Abstract
Morphogen gradients play pervasive roles in development, and understanding how they are established and decoded is a major goal of contemporary developmental biology. Here we examine how a Wingless (Wg) morphogen gradient patterns the peripheral specialization of the fly eye. The outermost specialization is the pigment rim; a thick band of pigment cells that circumscribes the eye and optically insulates the sides of the retina. It results from the coalescence of pigment cells that survive the death of the outermost row of developing ommatidia. We investigate here how the Wg target genes expressed in the moribund ommatidia direct the intercellular signaling, the morphogenetic movements, and ultimately the ommatidial death. A salient feature of this process is the secondary expression of the Wg morphogen elicited in the ommatidia by the primary Wg signal. We find that neither the primary nor secondary sources of Wg alone are able to promote ommatidial death, but together they suffice to drive the apoptosis. This represents an unusual gradient read-out process in which a morphogen induces its own expression in its target cells to generate a concentration spike required to push the local cellular responses to the next threshold response.
1. Introduction
Morphogen gradients play major roles in patterning developing tissues. Cells within a morphogen field can meter the ambient concentration and use this information to regulate gene transcription, and generate a field of cells patterned by distinct domains of gene expression. Many mechanisms have been described that allow the refinement and sharpening of the gene expression domains, and successful as these studies have been, they have not explained the progression from the arrayed gene expression patterns to the final “form” of the animal. In this study we examine the gradient read-out mechanism that patterns the periphery of the fly eye. This occurs relatively late in development, which allows the patterning to be followed from the initial presentation of the morphogen, through the transcriptional responses of the target tissues and subsequent cell interactions that result in the structured periphery of the functional adult eye.
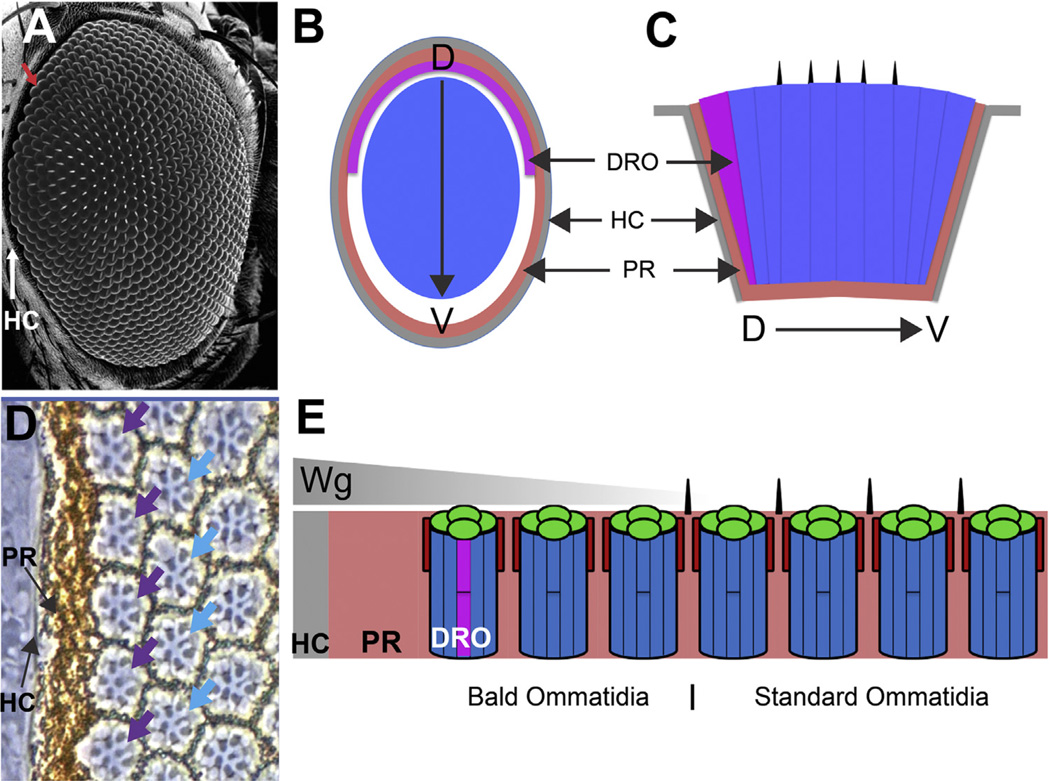
The Drosophila compound eye is made from many hundred subunit ommatidia arranged in a lattice array (Ready et al., 1976). The ommatidia in the main body of the eye are of a standard type but at the periphery there are three zones of specialization (Fig. 1). In the outer parts of the eye the ommatidia lack the standard mechanosensory bristles, and a halo of these bald ommatidia extends up to five ommatidial rows in to the eye (Fig. 1A). The R7/8 central photoreceptors in standard ommatidia have small rhabdomeres, and the two cells correlate their opsin expression in a defined manner (Huber et al., 1997; Papatsenko et al., 1997). At the periphery, the outermost row of ommatidia in the dorsal half of the eye are specialized polarized light-detecting dorsal rim ommatidia (DRO, Fig. 1B–E), in which the R7/8 photoreceptors have large rhabdomeres (Fig. 1D) and express the same Rh3 opsin (Fortini and Rubin, 1990). DRO do not occur in the ventral eye; here the outermost ommatidia are bald but otherwise standard (Fig. 1B and C) (Tomlinson, 2003; Wernet et al., 2003). Circumscribing the entire ommatidial array lies the pigment rim; a thick layer of pigment cells that is the most peripheral eye tissue, and acts as an optical insulating collar for the sides of the retina (Fig. 1B–E). Thus there are four domains in the retina: first and outermost is the pigment rim, second (specifically in the dorsal eye) are the bald DRO, third are bald standard ommatidia, and fourth are the standard bristle-bearing ommatidia.
Fig. 1.
Peripheral features of the fly eye. (A) Scanning EM image of adult fly eye. The array of facets and bristles are the most prominent features. The head capsule (HC) circumscribes the eye (white arrow), and the outermost ommatidia lack bristles (red arrow). (B) Schematic cross section through the eye highlighting: the head capsule (HC grey); the pigment rim (PR pink), and DRO (purple). The region of bald ommatidia is white, and the array of standard ommatidia throughout the main body of the eye is blue. (C) Schematic longitudinal section through the eye. (D) Phase contrast image of the dorsal peripheral eye. The head capsule is seen as a grey strip adjacent to the pigment rim. The first row of ommatidia are DRO (purple arrows) indicated by the large R7 rhabdomeres. In the second row, the R7 rhabdomeres are normal (blue arrows). (E) Schematic longitudinal section through the peripheral dorsal eye. The head capsule secretes Wingless (Wg) which diffuses (grey triangle) into the retina and directs the specializations.
The peripheral specializations are specified by Wingless (Wg-the fly Wnt-1) secreted by the presumptive head capsule that abuts and circumscribes the developing retina. Wg diffuses into the eye tissue from the head capsule beginning in the third larval instar and onward throughout pupal life. The peripheral patterning mechanism begins shortly after pupation when the diffusing Wg inhibits the transcription of the daughterless (da) and achaete (ac) genes in the outer regions of the retina (Cadigan et al., 2002). These genes encode proneural transcription factors; their absence prevents the formation of bristle precursors, and a bald halo is prefigured in the outer eye regions. At a similar time, Wg also induces the R7/R8 photoreceptors of the dorsal peripheral ommatidia to express the Homothorax (Hth) transcription factor which directs these cells to become DRO polarized light detectors (Wernet et al., 2003). The ventral eye fails to generate DRO because it does not express the requisite Iroquois transcription factors found in the dorsal eye. hth and da/ac are thus target genes of the Wg gradient, with ac/da repression being a lower threshold response than the activation of hth transcription, and accordingly the bald halo extends deeper into the eye than the DRO. The highest Wg threshold response is the generation of the pigment rim, which is the focus of this present work.
The formation of the pigment rim begins half way through the second day of pupation, and is intimately associated with the apoptosis of the outermost row of ommatidia (Wolff and Ready, 1991). In this mechanism the photoreceptors, cone cells and primary pigment cells (1°PCs) die, leaving the surrounding secondary/tertiary pigment cells (2°/3° PCs) to coalesce and form the pigment rim (Lim and Tomlinson, 2006). In addition to providing retinal optical insulation, the pigment rim likely serves two other purposes. First, the peripheral ommatidia frequently lack a full complement of ommatidial cells, and their death removes potentially defective units (Wolff and Ready, 1991). Second, the R1–6 photoreceptor axons project to the underlying optic lamina layer which contains cartridges arrayed in a one-to-one correspondence with the ommatidia of the retina. These axons do not target to their corresponding cartridge, but fan out and innervate the six surrounding cartridges (Braitenberg, 1967). However, at the edge of the eye there are no extra cartridges for the photoreceptors to innervate. This potential problem is solved by the death of the outer ommatidia which frees the most peripheral row of cartridges to be used by the outermost surviving row of ommatidia (see Meinertzhagen and Hanson, 1993 for a full exposition).
In previous work we described how Wg from the head capsule elicits target gene transcription in the moribund ommatidia. There are two cellular foci for these gene expressions; the cone cells and the 2°/3° PCs. Three transcription targets have been identified in the cone cells. First is wg itself, and thus the primary source of Wg from the head capsule elicits a secondary expression in the peripheral cone cells. Second, the cone cells express the three genes of the Snail family gene complex; snail (sna), escargot (esg) and worniu (wor), which encode functionally redundant helix-loop-helix transcription factors (Ashraf et al., 1999). Third, the cone cells also express notum, which encodes an α/β hydrolase (Gerlitz and Basler, 2002; Giraldez et al., 2002) that restricts Wg diffusion by deacylating it (Kakugawa et al., 2015; Zhang et al., 2015). The 2°/3°PCs express the snail family and notum genes (they do not express wg), and the photoreceptors and 1°PCs do not express any of these genes. Here we examine the roles played by the cone cells in directing the death of the ommatidia with specific reference to the roles played by Wg and the Snail-family transcription factors. We uncover a complex interplay between the cone cells and the other ommatidial cells; we infer a key role for the Snail family proteins in cone cell fall and death, and a critical role for Wg in triggering the apoptosis of the ommatidial cells. The Wg derived from the cone cells combines with the Wg from the head capsule to generate the requisite morphogen concentration for ommatidial death. Thus, we detect an unusual gradient decoding mechanism in which the morphogen elicits its own expression in its target cells to generate a local concentration surge.
2. Results
2.1. Pan-retinal Wg expression elicits pan-retinal peripheral patterning
When the death mechanism begins, the nuclei of the ommatidia are organized into three layers. Apically lie the cone cell nuclei (Fig. 2A), above the photoreceptor nuclei flanked by those of the 1° PCs (Fig. 2B). The lower layer contains the nuclei of the bristle group cells (Fig. 2C), along with the 2°/3° PCs (not stained in Fig. 2C). Since Wg from the head capsule prevents bristle formation, the peripheral ommatidia contain only 2°/3° PCs in the lower layer. At ~32 h after puparium formation (APF) the various gene expressions described above are detected, and at ~36 h APF the cone cells nuclei drop to the photoreceptor/1° PCs layer. At ~42 h APF apoptosis occurs in this layer and after this time, only the 2°/3° PCs remain. Observations of the behavior of the peripheral-most ommatidia are time consuming and difficult to make for two reasons. First, pupal retinas curve at the periphery, folding under the main body of the eye and making observations difficult. Second, some peripheral ommatidia do not contain the full complement of cells, and under these conditions it is difficult to know whether all cells have been accounted for in an analysis. Given these difficulties, we considered it impractical to investigate the mechanisms of pigment rim formation in situ, rather we adopted the approach of converting the entire eye into a pseudo-periphery and using this as the experimental tissue.
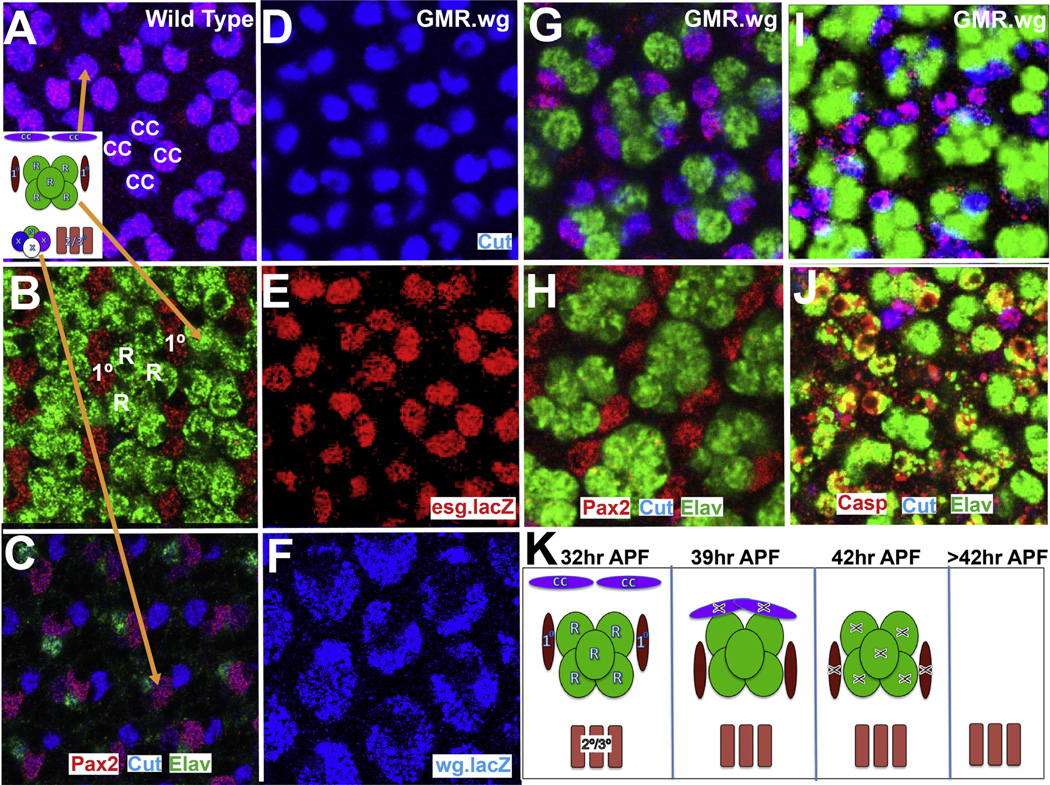
Fig. 2.
Features of wild type and GMR.wg pupal eyes. (A–C) The location of the nuclei in a 32 h APF wild type eye. (A) The cone cells are apical and express Cut (blue) and Pax2 (red). (B) Below them lie the photoreceptors labeled with Elav (green) flanked by the 1° PCs expressing Pax2 (red). (C) Basally lie the 2°/3° PCs (unlabeled) and the bristle group cells. (D–F) Cone cells in 32 h APF GMR.wg eye. (D) The cone cells express Cut (blue), (E) esg.lacZ (red), (F) and wg.lacZ (blue). (G, H) 36 h APF GMR.wg eye. (G) The cone cells fall to the photoreceptor layer. (H) The 1° PCs move more basally. (I, J) 42 h APF GMR.wg eye. (I) Death (Caspase) begins in the cone cells, followed by (J) the photoreceptors and 1° PCS. (K) Summary diagram.
In GMR.wg eyes, Wg is expressed at high chronic levels, and small bald eyes containing only pigment rim-like tissue results, suggesting that blanket Wg expression elicits peripheral patterning throughout the eye (Tomlinson, 2003). To investigate this further, we examined GMR.wg pupal eye development and observed the sequence of events that characterize the formation of the pigment rim. At ~32 h APF all cone cells expressed Wg and Snail family transcription factors (Fig. 2D, E and F). At ~36 h APF the cone cell nuclei fell to the apical level of the photoreceptor nuclei (Fig. 2G), and interestingly this resulted in the 1° PC nuclei shunting more basally (Fig. 2H). Two strata were now evident in the photoreceptor layer, with the cone cell nuclei overlying those of 1° PCs. At ~39 h APF the cone cell apoptosis began (Caspase expression Fig. 2I), shortly followed by the apoptosis of the 1 °PCs and photoreceptors, and by 42 h APF dying cells pervaded the retina (Fig. 2J). Since Wg inhibits the formation of the bristle precursors, there are no bristle cells in GMR.wg retinas, and the 2°/3° PCs are the only surviving cells (Fig. 2K). Thus in GMR.wg eyes all ommatidia execute the developmental program characteristic of the outermost ommatidial row, and hence peripheral patterning can be induced and examined in the main body of the eye.
2.2. The effects of Wg activation in cone cells throughout the retina
As Wg diffuses into the retina from the head capsule, it elicits a number of cellular/molecular responses, salient among which is the behavior of the cone cells: they express Wg and members of the Snail family transcription factors, and subsequently collapse and die. In this section we ask which aspects of these cone cell behaviors directly result from their activation of the Wg transduction pathway.
2.3. pros.Gal4 as a cone cell driver
To activate gene transcription in the developing cone cells, we needed a Gal4 driver expressed selectively in the cone cells at sufficient levels and at the correct times. Of the many potential driver lines only prospero.Gal4 (pros.Gal4) fulfilled these requirements; it is expressed robustly in cone cells from the third instar throughout pupal life (Fig. 3A), but it is also expressed in developing R7 photoreceptors and is therefore not strictly cone cell specific (Hayashi et al., 2008; Xu et al., 2000). However, since the sevenless (sev) mutation removes R7, this genetic background can be used when necessary to remove any confounding influences from R7 expression.
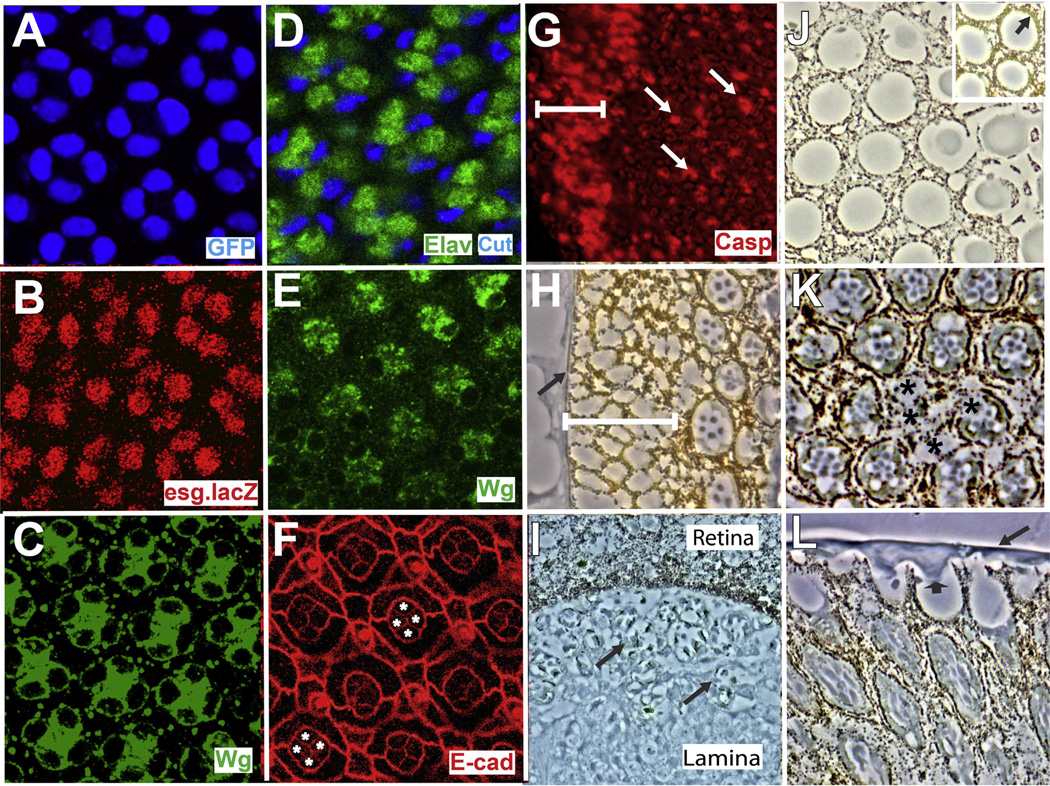
Fig. 3.
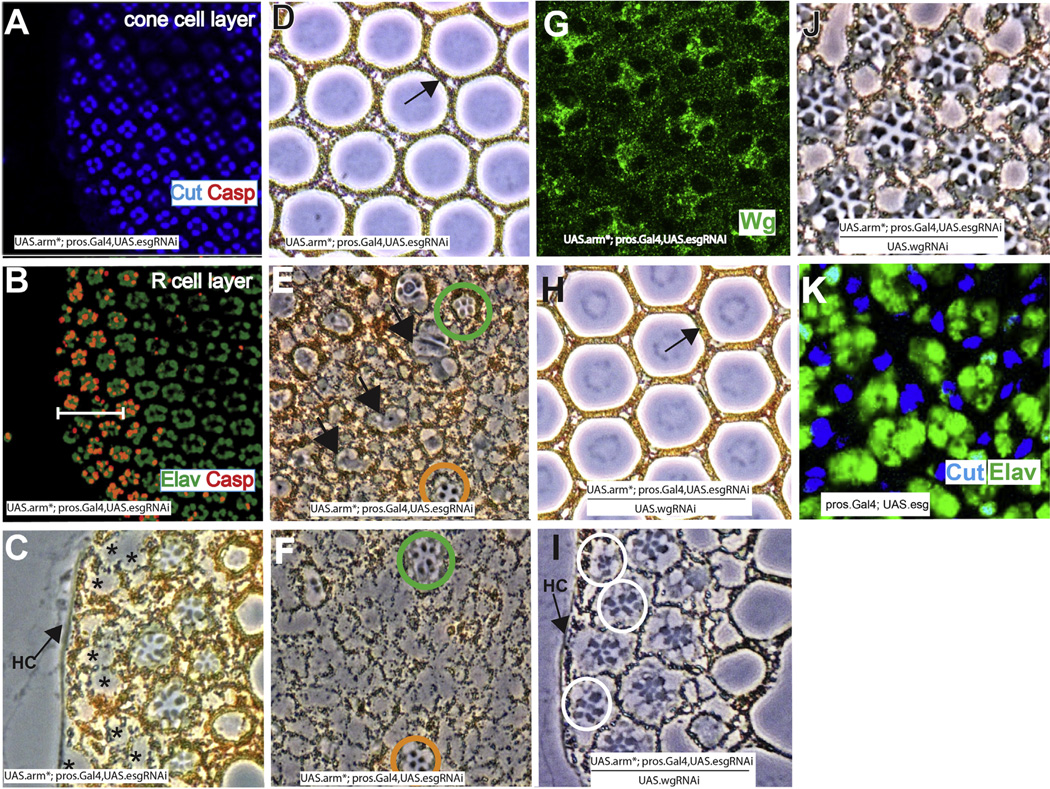
Features of pros-arm* eyes. (A) pros.Gal4 is expressed in the cone cells (GFP-blue). (B–L) Features of pros-arm* eyes. (B) All cone cells express esg.lacZ (red) and (C) Wg (green) at 32 h APF. (D) At 36 h APF the cone cell nuclei fall to the photoreceptor layer. (E–G) Images of 42 h APF retinas. (E) Wg expression (green) persists in the cone cells. (F) D/E-Cadherin staining (red) highlights the apical junctions and the profiles of the four cone cells (asterisks) are stained indicating intact adherens junctions. (G) Caspase staining (red) indicates a broad swath of apoptosis at the periphery (white bar). (H–L) Phase contrast images through pros-arm* eyes. (H) An expanded pigment rim (white bar) is evident. (I) A longitudinal section shows many photoreceptors (arrows) below the basal lamina of the retina. (J) Lens units are disrupted and lack 1° PCs. Inset shows a similar image from the posterior region of the same eye where 1° PCs are present (arrow). (K) The photoreceptor array is disrupted (asterisks indicate ommatidia completely or partially lacking photoreceptors). (L) A longitudinal section showing the continuous sheet of lens-like material overlying the lens units (arrow), and arrowhead indicates the protrusion into a pseudocone.
2.4. The effects of activating Wg transduction in the cone cells
N-terminal truncated Armadillo (Arm*) is a constitutive, cell autonomous activator of the Wg transduction pathway (Zecca et al., 1996). To evaluate the effects of Wg pathway activation in the cone cells we drove UAS.arm* with pros.Gal4 (hereafter pros-arm*). Cone cells in the periphery are exposed to Wg secreted from the head capsule from the third instar onward, and pros-arm* correspondingly provides Wg activation in the cone cells of all ommatidia from the third instar onward.
2.4.1. Analysis of pupal pros-arm* retinas
Cone cells of pros-arm* eyes behaved largely as in GMR.wg eyes. At ~32 h APF they expressed Wg and Snail family transcription factors (Fig. 3B and C), and at 36 h APF their nuclei dropped to the upper aspects of the photoreceptor layer (Fig. 3D). These cone cells persisted in their Wg expression albeit somewhat reduced (Fig. 3E) suggesting that the cone cells continue to release Wg after their nuclei have fallen to the photoreceptor layer.
Unlike GMR.wg ommatidia however, only limited apoptosis occurred in the main body of the retina at 42 h APF (evidenced by anti-Caspase expression-Fig. 3G white arrows). The identity of these dying cells is unclear, but we suspect that they may be 1° PCs (see Discussion). Most strikingly, in pros-arm* eyes there was a large swath of cell death in the peripheral regions (Fig. 3G white bar) extending 3 or more ommatidial rows. Sections through the peripheral regions of pros-arm* adult eyes showed a corresponding expanded pigment rim (Fig. 3H-white bar) lying between the head capsule and the array of ommatidia. Thus, in the main body of pros-arm* eyes, although the cone cell bodies fall to the photoreceptor layer and persist in Wg expression (Fig. 3E) only limited apoptosis occurs throughout the ommatidial array. But at the periphery, an extended region of fully-fledged ommatidial apoptosis occurs. This suggests that there is an influence present in the outer retina that can synergize with the effects of Wg pathway activation in the cone cells. Elsewhere, Snail family transcription factors regulate the stability of adherens junctions (Ohkubo and Ozawa, 2004), and we wondered whether the fall of the cone cell nuclei resulted from degradation of the cone cell adherens junctions. We stained 42 h APF pros-arm* pupal retinas with a D/E Cadherin antibody to highlight the adherens junctions and observed a relatively normal apical pattern, including those of the cone cells (Fig. 3F). Thus, a breakdown of the cone cell adherens junctions does not precede the fall of the cone cell nuclei, and we infer that the cone cell nuclei migrate to the photoreceptor layer in structurally stable cells.
2.4.2. Analysis of adult pros-arm* retinas
We next examined the ommatidia in the main body of pros-arm* adult eyes to understand the structural consequences resulting from Wg activation in pupal cone cells. In these eyes, the posterior most (up to five) columns of ommatidia are largely wild type, suggesting that pros.Gal4 does not drive strongly in this region, and in this, and all other descriptions of pros-arm* eyes, we ignore the posterior extreme and focus our attention to the more anterior tissue.
The lens units: Comparatively normal pseudocone cylinders are present in pros-arm* eyes (circular in cross section in Fig. 3J), notable by their absence of 1° PCs. Fig. 3J inset shows lens units from the posterior extreme where the ommatidia are largely wild type, and 1° PCs are evident (by their expression of the gold colored ommochrome pigment). In the more anterior lens units there are no 1° PCs, and we infer that here the sides of the pseudocones are supported by the 2°/3° PC lattice. Although the pseudocone structures are not severely disrupted, the overlying corneal lenses are. These are variable in structure, often uniting with neighboring lenses to form a continuous corneal sheet over the eye, and sometimes projecting rudely into the pseudocone space (Fig. 3L). Above the lenses a relatively normal bristle array was evident.
The photoreceptors: pros-arm* photoreceptors are variable in structure; sometimes individual photoreceptors or whole ommatidia can be missing (asterisks Fig. 3K). When present, they are frequently stunted and disfigured and largely confined to the more apical regions of the eye. Below the basal lamina of the eye, many photoreceptors are evident above the cell body layer of the lamina, having delaminated from the retina (Fig. 3I).
2.4.3. Removal of R7 from pros-arm* eyes
Since pros.gal4 also drives expression in R7s, we wished to check that the pros-arm* phenotypes did not result from influences mediated through R7s. We examined pros-arm* eyes in a sev mutant background and observed features closely similar to those described for pros-arm*. The cone cells expressed Wg and Snail family transcription factors and collapsed on schedule. The extensive peripheral death occurred, and the adult structure was essentially similar. Although we cannot rule out subtle effects, we infer that the major features of pros-arm* result from the effects of Wg pathway activation in the cone cells.
2.5. Abrogation of Wg release from pros-arm* cone cells
Activation of the Wg pathway in the cone cells results in their expression of Wg and Snail family transcription factors. In this section, we investigate the role played by Wg secretion from the cone cells by activating their Wg transduction (pros-arm*) and concomitantly abrogating their ability to release Wg by co-expressing a wgRNAi transgene.
2.5.1. Assessment of RNAi knockdown of Wg
To assess the efficacy of wg transcript knockdown we expressed Wg throughout the eye (GMR.Gal4; UAS.wg) and co-expressed UAS. wgRNAi. GMR-wg adult eyes are small and made almost entirely from pigment cells (Fig. 4A), but when wgRNAi was concomitantly supplied the eyes were normal except for being bald (indicating residual Wg activity). Thus we infer a significant but incomplete knockdown of wg gene function using the RNAi transgene.
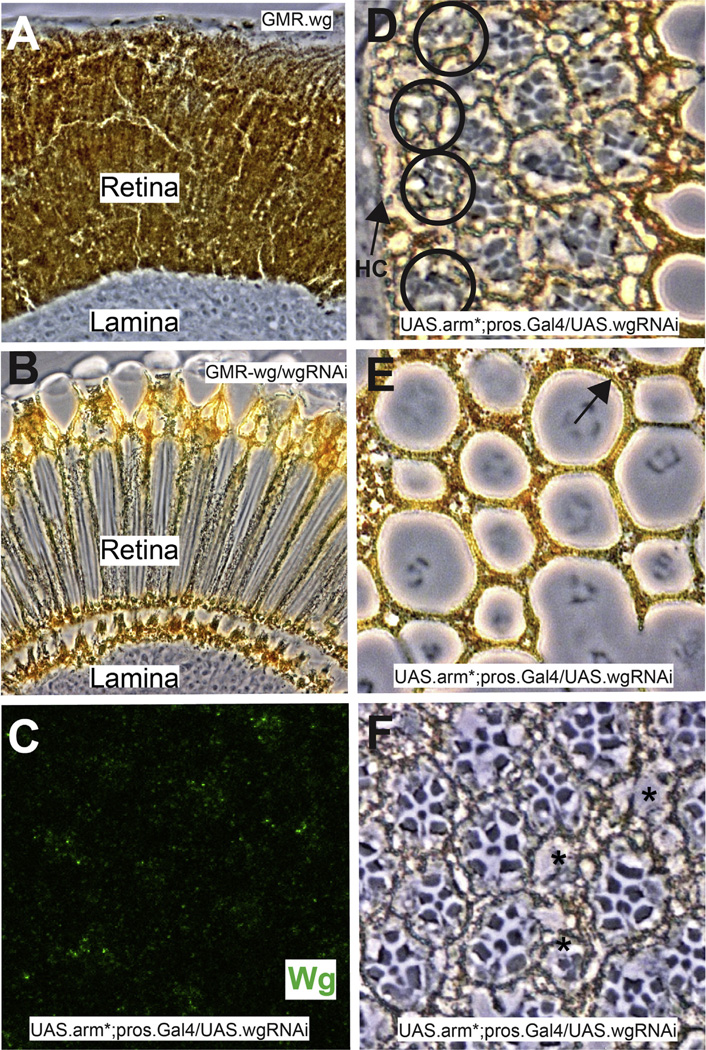
Fig. 4.
The effects of wgRNAi. (A–C) Demonstrations of the efficacy of wg RNAi. (A) Shows a longitudinal section through a GMR.wg eye in which the entire retina appears as pigment rim. (B) When wg RNAi is simultaneously expressed (GMR.Gal4; UAS.wg/UAS.wgRNAi) there is a rescue of the eye back to wild type structure. (C) Image of a Wg-stained (green) 32 h APF pros.Gal4 UAS.arm* UAS.wg-RNAi, showing dramatically reduced Wg expression (compare with Fig. 3C). (D–F) Images of adult pros.Gal4 UAS.arm* UAS.wg-RNAi eyes. (D) The most peripheral ommatidia survive (black circles) bearing disrupted photoreceptor arrays. (E) The lenses are disrupted but 1 °PCs are present (arrow). (F) The photoreceptors array is disrupted, but individual cells appear healthy.
2.5.2. Evaluation of pros-arm*/wgRNAi eyes
In pros.Gal4; UAS.arm*/UAS.wgRNAi eyes Wg levels were dramatically reduced in the cone cells (Fig. 4C), which fell to the photoreceptor layer on schedule. Thus, cone cells do not appear to require the expression of Wg for the fall of their nuclei. Sections through adult eyes showed three major differences from pros-arm*. First, the expanded pigment rim was lost. Indeed, frequently the ommatidial array extended up to the head capsule including the outermost ommatidia destined to die in wild type (Fig. 4D black circles). This result suggests that the expanded pigment rim seen in pros-arm* eyes critically depends upon the expression of Wg by the cone cells. Second, although the lens units were still disrupted, 1° PCs were rescued (Fig. 4E arrow) suggesting that 1° PC loss in pros-arm* is triggered by cone cell Wg expression. Third, the photoreceptor array was significantly rescued. Photoreceptors appeared healthier (Fig. 4C) and most extended the depth of retina. As in pros-arm*, many delaminated photoreceptors were evident beneath the basal lamina that correlate with “holes” in the photoreceptor array (Fig. 4F asterisks). Thus, reduction of Wg expression rescues the extended zone of peripheral cell death, restores 1° PCs, and removes the degenerate phenotype of the photoreceptors. Furthermore, we infer that the persistent disruption of the lens units and the delamination of photoreceptors results from the fall of the cone cell nuclei independent of Wg secretion.
2.6. Abrogating snail family gene expression in pros-arm* cone cells
In this section, we investigate the role played by Snail family transcription factors in the cone cells by activating Wg transduction in all cone cells and concomitantly knocking down snail family gene function. Although all three genes of the snail family complex are expressed in the cone cells, we discovered that expression of a single esgRNAi transgene dramatically rescued (detailed below) the structure of pros-arm* eyes (pros.Gal4; UAS.arm*/UAS.esgRNAi – hereafter pros-arm*/esgRNAi), and we infer that this RNAi transgene potently reduces snail family gene function.
2.6.1. Analysis of pupal pros-arm*/esg RNAi retinas
Examination of 42h APF pros-arm*/esg RNAi eyes showed a normal cone cell array that had neither collapsed, nor showed any evidence of apoptosis, even at the periphery (Fig. 5A). In contrast, the photoreceptor layer at this stage showed an extensive peripheral zone of apoptosis with little death in the main body of the eye (Fig. 5B). Thus, abrogation of snail family gene function in the cone cells of pros-arm* eyes prevents the fall of their nuclei but does not affect the extended band of apoptosis in the photoreceptors. The cone cells persisted in their expression of Wg (Fig. 5G), and we infer that as in pros-arm* eyes, this cone cell derived Wg synergizes with the signal from the periphery to direct the extended band of death in the photoreceptors. Why the cone cells do not die in this situation is addressed in the Discussion.
Fig. 5.
The effects of esgRNAi, wgRNAi and ectopic Esg expression. (A–B) Confocal images of a 42 h APF pros-arm* UAS.esgRNAi eye periphery. (A) The cone cell array (Cut-blue) is normal with no evidence of cell death. (B) Below the cone cell layer, multiple rows (white bar) of dying photoreceptors (Caspase-orange) are evident. (C–F) Phase contrast cross-sectional images through pros.Gal4 UAS.arm* UAS.esg-RNAi eyes. (C) Peripheral ommatidia are present lacking photoreceptors (asterisks) (D) Normal lenses with 1° PCs (arrow) are present. (E) Apically many ommatidia contain rhabdomere-like material (black arrows). (F) Deeper down this is no longer evident. Green and orange circles indicate ommatidia with healthy photoreceptors. (G) Staining of a 42 h APF pros-arm* UAS.esgRNAi eye showing persistent Wg expression (green) in the cone cells. (H–J) Phase contrast images through pros.Gal4 UAS.arm* UAS.esg-RNAi UAS.wg-RNAi eyes. (H) Wild type lens units, with 1° PCs (arrow). (I) Surviving peripheral ommatidia are present (white circles). (J) A normal photoreceptor array is formed. (K) Phase contrast image of a 28 h APF pros.Gal4 UAS.esg eye showing the cone cell nuclei (blue) prematurely fallen to the level of the photoreceptors (green).
2.6.2. Analysis of adult pros-arm*/esg RNAi retinas
Sections through pros-arm*/esg RNAi eyes showed normal lens units (including appropriately positioned 1° PCs-Fig. 5D arrow), suggesting that if the cone cells do not collapse then they cooperate in the organization of normally constructed lens units. Additionally, there was no photoreceptor delamination in these eyes, suggesting that maintenance of the cone cell array supplies a structural integrity that prevents the extrusion of photoreceptors beneath the basal lamina. Sections through the peripheral regions of these eyes showed lens units extending to the head capsule, with the outermost ones devoid of photoreceptors (Fig. 5C-asterisks). Sections through the main body photoreceptor layer showed variable phenotypes with more severe disruptions than those of pros-arm* eyes. The apical regions of the photoreceptor layer frequently showed wispy rhabdomere-like material (Fig. 5E arrows), but at deeper levels most ommatidia were devoid of photoreceptors (Fig. 5F). This rhabdomere-like tissue suggests that the photoreceptors began differentiation but then structurally degenerated, and we suspect that the chronic expression of Wg by the cone cells may account for this (see Discussion).
2.7. Concomitant abrogation of Wg and Snail family transcription factors Wg in pros-arm* cone cells
The previous sections suggested that Wg and Snail family transcription factors mediate separate behaviors of the cone cells. In this section, we abrogate both activities concomitantly in pros-arm* eyes. Cross sections through pros-arm*/wg RNAi/esg RNAi retinas showed almost completely wild type eyes: Bristles were arrayed normally, the lens units were normal with 1° PCs correctly positioned (Fig. 5H), and underlying photoreceptors were normal (Fig. 5J) with no basal delamination. Thus, removal/reduction of wg and snail family function negated almost all the defects of pros-arm* eyes, suggesting that these represent the key Wg target genes in the cone cells. Although these eyes appeared largely wild type, the outermost ommatidia survived adjacent to the head capsule (Fig. 5I white circles). Hence, peripheral ommatidia normally destined to die will not do so if Wg and Esg are removed/reduced from their cone cells, further highlighting the critical role these proteins play in the ommatidial death mechanism.
2.8. Ectopic Esg expression in cone cells
The above results suggest that a key role played by Esg expression in peripheral cone cells is to trigger the fall of the cone cell nuclei, and accordingly ectopic expression of Esg in cone cells throughout the main retina should induce the fall of all cone cell nuclei. To test this, we expressed UAS.esg using pros.Gal4 and observed an extensive but premature fall of the cone cell nuclei (Fig. 5K). This is consistent with Esg expression driving the collapse of the cone cells, but since it occurred prematurely and hence temporally de-coupled from the normal patterning mechanism, we did not examine the subsequent development of these ommatidia.
2.9. Additive effects of Wg derived from the head capsule and the cone cells
A striking feature of the pros-arm* eye is the expanded zone of peripheral ommatidial death in the ~42 h APF retinas (schematized in Fig. 6F). When Wg activity is abrogated in cone cells of these eyes (pros-arm*; wgRNAi), the extended zone of ommatidial death is lost, arguing that Wg released by the cone cells plays a critical role in promoting that death. Since this swath of death is restricted to the periphery we infer a synergizing influence in that region. We surmised that that influence was Wg secreted from the head capsule, but it was not technically possible to remove it and show the corresponding loss of the extended zone of pigment rim. Rather, we supplied extra Wg throughout pros-arm* eyes to determine whether this would trigger pan-retinal apoptosis.
Fig. 6.
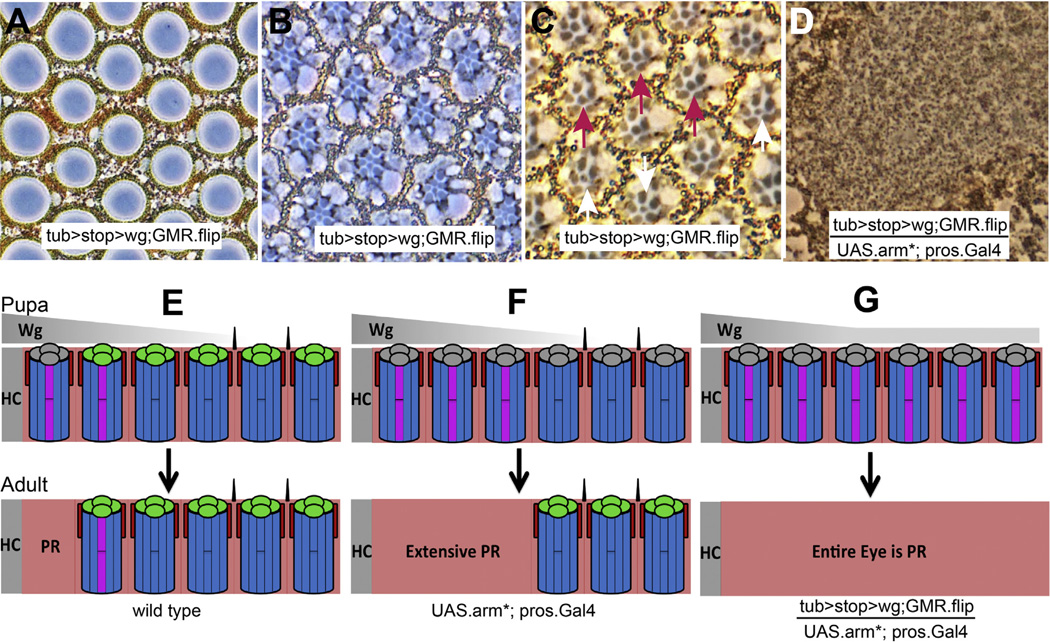
Wg from different sources triggers ommatidial death. (A–C) Phase contrast images through dorsal tub > stop > wg; GMR.flip eyes. (A) Normal lenses are formed. (B) Healthy photoreceptors at the R7 level (the enlargement of DRO R7 rhabdomeres is present but difficult to observe). (C) The R8s show a mixture of DRO (purple arrows pointing to R8s with large rhabdomeres) and wild type ommatidia (white arrows). (D) When pros-arm* is introduced, a pigment rim-like retina results. (E–G) Schematic summary of the effects of Wg secreted from different sources. The image shows the effects on the dorsal eye, and the indication that ommatidia are specified as DRO before they are undergo apoptosis is inferred from previous work (Tomlinson, 2003).
2.9.1. A method for supplying pan-retinal Wg expression
We crossed a tub.wg transgene carrying a flip-out cassette (tub > stop > wg) (Wehrli and Tomlinson, 1998) to GMR.flipase which resulted in selective excision of the cassette in cells behind the morphogenetic furrow and thereby provided eye-specific tub.wg expression. These tub.wg eyes had three informative features. First, they were bald, suggesting that the level of Wg was sufficient to inhibit bristle formation. Second, in the dorsal retina a mixture of DRO and normal ommatidia occurred (Fig. 6C) suggesting that the Wg level was around the threshold needed to specify DRO, but not high enough to achieve this robustly. Third, the lens units and the general structure of the eye (Fig. 6A, B and C) appeared wild type, suggesting that the Wg levels were insufficient to trigger the apoptosis. Using these features, we estimate the level of Wg expressed throughout tub.wg eyes to correspond to that normally found around the third ommatidial row from the head capsule: high enough to prevent the formation of the bristles, and around the levels where DRO are specified.
2.9.2. Synergy between pros-arm* and tub.wg
We then introduced pros-arm* into tub.wg eyes and observed an eye made entirely from pigment-rim like tissue (Fig. 6D). We thus conclude that by providing blanket levels of Wg approximating those corresponding to the outermost third ommatidial row, and by simultaneously activating Wg transduction in the cone cells, the conditions for ommatidial apoptosis are attained throughout the retina, and an eye made entirely from pigment rim tissue results (Fig. 6G). This argues that a summation of Wg derived from both the head capsule and the cone cells achieves the levels needed to trigger the ommatidial death.
2.10. The death of the photoreceptors
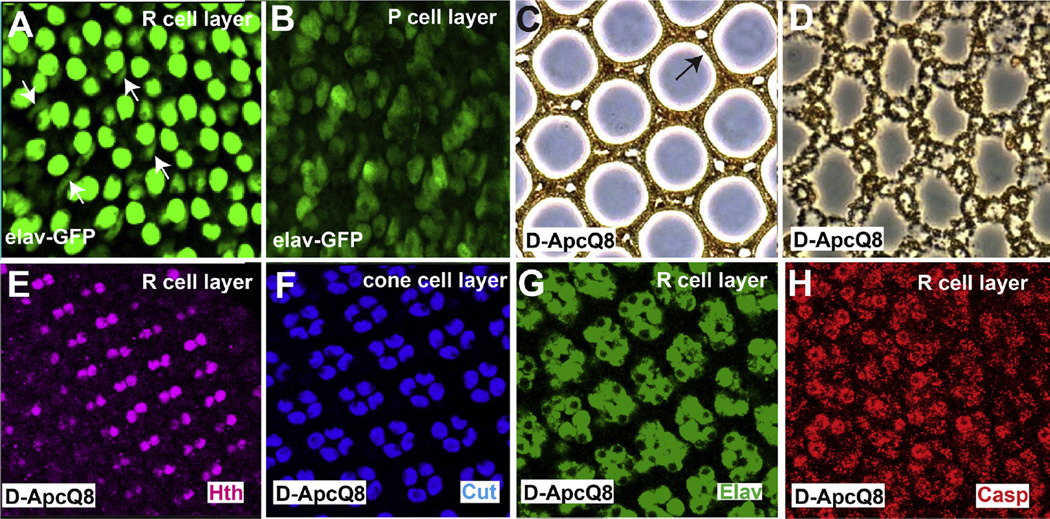
The experiments to date had focused on the roles played by the cone cells in orchestrating the death of the peripheral ommatidia. We next sought to activate the Wg pathway selectively in the photoreceptors to determine whether this would autonomously induce their death and whether it would influence the behavior of other ommatidial cells. We began using two separate Gal4 driver lines that we assumed would be photoreceptor specific: elav.Gal4 (Lin and Goodman, 1994) and long-GMR.Gal4 (Wernet et al., 2003). Both these drivers showed strong expression in the photoreceptors with weaker but evident expression in the 1° and 2°/3° PCs (Fig. 7 A and B), and were not useful for our purposes. We therefore examined eyes homozygous for the Drosophila APC (APCQ8) mutation which induces ectopic Wg pathway activation in the photoreceptors throughout the eye leading to their death (Ahmed et al., 1998). We examined the pupal development of these eyes and observed that ~32 h APF pupal discs showed a normal cone cell array (Fig. 7F) overlying a normal photoreceptor array in which all R7/8s of the dorsal region expressed Hth (Fig. 7E) indicating Wg pathway activation in these cells (Benchabane et al., 2008). At 42hrs APF extensive cell death occurred selectively in the photoreceptors (Fig.7G, H); all other cells types remained unaffected (Fig. 7F). Thus, in D-APCQ8 mutants the Wg pathway appears selectively activated in the photoreceptors, and these cells consequently die without affecting other cells of the ommatidia. Indeed, adult eyes have a normal bristle array, correctly structured lens units with appropriately positioned 1° PCs (Fig. 7C) and a normal 2°/3° PC array lacking photoreceptors (Fig. 7D).
Fig. 7.
Activation of the Wg pathway in the photoreceptors. (A, B) Confocal images through a 32 h APF Elav-Gal4; UAS.GFP retina. (A) Staining is seen in the photoreceptor nuclei, but clear weaker staining is evident in the 1° PCs (white arrows) and (B) 2°/3° PCs. (C, D) Sections though an adult APCQ8 retina. (C) Normal lenses form containing 1° PCs (arrow). (D) In the main retina the 2°/3° PCs lattice lacks photoreceptors. (E) Confocal image through pupal 32 h APF APCQ8 retina shows R7/8s expressing Hth (purple). (F–H) Confocal images of a 42 h APF APCQ8 retina. (F) A normal cone cell array is evident (Cut-blue). (G) The photoreceptors (Elav-green) express (H) high levels of Caspase (red).
3. Discussion
In this paper we use the Drosophila eye as a model system with which to study how morphogen gradients can be converted into sharply constrained tissue patterns. We examine the action of the Wg morphogen gradient and ask how the highest threshold response, the death of the peripheral ommatidia, is orchestrated.
3.1. Synergy between Wg derived from the head capsule and from the cone cells
Three observations argue that the secondary Wg expressed by the cone cells combines with the primary Wg from the head capsule to generate a sufficient concentration to kill the ommatidia. First, when the Wg pathway is activated in all cone cells (pros-arm*) there is an extended zone of apoptosis in the region where the primary Wg source is known to be high. Second, when the secondary Wg (that secreted by the cone cells) is removed the extended band of ommatidial death is lost. Third, when a level of Wg equivalent to that normally found in the peripheral regions is supplied to pros-arm* eyes all ommatidia now die. Thus, this represents a novel gradient read-out mechanism in which the primary morphogen (Wg derived from the head capsule) elicits a secondary morphogen expression (Wg expressed by the cone cells) in the target cells. Thereafter, the two sources unite to generate the high local morphogen concentration needed to direct the appropriate cell behaviors at that position.
3.1.1. Restriction of the cone cell responses to the outermost ommatidia
If there is a permissive zone in the periphery (~3 ommatidial rows) in which the ommatidia will die if cone cell Wg expression occurs, then this raises the question of how the cone cells responses are normally tightly restricted to the peripheral-most row of ommatidia to ensure that only these ommatidia die. Below we discuss the mechanisms likely responsible for this restriction.
The high threshold of the ommatidial response: We surmise that the cone cells have a high threshold response to the morphogen, and the initial responses to the primary Wg source (diffusing from the head capsule) is restricted to the outermost ommatidia. However, it can be envisioned that the secondary Wg secreted by the outer cone cells could diffuse and elicit the same output in the next ommatidial row, and an extreme view could see a relay mechanism in which even more internal rows of ommatidia could express Wg in their cone cells.
The role played by Notum: The expression of Notum is similar to Snail family transcription factors in that it is expressed in the cone cells and 2°/3° PCs of the outermost ommatidia, and since Notum functions to inhibit the free diffusion of Wg, it likely acts to prevent Wg diffusion into more interior ommatidia. Indeed we previously showed that in notum mutant clones the zone of death expanded out into more interior rows (Lim and Tomlinson, 2006). Thus we see Notum (and other mechanisms for preventing Wg diffusion) as playing a critical role in restricting the ommatidial death to the outermost row of ommatidia.
Combining the high threshold response with the restriction of Wg diffusion: Consider the primary Wg diffusing from the head capsule. It enters the outer row of ommatidia and is of sufficient concentration to elicit the appropriate responses (the various expressions in the cone cells and 2°/3° PCs) but not at a level high enough to kill the ommatidia. The cone cells of the outermost row now begin to secrete the secondary Wg, but the concomitant expression of Notum by the cone cells and 2°/3° PCs of these ommatidia provide a barrier to the movement of both the primary and secondary sources of Wg. This restriction of Wg movement not only protects the more internal ommatidia, but ensures that the high levels of morphogen are constrained in the outermost ommatidia to provide the requisite signal for apoptosis.
3.2. Individual cell type behaviors in pigment rim formation
In addition to uncovering the synergy between the Wg derived from the head capsule and the cone cells, we have also detected a number of phenomena relating to the behavior of the various cell types which we discuss below.
3.2.1. Behavior of the cone cells
The early cone cell death: Following the collapse of the cone cells, the ommatidial apoptosis program begins with the death of cone cells themselves, followed~two hours later by the other ommatidial cells. This precocious cone cell death may represent a lower apoptosis threshold for these cells, but we note that they are sources of Wg secretion and likely experience autocrine and paracrine (between cone cells of the same ommatidium) Wg signaling as they collapse, and as such are more likely to reach the critical Wg activation level before the other cells.
The cone cell immunity to death: In pros-arm* eyes, in which all cone cell nuclei fall to the photoreceptor layer and express Wg, there is a wide swath of extended death at the periphery in which all cells of the ommatidia die (including the cone cells). But upon prevention of the cone cell nuclear fall by the expression of esg RNAi, the cone cells survive while the photoreceptors in the extended peripheral zone still die. In these ommatidia, levels of Wg needed to drive apoptosis are achieved, but the cone cells appear invulnerable to it. Whether this invulnerability results from the absence of Snail family transcription factors needed to prime the cone cells for the death signal, or whether by remaining in the apical location they somehow avoid the full level of Wg exposure remains unclear.
The fall of the cone cell nuclei: The maintenance of cone cell cell-bodies in the appropriate apical location is seemingly critical for the ommatidial stability and integrity, as their fall leads to the disruption of corneal lens units and delamination of photoreceptors. This fall appears to be directed by their expression of Snail family transcription factors. In pros-arm* eyes, the expression of esg.RNAi prevents the fall, and correspondingly the ectopic expression of esg in otherwise wild type cone cells engenders their nuclear fall (albeit prematurely). We wondered whether the fall of the cone cell nuclei resulted from a wholesale collapse of the apical junctions of the cone cells, but D/E-cadherin staining showed a normal apical junction pattern many hours after the nuclei had migrated basally. Thus it does not appear that the cone cells nuclei move basally because the cells lose their apical attachments, rather we infer that expression of the Snail family transcription factors reprograms some other behaviors of the cone cells. Such a behavior could be a switch in cell-type affinity. If cone cells normally maintain an apical location by adhesive differences with the photoreceptors, and if these adhesive differences are switched, then cone cell plasma membranes will then preferentially move to the photoreceptor layer. Since the nucleus defines the site of maximum cell body profile with corresponding maximum membrane area, then the fall of the nuclei may simply result from the cone cells acquiring an adhesive affinity with the photoreceptors. Other mechanisms can also be envisaged, in which, for example, motor machinery of the cell is used to reposition the cone cell nuclei in the more basal location.
3.2.2. The 1° PCs
An appropriate Gal4 driver line is not available to activate gene expression selectively in the 1° PCs, and the mechanism of their death remains unresolved. In GMR.wg eyes we observe their death coincident with the photoreceptors (following the apoptosis of the cone cells) and we surmise that it is the high level of Wg derived from head capsule and the cone cells that directs their death. However, there are a number of indications from that offer clues to a more nuanced understanding of their behavior. Initially the nuclei of the 1° PCs flank the clustered photoreceptor nuclei in their more apical region, but when the cone cell nuclei fall, those of the 1° PCs are shunted more basally. This movement deeper into the photoreceptor layer may play a role in their death. A similar argument can be made from the analysis of pros-arm* eyes in which 1° PCs are lost, but when Snail family transcription factors are removed from this background, the cone cell nuclei do not fall, and the 1° PCs do not die. Hence the 1° PCs behave in a similar manner as the cone cells; if their position is maintained they do not die even though ambient Wg concentrations are sufficient for their death. This may indicate a general principle; that cells need to be in the correct topological position to experience the death signal.
Furthermore, in pros-arm* eyes, the cone cell nuclear fall is accompanied by the loss of the 1° PCs even though the cone cells themselves do not die. The removal of Wg expression from the pros-arm* cone cells rescues the 1° PCs indicating that their loss is normally triggered by the cone cell Wg expression, and we suspect that the low-level apoptosis seen in the main body of pros-arm* eyes may represent the death of the 1° PCs. If this is the case, then this suggests that the 1° PCs have a lower threshold Wg response for their apoptosis than the cone cells and photoreceptors.
3.2.3. The photoreceptors
The death of the photoreceptors appears to simply require the additive of effects of the two Wg sources to trigger their death. But another feature has emerged from these studies – the idea that chronic exposure to sub-lethal levels of Wg triggers photoreceptor degeneration. Consider pros-arm*/esgRNAi eyes; here the photoreceptor death occurs only at the widened zone of peripheral apoptosis, but in the main body of the adult eyes ommatidia show degenerate rhabdomere-like tissue in the apical retinas. The presence of rhabdomere-like tissue suggests the differentiation and subsequent degeneration of the photoreceptors leaving them alive but in a runtish condition. Since this phenomenon is Wg dependent (it is absent when wgRNAi is additionally included) we infer that the persistent Wg expression from the cone cells chronically signals to the photoreceptors. Indeed, when we examined GMR.wg/GMR.P35 eyes (in which the apoptosis mechanism is suppressed and the photoreceptors are therefore subject to chronic Wg exposure), a similar degenerate phenotype occurred. This observation suggests another function for the removal of the outermost row of ommatidia: if they were not removed, chronic exposure to high levels of Wg emanating from the head capsule would lead them to deteriorate into a runtish condition.
3.2.4. The timing mechanism
A striking feature of the peripheral patterning mechanism is the timing aspect. The peripheral ommatidia are exposed to head capsule-derived Wg from the time of their birth. And yet they only respond to this Wg signal at defined times. The first occurs shortly after pupation when ac/da transcription is repressed and hth expression is induced. This corresponds with the surge in ecdysone expression that occurs in the animals at this time (Riddiford, 1993). The second response is the death of ommatidia at 42 h APF and this mechanism is closely tied with the large peak of ecdysone expression that occurs in the second day of pupation (Riddiford, 1993). Thus, we speculate that Wg provides the spatial signal for peripheral patterning, but that the hormone system of the fly provides the temporal cue that determines when the spatial information can be utilized.
4. Conclusion
The periphery of the fly eye is an excellent model system with which to study how morphogen gradients are decoded into discrete tissue types, and here we delved into the mechanism that precisely restricts the spatial positioning of one of those tissue types. We have uncovered an intricate mechanism in which initial threshold responses lead to the local boosting of the morphogen signal while at the same time upregulating mechanisms to prevent the spread of the morphogen. We also provide evidence to support the idea that appropriate spatial, temporal and topological context is required for the peripheral ommatidia to undergo developmental apoptosis.
5. Materials and methods
5.1. Drosophila genetics
All crosses and staging were performed at 25 °C. Pupal development was expressed as hours after puparium formation (APF) where white pre-pupae were defined as 0 h APF. Stocks used were: GMR.wg ((Wehrli and Tomlinson, 1998)), Tub-α1 > w + > wg (Wehrli and Tomlinson, 1998) UAS-GFP (Johnston and Sanders, 2003), UAS-arm* (Zecca et al., 1996) esg-LacZ, wg-lacZ, UAS-Esg-RNAi (TRiP stock BL# 28514), pros-Gal4 (gift of Tiffany Cook) LongGMR-Gal4 (Wernet et al., 2003) (All from Bloomington Stock Center). GMR.flip (generated by Y. Mavromatakis), UAS-wg-RNAi (Gift from G. Struhl), d-APCQ8, elav(C155)-Gal4 (Ahmed et al., 1998).
5.2. Immunostaining
Standard immunostaining was performed. Primary antibodies: mouse anti-Cut, mouse anti-Wg, rat anti-Elav, anti-D/E Cadherin (University of Iowa Hybridoma Bank); rabbit anti-cleaved Caspase-3 (Cell Signaling Technologies), rabbit anti-beta-gal (Cappell), rabbit anti-GFP, mouse anti-GFP (Molecular Probes); guinea pig anti-Hth (gift from R. Mann) Secondary antibodies: Alexa Fluor 488, 555, and 647 (Molecular Probes). Image analysis – Immunofluorescence images were taken on a Leica SP5 confocal microscope, and edited using Adobe Photoshop software.
5.3. Adult eye sectioning
Histological preparation of adult eyes was performed as described previously (Tomlinson, 2003). The adult eye sections were imaged using phase contrast microscopy, and edited using Adobe Photoshop software.
Acknowledgments
We thank Yannis Mavromatakis for cloning the GMR.flipase transgene, and Jason Rudas for performing germ line transformation and other critical support to the project. We thank Tiffany Cook for sharing the unpublished pros-Gal4 line, and Yashi Ahmed, Claude Desplan, Richard Mann, Gary Struhl and the Bloomington Stock Center for fly stocks and the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi flies. This work was supported by grants R01EY023635 and R01EY012536 to AT.
References
- Ahmed Y, Hayashi S, Levine A, Wieschaus E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 1998;93:1171–1182. doi: 10.1016/s0092-8674(00)81461-0. [DOI] [PubMed] [Google Scholar]
- Ashraf SI, Hu X, Roote J, Ip YT. The mesoderm determinant snail collaborates with related zinc-finger proteins to control Drosophila neurogenesis. EMBO J. 1999;18:6426–6438. doi: 10.1093/emboj/18.22.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchabane H, Hughes EG, Takacs CM, Baird JR, Ahmed Y. Adenomatous polyposis coli is present near the minimal level required for accurate graded responses to the Wingless morphogen. Development. 2008;135:963–971. doi: 10.1242/dev.013805. [DOI] [PubMed] [Google Scholar]
- Braitenberg V. Patterns of projection in the visual system of the fly. I. Retinalamina projections. Exp. Brain Res. 1967;3:271–298. doi: 10.1007/BF00235589. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Jou AD, Nusse R. Wingless blocks bristle formation and morphogenetic furrow progression in the eye through repression of Daughterless. Development. 2002;129:3393–3402. doi: 10.1242/dev.129.14.3393. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Rubin GM. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev. 1990;4:444–463. doi: 10.1101/gad.4.3.444. [DOI] [PubMed] [Google Scholar]
- Gerlitz O, Basler K. Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev. 2002;16:1055–1059. doi: 10.1101/gad.991802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Copley RR, Cohen SM. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev. Cell. 2002;2:667–676. doi: 10.1016/s1534-5807(02)00180-6. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Xu C, Carthew RW. Cell-type-specific transcription of prospero is controlled by combinatorial signaling in the Drosophila eye. Development. 2008;135:2787–2796. doi: 10.1242/dev.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Schulz S, Bentrop J, Groell C, Wolfrum U, Paulsen R. Molecular cloning of Drosophila Rh6 rhodopsin: the visual pigment of a subset of R8 photoreceptor cells 1. FEBS Lett. 1997;406:6–10. doi: 10.1016/s0014-5793(97)00210-x. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Sanders AL. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat. Cell Biol. 2003;5:827–833. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- Kakugawa S, Langton PF, Zebisch M, Howell SA, Chang TH, Liu Y, Feizi T, Bineva G, O’Reilly N, Snijders AP, Jones EY, Vincent JP. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519:187–192. doi: 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Tomlinson A. Organization of the peripheral fly eye: the roles of Snail family transcription factors in peripheral retinal apoptosis. Development. 2006;133:3529–3537. doi: 10.1242/dev.02524. [DOI] [PubMed] [Google Scholar]
- Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Hanson TE. The development of the optic lobe. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1363–1491. [Google Scholar]
- Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J. Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- Papatsenko D, Sheng G, Desplan C. A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development. 1997;124:1665–1673. doi: 10.1242/dev.124.9.1665. [DOI] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Horm. Drosoph. Dev. 1993:899–939. [Google Scholar]
- Tomlinson A. Patterning the peripheral retina of the fly: decoding a gradient. Dev. Cell. 2003;5:799–809. doi: 10.1016/s1534-5807(03)00326-5. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Tomlinson A. Independent regulation of anterior/posterior and equatorial/polar polarity in the Drosophila eye; evidence for the involvement of Wnt signaling in the equatorial/polar axis. Development. 1998;125:1421–1432. doi: 10.1242/dev.125.8.1421. [DOI] [PubMed] [Google Scholar]
- Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267–279. doi: 10.1016/s0092-8674(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Xu C, Kauffmann RC, Zhang J, Kladny S, Carthew RW. Overlapping activators and repressors delimit transcriptional response to receptor tyrosine kinase signals in the Drosophila eye. Cell. 2000;103:87–97. doi: 10.1016/s0092-8674(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cheong SM, Amado NG, Reis AH, MacDonald BT, Zebisch M, Jones EY, Abreu JG, He X. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev. Cell. 2015;32:719–730. doi: 10.1016/j.devcel.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]