Abstract
Inositol triphosphate (IP3) is an important second messenger that participates in signal transduction pathways in diverse cell types including hippocampal neurons. Stimulation of phospholipase C in response to various stimuli (hormones, growth factors, neurotransmitters, neurotrophins, neuromodulators, odorants, light, etc) results in hydrolysis of phosphatidylinositol 4, 5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, and leads to the production of IP3 and diacylglycerol. Binding of IP3 to the IP3 receptor (IP3R) induces Ca2+ release from intracellular stores and enables the initiation of intracellular Ca2+-dependent signaling. Here we describe a procedure for the measurement of cellular IP3 levels in tissue homogenates prepared from rat hippocampal slices.
Materials and Reagents
Syringes (1 ml and 60 ml) and needles (24 and 27 gauge) (Becton Dickinson, catalog numbers: 309659 and 309653)
Single edge razor blade (IDL TechniEdge, catalog number: 05-091C)
Double edge razor blade (Electron Microscopy Sciences, catalog number: 72002-10)
Costar Netwell Insert with 440 μm polyester mesh (Corning, catalog number: 3478)
Corning assay plate, black 384 well with non-binding surface (Corning, catalog number: 3575)
-
Conical tube (15 ml) (BD Biosciences, catalog number: REF352196)
Note: Currently, it is “Thermo Fisher Scientific, Falcon™, catalog number: REF352196”.
Micro-centrifuge tubes (1.5 ml) (VWR International, catalog number: 20170-038)
Aerosol barrier pipette tips (30 μl and 200 μl) (Thermo Fisher Scientific, catalog numbers: 02-707-33 and 02-707-42)
Disposable transfer pipette (Thermo Fisher Scientific, catalog number: 13-711-9AM)
-
Integrid Petri dishes (Becton Dickinson, Falcon, catalog number: 351112)
Note: Currently, it is “Thermo Fisher Scientific, Falcon™, catalog number: 351112”.
Adult (47–57 days old) male and female Sprague Dawley rats (Harlan Laboratories)
Hit-Hunter IP3 fluorescence polarization assay kit (DiscoveRx Corporation, catalog number: 90-0037)
70% Perchloric acid (HClO4) (Sigma-Aldrich, catalog number: 244252)
Complete protease inhibitor cocktail tablets (Roche Diagnostics, catalog number: 11697498001)
Quick Start Bovine Serum Albumin (BSA) standard (Bio-Rad Laboratories, catalog number: 500-0206)
Protein assay reagent (Bio-Rad Laboratories, catalog number: 500-0006)
Sodium pentobarbital solution (Virbac, catalog number: 710101)
Calcium chloride solution (CaCl2) (Sigma-Aldrich, catalog number: 21115)
Magnesium chloride solution (MgCl2) (Sigma-Aldrich, catalog number: M1028)
Super glue (Super Glue Corporation, catalog number: SGP3)
70% ethanol (Thermo Fisher Scientific, Decon labs™, catalog number: 04-355-305)
95% O2/5% CO2 gas mixture (Airgas, catalog number: X02OX95C2003102)
NaCl
NaHCO3
Dextrose
KCl
NaH2PO4
HEPES (pH 7.4)
EGTA
Sodium orthovanadate
NaF
Sodium pyrophosphate
PMSF
Artificial cerebrospinal fluid (aCSF) (see Recipes)
Sucrose aCSF (s-aCSF) (see Recipes)
HEPES homogenization buffer (see Recipes)
Equipment
Scissors (Fine science tools, catalog numbers: 14000-12 and 14060-10)
Forceps (Stoelting, catalog numbers: 52104-37 and 52104-35)
Hemostat (Fine science tools, catalog number: 13008-12)
Spatula (Thermo Fisher Scientific, catalog number: S50822)
Accuspin micro 17R microcentrifuge (Thermo Fisher Scientific, catalog number: 13-100-676)
Biophotometer (Eppendorf AG, catalog number: 6133000010)
Leica VT1200S vibrating blade microtome (Leica Biosystems Nussloch GmbH, catalog number: 1491200S001)
Isotemp 105 water bath (Thermo Fisher Scientific, catalog number: S63077Q)
Qsonica Q500 sonicator (Thermo Fisher Scientific, catalog number: 15-338-282)
Synergy 4 multimode microplate reader (BioTek Instruments)
Vapor pressure osmometer 5520 (Wescor)
Accumet AB15 plus pH meter (Thermo Fisher Scientific, catalog number: 13-636-AB15PC)
Rotator (Labline Instruments)
Micropipettes (2–20 μl, 20–200 μl and 100–1,000 μl) (Capitol Scientific, Eppendorf, catalog numbers: 3120000038, 3120000054 and 3120000062)
Glass plate (Electron Microscopy Sciences, catalog number: 70312-22)
Glass staining dish (Newcomer supply, catalog number: 6816A)
Coarse fritted glass bubbler (Corning, catalog number: 39533-12EC)
Procedure
A. Preparation and treatment of transverse hippocampal slices
-
Before starting the procedure
Place ~ 200 ml s-aCSF per rat at −80 °C for approximately 40 min to form a slush (if s-aCSF is pre-chilled at 4 °C slush will be formed more quickly, in 15–20 min).
Place Netwell insert into a glass staining dish with 350 ml regular aCSF (constantly oxygenating with 95% O2/5% CO2 mixture using a coarse glass bubbler) and put the dish into a water bath at 34–35 °C 20 min prior to slice preparation.
Pour 20–30 ml s-aCSF slush in a small glass beaker, place the beaker on ice and oxygenate with 95% O2/5% CO2 mixture using a coarse glass bubbler for 10–15 min.
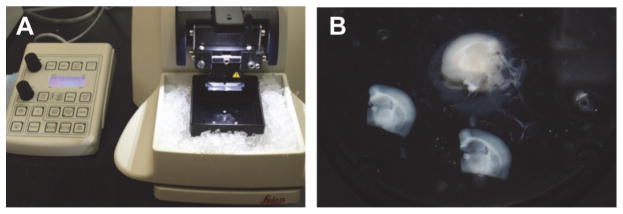
Pre-chill the microtome stage by placing ice in the ice chamber and attach the double edge razor blade to the blade holder (Figure 2A).
Bend the tip of a 27 gauge needle (~45 degrees) using forceps and place it on a 1 ml syringe.
Deeply anesthetize rats with sodium pentobarbital (125 mg/kg, i.p.) and perform laparotomy using surgical scissors and forceps until the heart is completely exposed. Clamp the tip of the sternum with a hemostat and place the hemostat over the head.
-
Perfuse transcardially with ice-cold oxygenated (95% O2/5% CO2) s-aCSF (~50 ml/rat).
Note: Estimated time to carry out step A3 is approximately 25 sec.
-
Remove the head immediately, quickly make an incision in the middle of the scalp, pull aside the skin and cut through the skull along the midline suture of the parietal plates. Remove the skull plates, carefully extract the brain using a spatula and transfer it into oxygenated and pre-chilled s-aCSF prepared in the step A1c for about one minute.
Note: Estimated time to carry out step A4 is less than a minute.
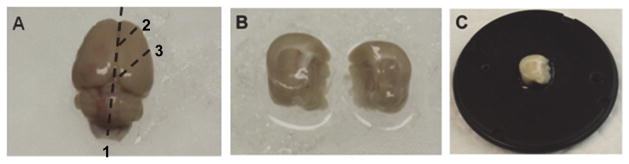
Place the brain on a glass plate on ice (Figure 1A). Hemisect the brain along the longitudinal fissure (Figure 1A, dashed line 1) and make two 45 degree angle cuts relative to the coronal plane (Figure 1A, dashed lines 2 and 3) for each hemisphere, producing tissue blocks containing the hippocampus (Figure 1B).
Place a small drop of super glue in the center of the mounting disk, carefully place one hemisphere on the drop of glue with rostral side up (Figure 1C) and transfer to the sectioning stage of the vibrating microtome (Figure 2A).
Immediately cover the tissue with s-aCSF slush and cut 300 μm sections (Figure 2B).
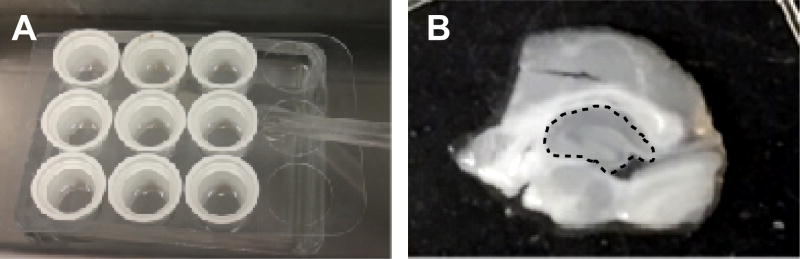
Transfer the sections into separate wells of the Netwell insert (Figure 3A) using a disposable transfer pipette and let the sections recover at 34–35 °C in oxygenated regular aCSF for 30–60 min. Make sure the sections are resting flat on the mesh of each insert and are not floating or moving.
After recovery, the slices are ready for experimental treatment. Treat slices in oxygenated regular aCSF either with vehicle or a pharmacological agent of interest. Treatment times should be determined empirically (seconds to minutes). Slices from each animal should be distributed evenly between vehicle and treatment groups. We pool four slices for each sample, which typically yields 300–400 μg total protein per sample.
After treatment, using a disposable transfer pipette, immediately transfer slices into the Integrid Petri dish with ~20 ml of 0.2 N perchloric acid solution made from 70% perchloric acid in regular aCSF to stabilize IP3 and incubate at room temp for 2 min. Quickly replace perchloric acid-aCSF with regular aCSF and place the Integrid Petri dish on ice.
Quickly dissect hippocampi from each slice in regular aCSF on ice (Figure 3B) using a 1 ml syringe with a bent needle and, using the needle tip, quickly transfer each hippocampus into a 1.5 ml microcentrifuge tube with 300 μl ice cold HEPES homogenization buffer containing protease inhibitors (1 tablet per 25 ml buffer).
Figure 2.
A. Leica VT1200S vibrating blade microtome with stage pre-chilled with ice. B. Two transverse slices (300 μm) that were cut using the microtome, and the remaining tissue block.
Figure 1.
A. Perfused rat brain on a glass plate on ice. The dashed lines indicate where cuts are made and numbers indicate the order of the cuts. B. Blocked brain with the two hemispheres separated. C. One hemisphere of the brain mounted on the mounting disk of a Leica VT1200S vibrating microtome.
Figure 3.
A. Slice recovery and treatment set-up. B. A single slice is shown on the Integrid Petri dish before dissection of the hippocampus. Hippocampus is outlined by a dashed line in the middle of the slice.
B. Tissue homogenization and protein quantification
Homogenize the tissue on ice using a sonicator (2 times, 3 sec pulses, using the 40% amplitude setting), keeping the microcentrifuge tube steady and the sonicator tip submerged half way into the liquid in order to avoid bubbling.
Centrifuge the tissue homogenates [3,000 rpm (1,000 x g) at 4 °C for 10 min] to remove unbroken cells and nuclei. Discard the pellet.
Measure protein concentration in each sample using a Bradford assay. Briefly, prepare standards with known BSA (1 mg/ml stock) concentrations and unknown samples in double distilled water. Add Bradford protein assay reagent and incubate at room temp for 5 min. Measure absorbance at 595 nm using a spectrophotometer. Build a standard curve using absorbance values from the standards and determine protein concentration for each sample based on the standard curve.
Adjust protein concentration in each sample to the same value (e.g. 2 μg/μl) using ice cold HEPES homogenization buffer with protease inhibitors.
C. Measuring IP3 levels
Spray down the bench surface with 70% ethanol and clean it thoroughly. Make sure to carry out the IP3 assay in a dust free environment.
Thaw the IP3 assay kit reagents completely and equilibrate to room temp before use.
Dilute the IP3 standard (20 μM stock) to prepare ten 3-fold serial dilutions using the IP3 Standard Dilution Buffer. For the blank, use the IP3 Standard Dilution Buffer.
Pipette 10 μl of the blank, standards, and each sample (20 μg protein per sample) in triplicate into wells of the 384-well assay plate with non-binding surface using barrier tips.
Add 10 μl IP3 tracer to each well, followed by 20 μl IP3 binding protein and mix well by pipetting up and down several times.
Carefully examine the plate for bubbles. If bubbles form, pop them with barrier pipette tips. Avoid cross-contamination by using new tip for each well. Incubate the plate for 30 min at room temp in the dark on a rotator with gentle mixing (~60 rpm).
Read the fluorescence polarization signal of the IP3 tracer with a multimode microplate reader and a fluorescence polarization filter, using 485 nm excitation and 530 nm emission wavelengths.
Plot a standard curve and calculate the IP3 concentration in each sample (nM range in the hippocampus). In addition to absolute concentration values, IP3 levels for each vehicle and experimental group can also be reported relative to baseline (no treatment control).
Recipes
-
Artificial cerebrospinal fluid (aCSF, should be made fresh)
126 mM NaCl
26 mM NaHCO3
10 mM dextrose
3 mM KCl
1.25 mM NaH2PO4
2 mM CaCl2
1 mM MgCl2
Adjust pH to 7.4 using either 2 N HCl or 5 N NaOH
Measure osmolarity (300–310 mOsm)
-
Sucrose aCSF (s-aCSF, should be made fresh)
75 mM sucrose
75 mM NaCl
25 mM NaHCO3
15 mM dextrose
2.4 mM sodium pyruvate
2 mM KCl
1.3 mM ascorbic acid
1.25 mM NaH2PO4
3 mM MgCl2
0.5 mM CaCl2
Adjust pH to 7.4 using either 2 N HCl or 5 N NaOH
-
HEPES homogenization buffer (can be stored at 4 °C up to three months)
4 mM HEPES (pH 7.4)
320 mM sucrose
2 mM EGTA
1 mM sodium orthovanadate
50 mM NaF
10 mM sodium pyrophosphate
20 mM glycerophosphate
0.1 mM PMSF
Add protease inhibitor cocktail just before use in 15 ml conical tube
Acknowledgments
This research was supported by National Institutes of Health Grant R01 NS037324, the Office for Research on Women’s Health, and the Northwestern University High Throughput Analysis Facility.
References
- 1.Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J Neurosci. 2015;35(32):11252–11265. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]