic approaches for paediatric tuberculosis by use of different specimen types, culture methods, and PCR: a prospective case–control study. Lancet Infect Dis. 2010;10:612–620. doi: 10.1016/S1473-3099(10)70141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Link-Gelles R., Moultrie H., Sawry S., Murdoch D., Van Rie A. Tuberculosis immune reconstitution inflammatory syndrome in children initiating antiretroviral therapy for HIV infection: a systematic literature review. Pediatr Infect Dis J. 2014;33:499–503. doi: 10.1097/INF.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes R.C., de Araújo L.C., Medina-Acosta E. Reduced rate of adverse reactions to the BCG vaccine in children exposed to the vertical transmission of HIV infection and in HIV-infected children from an endemic setting in Brazil. Eur J Pediatr. 2009;168:691–696. doi: 10.1007/s00431-008-0822-y. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization BCG Vaccine Position paper. Wkly Epidemiol Rec. 2004;79:27–38. [PubMed] [Google Scholar]
- 19.Hesseling A.C., Rabie H., Marais B.J., et al. Bacille Calmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;42:548–558. doi: 10.1086/499953. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Global Advisory Committee on Vaccine safety 29–30 November 2006. Wkly Epidemiol Rec. 2007;82:18–23. [PubMed] [Google Scholar]
- 21.World Health Organization Report 2011. Global tuberculosis control. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf [accessed 16.12.13].