ABSTRACT
In Bacillus subtilis, the dl-endopeptidase LytE is responsible for lateral peptidoglycan hydrolysis during cell elongation. We found that σI-dependent transcription of lytE is considerably enhanced in a strain with a mutation in ltaS, which encodes a major lipoteichoic acid (LTA) synthase. Similar enhancements were observed in mutants that affect the glycolipid anchor and wall teichoic acid (WTA) synthetic pathways. Immunofluorescence microscopy revealed that the LytE foci were considerably increased in these mutants. The localization patterns of LytE on the sidewalls appeared to be helix-like in LTA-defective or WTA-reduced cells and evenly distributed on WTA-depleted or -defective cell surfaces. These results strongly suggested that LTA and WTA affect both σI-dependent expression and localization of LytE. Interestingly, increased LytE localization along the sidewall in the ltaS mutant largely occurred in an MreBH-independent manner. Moreover, we found that cell surface decorations with LTA and WTA are gradually reduced at increased culture temperatures and that LTA rather than WTA on the cell surface is reduced at high temperatures. In contrast, the amount of LytE on the cell surface gradually increased under heat stress conditions. Taken together, these results indicated that reductions in these anionic polymers at high temperatures might give rise to increases in SigI-dependent expression and cell surface localization of LytE at high temperatures.
IMPORTANCE The bacterial cell wall is required for maintaining cell shape and bearing environmental stresses. The Gram-positive cell wall consists of mesh-like peptidoglycan and covalently linked wall teichoic acid and lipoteichoic acid polymers. It is important to determine if these anionic polymers are required for proliferation and environmental adaptation. Here, we demonstrated that these polymers affect the expression and localization of a peptidoglycan hydrolase LytE required for lateral cell wall elongation. Moreover, we found that cell surface decorations with teichoic acid polymers are substantially decreased at high temperatures and that the peptidoglycan hydrolase is consequently increased. These findings suggest that teichoic acid polymers control lateral peptidoglycan hydrolysis by LytE, and bacteria drastically change their cell wall content to adapt to their environment.
INTRODUCTION
The cell wall (CW) of Gram-positive bacteria is responsible for maintaining cell shape and bearing environmental stress. The CW consists of mesh-like peptidoglycan (PG) and covalently linked anionic polymers such as teichoic acids (TAs) (1). PG has a basic mesh-like structure composed of long glycan strands cross-linked by peptide side chains. Anionic polymers are either covalently attached to the PG (wall teichoic acid [WTA]) or anchored to the membrane lipids (lipoteichoic acid [LTA]) (1, 2). In Bacillus subtilis 168, the WTAs are composed of major and minor forms, and both are tethered to peptidoglycan via a linkage unit (3). The main chains of the major and minor WTAs consist of glycerol phosphate (GroP) and glucosyl-N-acetylgalactosamine 1-phosphate (GlcGalNAcP) repeats, respectively.
LTA in B. subtilis consists of poly(GroP) polymers linked on the cytoplasmic membrane via a glycolipid anchor, diglycosyl-diacylglycerol (Glc2-DAG) (4, 5). The anchor synthesis is catalyzed by the glycosyltransferase UgtP, and the main chain is mainly synthesized by LtaS (5). The loss of LtaS activity in B. subtilis impacts cell division, cell morphogenesis, and divalent cation homeostasis (6). In addition to these phenotypes, a quadruple ltaS homolog mutant shows a loss of LTA (7), an aberrant twisted morphology, slower growth (6), and reduced adsorption of rare earth elements (8, 9). Moreover, we reported that σD-dependent transcription of lytF, which encodes a dl-endopeptidase that functions in cell separation, is reduced in an ltaS mutant and is nearly absent in multiple mutants of ltaS and its homologs (10). Interestingly, we also found that lytF transcription was repressed in tagO-null mutant cells, which lack WTA. These results indicate that LTA and WTA, which are similar anionic polymers with a poly(GroP) backbone located on the B. subtilis cell surface, are required for temporal σD-dependent lytF expression. Moreover, both WTA and LTA hinder LytF localization in the cylindrical part of the cell (10, 11). In addition to septum PG digestion by LytF and CwlS, sidewall PG hydrolysis, which is required for nascent PG incorporation, is catalyzed by LytE and CwlO during vegetative growth (12, 13). Both lytE and cwlO are in the WalRK regulon (14). In addition, lytE transcription is regulated by σI, which is required for heat shock adaptation (15, 16) and is enhanced at high temperatures (17). Moreover, the dl-endopeptidase activity of LytE is essential for survival at high temperatures (17). It is thought that LytE digests not only septum PG but also sidewall PG to allow newly synthesized PG precursors to be incorporated (12). The sidewall localization of LytE appears to be governed by an interaction between the C-terminal catalytic domain of LytE and the actin homologue MreBH (12). Furthermore, it has been reported that a cwlO lytE double mutant strain is not viable and that cells lacking LytE and depleted for CwlO exhibit defects in lateral CW synthesis and cell elongation (14). In addition, recent reports have revealed that CwlO is also localized along the sidewall (13, 18). Interestingly, the localization and activity of CwlO are controlled by an ABC transporter-like component, FtsEX, located on the cytoplasmic membrane (18, 19).
Previous reports have shown that cells lacking LTA grow slowly and have an aberrant twisted chained morphology (6, 10). In addition, when WTA was abolished, cells grew slowly, showed a swelled morphology, and formed clumps (20). Thus, we presumed that the loss of LTA or WTA affects not only cell division and separation but also lateral CW elongation. In this study, we investigated the effects of the anionic polymers LTA and WTA on the expression and localization of the dl-endopeptidase LytE, which is required for lateral PG hydrolysis during cell elongation. Moreover, we found that cell surface decorations with LTA and WTA are decreased at high temperatures.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table S1 in the supplemental material. For the localization analysis of a FLAG tag fusion of LytE, B. subtilis WEC, a double mutant strain of wprA and epr without any antibiotic resistance genes (11), was used as the parent strain throughout this study. B. subtilis strains were grown in Luria-Bertani (LB) medium (21) at 37°C unless otherwise noted. When necessary, chloramphenicol, kanamycin, spectinomycin, tetracycline, and erythromycin were added at final concentrations of 5, 5, 100, 10, and 0.3 μg/ml, respectively. To culture the conditional mutants of ltaS homologues or tagO, isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to the culture medium. Escherichia coli strains were cultured in LB medium at 37°C, and, when necessary, ampicillin was added at a final concentration of 100 μg/ml. E. coli JM109 and C600 cells were used for plasmid construction and multimeric plasmid DNA preparation, respectively. The DNA manipulations and E. coli transformation were performed using the standard methods (21). The B. subtilis transformation was also carried out based on the conventional transformation method (22).
Plasmid and strain construction.
The plasmids and primers used in this study are listed in Tables S2 and S3, respectively, in the supplemental material. After digestion with restriction enzymes, all DNA fragments were fractionated using agarose gel electrophoresis and purified with the QIAquick gel extraction kit (Qiagen), according to the manufacturer's instructions. The nucleotide sequences of all inserts amplified by PCR were confirmed by sequencing.
To construct a lytE-6×FLAG fusion gene at the lytE locus, the 3′-flanking region of lytE was obtained by digesting pCA3FLCF (23) with HindIII and XbaI. The fragment was subcloned into the corresponding sites of pCA6×FLAG (11) to generate pCA6FLLE. This plasmid was transformed into B. subtilis to obtain strain WECF6FL (wprA epr lytE-6×FLAG).
To construct the PlytE-lacZ, PmreBH-lacZ, and PcwlO-lacZ fusions at the amyE locus, the 5′-flanking regions of lytE, mreBH, and cwlO were amplified with two sets of primers, PlytE-Ef and PlE-SmSr for lytE, PmreBHLZ-Ef and PmreBHLZ-Smr for mreBH, and PcwlO-Ef and PcwlO-SaSr for cwlO using B. subtilis 168 chromosomal DNA (chrDNA) as the template. After the amplified lytE, mreBH, and cwlO fragments were digested with EcoRI and SmaI, they were cloned into the corresponding sites of pDHAFBLZ (24) to generate pPlELZ, pPmreBHLZ, and pPCOLZ, respectively. The plasmids were linearized with ScaI and transformed into B. subtilis to generate strains YK3001 (PlytE-lacZ), YK3003 (PmreBH-lacZ), and YK3004 (PcwlO-lacZ).
To construct disruption mutants in sigI and sigH, the internal regions of these genes were amplified with primers SIGId-Hf and SIGId-Br for sigI and SH-HF and SH-BR for sigH using B. subtilis 168 chrDNA as the template. After digestion with HindIII and BamHI, the sigI and sigH fragments were cloned into the corresponding sites of pM4ΔZ (10) to generate pM4ΔZsigId and pM4ΔZsigHd, respectively. The resulting plasmids were transformed into B. subtilis to generate strains sigIdΔZ (sigI::pM4ΔZsigId) and sigHdΔZ (sigH::pM4ΔZsigHd).
To construct an mreBH mutant, a DNA fragment containing the upstream and downstream regions of mreBH was amplified with primers mreBHf-salf and mreBHb-smr using pBmreBHfb (11) as the template. After digestion with SalI and SmaI, the fragment was cloned into the corresponding sites of pMAD (25) to generate pMADmreBHfb. To obtain an mreBH mutant (TK2202) without any antibiotic resistance genes, we used a previously described efficient allelic replacement method (25). The mreBH locus of the clean mutant was checked by PCR and sequencing with primers mreBH-UP and mreBH-DN.
To construct a cwlO-null mutant, an internal region of cwlO was amplified by PCR using B. subtilis 168 chrDNA as the template and primers pQEvcE-Fw and KR-YVCE. The amplified fragment was digested with EcoRI and KpnI, and the digested fragment was cloned into the corresponding sites of pBluescript II SK(+) to generate pBlue-cwlO. The kanamycin resistance cassette of pDG780 (digested with HindIII) was cloned into the HindIII site of pBlue-cwlO to obtain pBlue-ΔcwlO. The resulting plasmid was linearized with BamHI and transformed into B. subtilis to obtain the OKD (cwlO::kan) strain.
β-Galactosidase assay.
After the cells were cultured at 37°C, samples were withdrawn at various time points to assay the β-galactosidase activity. The β-galactosidase activity (expressed as units per milligram of protein) was measured and calculated as described by Shimotsu and Henner (26). One unit of β-galactosidase activity was defined as the amount of enzyme necessary to release 1 nmol of 2-nitrophenol from O-nitrophenyl-β-d-galactopyranoside (ONPG) in 1 min at 28°C. The assay was performed at least three times independently.
Immunofluorescence microscopy.
The sample preparation for immunofluorescence microscopy (IFM) was described previously (10, 23) and used with minor modifications, as follows. An anti-DYKDDDDK tag monoclonal antibody (1:400; Wako Pure Chemical Industries) was used as the primary antibody, and an Alexa Fluor 555 F(ab′)2 fragment of goat anti-mouse IgG (H+L) (1:1,200; Life Technologies) was used as the secondary antibody. The lysozyme treatment just before the sample was spotted onto a poly-l-lysine-coated microscope slide (23) was omitted to avoid exfoliation of the LytE-6×FLAG protein. Fluorescence microscopy was performed as described previously (10) with an Axio Imager M1 microscope, a Plan-Apochromat Fluorite differential interference objective (magnification, ×63; numerical aperture, 1.4), and standard filter sets for visualizing rhodamine (for Cy3 or Alexa Fluor 555). The exposure time was 0.04 s for differential interference contrast microscopy and 0.005 to 0.1 s (gain 2) for Alexa Fluor 555. The cells were photographed with a charge-coupled device camera (AxioCam MRm; Carl Zeiss) using AxioVision software (version 4.6; Carl Zeiss). The three-dimensional deconvolution utility of the AxioVision software was used for z-axis imaging. All images were processed with AxioVision and Adobe Photoshop. ImageJ v.1.47 (National Institutes of Health) was used for a quantitative analysis. Fluorescence was quantified as described in a previous report (18). Fluorescent images were basically taken with the same acquisition settings, but the exposure time was appropriately varied so that the fluorescence was not saturated. The averaged value of the fluorescence intensity was calculated in segments of equal size on the lateral walls or the septa of the cells (>50 cells).
SDS-PAGE and Western blot analysis of the cell surface proteins and LTA.
Cell surface proteins were prepared as described previously (27). Briefly, 200 μl of 2× SDS-PAGE sample buffer (28) was added to the cell pellet, corresponding to 1 unit at optical density at 600 nm (OD600). The cell suspension was boiled for 10 min, and the proteins were separated by SDS-PAGE in 12% (wt/vol) polyacrylamide gels as described by Laemmli (28). After electrophoresis, Western blot analysis of the LytE-6×FLAG fusion protein was performed as described previously (23). An anti-DYKDDDDK tag monoclonal antibody (Wako Pure Chemical Industries) was used as the primary antibody, and an anti-mouse IgG, horseradish peroxidase (HRP)-linked whole antibody (GE Healthcare) was used as the secondary antibody (both at 1:10,000 for FLAG-specific detection as described previously) (10). For the Western blot analysis of LTA, the sample preparation, SDS-PAGE, and blotting were performed as described previously (7). A humanized monoclonal LTA antibody (Biosynexus Incorporated, Gaithersburg, MD) was used as the primary antibody, and HRP-conjugated polyclonal rabbit anti-human IgA, IgG, IgM, Kappa, and Lambda antibodies (DakoCytomation) were used as the secondary antibodies (1:10,000 dilutions for LTA detection). Chemiluminescence was detected using the Western Lightening ECL Pro kit (PerkinElmer), according to the manufacturer's instructions.
Preparation of CW, PG, and WTA.
The cell wall (CW) and peptidoglycan (PG) of the B. subtilis strains were prepared as described previously (11, 29, 30). The amount of PG was calculated by measuring the OD540 value. One (OD540/ml) unit is equivalent to 6.45 mg/ml of PG (11). Cell wall teichoic acid (WTA) was extracted using two methods with either trichloroacetic acid (TCA) or NaOH (31). The phosphorus content was determined by methods described in previous reports (32, 33) and was converted into the amount decorating 1 mg of PG. Polyacrylamide gel electrophoresis of WTA was performed as described previously (31). After electrophoresis, WTA was visualized by the alcian blue-silver stain method (34).
RESULTS
lytE transcription is enhanced in mutants defective for LTA or WTA.
As described in our previous report, σD-dependent transcription of lytF was repressed in mutants lacking LTA or WTA (10). B. subtilis produces another dl-endopeptidase, LytE, which is responsible for not only cell separation but also cell elongation during vegetative growth (12, 23, 35, 36). Interestingly, lytE transcription is enhanced when the ltaS gene is disrupted. The β-galactosidase activity driven by the lytE promoter was at least 2-fold higher in the ltaS mutant than in wild-type B. subtilis 168 cells (Fig. 1A). Previous reports demonstrated that the lytE gene has two promoters, PsigH and PsigI (17, 35). Here, we tried to determine which promoter is required for induction of lytE expression in ltaS-deficient cells. To address this, we introduced a sigI mutation into an ltaS-null mutant background. As a result, lytE induction was almost completely lost (see Fig. S1B in the supplemental material). In contrast, a sigH mutation in the ltaS mutant had little effect on lytE transcription during early vegetative growth. These results suggest that σI-dependent lytE transcription is elevated by the ltaS mutation. Moreover, transcription from PlytE was slightly affected in a triple mutant strain with mutations in three ltaS homologues (yfnI, yqgS, and yvgJ) (Fig. 1A), suggesting that the LTA polymer synthesized by LtaS is a main regulator of vegetative lytE transcription. We also examined lytE expression in strains harboring mutations in pgcA, gtaB, and ugtP, which are involved in the glycolipid anchor biosynthesis pathway (4, 37–39). As shown in Fig. S1A in the supplemental material, lytE transcription was clearly increased in the pgcA, gtaB, and ugtP mutants compared with the expression in the wild-type strain.
FIG 1.
Effect of mutations in genes involved in the teichoic acid biosynthetic pathway or the modification pathway on lytE transcription. Cultures were inoculated at a starting optical density at 600 nm (OD600) of 0.001, unless otherwise noted. Growth (OD600) and β-galactosidase activity are represented by dotted and solid lines, respectively. The experiments were performed three times with similar results. (A) β-Galactosidase activity of PlytE-lacZ in the wild type and ltaS homologue mutants: YK3001 (wild type; ○), YA1715 (ltaS; □), YK2307 (ltaS yfnI; ⬥), YK2236 (ltaS yfnI yqgS; △), YK2164 (ltaS yfnI yqgS yvgJ; ▲), and YK2157 (yfnI yqgS yvgJ; ■). For the multiple mutants, cultures were inoculated at OD600 of 0.002 (YA1715, YK2307, and YK2236) or 0.003 (YK2164) because they grew slowly. (B) β-Galactosidase activity of PlytE-lacZ in the wild type and WTA biosynthesis mutants: YK3001 (wild type; ○), YK2234 (tagE; ×), YK2377 (ggaAB; □), and YK2145 (tagO; △). For the tagO-null mutant (YK2145), the culture was inoculated at a starting OD600 of 0.005 because it grew slowly.
Next, we tested whether multiple mutations in ltaS and its homologues influence lytE expression. As shown in Fig. 1A, the peak lytE expression in a double ltaS yfnI mutant was reduced and occurred earlier during vegetative growth than the expression in an ltaS single mutant. This suggests that the longer LTA polymers produced by YfnI in the ltaS mutant cells (7) also affect lytE transcription. In addition, the transcriptional activities from the PlytE promoter in triple ltaS yfnI yqgS and quadruple mutants were slightly enhanced compared with the expression in the ltaS yfnI double mutant (Fig. 1A). Moreover, lytE transcription in a tagO-null mutant, in which WTA is absent, was approximately 4-fold higher than that in the wild-type strain (Fig. 1B). Likewise, transcription of mreBH, which is another σI-dependent gene (40), was enhanced in the LTA and WTA mutants (see Fig. S2A in the supplemental material). These results suggest that impairment of cell surface decoration by anionic polymers greatly influences σI-dependent lytE transcription. In contrast, lytE transcription was barely altered in cells absent minor WTA (ggaAB mutant) and glycosylation (tagE mutant) and only slightly reduced in cells missing d-alanylation (dltA mutant) (Fig. 1B; see also Fig. S1A in the supplemental material).
Both LTA and WTA control the cell surface localization of LytE.
We showed that LTA or WTA deficiency results in considerable enhancement of lytE transcription from the σI-dependent promoter (Fig. 1; see also Fig. S1A and B in the supplemental material). Next, we examined whether these enhancements affect the cell surface localization of LytE. To test this, we analyzed the localization of 6×FLAG-tagged LytE in the cell surface fraction by Western blotting. The results clearly showed a larger amount of LytE in the cell surface fraction of the ltaS mutant than in that of wild-type cells and the amounts of LytE in the double, triple, and quadruple mutants were gradually increased compared to that in the ltaS single mutant (Fig. 2A). To confirm these observations, we carried out an immunofluorescence microscopy (IFM) experiment with cells expressing the LytE-6×FLAG fusion protein. As described in previous reports (12, 13), LytE localized not only to the septum and the poles but also to the sidewall in a helix-like manner across the long axis (Fig. 2B and C). Interestingly, a considerable increase in the LytE foci was observed in the ltaS mutant (Fig. 2B), and the helix-like pattern was not altered (Fig. 2C). In addition, the signal intensities of LytE foci in the multiple gene mutants were slightly increased compared to that in the ltaS single mutant (Fig. 2B), which was consistent with the results of the Western blot analysis (Fig. 2A). However, the localization patterns in the multiple mutants have appeared not to alter compared to that in the ltaS mutant (Fig. 2C). Next, we examined LytE localization in the glycolipid anchor biosynthesis pathway mutants. In the ugtP mutant, the amount of LytE-6×FLAG on the cell surface was higher than that in the wild-type strain (Fig. 2A). Likewise, IFM showed that the LytE foci in the ugtP mutant were stronger than those in the wild-type cells (Fig. 2D). Very similar increases in the LytE amount were observed in the gtaB and pgcA mutants (Fig. 2A and D). Moreover, a large amount of LytE-6×FLAG was detected in tagO-null mutant cells lacking WTA (Fig. 3A). An IFM image of the tagO-null mutant showed that the LytE foci are fairly evenly distributed on the cell surface (Fig. 3B). However, it was difficult to clearly observe the LytE localization in the tagO-null mutant since these cells formed clumps. Therefore, we performed similar localization analyses with a tagO conditional mutant strain. The results clearly showed that the LytE levels gradually increased as TagO expression was reduced (Fig. 3A). Moreover, we found that the increased fluorescence of the LytE foci were unevenly localized on the TagO-reduced cell surfaces (0.08 and 0.04 mM IPTG) (Fig. 3C), but the foci were fairly evenly distributed on the TagO-depleted cell surfaces (0 mM IPTG) (Fig. 3C). Taking our results together, we concluded that both LTA and WTA affect the amount of LytE on the cell surface at the level of transcription and localization.
FIG 2.
Subcellular localization of LytE-6×FLAG in LTA mutants. (A) Western blot analysis of LytE-6×FLAG in the LTA mutants. The cultures were inoculated at a starting OD600 of 0.001. For slow-growing strains, the initial OD600 value was 0.002 (KY1215, YK1637, and YK2369) or 0.003 (RY2173). After incubation for 3 h (to an OD600 of 0.3 to 0.5), the cells were harvested. Wild type, WECF6FL; yfnI yqgS yvgJ, RY1610; ltaS, KY1215; ltaS yfnI, YK1637; ltaS yfnI yqgS, YK2369; ΔLTA, RY2173 (ltaS yfnI yqgS yvgJ); pgcA, JK2525; gtaB, JK2526; ugtP, JK2527; dltA, JK2528. Proteins from the cell surface were isolated from the equivalent of 0.0125 OD600 cells in each lane and were separated on a 12% polyacrylamide gel. The molecular masses of the protein standards (Bio-Rad) are indicated on the left. (B) Immunofluorescence microscopy (IFM) of LytE-6×FLAG in WECF6FL (wild-type), RY1610 (yfnI yqgS yvgJ), KY1215 (ltaS), YK1637 (ltaS yfnI), YK2369 (ltaS yfnI yqgS), and RY2173 (ΔLTA, ltaS yfnI yqgS yvgJ) cells. The culture conditions and sampling time points were similar to those used for the Western blot analysis in panel A. The exposure time for the Alexa Fluor 555 images (gain 2) was 100 ms. Bar, 10 μm. For quantification of the fluorescence intensities, fluorescent images were taken with the same acquisition settings (gain 2, exposure time of 25 ms). The averaged values of the fluorescence intensity were calculated in small segments of equal size on the lateral walls. The relative fluorescence intensities to the wild-type cells together with the calculated standard deviations for the LytE-6×FLAG foci on the lateral wall are shown below the fluorescence images. (C) Deconvolution images of LytE-6×FLAG in LTA mutants. The exposure times were 100 ms for the wild type and 25 ms for the LTA mutants (gain 2). After deconvolution of the dotted rectangle regions in panel B, three sections (top, middle, and bottom) at different levels along the z axis are shown. Bar, 5 μm. (D) Subcellular localization of LytE-6×FLAG in glycolipid anchor synthesis mutant strains JK2525 (pgcA), JK2526 (gtaB), and JK2527 (ugtP) or the d-alanylation mutant strain JK2528 (dltA). The exposure time for the Alexa Fluor 555 images (gain 2) was 100 ms. Bar, 10 μm. For quantification of the fluorescence intensities, fluorescent images were taken with the same acquisition settings (gain 2, exposure time of 25 ms). The relative fluorescence intensities with standard deviations for the LytE-6×FLAG foci on the lateral wall are shown below the fluorescence images.
FIG 3.
Subcellular localization of LytE-6×FLAG in WTA mutants. (A) Western blot analysis of LytE-6×FLAG in WTA mutants. Cultures were inoculated at a starting OD600 of 0.001 except for YK2141, which was inoculated at 0.005. After incubation for 3 h (to an OD600 of 0.3 to 0.5), the cells were harvested and fixed. Wild type, WECF6FL; ggaAB, JK2530; tagE, JK2529; tagO, YK2141; Pspac-tagO, YK2133 (0, 0.04, 0.08, 0.12, and 0.15 mM IPTG); ΔLTA, RY2173 (ltaS yfnI yqgS yvgJ). Proteins from the cell surface in each lane were the equivalent of 0.0125 OD600 cells. The molecular masses of the protein standards (Bio-Rad) are indicated on the left. (B) IFM analyses of LytE-6×FLAG in strains WECF6FL (wild type), JK2530 (ggaAB), JK2529 (tagE), and YK2141 (tagO). The culture conditions and sampling time points were similar to those used for the Western blot analysis in panel A. The exposure time for the Alexa Fluor 555 image (gain 2) is shown in each panel. Bars, 10 μm. For quantification of the fluorescence intensities, fluorescent images were taken with the different exposure times (5 or 100 ms) as described in the images since the LytE foci were strong in the tagO-null mutant cells. The relative fluorescence intensities with standard deviations for the LytE-6×FLAG foci on the lateral wall are shown below the fluorescence images. (C) IFM observations and deconvolution images of LytE-6×FLAG in a TagO-depletion experiment. An IPTG-inducible tagO conditional mutant (YK2133, Pspac-tagO) was inoculated at an OD600 of 0.001 into LB medium with or without IPTG. After incubation for 3 h, cells were harvested and fixed. The exposure time for the Alexa Fluor 555 image (gain 2) is shown in each panel. For quantification of the fluorescence intensities, fluorescent images were taken with the different exposure times (5 or 100 ms) as described in the images since the LytE foci were strong in the TagO-depleted (0 and 0.04 mM IPTG) cells. The relative fluorescence intensities with standard deviations for the LytE-6×FLAG foci on the lateral wall are shown below the fluorescence images. After deconvolution analysis of the dotted rectangle regions in the upper panels, the middle sections on the z axis (DEC) are shown. Bars, 5 μm.
Increased LytE localization in the ltaS mutant is largely independent of MreBH and is suppressed by YqgS induction.
A previous report showed that sidewall LytE localization depends on the physiological interaction between the C-terminal dl-endopeptidase domain of LytE and the actin homologue MreBH (12). In addition, both lytE and mreBH are in the SigI regulon (17, 40). In the previous section, we showed that σI-dependent transcriptions of lytE and mreBH were activated in an ltaS mutant (see Fig. S1B and S2A in the supplemental material). In contrast, an mreBH mutation did not affect lytE transcription (T. Kondo, unpublished data). Thus, we examined whether the increased LytE localization along the sidewall of the ltaS mutant is controlled in an MreBH-dependent manner. Very interestingly, the amount of LytE in the cell surface fraction of the ltaS mreBH double mutant was very similar to that of the ltaS mutant (Fig. 4A). In addition, no distinguishable difference was observed in the localization patterns along the sidewall in these mutants (Fig. 4B). Only the LytE foci along the sidewall in the ltaS mreBH double mutant were slightly reduced compared to the foci in the ltaS mutant, whereas the foci at the septum and poles were slightly increased (Fig. 4B). Taken together, these results appear to suggest that increased LytE localization in the ltaS mutant might be largely governed in an MreBH-independent manner.
FIG 4.
MreBH dependence of LytE localization and complementation test in the ltaS mutant background. (A) Western blot analysis of LytE-6×FLAG in the mreBH mutant background. Cultures were inoculated at a starting OD600 of 0.002 into LB medium. After incubation for 3 h (to an OD600 of 0.3 to 0.4), the cells were harvested. Lane 1, KY1215 (ltaS); lane 2, YK2363 (ltaS mreBH). Proteins from the cell surface in each gel lane were equivalent to that from 0.025 OD600 cells. The molecular masses of the protein standards (Bio-Rad) are indicated on the left. (B) Localization patterns of LytE-6×FLAG in KY1215 (ltaS) and YK2363 (ltaS mreBH). Culture conditions and sampling points were similar to those of the Western blot analysis in panel A. The exposure time was 100 ms (gain 2) for the Alexa Fluor 555 images. For quantification of the fluorescence intensities, fluorescent images were taken with the same acquisition settings (gain 2, exposure time of 25 ms). The relative fluorescence intensities to the ltaS mutant cells together with the calculated standard deviations for the LytE-6×FLAG foci on the lateral wall (LW) and septum and poles (SP) are indicated below the IFM images. Bar, 10 μm. (C) Western blot analysis of LytE-6×FLAG in the complementation experiment. Cultures were inoculated at a starting OD600 of 0.001 into LB medium without IPTG, WECF6FL (wild type), and KY1215 (ltaS), or with 0.5 mM IPTG, KY1202 (Pspac-ltaS), JK2520 (ltaS Pspac-yfnI), JK2524 (ltaS Pspac-yvgJ), and JK2522 (ltaS Pspac-yqgS). After incubation for 3 h (OD600 of 0.4 to 0.5), the cells were harvested. Proteins from the cell surface in each lane were the equivalent of that in 0.025 OD600 cells. The molecular masses of the protein standards (Bio-Rad) are indicated on the left. Lane 1, WECF6FL (wild type); lane 2, KY1215 (ltaS); lane 3, KY1202 (Pspac-ltaS); lane 4, JK2520 (ltaS Pspac-yfnI); lane 5, JK2524 (ltaS Pspac-yvgJ); lane 6, JK2522 (ltaS Pspac-yqgS). (D) LytE localization in strains carrying IPTG-inducible ltaS homologous genes. The culture conditions and sampling points were similar to those of the Western blot analysis in panel C. The exposure time was 100 ms (gain 2) for the Alexa Fluor 555 images. Bar, 10 μm. For quantification of fluorescence intensity, fluorescent images were taken with the same acquisition settings (gain 2, exposure time of 25 ms). Relative fluorescence intensities to the wild-type cells together with the calculated standard deviations for the LytE-6×FLAG foci on the lateral wall (LW) and septum and poles (SP) are indicated below the IFM images.
Our previous report revealed that artificial expression of YqgS, which has both LTA primase and LTA synthase activities (7), in an ltaS mutant restored the normal rod-shape morphology, SigD-dependent expression, and septal localization of LytF (10). Thus, we examined whether artificial expression of YqgS also suppressed the enhanced SigI-dependent expression and lateral localization of LytE in the ltaS mutant. The results indicated that the transcription level of lytE in YqgS-induced cells in an ltaS genetic background was suppressed to that in wild-type cells but those in YfnI- and YvgJ-induced cells were not (see Fig. S2B in the supplemental material). In addition, Western blotting and IFM analyses indicated that the amount of localized LytE in the ltaS genetic background was restored only by YqgS induction (Fig. 4C and D). Taken together, these results clearly indicate that the LtaS paralog YqgS is able to complement the physiological roles of LtaS in lateral PG hydrolysis.
Cell surface decorations with LTA and WTA were decreased at high temperatures.
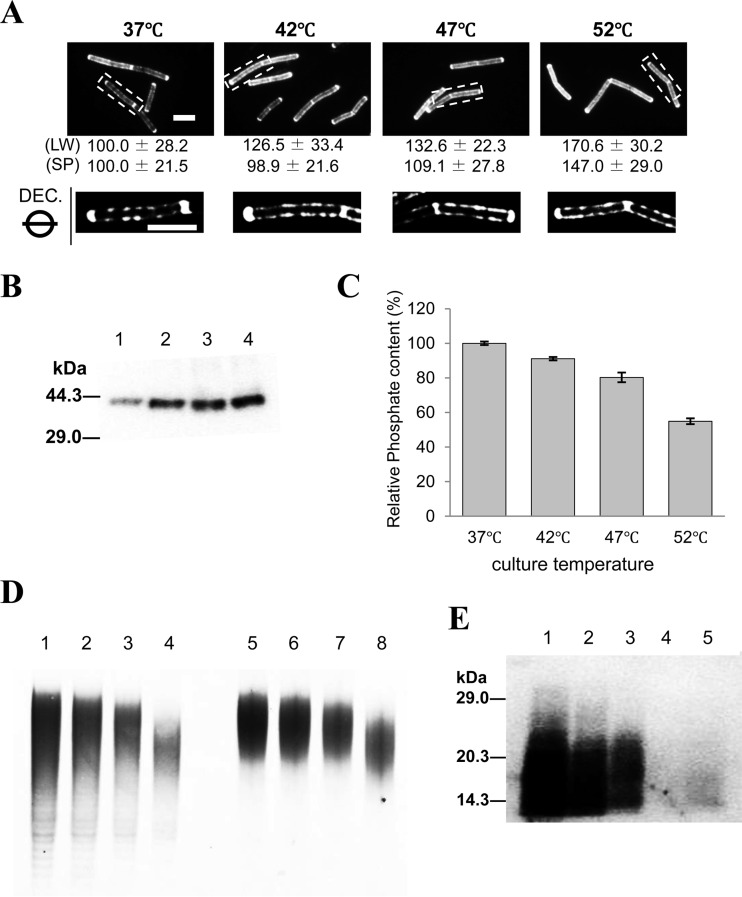
In this report, we found that defects in LTA or WTA enhance SigI-dependent lytE transcription (Fig. 1; see also Fig. S1B in the supplemental material). A similar enhancement of lytE transcription has been reported under heat stress (17, 41). Therefore, we assessed LytE localization at high temperatures and found that the amount of LytE in the cell surface fractions gradually increased under heat stress (Fig. 5B). Moreover, IFM showed that the LytE foci on the sidewall at 52°C were obviously stronger than those at 37°C and were gradually enhanced as the culture temperature increased (Fig. 5A). The localization pattern of LytE at 52°C appeared to resemble the patterns in the ltaS mutant and the TagO-reduced cells (Fig. 2B and C and 3C). These results suggest that cell wall decorations by LTA or WTA might be reduced at high temperatures. To verify this, the amount of phosphate in the CW was measured when cells were grown at different temperatures. The results showed that the amounts of phosphate in the CW at 42°C and 47°C were lower than that at 37°C and the amount at 52°C was considerably (approximately 45%) lower (Fig. 5C). To confirm these findings, we performed a native PAGE analysis of the WTA polymer. As shown in Fig. 5D, the length and amount of the WTA polymer from cells cultured under heat stress were lower than those from cells grown at 37°C. Surprisingly, in addition to the reduced WTA, Western blot analysis of LTA showed that LTA decreased as the culture temperature increased (Fig. 5E). Notably, LTA in cells cultured at 52°C was mostly lost. These results indicate that cell surface decorations with WTA and LTA are clearly lower when cells are cultured at high temperatures.
FIG 5.
LytE localization and cell wall decoration by WTA and LTA at high temperature. (A) IFM observations and deconvolution images of LytE-6×FLAG at different temperatures. Cells in mid-exponential phase (OD600 of 0.6) were fixed and used for IFM. The exposure time was 100 ms (gain 2) for the Alexa Fluor 555 images. The relative fluorescence intensities to the cells at 37°C together with the calculated standard deviations for the LytE-6×FLAG foci on the lateral wall (LW) and septum and poles (SP) are indicated below the IFM images. Deconvolution is performed using the dotted rectangle regions in the upper panels, and the middle sections along the z axis (DEC.) are shown. Bars, 5 μm. (B) Western blot analysis of LytE-6×FLAG at different temperatures. The culture conditions and sampling points were same as those for IFM in panel A. Cell surface proteins were extracted from the B. subtilis WECF6FL cells cultured at 37°C (lane 1), 42°C (lane 2), 47°C (lane 3), and 52°C (lane 4). Proteins from the cell surface were equivalent to 0.025 OD600 cells in each lane. The molecular masses of the protein standards (Bio-Rad) are indicated on the left. (C) Phosphate content in the cell wall at different culture temperatures. Cell wall preparation and phosphate content analysis were performed as described in the Materials and Methods. The relative amounts of phosphate in the CW prepared from cells cultured at 37°C are represented as the percent phosphate contents with standard deviations coming from three independent experiments. (D) PAGE analysis of WTA. WTA samples were prepared from B. subtilis 168 cells cultured at 37°C (lanes 1 and 5), 42°C (lanes 2 and 6), 47°C (lanes 3 and 7), and 52°C (lanes 4 and 8) as described in the Materials and Methods. WTA was extracted with trichloroacetic acid (lanes 1 to 4) or with NaOH (lanes 5 to 8). WTA samples extracted from the CW corresponded to 19 μg (lanes 1 to 4) and 9.8 μg (lanes 5 to 8) of PG loaded onto each lane of a 15% polyacrylamide gel. (E) Western blot analysis of LTA. LTA was extracted from B. subtilis 168 cells cultured at 37°C (lane 1), 42°C (lane 2), 47°C (lane 3), and 52°C (lane 4 and 5). LTA samples were the equivalent of 0.25 (lanes 1 to 4) or 0.5 (lane 5) OD600 cells in each lane, respectively. The molecular masses of the protein standards (Bio-Rad) are indicated on the left.
DISCUSSION
In this study, we found that lytE transcription was considerably increased in a strain containing a mutation in ltaS, which encodes a principal LTA synthase, as well as in strains containing multiple mutations in ltaS and its homologs (Fig. 1A). The enhancement of lytE transcription observed in the ltaS mutant appeared to be regulated in the σI-dependent manner (see Fig. S1B in the supplemental material). A similar enhancement was observed in single mutants of ugtP, gtaB, and pgcA, which encode the enzymes required for synthesis of the LTA glycolipid anchor (see Fig. S1A in the supplemental material), or tagO, which encodes the first enzyme required for synthesis of the linkage unit of WTA (Fig. 1B). These findings suggest that defects in the poly(GroP) main chains of LTA and WTA lead to considerable activation of σI-dependent transcription. Interestingly, transcription of cwlO (YK3004), which encodes another dl-endopeptidase required for cell elongation, was little altered in the ltaS mutant (YK2237) (Y. Kiriyama, unpublished data). This divergence on transcription between lytE and cwlO in the ltaS mutant may result from a difference in σI dependence (42). In cells lacking LTA or WTA, the lateral PG hydrolytic activity of LytE might be insufficient during cell elongation. Therefore, these cells may respond by upregulating lytE transcription. A previous report revealed that the Mg2+ dependence of an mbl mutant is suppressed by mutations in ltaS and rsgI, which encodes an anti-sigma factor for SigI (43). In this report, we found that a mutation in ltaS enhanced σI-dependent transcription of lytE and mreBH (Fig. 1A; see also Fig. S1B and S2A in the supplemental material). Since both mutations occur in an activation of the SigI regulon, the ltaS mutation appears to suppress the lethal phenotype of the mbl mutant. In addition, enhancement of lytE expression in the ltaS mutant was only suppressed by artificial induction of another LTA synthase, YqgS (Fig. 4C and D; see also Fig. S2B in the supplemental material), which produces normal-length LTA polymer as LtaS (7). In contrast, it has been reported that the LTA synthase YfnI synthesizes the longer LTA polymer and that LTA primase YvgJ transfers one glycerol phosphate subunit onto the glycolipid anchor (7). In our previous study, we showed that YqgS induction completely restored σD-dependent expression and septal localization of LytF, which functions as a principal vegetative cell separation enzyme, in an ltaS mutant (10). These results appear to suggest that induction of YqgS in ltaS mutant cells complements not only the LytF-induced cell separation but also the lateral PG hydrolysis catalyzed by LytE.
Transcriptional enhancement of lytE in LTA- or WTA-defective mutants caused the increased amount of LytE localized on the cell surface (Fig. 2 and 3). This elevation in LytE may result from a synergistic effect of both transcriptional enhancement and an increased LytE-attachable region. Interestingly, the patterns of LytE localization were helix-like in cells lacking LTA or reduced WTA (Fig. 2C and 3C) and evenly distributed in cells absent WTA (Fig. 3B and C). These localization patterns resemble those of another dl-endopeptidase, LytF. Our previous reports revealed that LytF was localized to the septum and poles in the wild-type strain (23) and was also localized to the sidewalls in LTA- or WTA-deficient mutants (10, 11). The N-terminal LysM motifs of LytF are required for specific attachment to PG, and both LTA and WTA control the sidewall localization of LytF (10, 11). In addition, Hashimoto et al. (13) reported that chimeric proteins containing the N-terminal LysM domain of LytE and the C-terminal dl-endopeptidase domains of LytF or CwlS complemented the important functions of LytE in lateral PG hydrolysis. Since cell wall attachment of LytE also appears to depend on three tandem LysM motifs in the N-terminal cell wall-binding domain, LytE localization may be controlled by LTA and WTA in the same manner as LytF. This idea is supported by the finding that an mreBH mutation barely affected LytE localization in the ltaS mutant (Fig. 4B), suggesting that LytE might be localized to the sidewall in a largely MreBH-independent manner when ltaS is absent. Actually, the LytE foci along the sidewall were slightly reduced in the ltaS mreBH double mutant, whereas the foci at the septum and poles were slightly increased compared with these foci in the ltaS mutant (Fig. 4B). This suggests that the specific interaction between LytE and MreBH is dispensable for the localization and function of LytE in the ltaS mutant cells. As support for this presumption, we found that a cwlO ltaS mreBH triple mutant (JK2535) exhibited growth similar to that of a cwlO ltaS double mutant (JK2532) (J. Kasahara, unpublished data). This result indicates that the increased LytE observed along the sidewall in the triple mutant is fully functional for the lateral PG hydrolysis required for cell elongation in the absence of MreBH. These observations appear to be supported by a previous report that depletion of either CwlO or FtsEX in cells lacking MreBH did not affect cell growth (19).
It is thought that the SigI-dependent genes are mainly involved in heat stress responses (15, 16). Notably, SigI is required for most lytE transcription, and the dl-endopeptidase activity of LytE is essential for survival under heat stress (17, 41). In this report, we found that loss of the poly(GroP) main chains of LTA or WTA at 37°C affects the expression of lytE and mreBH, which are part of the SigI regulon (Fig. 1; see also Fig. S2A in the supplemental material). In addition, the amount of LytE on the wild-type cell surface gradually increased as the culture temperature increased (Fig. 5A and B). Moreover, the LytE localization pattern at high temperatures is very similar to that in LTA-deficient or WTA-reduced cells (Fig. 2C, 3C, and 5A). These observations suggest that cell surface decorations with LTA and/or WTA might be reduced at high temperatures. Actually, the amount of phosphorus in CW prepared from cells cultured at 52°C was considerably lower (approximately 55%) than that from cells cultured at 37°C (Fig. 5C). The reduced amount of phosphorus appeared to result from decreases in both the total amount and the length of WTA polymer (Fig. 5D). Moreover, LTA in wild-type cells was gradually reduced at increased temperatures and was nearly lost at 52°C (Fig. 5E). Taken together, these findings strongly suggest that transcriptional enhancement of the SigI regulon at high temperatures may result from reduced cell surface decorations with WTA and LTA. In addition, it appears that B. subtilis cells drastically change their cell wall content to adapt to the high temperature conditions. More detailed studies are needed to explain why the cell surface decorations with LTA and WTA affect expression of the σI-dependent transcription and are decreased at high temperatures.
Supplementary Material
ACKNOWLEDGMENTS
We thank John F. Kokai-Kun from Biosynexus Incorporated (Gaithersburg, MD) for the gift of the humanized monoclonal LTA antibody. We also thank the members of our research group for helpful advice.
Funding Statement
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (23580107) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to Hiroki Yamamoto.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00003-16.
REFERENCES
- 1.Foster SJ, Popham DL. 2001. Structure and synthesis of cell wall, spore cortex, teichoic acids, S-layers, and capsules, p 21−41. In Sonenshein AL, Losick R, Hoch JA (ed), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology Press, Washington, DC. [Google Scholar]
- 2.Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol Biol Rev 67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarevic V, Pooley HM, Mauël C, Karamata D. 2002. Teichoic and teichuronic acids from Gram-positive bacteria, p 465–492. In Vandamme EJ, DeBaets S, Steinbüchel A (ed), Biopolymers, polysaccharides I: polysaccharides from prokaryotes, vol 5 Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 4.Gründling A, Schneewind O. 2007. Genes required for glycolipid synthesis and lipoteichoic acid anchoring in Staphylococcus aureus. J Bacteriol 189:2521–2530. doi: 10.1128/JB.01683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichmann NT, Gründling A. 2011. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett 319:97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schirner K, Marles-Wright J, Lewis RJ, Errington J. 2009. Distinct and essential morphogenic functions for wall- and lipo-teichoic acids in Bacillus subtilis. EMBO J 28:830–842. doi: 10.1038/emboj.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wörmann ME, Corrigan RM, Simpson PJ, Matthews SJ, Gründling A. 2011. Enzymatic activities and functional interdependencies of Bacillus subtilis lipoteichoic acid synthesis enzymes. Mol Microbiol 79:566–583. doi: 10.1111/j.1365-2958.2010.07472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriwaki H, Koide R, Yoshikawa R, Warabino Y, Yamamoto H. 2013. Adsorption of rare earth ions onto the cell walls of wild-type and lipoteichoic acid-defective strains of Bacillus subtilis. Appl Microbiol Biotechnol 97:3721–3728. doi: 10.1007/s00253-012-4200-3. [DOI] [PubMed] [Google Scholar]
- 9.Moriwaki H, Yamamoto H. 2013. Interactions of microorganisms with rare earth ions and their utilization for separation and environmental technology. Appl Microbiol Biotechnol 97:1–8. doi: 10.1007/s00253-012-4519-9. [DOI] [PubMed] [Google Scholar]
- 10.Kiriyama Y, Yazawa K, Tanaka T, Yoshikawa R, Yamane H, Hashimoto M, Sekiguchi J, Yamamoto H. 2014. Localization and expression of the Bacillus subtilis dl-endopeptidase LytF are influenced by mutations in LTA synthases and glycolipid anchor synthetic enzymes. Microbiology 160:2639–2649. doi: 10.1099/mic.0.080366-0. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Miyake Y, Hisaoka M, Kurosawa S, Sekiguchi J. 2008. The major and minor wall teichoic acids prevent the sidewall localization of vegetative dl-endopeptidase LytF in Bacillus subtilis. Mol Microbiol 70:297–310. doi: 10.1111/j.1365-2958.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- 12.Carballido-López R, Formstone A, Li Y, Ehrlich SD, Noirot P, Errington J. 2006. Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev Cell 11:399–409. doi: 10.1016/j.devcel.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Ooiwa S, Sekiguchi J. 2012. Synthetic lethality of the lytE cwlO genotype in Bacillus subtilis is caused by lack of d,l-endopeptidase activity at the lateral cell wall. J Bacteriol 194:796–803. doi: 10.1128/JB.05569-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisicchia P, Noone D, Lioliou E, Howell A, Quigley S, Jensen T, Jarmer H, Devine KM. 2007. The essential YycFG two-component system controls cell wall metabolism in Bacillus subtilis. Mol Microbiol 65:180–200. doi: 10.1111/j.1365-2958.2007.05782.x. [DOI] [PubMed] [Google Scholar]
- 15.Zuber U, Drzewiecki K, Hecker M. 2001. Putative sigma factor SigI (YkoZ) of Bacillus subtilis is induced by heat shock. J Bacteriol 183:1472–1475. doi: 10.1128/JB.183.4.1472-1475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asai K, Ootsuji T, Obata K, Matsumoto T, Fujita Y, Sadaie Y. 2007. Regulatory role of RsgI in sigI expression in Bacillus subtilis. Microbiology 153:92–101. doi: 10.1099/mic.0.29239-0. [DOI] [PubMed] [Google Scholar]
- 17.Tseng CL, Chen JT, Lin JH, Huang WZ, Shaw GC. 2011. Genetic evidence for involvement of the alternative sigma factor SigI in controlling expression of the cell wall hydrolase gene lytE and contribution of LytE to heat survival of Bacillus subtilis. Arch Microbiol 193:677–685. doi: 10.1007/s00203-011-0710-0. [DOI] [PubMed] [Google Scholar]
- 18.Domínguez-Cuevas P, Porcelli I, Daniel RA, Errington J. 2013. Differentiated roles for MreB-actin isologues and autolytic enzymes in Bacillus subtilis morphogenesis. Mol Microbiol 89:1084–1098. doi: 10.1111/mmi.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meisner J, Montero Llopis P, Sham LT, Garner E, Bernhardt TG, Rudner DZ. 2013. FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis. Mol Microbiol 89:1069–1083. doi: 10.1111/mmi.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Elia MA, Millar KE, Beveridge TJ, Brown ED. 2006. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J Bacteriol 188:8313–8316. doi: 10.1128/JB.01336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 22.Anagnostopoulos C, Spizizen J. 1961. Requirements for transformation in Bacillus subtilis. J Bacteriol 81:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto H, Kurosawa S, Sekiguchi J. 2003. Localization of the vegetative cell wall hydrolases LytC, LytE, and LytF on the Bacillus subtilis cell surface and stability of these enzymes to cell wall-bound or extracellular proteases. J Bacteriol 185:6666–6677. doi: 10.1128/JB.185.22.6666-6677.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto H, Mori M, Sekiguchi J. 1999. Transcription of genes near the sspE locus of the Bacillus subtilis genome. Microbiology 145(Part 8):2171–2180. doi: 10.1099/13500872-145-8-2171. [DOI] [PubMed] [Google Scholar]
- 25.Arnaud M, Chastanet A, Débarbouillé M. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, Gram-positive bacteria. Appl Environ Microbiol 70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimotsu H, Henner DJ. 1986. Modulation of Bacillus subtilis levansucrase gene expression by sucrose and regulation of the steady-state mRNA level by sacU and sacQ genes. J Bacteriol 168:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto H, Hashimoto M, Higashitsuji Y, Harada H, Hariyama N, Takahashi L, Iwashita T, Ooiwa S, Sekiguchi J. 2008. Post-translational control of vegetative cell separation enzymes through a direct interaction with specific inhibitor IseA in Bacillus subtilis. Mol Microbiol 70:168–182. doi: 10.1111/j.1365-2958.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Fein JE, Rogers HJ. 1976. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol 127:1427–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeHart HP, Heath HE, Heath LS, LeBlanc PA, Sloan GL. 1995. The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus. Appl Environ Microbiol 61:1475–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollack JH, Neuhaus FC. 1994. Changes in wall teichoic acid during the rod-sphere transition of Bacillus subtilis 168. J Bacteriol 176:7252–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ames BN. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118. doi: 10.1016/0076-6879(66)08014-5. [DOI] [Google Scholar]
- 33.Harwood CR, Hancock IC. 1990. The Bacillus cell envelope and secretion. John Wiley & Sons Ltd., New York, NY. [Google Scholar]
- 34.Min H, Cowman MK. 1986. Combined alcian blue and silver staining of glycosaminoglycans in polyacrylamide gels: application to electrophoretic analysis of molecular weight distribution. Anal Biochem 155:275–285. doi: 10.1016/0003-2697(86)90437-9. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa S, Hara Y, Ohnishi R, Sekiguchi J. 1998. Regulation of a new cell wall hydrolase gene, cwlF, which affects cell separation in Bacillus subtilis. J Bacteriol 180:2549–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margot P, Wahlen M, Gholamhoseinian A, Piggot P, Karamata D. 1998. The lytE gene of Bacillus subtilis 168 encodes a cell wall hydrolase. J Bacteriol 180:749–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarevic V, Soldo B, Médico N, Pooley H, Bron S, Karamata D. 2005. Bacillus subtilis alpha-phosphoglucomutase is required for normal cell morphology and biofilm formation. Appl Environ Microbiol 71:39–45. doi: 10.1128/AEM.71.1.39-45.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varón D, Boylan SA, Okamoto K, Price CW. 1993. Bacillus subtilis gtaB encodes UDP-glucose pyrophosphorylase and is controlled by stationary-phase transcription factor sigma B. J Bacteriol 175:3964–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorasch P, Wolter FP, Zähringer U, Heinz E. 1998. A UDP glucosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1,2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products. Mol Microbiol 29:419–430. doi: 10.1046/j.1365-2958.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 40.Tseng CL, Shaw GC. 2008. Genetic evidence for the actin homolog gene mreBH and the bacitracin resistance gene bcrC as targets of the alternative sigma factor SigI of Bacillus subtilis. J Bacteriol 190:1561–1567. doi: 10.1128/JB.01497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang WZ, Wang JJ, Chen HJ, Chen JT, Shaw GC. 2013. The heat-inducible essential response regulator WalR positively regulates transcription of sigI, mreBH and lytE in Bacillus subtilis under heat stress. Res Microbiol 164:998–1008. doi: 10.1016/j.resmic.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Salzberg LI, Powell L, Hokamp K, Botella E, Noone D, Devine KM. 2013. The WalRK (YycFG) and σI RsgI regulators cooperate to control CwlO and LytE expression in exponentially growing and stressed Bacillus subtilis cells. Mol Microbiol 87:180–195. doi: 10.1111/mmi.12092. [DOI] [PubMed] [Google Scholar]
- 43.Schirner K, Errington J. 2009. The cell wall regulator σI specifically suppresses the lethal phenotype of mbl mutants in Bacillus subtilis. J Bacteriol 191:1404–1413. doi: 10.1128/JB.01497-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.