ABSTRACT
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), remains a significant cause of morbidity and mortality worldwide, despite the availability of a live attenuated vaccine and anti-TB antibiotics. The vast majority of individuals infected with M. tuberculosis develop an asymptomatic latent infection in which the bacterium survives within host-generated granulomatous lesions in a physiologically altered metabolic state of nonreplicating persistence. The granuloma represents an adverse environment, as M. tuberculosis is exposed to various stressors capable of disrupting the essential constituents of the bacterium. In Gram-negative and Gram-positive bacteria, resistance to cell envelope stressors that perturb the plasma membrane is mediated in part by proteins comprising the phage shock protein (Psp) system. PspA is an important component of the Psp system; in the presence of envelope stress, PspA localizes to the inner face of the plasma membrane, homo-oligomerizes to form a large scaffold-like complex, and helps maintain plasma membrane integrity to prevent a loss of proton motive force. M. tuberculosis and other members of the Mycobacterium genus are thought to encode a minimal functional unit of the Psp system, including an ortholog of PspA. Here, we show that Rv2744c possesses structural and physical characteristics that are consistent with its designation as a PspA family member. However, although Rv2744c is upregulated under conditions of cell envelope stress, loss of Rv2744c does not alter resistance to cell envelope stressors. Furthermore, Rv2744c localizes to the surface of lipid droplets in Mycobacterium spp. and regulates lipid droplet number, size, and M. tuberculosis persistence during anaerobically induced dormancy. Collectively, our results indicate that Rv2744c is a bona fide ortholog of PspA that may function in a novel role to regulate lipid droplet homeostasis and nonreplicating persistence (NRP) in M. tuberculosis.
IMPORTANCE Mycobacterium tuberculosis is the causative agent of tuberculosis, a disease associated with significant morbidity and mortality worldwide. M. tuberculosis is capable of establishing lifelong asymptomatic infections in susceptible individuals and reactivating during periods of immune suppression to cause active disease. The determinants that are important for persistent infection of M. tuberculosis or for reactivation of this organism from latency are poorly understood. In this study, we describe our initial characterizations of Rv2744c, an ortholog of phage shock protein A (PspA) that regulates the homeostasis of lipid bodies and nonreplicating persistence in M. tuberculosis. This function of PspA in M. tuberculosis is novel and suggests that PspA may represent a unique bacterial target upon which to base therapeutic interventions against this organism.
INTRODUCTION
Mycobacterium tuberculosis is the causative agent of the respiratory disease tuberculosis (TB) and is a human-specific pathogen of global importance. This bacterium is responsible for >9.6 million new cases of TB and 1.5 million deaths annually (1), ranking TB as the leading cause of death in the world due to an infectious agent, alongside HIV/AIDS. A key aspect of the M. tuberculosis life cycle is its ability to establish asymptomatic latent infections and reactivate to cause active disease and transmission to new hosts (reviewed in reference 2). During latency, M. tuberculosis exists in a state of nonreplicating persistence (NRP), a poorly understood physiological state in which the bacterium can utilize alternative carbon sources and energy-generating pathways for long-term survival within the host (3–6). While a live attenuated vaccine for TB is available (Mycobacterium bovis bacillus Calmette-Guérin [BCG]), its efficacy at preventing adult pulmonary TB and thus M. tuberculosis retransmission is highly varied (7, 8). Furthermore, M. tuberculosis exhibits increased phenotypic resistance to anti-TB antibiotics during NRP (2, 9), making it more difficult to kill this organism in latently infected individuals. Therefore, new strategies and/or therapeutics are urgently needed to help eradicate TB. This will require an improved understanding of the mechanisms utilized by M. tuberculosis to persist and/or reactivate within the host.
A hallmark of latent M. tuberculosis infection is the formation of host granulomatous lesions at sites of infection (10). Within the granuloma, M. tuberculosis is exposed to numerous environmental and/or biological “stressors” that alone, or in combination, are capable of damaging essential constituents of the bacterium. These stressors include low oxygen tension (5), limited nutrient availability (11), reactive oxygen and nitrogen species (12), and cell envelope-perturbing agents (13, 14). The recognition of and successful adaptation to these stressors in M. tuberculosis are mediated by the activities of regulatory proteins, including two-component signal transduction systems, extracytoplasmic function sigma factors, serine-threonine protein kinases, and other stress-responsive elements. MprAB, SigE, and PknB are three such regulatory factors that collectively mediate the resistance of M. tuberculosis to cell envelope stressors (reviewed in references 15 and 16). MprAB is a two-component signal transduction system that recognizes misfolded or unfolded proteins in the extracytoplasmic compartment (17). This system regulates a diverse set of >300 genes, including determinants encoding sigma factors, regulatory proteins, chaperones, proteases, and metabolic enzymes (18). SigE is an extracytoplasmic function sigma factor that controls a regulon of >70 genes in response to a variety of cell envelope-perturbing agents (19, 20). Finally, PknB is a serine-threonine protein kinase that regulates cell envelope stress resistance through posttranslational modifications (phosphorylation) of target substrates (21, 22). Recent evidence indicates that the stress-responsive pathways controlled by MprAB, SigE, and PknB are integrated at multiple levels. For example, MprAB directly regulates sigE expression (18), while SigE directly regulates the operon encoding MprA-MprB-PepD-MoaB2 (20). Similarly, PknB phosphorylates the SigE anti-sigma factor RseA, leading to the degradation of RseA by the ClpC1P2 proteases and the subsequent release of SigE for downstream gene regulation, including at the mprAB operon (21). However, in addition to this higher level integration, a subset of downstream determinants within each regulon are shared and are directly or indirectly coregulated by MprAB, SigE, and PknB. One such locus is the four-gene operon encoding Rv2745c (ClgR)-Rv2744c-Rv2743c-Rv2742c (20, 21, 23), which recently was predicted to encode a minimal functional unit of an M. tuberculosis phage shock protein (Psp) system (24).
In Gram-negative and Gram-positive bacteria, the Psp system helps maintain homeostasis of the plasma membrane under conditions of cell envelope stress (25). PspA is a key component of the Psp system, acting to repress or activate the Psp response depending on the stress status of the cell. In the absence of stress, PspA localizes to the cytoplasmic compartment, where it binds to and negatively regulates PspF, a transcription factor controlling the psp operon (pspABCDE) and other PspA regulon members (26–28). In the presence of stress, PspA dissociates from PspF, relocalizes to the inner face of the plasma membrane, binds to other membrane-associated components of the Psp system, and homo-oligomerizes to form a higher-order scaffold-like structure to physically stabilize the plasma membrane and help prevent the loss of proton motive force (29). In M. tuberculosis, Rv2744c has been predicted to encode an ortholog of PspA (30). Rv2744c contains a PspA/IM30 domain, and it has been shown to interact with itself (31) and other components of the proposed M. tuberculosis Psp system, including transcription factor Rv2745c (ClgR) and Rv2743 (a protein of unknown function) (24). However, apart from these observations, the function and/or role of Rv2744c have yet to be elucidated. In this report, we present evidence demonstrating that Rv2744c shares structural and physical features consistent with its designation as a PspA family member. However, while Rv2744c is upregulated in M. tuberculosis upon exposure to cell envelope stress, loss of this determinant does not alter resistance to cell envelope perturbants. Furthermore, Rv2744c localizes to the surface of lipid droplets (LDs) and regulates the characteristics of these quasiorganelles, including their number and size. In addition, dysregulation of Rv2744c production negatively impacts the survival of M. tuberculosis in vitro under conditions of NRP. Based on these observations, we propose that Rv2744c is a PspA ortholog that may function in a novel manner in M. tuberculosis relative to other archetypical PspA family members.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
All strains, plasmids, and mycobacteriophages used in the study are described in Table 1. Escherichia coli TOP10 or DH5α was used for cloning. E. coli BL21(DE3)/pLysS (EMD Millipore, Billerica, MA) was used to overexpress and purify recombinant proteins following induction with isopropyl-β-d-thiogalactopyranoside (IPTG) (Thermo Fisher Scientific, Waltham, MA). E. coli BTH101 was used for bacterial adenylate cyclase two-hybrid assays. E. coli HB101 was used as a recipient for transduction with in vitro-packaged lambda phages. E. coli strains were grown in Luria-Bertani (LB) broth, LB agar (Thermo Fisher Scientific), or MacConkey base agar medium (BD, Franklin Lakes, NJ) containing maltose as the sole carbon source. The medium was supplemented with 100 μg/ml ampicillin (Thermo Fisher Scientific), 50 μg/ml kanamycin sulfate (Thermo Fisher Scientific), 150 μg/ml hygromycin B (AG Scientific, San Diego, CA), or 25 μg/ml chloramphenicol (Thermo Fisher Scientific) when necessary. The Mycobacterium strains used in this study were from ATCC (Manassas, VA) and are derivatives of Mycobacterium tuberculosis H37Rv (ATCC 27294) or Mycobacterium smegmatis mc2155 (ATCC 700084). For routine culturing, Mycobacterium strains were grown with shaking (150 rpm) at 37°C in Middlebrook 7H9 broth (BD) or on Middlebrook 7H10 agar medium (BD) supplemented with 0.5% glycerol, 10% albumin-dextrose-catalase (ADC) or oleic acid-ADC (OADC) (BD), and 0.05% Tween 80 (Sigma, St. Louis, MO), unless otherwise noted. To prepare lipid droplets, Mycobacterium strains were grown with shaking at 37°C in Sauton's medium (32). When required, the mycobacterial medium was supplemented with 25 μg/ml kanamycin sulfate (Thermo Fisher Scientific), 50 μg/ml hygromycin B (AG Scientific), and/or 10 ng/ml anhydrotetracycline (Sigma). Mycobacteriophage production and transduction of M. tuberculosis H37Rv were described previously (33). Electrocompetent cells of M. tuberculosis were prepared as described previously (34).
TABLE 1.
Strains and plasmids used in this study
| Strain, plasmid, or mycobacteriophage | Genotype or descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | F− ϕ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ thi-1 gyrA96 relA1 | Lab collection |
| E. coli TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL str endA1 nupG | Lab collection |
| E. coli BL21(DE3)/pLysS | F− ompT hsdSB(rB− mB−) gal dcm (DE3); contains pLysS | Lab collection |
| E. coli HB101 | F− Δ(gpt-proA)62 leuB6 glnV44 ara-14 galK2 lacY1 Δ(mcrC-mrr) rpsL20 str xyl-5 mtl-1 recA13 thi-1 | Lab collection |
| M. tuberculosis H37Rv | Laboratory strain | ATCC 27294 |
| M. smegmatis mc2155 | Laboratory strain | ATCC 700084 |
| TCZ1441 | M. smegmatis containing pTZ1001 | Lab collection |
| TCZ1820 | E. coli BL21(DE3)/pLysS containing pTZ1168 | This study |
| TCZ2054 | M. smegmatis ΔMSMEG_2695 | This study |
| TCZ2365 | M. smegmatis ΔMSMEG_2695 containing pTZ1227 | This study |
| TCZ2455 | M. smegmatis ΔMSMEG_2695 attB::pTZ1343 containing pTZ1227 | |
| TCZ2469 | M. smegmatis ΔMSMEG_2695 containing pTZ1167 | This study |
| TCZ2654 | M. smegmatis ΔMSMEG_2695 containing pTZ1489 | This study |
| TB284 | M. tuberculosis H37Rv ΔRv2744c | This study |
| TB303 | M. tuberculosis H37Rv ΔRv2744c containing pTZ1227 | This study |
| TB314 | M. tuberculosis H37Rv ΔRv2744c containing pTZ1463 | This study |
| Plasmids | ||
| CT158 | pMV261 containing GFPmut3 gene; Kanr | Eric Rubin |
| pCR2.1-TOPO | 3.9-kb plasmid for cloning PCR products; Ampr Kanr | Thermo Fisher Scientific |
| pET24b | 3.5-kb plasmid allowing N-terminal protein fusion to 6×-His; Ampr | EMD Biosciences |
| pKT25 | 3.4-kb plasmid allowing N-terminal protein fusion to T25 fragment; Kanr | Euromedex |
| pSE100 | TetR-regulated protein expression vector; Hygr | 37 |
| pTZ898 | pYUB854 containing sacB from pYUB870; Kanr | 31 |
| pTZ1001 | pMV261 expressing gfp; Kanr | Lab collection |
| pTZ1167 | pSE100 expressing 3×FLAG-6×His; Hygr | This study |
| pTZ1168 | pET-24b containing Rv2744c coding sequence cloned into NheI-NotI site; Kanr | 31 |
| pTZ1182 | pUT18c expressing Rv2744c coding sequence; Ampr | 31 |
| pTZ1183 | pUT18C containing Rv2744c coding sequence; Ampr | 31 |
| pTZ1185 | pKT25 expressing Rv2744c coding sequence; Kanr | 31 |
| pTZ1227 | pSE100 expressing 3×FLAG-Rv2744c-6×His; Hygr | This study |
| pTZ1231 | pTZ898 containing MS_2695 deletion sequence cloned into XbaI site; Hygr | This study |
| pTZ1246 | pYUB854 containing 1.0-kb fragments upstream and downstream of Rv2744c flanking Hygr cassette; Hygr | This study |
| pTZ1343 | pMV361 expressing tetR; Kanr | This study |
| pTZ1345 | pKT25 expressing Rv2745c (clgR) coding sequence; Ampr | This study |
| pTZ1346 | pUT18c expressing Rv2745c (clgR) coding sequence; Ampr | This study |
| pTZ1364 | pUT18 expressing Rv2745c (clgR) coding sequence; Ampr | This study |
| pTZ1379 | pUT18c expressing Rv2743c coding sequence; Ampr | This study |
| pTZ1380 | pUT18c expressing Rv2743c coding sequence; Ampr | This study |
| pTZ1463 | pSE100 containing Rv2744c-GFPmut3 gene; Hygr | This study |
| pTZ1489 | pSE100 expressing Rv2744c; Hygr | This study |
| pUT18 | 3.0-kb plasmid allowing C-terminal protein fusion to T18 fragment; Ampr | Euromedex |
| pUT18C | 3.0-kb plasmid allowing N-terminal protein fusion to T18 fragment; Ampr | Euromedex |
| pYUB854 | 3.9-kb cosmid for generating gene deletions in Mycobacterium; carries Hygr cassette flanked by multiple cloning sites and λpac site; Hygr | 33 |
| Mycobacteriophages | ||
| phAE87 | Derivative of conditionally replicating mycobacteriophage PH101(ts) | 33 |
| phTZ1246 | phAE87 derivative containing pTZ1246 | This study |
Kanr, kanamycin resistant; Ampr, ampicillin resistant; Hygr, hygromycin resistant.
DNA manipulations.
Restriction enzyme digestion, cloning, subcloning, and DNA electrophoresis were done according to standard techniques (35). Oligonucleotides and primers were synthesized by Eurofins MWG Operon (Huntsville, AL) and are listed in Table S1 in the supplemental material. PCR was performed using Platinum Pfx or Taq polymerase (Thermo Fisher Scientific, Waltham, MA). All amplified products were first cloned into pCR2.1-TOPO (Thermo Fisher Scientific) and sequenced (MCLAB, San Francisco, CA) to confirm the absence of mutations. Cloned inserts were then removed by restriction enzyme digestion (NEB, Ipswich, MA), resolved on a 1% agarose gel, recovered by gel purification, and subcloned into the appropriate vector. Ligations were performed using T4 DNA ligase (NEB). When necessary, plasmid DNA was treated with Antarctic phosphatase (NEB) to prevent religation of the vector ends. Plasmid DNA was prepared using the QIAprep spin miniprep kit (Qiagen, Venlo, the Netherlands), as recommended by the manufacturer. DNA fragments were purified using either the QIAquick gel extraction kit or QIAquick PCR purification kit (Qiagen).
DNA and RNA extraction and RT-PCR.
Mycobacterial genomic DNA was isolated as described previously (34). For isolation of RNA, M. tuberculosis or M. smegmatis was grown in 7H9 or Sauton's broth to mid-log phase (optical density at 600 nm [OD600], 0.5). RNA was fixed by treating cultures with RNAlater (Thermo Fisher Scientific, Waltham, MA) at 4°C prior to processing. Bacteria were collected by centrifugation, washed twice with phosphate-buffered saline (PBS), and then mechanically disrupted in the presence of RNA-Bee (Tel-Test, Friendswood, TX) by bead beating. RNA was then extracted with chloroform, precipitated with isopropanol, and stored at −20°C until use. The resulting RNA was suspended in diethylpyrocarbonate (DEPC)-treated water, treated with Turbo DNase (Thermo Fisher Scientific) to remove contaminating genomic DNA, and purified using an RNeasy mini prep column (Qiagen, Venlo, the Netherlands). For reverse transcription-PCR (RT-PCR), cDNA from M. tuberculosis or M. smegmatis strains was prepared by incubating 500 ng of total RNA with random M. tuberculosis decamers (36) in a reverse transcription reaction mixture containing SuperScript III reverse transcriptase (Thermo Fisher Scientific). To test for the presence of DNA contamination, identical reaction mixtures lacking SuperScript III reverse transcriptase were also run. The reaction mixtures included primer sets that amplified 100- to 150-bp fragments from specific coding sequences. Amplification conditions consisted of 40 rounds of denaturation at 95°C for 30 s and annealing/extension at 60°C for 15 s using iQ SYBR green Supermix (Bio-Rad, Hercules, CA). The reactions were run on an iCycler iQ real-time PCR detection system (Bio-Rad). Single PCR products were confirmed by performing a postamplification melting curve analysis for each reaction. For relative quantification of transcript levels, the expression of target genes was normalized to the gene encoding the constitutively expressed SigA sigma factor. Absolute quantification of transcript levels was performed by preparing a standard curve for each primer set using 10-fold serial dilutions of chromosomal DNA as the template. The transcript number of the desired gene was then normalized to sigA, whose expression was set at 1.0.
Protein extraction, SDS-PAGE, and Western blotting.
Proteins were extracted from E. coli or Mycobacterium spp. by French press or bead-beating. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), protein-containing fractions were incubated with 2× SDS-PAGE loading dye and denatured for 5 min at 95°C. The proteins were then separated on 12% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes using the Trans-Blot Turbo RTA transfer kit and Trans-Blot Turbo transfer system (Bio-Rad, Hercules, CA). For Western blotting, membranes were blocked in TTBS (20 mM Tris-HCl [pH 7.5], 500 mM NaCl, 0.5% Tween 20) containing 5% skim milk for 1 h and probed with antiserum diluted in TTBS overnight at 4°C. The primary antibodies included anti-His (1:1,500) (Covance, Princeton, NJ), anti-FLAG (1:3,000) (Sigma, St. Louis, MO), anti-MprB (1:50,000) (Covance), anti-GroES (1:3,000) (BEI Resources, National Institute of Allergy and Infectious Diseases [NIAID], NIH), and anti-green fluorescent protein (anti-GFP) (1:5,000) (Covance). Membranes were then washed in TTBS and incubated for 2 h at room temperature with goat anti-mouse IgG (1:5,000) (Thermo Fisher Scientific, Waltham, MA) or goat anti-rabbit IgG (1:5,000) secondary antibody conjugated to horseradish peroxidase. Blots were developed using the SuperSignal West Pico chemiluminescent substrate kit (Thermo Fisher Scientific) and visualized on CL-Xposure X-ray film (Thermo Fisher Scientific).
Plasmid construction.
To generate the vector for Rv2744c protein production, Rv2744c was amplified from M. tuberculosis genomic DNA by PCR using primers Rv2744cFWD-NheI and Rv2744cRev4-NotI. The product was cloned into pCR2.1-TOPO, digested with NheI and NotI, and subcloned into the same sites of pET24b. The resulting plasmid (pTZ1168) was then transformed into E. coli BL21(DE3)/pLysS to generate TCZ1820. To generate the vector for MS_2695 deletion, 1.0-kb regions upstream and downstream of MS_2695 were individually amplified from M. smegmatis mc2155 genomic DNA using the primer pairs Ms2695kupF and Ms2695kupR, and Ms2695kdnF and Ms2695kdnR. The resulting PCR products were then purified and assembled into a contiguous fragment by overlap PCR using primers Ms2695kupF and Ms2695kdnR. The product was cloned into pCR2.1-TOPO, released following digestion with XbaI, and subcloned into the XbaI site of pTZ898 (31) to generate pTZ1231. To generate the vector for Rv2744c deletion, 1.0-kb regions upstream and downstream of the Rv2744c coding sequence were PCR amplified from M. tuberculosis H37Rv genomic DNA using the primer pairs Rv2744kupF and Rv2744kupR2, and Rv2744kdnF2 and Rv2744kdnR2, respectively. The resulting products were cloned into pCR2.1-TOPO, released following digestion with SpeI-XhoI and XbaI-EcoRI, respectively, and fragments directionally subcloned into the corresponding sites flanking the res-hyg-res cassette present in pYUB854 (33). The resulting plasmid, pTZ1246, was then used to prepare high-titer transducing phage for allelic exchange. To generate the Rv2744c complementation plasmid, the Rv2744c coding sequence along with N-terminal 3× FLAG and C-terminal 6×His epitope tags were amplified from pTZ1171 (31) using primers pET24fwd-PstI and pET24rev-HindIII, cloned into pCR2.1-TOPO, released by digestion with PstI-HindIII, and directionally subcloned into the corresponding sites of Mycobacterium expression construct pSE100 (37), resulting in pTZ1227. The untagged wild-type allele of Rv2744c was also amplified from M. tuberculosis genomic DNA using primers Rv2744cFpSE100PstI and Rv2744cRpSE100EcoRV, cloned into pCR2.1-TOPO, released by digestion with PstI-EcoRV, and directionally subcloned into the corresponding sites of pSE100, resulting in pTZ1489. To generate the vector producing TetR, the tet(R) coding sequence was amplified from pMC1s (37) using primers TetRF-EcoRI and TetRR-ClaI, cloned into pCR2.1-TOPO, released following digestion with EcoRI and ClaI, and directionally cloned into the corresponding sites of pMV361, resulting in pTZ1343. Finally, to generate the vector producing Rv2744c-GFP, the coding sequence for Rv2744c was amplified from M. tuberculosis genomic DNA using primers 2744cF-OVLP and 2744cR-OVLPGFPm3 and the coding sequence for GFP mutant GFPmut3 was amplified from CT158 (a kind gift from Eric Rubin) using primers GFPm3F-OVLP and GFPm3R-OVLP. The two fragments were joined by overlapping PCR using primers 2744cF-OVLP and GFPm3R-OVLP, and the resulting product was cloned into pCR2.1-TOPO, released by digestion with PacI, and subcloned into the corresponding site of pSE100. The resulting vector, pTZ1463, was then electroporated into M. tuberculosis ΔRv2744c, resulting in TB314.
Purification of Rv2744c.
M. tuberculosis Rv2744c was purified from E. coli using Ni affinity chromatography. Briefly, TCZ1820 was grown in LB with selection at 37°C until cells reached mid-exponential phase. Protein was then induced with 1.0 mM IPTG for 3 h at 37°C. Following induction, cultures were centrifuged and the resulting bacterial pellet suspended in lysis buffer (50 mM Na2HPO4 [pH 8.0], 300 mM NaCl, 10 mM imidazole, 3 μg DNase/ml, 3 μg RNase/ml, and protease inhibitor cocktail [Sigma, St. Louis, MO]) before lysis with a French pressure cell. The total cell lysate was then clarified by centrifugation at 25,000 × g for 30 min, rocked with nickel-nitrilotriacetic acid (NTA)-agarose (Qiagen, Venlo, the Netherlands) for 90 min at 4°C, and then processed by batch purification. Bound protein was washed with binding buffer (50 mM Na2HPO4 [pH 8.0], 300 mM NaCl, 10 mM imidazole) and then wash buffer (50 mM Na2HPO4 [pH 8.0], 300 mM NaCl, 25 mM imidazole). Finally, NTA resin was sequentially incubated with 1-ml aliquots of elution buffer (50 mM Na2HPO4 [pH 8.0], 300 mM NaCl, 150 mM imidazole) to remove protein from the resin. Protein purity was determined by subjecting recovered fractions to SDS-PAGE and staining with Coomassie brilliant blue (Thermo Fisher Scientific, Waltham, MA).
Sucrose gradient ultracentrifugation.
The molecular mass of purified Rv2744c was determined by sucrose gradient ultracentrifugation. Briefly, a Gradient Master (BioComp Instruments, Fredericton, Canada) was used to generate linear 10% to 50% sucrose gradients prepared in protein elution buffer. Approximately 0.5 mg of purified Rv2744c-His, or protein standards, including bovine serum albumin (66 kDa), β-amylase (200-kDa), thyroglobulin (669 kDa), and blue dextran (2,000 kDa) (all from Sigma, St. Louis, MO), were layered on top of individual gradients and enriched by ultracentrifugation in a SW55 Ti rotor (Beckman Coulter, Brea, CA) at 100,000 × g for 16 h at 4°C. Half-milliliter fractions were then removed sequentially from the tops of tubes and collected to quantify the amount of protein at A280 using a NanoDrop (Thermo Fisher Scientific, Waltham, MA) or to determine protein identity by SDS-PAGE, followed by Western blotting.
Bacterial two-hybrid assays.
Specific protein-protein interactions were investigated using the bacterial two-hybrid system BACTH (Euromedex, Souffelweyersheim, France), as per the manufacturer's directions. BACTH vectors expressing Rv2744c have been described, including pTZ1182, pTZ1183, and pTZ1185 (31). To generate BACTH vectors expressing Rv2745c (clgR) or Rv2743c, the coding sequence for these genes was PCR amplified from M. tuberculosis H37Rv genomic DNA using the primer pairs 2745cXbaI-F2H and 2745cKpnI-R2H, and FBamH1Rv2743c and RKpn1Rv2743c, respectively. The resulting products were cloned into pCR2.1-TOPO, released using XbaI-KpnI (for Rv2745c) or BamHI-KpnI (for Rv2743c), and directionally subcloned into BACTH vectors pKT25, pUT18, or pUT18c digested with the same enzymes, resulting in pTZ1345, pTZ1346, pTZ1364, pTZ1379, and pTZ1380. Vector combinations were then cotransformed into the E. coli BTH101 reporter strain, and the isolated transformants were streaked onto MacConkey/maltose agar medium containing ampicillin and kanamycin. Plates were incubated at 30°C for 48 to 72 h, and bacteria were subsequently screened for the presence of a red colony phenotype indicative of protein-protein interactions. Two-hybrid vectors expressing DNA sequences coding for leucine zipper motifs (T25-zip and T18-zip) were included as positive controls, while negative controls were performed with the empty T18 or T25 plasmids.
Homology modeling, BLAST searches, and phylogenetic analyses.
PspA orthologs were identified via PSI-BLAST search (blast.ncbi.nlm.nih.gov) using the M. tuberculosis Rv2744c, Escherichia coli PspA, or Arabidopsis thaliana Vipp1 protein sequence as a query. Searches were directed at bacteria phyla that included Cyanobacteria, Actinobacteria, Spirochaetes, Fusobacteria, Acidobacteria, and Proteobacteria. The proteins were considered potential PspA orthologs and included in phylogenetic analyses if they either had an E value of <1 × 10−3 or possessed a PspA/IM30 domain. Representative hits were aligned using Clustal W (38) and analyzed for sequence homology via MegAlign (Lasergene software; DNAStar, Madison, WI) to generate a phylogenetic tree. De novo protein modeling was carried out using the Rosetta 3.5 ab initio protein modeling application (rosettacommons.org) for selection of the lowest-energy models (according to the Rosetta scoring function) from >10,000 predicted structures for both M. tuberculosis Rv2744c and E. coli PspA.
Construction of M. smegmatis and M. tuberculosis deletion mutants and complemented strains.
The coding sequence for MS_2695 in M. smegmatis mc2155 was deleted using a standard two-step homologous recombination procedure, as described previously (15). Briefly, pTZ1231 was electroporated into wild-type M. smegmatis mc2155, and hygromycin-resistant (Hygr) transformations selected. Following confirmation of pTZ1231 integration, the resulting merodiploids were grown in 7H9 broth without selection, diluted, and plated onto 7H10 agar medium containing 10% sucrose to identity derivatives that had undergone the second crossover event. Recombinants that were sucrose resistant and hygromycin sensitive were then screened by PCR to confirm the loss of MS_2695 using primers Sm2695F and Sm2695R, resulting in strain TCZ2054. The unmarked M. tuberculosis ΔRv2744c mutant was generated by specialized transduction, essentially as described previously (33). Briefly, pTZ1246 was digested with PacI, ligated to PacI-digested concatemers of phAE87, and packaged into λ heads using the GIGAPackIII in vitro λ-packaging system (Agilent Technologies, Santa Clara, CA). The resulting phage particles were transduced into E. coli HB101, and hygromycin-resistant transformants were selected. Phasmid DNA was prepared from pooled transductants, electroporated into M. smegmatis mc2155 at the permissive temperature of 30°C, and the resulting plaques were purified and screened at the nonpermissive temperature of 38°C to verify the temperature-sensitive phenotype. High-titer specialized transducing phage lysates were then prepared and used to transduce M. tuberculosis H37Rv at a nonpermissive temperature using a multiplicity of infection of 10:1 (phage to bacteria). Hygromycin-resistant transductants were grown for 3 to 4 weeks, streak isolated onto selective medium, and screened for the desired allelic exchange event by PCR using primers Rv2744cF100 and Rv2744cR100. Confirmed deletion mutants were unmarked by electroporation with pYUB870 (33). Following counterselection on medium containing 10% sucrose, the resulting sucrose-resistant, kanamycin-sensitive, and hygromycin-sensitive recombinants were screened for unmarking of the Rv2744c mutation by PCR using primers Rv2744cF100 and Rv2744cR100, resulting in strain TB284. To complement the unmarked deletions in MS_2695 and Rv2744c, pTZ1227 was electroporated into TCZ2054 or TB284, resulting in strain TCZ2365 or TB303, respectively. Expression of 3×FLAG-Rv2744c-His in these strains was then confirmed by Western blotting using α-FLAG and α-His antibodies.
In vitro survival assays.
The sensitivity of Mycobacterium spp. to in vitro stressors was assessed using standard survival assays or MIC growth assays. M. tuberculosis or M. smegmatis derivatives were grown to mid-log phase in 7H9 medium, diluted in fresh medium to an OD600 of 0.02, and exposed to specific stressors, including SDS (0.5% for M. tuberculosis and 0.1% for M. smegmatis), H2O2 (1.25 mM to 80 mM), thioridazine (4 μg/ml to 256 μg/ml), vancomycin (0.5 μg/ml to 32 μg/ml), ethanol (0.3% to 5.0%), and NaCl (500 mM and 1 M). Bacterial survival was then measured by removing aliquots at specific times and plating for viable CFU on agar medium (survival assay) or by measuring culture growth at specific times postexposure (MIC assay) using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). To assess the survival of M. tuberculosis derivatives during NRP, strains were grown using the rapid anaerobic dormancy (RAD) model, with slight modifications (39). Briefly, wild-type M. tuberculosis H37Rv, TB284, or TB303 was grown in Sauton's medium to mid-exponential phase and diluted to an OD600 of 0.05 in Sauton's medium. A 6.7-ml aliquot of culture was then dispensed into individual 16 by 100-mm blood collection tubes (product no. 366441; BD, Sparks, MD), each containing a 3 by 10-mm micro-stir bar (product no. 1451365; Thermo Fisher Scientific, Waltham, MA). The tubes were tightly sealed with rubber stoppers, resulting in a defined headspace of 0.5 within each tube. Control tubes lacking bacteria or containing 9 μg/ml methylene blue to monitor oxygen depletion in the culture medium were also included for each strain assayed. The cultures were stirred at 200 rpm on a four-position micro-stir plate (Wheaton, Millville, NJ) that was placed inside a 37°C incubator. At defined time points, individual sacrificial tubes were opened and the CFU quantified on 7H10 agar medium. For each time point, three sacrificial tubes were processed for each strain assayed. Nutrient stress was induced by growing M. tuberculosis cultures to mid-log phase in Sauton's medium, washing in PBS, and then incubating bacteria in 1× PBS containing 0.05% tyloxapol (Thermo Fisher Scientific) at 37°C with shaking. At various times poststarvation, aliquots were removed, diluted, and plated on agar medium to quantify viable bacteria.
Lipid droplet purification, subcellular fractionation, and protein localization.
Lipid droplets (LDs) were isolated from 1.0-liter cultures of M. smegmatis derivatives after growth in Sauton's medium for 1 week at 37°C with shaking, in accordance with previously described protocols (27, 40, 41). Bacteria were pelleted, suspended in 20 ml of buffer A (25 mM tricine, 250 mM sucrose [pH 7.8]), and then incubated on ice for 30 min. Bacteria were lysed via French press, and bacterial debris was removed following centrifugation for 10 min at 3,700 × g. Sixteen milliliters of the resulting total cell lysate was then equally divided into two ultracentrifuge tubes, and 2 ml of buffer B (20 mM HEPES, 100 mM KCl, 2 mM MgCl2 [pH 7.4]) was carefully layered on top. Tubes were then spun at 100,000 × g for 1 h at 4°C using an SW-41 rotor, and the buoyant upper layer containing LDs was removed, transferred to a new ultracentrifuge tube, mixed with 2 to 5 ml of buffer B, and centrifuged for an additional 1 h at 100,000 × g and 4°C using an SW-55 Ti rotor. LDs were again removed, placed into an Eppendorf tube, washed 3 times with 0.2 ml of buffer B, and pelleted in a tabletop centrifuge at maximum speed. The isolated LDs were then suspended in buffer B for use in downstream applications. To isolate cytoplasmic proteins, the solution underlying the LD layer following the first ultracentrifugation step was removed, transferred to new ultracentrifuge tubes, and centrifuged for an additional hour at 100,000 × g and 4°C using an SW-55 Ti rotor to pellet residual membrane-associated proteins. The resulting supernatant was removed, transferred to a new tube, and designated the cytoplasmic fraction. Finally, to isolate membrane proteins, the insoluble pellet remaining in the bottom of the tube following the first ultracentrifugation was washed 3 times with PBS containing 0.05% Tween 20 and then solubilized in PBS containing 1% Triton X-100 overnight at 4°C with gentle rocking. The resulting material was designated the membrane fraction.
Triglyceride quantification of LDs.
To quantify the triacylglycerol concentration in LDs, total protein concentration of the isolated LDs was first determined using a Qubit protein assay kit (Thermo Fisher Scientific, Waltham, MA). Samples were then adjusted so that the final protein concentration was 25 μg/ml. Ten microliters of each sample was processed using a serum triglyceride kit (Cell BioLabs, San Diego, CA), as per manufacturer's recommendations, and run alongside triglyceride standards that were prepared in parallel. After incubation, plates were read at an absorbance of 570 nm, values from replicate wells were averaged, and the triacylglycerol concentration was calculated.
Epifluorescence, immunofluorescence, and electron microscopy.
Epifluorescence microscopy was used to visualize Rv2744c-GFP in M. tuberculosis ΔRv2744c. Briefly, TB314 was grown in Sauton's medium for 1 week. Bacteria were then pelleted and suspended in 4% paraformaldehyde (PFA) for 1 h at 4°C to fix the bacteria. Fixed bacteria were washed 3 times with PBS, and aliquots were plated on agar medium to confirm culture sterilization. Once confirmed, samples were removed from the biosafety level 3 (BSL3) laboratory and incubated with 1 mg/ml Nile red for 1 h in the dark at room temperature to stain the LDs. Bacteria were then washed 3 times in PBS and mounted on slides using a glass coverslip and ProLong Gold antifade reagent (Thermo Fisher Scientific, Waltham, MA). To visualize LDs by immunofluorescence, LDs were isolated from TCZ2365, as described previously, and then immobilized onto glass slides coated with 0.01% poly-l-lysine. LDs were fixed with 4% PFA for 15 min at room temperature, washed 3 times in PBS, permeabilized with 0.1% Triton X-100 for 1 h at room temperature, washed 3 times in PBS, and then blocked with PBS containing 10% bovine serum albumin (BSA) for 1 h at room temperature. LDs were incubated with a 1:1,000 dilution of anti-FLAG antibody (Sigma, St. Louis, MO) for 1 h at room temperature, washed 3 times in PBS–10% BSA, and then incubated with a 1:2,000 dilution of anti-mouse IgG–Alexa Fluor 488 (Thermo Fisher Scientific) for 1 h at room temperature. Finally, LDs were washed 3 times in PBS–10% BSA and then 3 times in PBS. Coverslips were mounted onto glass slides using the ProLong Gold antifade reagent. Bacteria and LDs were observed with a Nikon CFI Plan Apochromat VC 100×/1.40-numerical-aperture oil immersion lens mounted on a Nikon Eclipse 80i upright microscope outfitted with a multifluorescence filter set and Nikon DS-2MBW monochromatic digital camera (Nikon, Inc., Melville, NY). Images were captured using the NIS-Elements software (Nikon, Inc.). For electron microscopy (EM) of LDs within bacteria, M. smegmatis derivatives were grown in Sauton's medium for 1 week, pelleted, washed 2 times in 100 mM sodium cacodylate buffer, and fixed in a 4% glutaraldehyde–2% PFA solution overnight. Samples were then immobilized into epoxy resin and sections viewed on a Hitachi H600 transmission electron microscope (TEM) equipped with an AMT digital camera. For EM of purified Rv2744c-His, an aliquot of protein from fraction 17 of the sucrose gradient was diluted to 1 mg/ml. Protein was adsorbed onto ionized Formvar/copper 400 mesh grids, stained with 2% uranyl acetate, and imaged on the Hitachi H600 TEM at an accelerating voltage of 75 kV with a magnification from ×100,000 to ×300,000.
Statistical analysis.
Statistical differences were determined using Student's t test. A P value of <0.05 was considered significant.
RESULTS
Rv2744c is a member of a novel clade of PspA-like proteins.
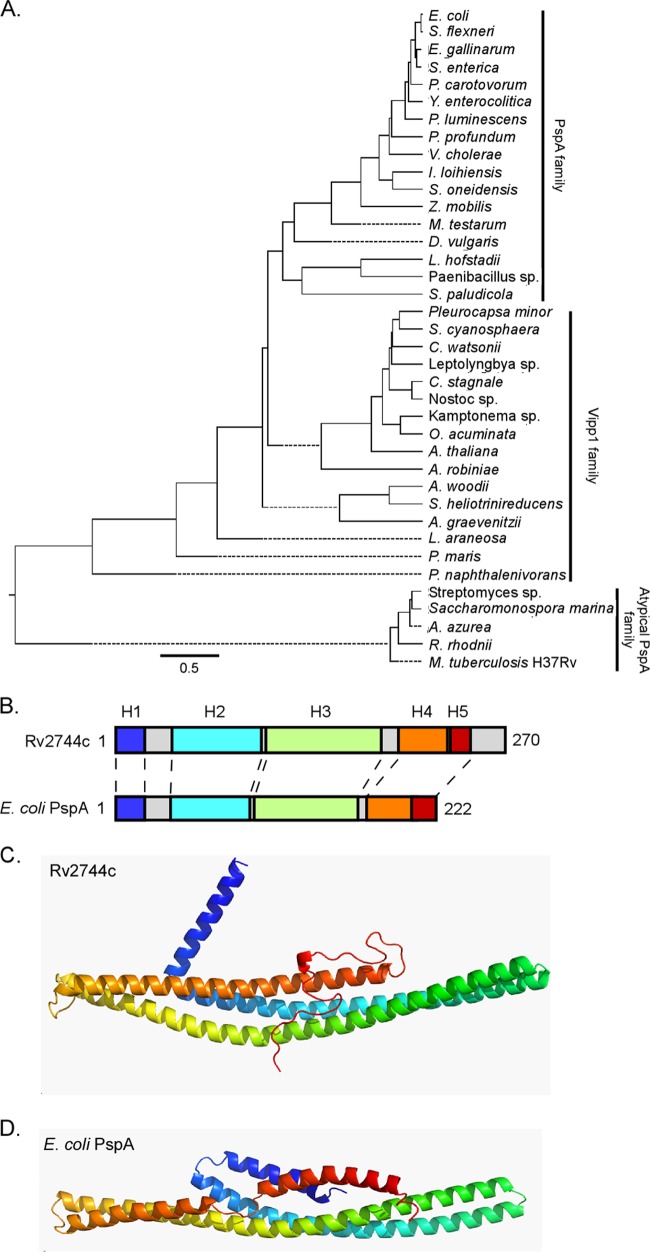
PSI-BLAST analysis indicates that M. tuberculosis Rv2744c contains a PspA/IM30 domain similar to that of other established PspA family members. Furthermore, Rv2744c lies in an operon along with other genes thought to encode a minimal functional unit of a Mycobacterium Psp system (24). To begin investigating whether Rv2744c possesses characteristics consistent with its designation as a PspA family member, Rv2744c was analyzed by bioinformatics. First, the amino acid sequence of Rv2744c was aligned to PspA family members from a representative group of organisms in which PspA had been characterized and/or identified (27, 42–46). While M. tuberculosis Rv2744c exhibited 86% and 76% sequence identity to the PspA ortholog present in Mycobacterium smegmatis and Rhodococcus jostii sp. strain RHA1, respectively (see Fig. S1 in the supplemental material), the sequence identities between Rv2744c and PspA orthologs in Escherichia coli, Yersinia enterocolitica, Streptomyces lividans, and Arabidopsis thaliana were <20% (see Fig. S1). Next, M. tuberculosis Rv2744c, E. coli PspA, and A. thaliana Vipp1 (vesicle inducing protein in plastids, a PspA ortholog in plants and photosynthetic bacteria), were used in individual BLAST searches to identify high-scoring PspA-like proteins from the general protein database. Amino acid sequences from protein hits were then compared using Clustal W alignment (DNAStar, Madison, WI) to determine the extent of conservation between PspA family members. PspA family members segregated into three distinct clades, with M. tuberculosis Rv2744c comprising an atypical PspA clade more closely related to A. thaliana Vipp1 than to E. coli PspA (Fig. 1A). Finally, it has been reported that PspA family members possess a conserved secondary structure containing 4 to 6 α-helical domains (28, 47). To investigate whether M. tuberculosis Rv2744c shared this characteristic, Rv2744c was modeled using PsiPred and other secondary structure prediction programs alongside E. coli PspA (48). Similar to PspA, the secondary structure of Rv2744c was predominantly α-helical, consisting of 5 distinct α-helical domains (Fig. 1B). Interestingly, Rv2744c contained an extra C-terminal region that was not present in E. coli PspA (Fig. 1B; see also Fig. S1 in the supplemental material). M. tuberculosis Rv2744c and E. coli PspA were also modeled using Rosetta ab initio application (49) due to the absence of structural data on PspA family members. This analysis produced models in which secondary and tertiary structures were conserved between Rv2744c and E. coli PspA, including a central helix-helix region (Fig. 1C and D). Thus, M. tuberculosis Rv2744c shares conservation in its predicted secondary and tertiary structures with E. coli PspA even though the primary amino acid sequence of these proteins is poorly conserved.
FIG 1.
Phylogenetic and modeling analysis of distantly related PspA family members. (A) Phylogenetic tree generated from representative protein BLAST search results with Rv2744c (M. tuberculosis), PspA (E. coli), and Vipp1 (A. thaliana) against bacterial and plant families, including Cyanobacteria, Actinobacteria, Spirochaetes, Fusobacteria, Acidobacteria, and Proteobacteria. Specific species analyzed included Escherichia coli, Shigella flexneri, Enterococcus gallinarum, Salmonella enterica, Pectobacterium carotovorum, Yersinia enterocolitica, Photorhabdus luminescens, Photobacterium profundum, Vibrio cholerae, Idiomarina loihiensis, Shewanella oneidensis, Zymomonas mobilis, Mastigocoleus testarum, Desulfovibrio vulgaris, Leptotrichia hofstadii, Paenibacillus sp., Sideroxydans paludicola, Pleurocapsa minor, Stanieria cyanosphaera, Crocosphaera watsonii, Cylindrospermum stagnale, Nostoc sp., Otoba acuminata, Arabidopsis thaliana, Actinospica robiniae, Acetobacterium woodii, Slakia heliotrinireducens, Actinomyces graevenitzii, Lentisphaera araneosa, Planctomyces maris, Polaromonas naphthalenivorans, Streptomyces lividans, Saccharomonospora marina, Amycolatopsis azurea, Rhodococcus rhodnii, and Mycobacterium tuberculosis. Representative proteins were selected for comparison if they either had an E value of <1 × 10−3 or were denoted as a phage shock protein A ortholog. Primary amino acid sequences were aligned via Clustal W alignment, and a phylogenetic tree was generated using MegAlign. Selected sequences were segregated into one of three major subgroups: PspA family, Vipp1 family, or atypical PspA family. (B) Despite their relatively low homology to each other, the PsiPred secondary structure prediction for Rv2744c and E. coli PspA revealed largely α-helical structures with similar organization (indicated as H1 to H5). Individual α-helical domains are denoted by color and are coded as described for panels C and D. The number of amino acids comprising each protein is also indicated. (C and D) Rv2744c (C) and E. coli PspA (D) were modeled via the Rosetta ab initio folding protocol and were colored N terminus (blue) to C terminus (red). Despite divergence in their amino acid sequences, the proteins appear to adopt similar folds composed of a central helix-helix domain.
Rv2744c forms homo-oligomers of high molecular weight and distinct structure.
A common characteristic of PspA family members is their ability to homo-oligomerize and form structured complexes of high molecular weight (42, 50–52). E. coli PspA forms a 36-mer spheroid-like homo-oligomeric complex consisting of 4 stacked rings of PspA 9-mers (42). Similarly, PspA from Burkholderia pseudomallei homo-oligomerizes to form ring-shaped complexes, rod-like species of various lengths, and mesh-like structures (51). As M. tuberculosis Rv2744c interacts with itself in vivo (31), we next determined whether Rv2744c also formed homo-oligomeric complexes in vitro. To facilitate the purification and detection of Rv2744c, a histidine-tagged variant (Rv2744c-His) was generated and used for these assays. Rv2744c-His was produced at high levels in E. coli and could be purified from the soluble fraction to near homogeneity (Fig. 2A). Next, Rv2744c-His was resolved by size exclusion chromatography to determine its mass; however, Rv2744c-His was unable to migrate in and/or around the S300 Sephacryl column matrix (data not shown), indicating that Rv2744c either forms a large aggregate and/or it has an extended conformation that is not conducive to column chromatography. To circumvent this problem, Rv2744c was resolved on a 10% to 50% continuous sucrose gradient alongside protein standards of known mass. Following ultracentrifugation, Rv2744c-His localized predominantly in fractions 17 to 19 (Fig. 2B and C). Rv2744c-His was also detected in fractions 21 to 23 (Fig. 2B and C). This banding pattern places the mass of Rv2744c-His between the protein standards thyroglobulin (669 kDa) and blue dextran (>2,000 kDa). To determine whether Rv2744c-His present in fractions 17 to 19 formed a structured complex, an aliquot of fraction 17 from the sucrose gradient was visualized by negative-stain electron microscopy. The protein present in this fraction formed higher-ordered homo-oligomeric complexes consisting of individual spheroids and organized tube-like structures (Fig. 2D and E). These structures strongly resembled those seen with other characterized PspA family members (51, 52). Collectively, these data, along with the bioinformatic studies, suggest that Rv2744c possesses structural and physical characteristics consistent with its designation as a PspA family member.
FIG 2.
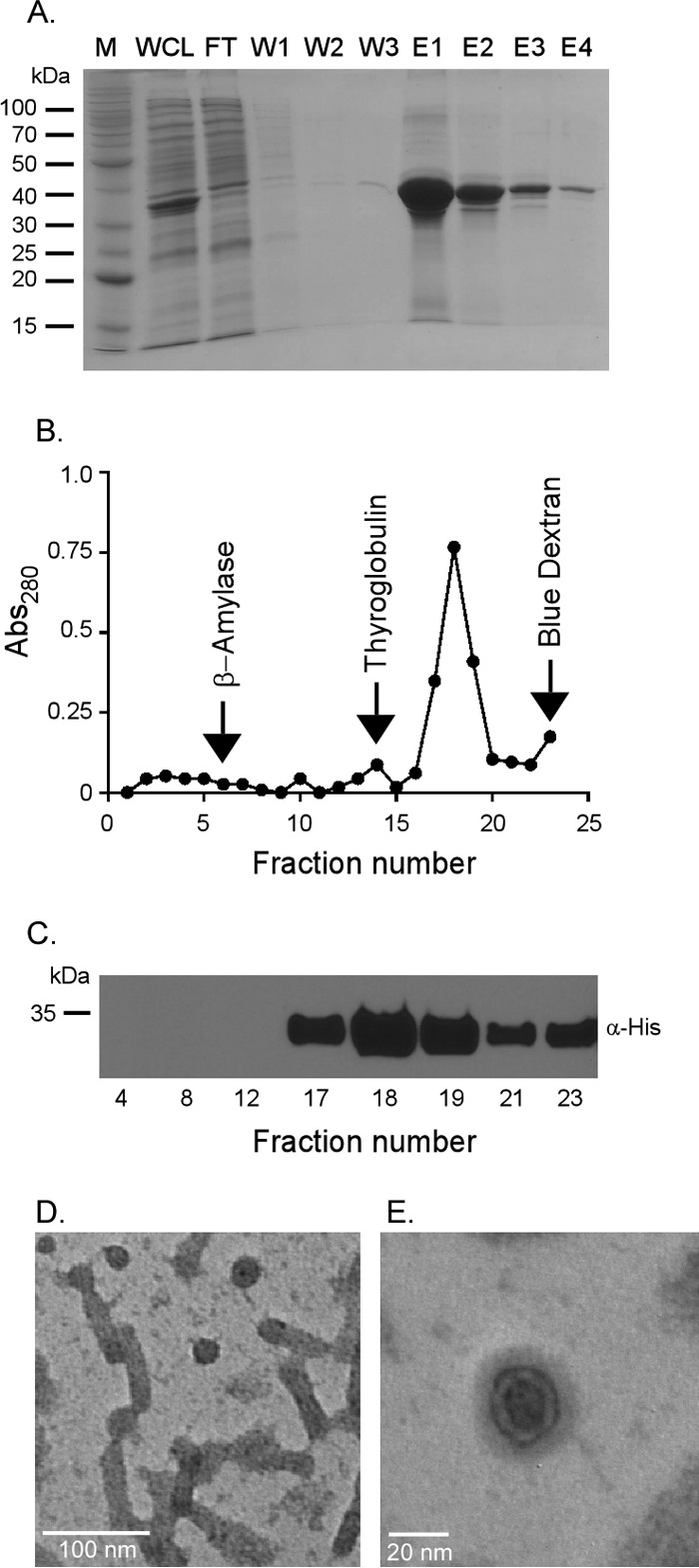
Rv2744c forms higher-order homo-oligomeric structures in vitro. (A) Rv2744c-His was purified via Ni affinity chromatography. Protein fractions were resolved by SDS-PAGE to confirm purity (M, molecular mass marker; WCL, whole-cell lysate; FT, flowthrough; W1 to W3, washes 1 to 3; E1 to E4, elutions 1 to 4). (B) Purified Rv2744c-His was resolved on a continuous 10 to 50% sucrose gradient by ultracentrifugation for 16 h at 4°C and 100,000 × g. Fractions were removed from the tops of the gradient tubes, and the absorbance at 280 nm (Abs280) was quantified via spectrophotometer to determine protein abundance. Protein standards were also separated in parallel and included β-amylase (200 kDa), thyroglobulin (669 kDa), and blue dextran (2,000 kDa). (C) Fractions containing protein were resolved by SDS-PAGE and probed by Western blotting using mouse anti-His antibody in order to confirm the presence of Rv2744c-His. Only selected fractions are shown. (D and E) Purified Rv2744c isolated from fraction 17 of the sucrose gradient was examined via negative-stain electron microscopy at a magnification of ×100,000 (D) or ×300,000 (E). Regular organized structures of small spheroids and tubular structures with similar diameters were observed.
Rv2744c-MS_2695 is upregulated by, but does not mediate resistance to, cell envelope stressor SDS.
To begin investigating the importance of PspA in Mycobacterium physiology, unmarked deletion mutations were generated in the coding sequences for Rv2744c or its homolog in M. smegmatis, MS_2695 (Fig. 3A). PCR analyses confirmed the loss of these genes in both mutant strains (Fig. 3B). To verify that loss of Rv2744c or MS_2695 did not impact Mycobacterium growth, wild-type and mutant strains of M. tuberculosis or M. smegmatis were cultured in standard laboratory medium, and replication kinetics were monitored. The deletion of Rv2744c or MS_2695 did not alter growth characteristics in either M. tuberculosis (Fig. 3C) or M. smegmatis (Fig. 3D). To determine whether Rv2744c or MS_2695 was regulated in response to cell envelope stress, gene expression levels were measured in wild-type M. tuberculosis or M. smegmatis by reverse transcription-quantitative PCR (qRT-PCR) following exposure to 0.05% SDS for 90 min (18). Rv2744c or MS_2695 expression levels were first normalized to the housekeeping gene sigA, whose expression is constitutive, and then compared to untreated controls, whose expression was set to 1.0. Exposure of M. tuberculosis to SDS induced Rv2744c expression by ∼3.0-fold (Fig. 3E), while the expression of MS_2695 was upregulated by >40-fold in M. smegmatis following SDS exposure (Fig. 3F). Finally, the importance of Rv2744c and MS_2695 for cell envelope stress resistance was assessed by exposing wild-type and mutant strains to bactericidal concentrations of SDS. Bacterial survival was monitored over time by plating for CFU. While exposure to SDS reduced the viability of M. tuberculosis (Fig. 3G) and M. smegmatis (Fig. 3H) over time, the loss of Rv2744c or MS_2695 did not increase sensitivity of either species to SDS. Similar results were obtained following exposure of Mycobacterium strains to other cell stressors known to alter the survival of ΔpspA mutants in other bacterial species, including heat shock, acidic or alkaline conditions, osmotic stress, and vancomycin exposure (data not shown) (26, 44, 45, 53). Thus, Rv2744c and MS_2695 are responsive to, but do not mediate resistance against, the cell envelope stressor SDS in M. tuberculosis or M. smegmatis.
FIG 3.
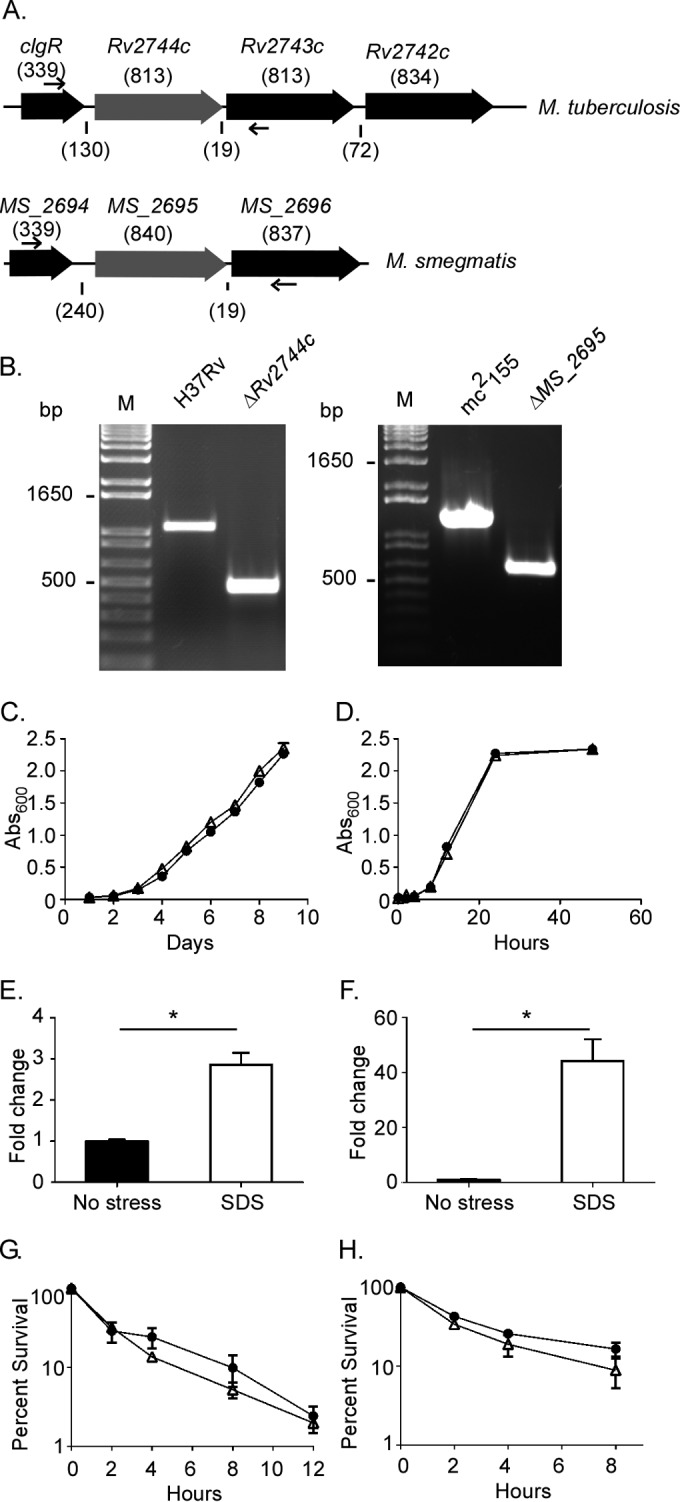
Rv2744c and MS_2695 are upregulated by, but do not mediate protection against, extracellular stress. (A) Organization of loci containing Rv2744c or MS_2695 in M. tuberculosis or M. smegmatis, respectively. The numbers above the genes refer to gene length (in base pairs), and the numbers below the genes refer to the distance (in base pairs) between genes. Unmarked deletion mutations were generated via homologous recombination in Rv2744c or MS_2695. (B) Loss of Rv2744c or MS_2695 was confirmed by PCR using primers flanking the gene (small arrows in panel A). M, 1-kb plus DNA ladder. (C and D) Growth of the wild type (●) or ΔRv2744c or MS_2695 mutants (△) of M. tuberculosis (C) or M. smegmatis (D) was assessed in 7H9 medium supplemented with OADC (M. tuberculosis) or ADC (M. smegmatis) and containing 0.05% Tween 20. (E and F) The expression of Rv2744c (E) or MS_2695 (F) was measured in the absence of or following exposure to 0.05% SDS for 90 min. The expression of Rv2744c or MS_2695 was measured via qRT-PCR and expressed as the ΔΔCT relative to the control without SDS. (G and H) The survival of the wild type (●) or ΔRv2744c or MS_2695 mutants (△) was assessed following exposure of M. tuberculosis to 0.5% SDS (G) or exposure of M. smegmatis to 0.1% SDS (H). Bacterial survival was quantified by plating serial 10-fold serial dilutions to determine the CFU per milliliter. All error bars represent the standard error of the results from three replicates (*, P < 0.05).
Rv2744c localizes to lipid droplets produced by M. smegmatis and M. tuberculosis.
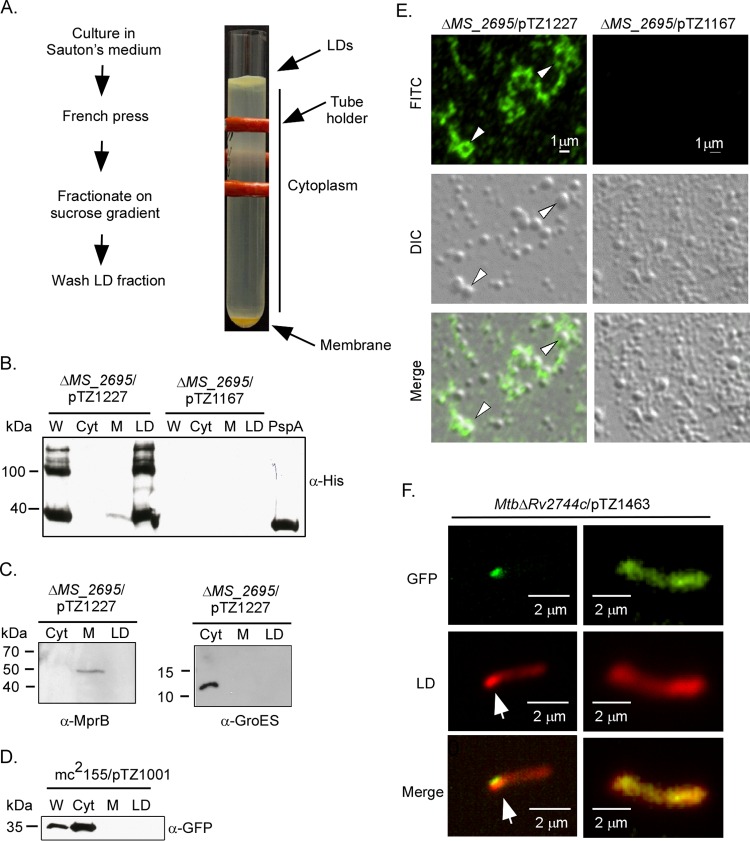
Recently, a PspA ortholog was identified in the lipid droplet (LD) proteome of Rhodococcus jostii sp. RHA1 (27), an organism from the same suborder as Mycobacterium. LDs are quasiorganelles that contain a neutral fatty acid core, such as triacylglycerol, surrounded by a single phospholipid monolayer and proteins (reviewed in reference 54). As M. tuberculosis Rv2744c shares high sequence identity with PspA from Rhodococcus (see Fig. S1 in the supplemental material), we reasoned that Rv2744c may also localize to LDs in Mycobacterium species. To test this possibility, an Rv2744c variant containing a 3×FLAG epitope tag at the N terminus and a 6×His epitope tag at the C terminus of the protein was generated and produced in M. smegmatis ΔMS_2695 under conditions promoting LD production (Fig. 4A) (55). LDs, along with other protein-containing fractions, were then isolated by lysing bacteria via French press and subjecting total cell lysates to ultracentrifugation (27). LDs form a buoyant layer in buffer containing sucrose, while membrane proteins pellet and the soluble cytoplasmic proteins fractionate between these two layers (Fig. 4A). When assayed by Western blotting, Rv2744c localized predominantly to the LD fraction (Fig. 4B). In contrast, the sensor kinase MprB and chaperone GroEL localized to the membrane and cytoplasmic compartments, respectively, as expected (Fig. 4C). To further confirm that Rv2744c localization to LDs was not an artifact of the purification process, an M. smegmatis derivative overproducing GFP was grown under the same conditions and processed in parallel. While this strain produced LDs (data not shown), GFP localized exclusively to the cytoplasmic compartment (Fig. 4D). Rv2744c localization to LDs was also confirmed using immunofluorescence microscopy. For these studies, LDs were isolated from M. smegmatis ΔMS_2695 producing 3×FLAG-Rv2744c-6×His. LDs were then immobilized onto poly-l-lysine-coated glass slides and visualized by immunofluorescence microscopy following labeling with anti-FLAG monoclonal antibody and anti-IgG secondary antibody conjugated to Alexa Fluor 488. Rv2744c was associated with the surface of LDs, including a subset of LDs whose surfaces were completely surrounded by Rv2744c (Fig. 4E). As expected, this pattern of immunofluorescence was not observed in LDs isolated from the M. smegmatis ΔMS_2695 mutant carrying the vector-only control (Fig. 4E). Finally, the ability of Rv2744c to associate with LDs in M. tuberculosis was examined. For these studies, Rv2744c colocalization to LDs was investigated within fixed bacteria, as it was not possible to isolate LDs directly from M. tuberculosis due to biosafety reasons. Here, GFP was fused to the C terminus of Rv2744c, cloned into pSE100, and transformed into M. tuberculosis ΔRv2744c. The resulting recombinant strain was then grown under conditions promoting LD production, fixed, incubated with Nile red to stain neutral fatty acids within LDs, and visualized by epifluorescence microscopy (56, 57). Rv2744c can be seen associating with clusters of LDs produced in M. tuberculosis (Fig. 4F). Thus, Rv2744c localizes to and associates with LDs produced by M. smegmatis and M. tuberculosis.
FIG 4.
Rv2744c localizes to lipid droplets in M. smegmatis. (A) Schematic of protocol used to isolate LDs from M. smegmatis strains and image of discontinuous sucrose gradient containing the buoyant lipid droplet layer floating on top, the region containing cytoplasmic proteins, and the pellet containing membrane-associated proteins. The red structure is a tube holder. (B) M. smegmatis ΔMS_2695 producing 3×FLAG-Rv2744c-6×His (pTZ1227) or M. smegmatis ΔMS_2695 carrying the empty vector control (pTZ1167) was fractionated via sucrose gradient centrifugation, and recovered aliquots were probed for 3×FLAG-Rv2744c-6×His by Western blotting. W, whole-cell lysate; Cyt, cytosol; M, membrane. The far-right lane contains recombinant Rv2744c-6×His purified from E. coli and included as a loading control. (C) M. smegmatis ΔMS_2695 producing 3×FLAG-Rv2744c-6×His was also probed with antibodies to sensor kinase MprB (membrane protein) and chaperone GroES (cytoplasmic protein) to confirm the integrity of our subcellular fractionation procedure. (D) M. smegmatis producing GFP (mc2155/pTZ1001) was cultured and processed as described above to isolate LDs. Fractions were then probed for GFP, showing proper cytosolic localization. (E) Immunofluorescence of LDs prepared from M. smegmatis ΔMS_2695 producing 3×Flag-Rv2744c-6×His (left) or M. smegmatis ΔMS_2695 carrying the vector-only control (right). Arrowheads indicate LDs in which Rv2744c completely surrounds the vesicle. FITC, fluorescein isothiocyanate channel; DIC, differential interference contrast used to observe LDs; merge, FITC and DIC channels combined. (F) Epifluorescence microscopy showing association of PspA-GFPmut3 fusion protein (FITC channel) with a region containing a cluster of Nile red-stained lipid droplets (tetramethyl rhodamine isothiocyanate [TRITC] channel). Arrows indicate the region of lipid droplet accumulation within the bacterial cytoplasm.
Rv2744c/MS_2695 regulates the size and number of LDs produced by Mycobacterium species.
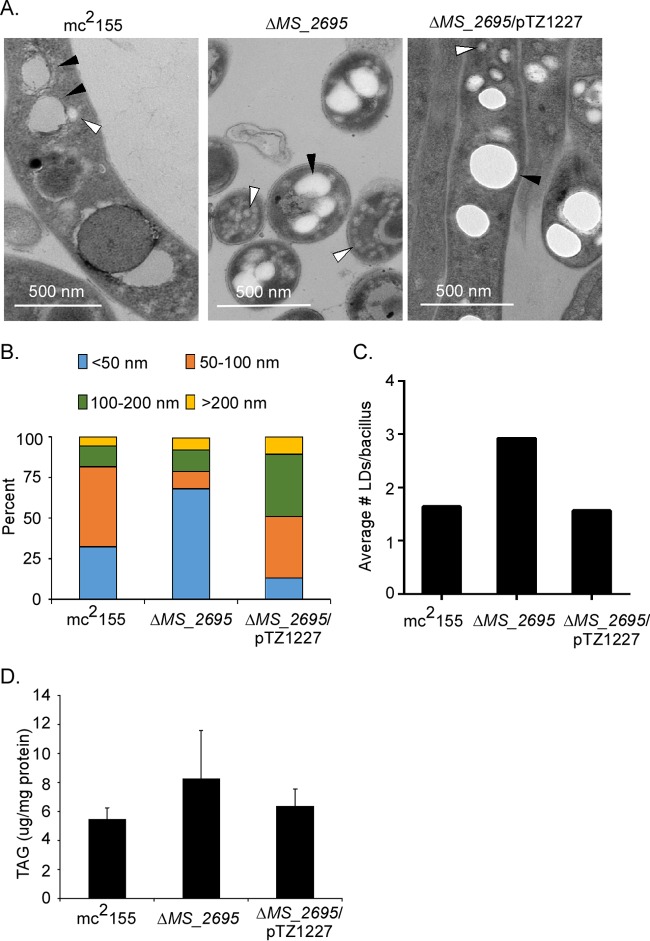
Given that Rv2744c formed large homo-oligomeric complexes in vitro and localized to LDs in vivo, we next assessed whether Rv2744c regulated aspects of LD homeostasis. To investigate this possibility, wild-type M. smegmatis mc2155, the M. smegmatis ΔMS_2695 mutant, or the M. smegmatis ΔMS_2695 mutant overproducing the epitope-tagged form of Rv2744c (pTZ1227) was grown under conditions promoting LD production. Strains were fixed, stained, and visualized using transmission electron microscopy. Electron-lucent LDs were observed in all three strains (Fig. 5A), demonstrating that Rv2744c/MS_2695 is not necessary for LD formation. Next, the impact of Rv2744c/MS_2695 on LD size was examined. For these studies, the diameters of >300 LDs from >200 bacteria present in >20 different microscopic fields were quantified for each strain, using a counter blinded to the strain genotype. The M. smegmatis ΔMS_2695 mutant exhibited an increased number of LDs possessing a smaller diameter (<50 nm) compared to that of the wild-type control (Fig. 5B). In contrast, overproduction of the tagged Rv2744c variant in the M. smegmatis ΔMS_2695 mutant (TCZ2365) led to an increased number of LDs possessing a larger diameter (≥100 nm) compared to that of the wild-type parent (Fig. 5B). The observed increase in LD diameter was not an artifact of the epitope tags present on Rv2744c, as a similar phenotype was observed with an M. smegmatis ΔMS_2695 mutant expressing the wild-type Rv2744c allele from the same vector (TCZ2654) (see Fig. S2A in the supplemental material).
FIG 5.
Deletion of MS_2695 or overproduction of Rv2744c results in altered lipid droplet morphology. (A) Transmission electron micrographs of M. smegmatis mc2155, M. smegmatis ΔMS_2695, and M. smegmatis ΔMS_2695 strains expressing Rv2744c after 1 week's growth in Sauton's medium. White arrowheads, small LDs; black arrowheads, large LDs. Note that the panel displaying M. smegmatis ΔMS_2695 is from a longitudinal cross-section to allow better visualization of the smaller LDs produced. (B) Stacked bar graph depicting size distribution of LDs in each strain. Graphed values are representative of the percentage of LDs of each diameter category among the total lipid droplets measured per strain. (C) The average number of lipid droplets observed per bacillus was determined, revealing that the ΔMS_2695 strain contained nearly double the number of LDs per bacillus compared to the wild-type and complemented counterparts. (D) The triacylglycerol (TAG) content present in LDs isolated from each strain was quantified relative to a standard curve and normalized to the total protein concentration of isolated LDs. The total protein concentrations of isolated LDs were similar between strains.
To further confirm that Rv2744c regulated LD size, TCZ2365 was transformed with an integrating plasmid constitutively producing the TetR repressor. The operator sequence for tet(R) is positioned between the promoter and Rv2744c coding sequence present on pTZ1227, allowing Rv2744c expression in this new strain (TCZ2455) to be regulated by anhydrotetracycline (ATc), which can be included in the growth medium. TCZ2455 was grown under conditions inducing LD production either in the absence or presence of 10 nM ATc, fixed, and processed for electron microscopy. The size of LDs was then quantified and compared to the size distribution of LDs observed in wild-type M. smegmatis mc2155, the M. smegmatis ΔMS_2695 mutant (TCZ2054), and TCZ2365 (Fig. 5B). Rv2744c and MS_2695 expression levels in these strains were also quantified by qRT-PCR relative to a standard curve, so that Rv2744c and MS_2695 expression levels could be directly correlated with LD size. In the absence of ATc, TCZ2455 expressed a small amount of Rv2744c, likely due to the inability of TetR to completely repress the expression of this determinant from the pTZ1227 multicopy plasmid (see Fig. S2B in the supplemental material). Interestingly, this level of Rv2744c expression was similar to that observed in wild-type M. smegmatis mc2155 (see Fig. S2B) and resulted in LDs that had a similar size distribution to that observed in the wild-type parent (compare Fig. S2C in the supplemental material with Fig. 5B). In contrast, the incubation of TCZ2455 in the presence of 10 nM ATc increased Rv2744c expression to levels similar to those observed in the Rv2744c overexpression strain TCZ2365 (see Fig. S2B). Furthermore, the size distribution of LDs produced in TCZ2455 under these conditions was similar to that produced by TCZ2365 (compare Fig. S2C with Fig. 5B). Taken together, these results suggest that LD size distribution is directly correlated to the amount of Rv2744c produced within the bacteria.
Apart from altering LD diameter, the deletion of MS_2695 also increased the average number of LDs observed by ∼2-fold compared to that in wild-type M. smegmatis or the M. smegmatis ΔMS_2695 mutant overproducing Rv2744c (Fig. 5C). Finally, the importance of Rv2744c and MS_2695 for triacylglycerol (TAG) incorporation into LDs was assessed. Here, LDs were isolated from wild-type and mutant M. smegmatis derivatives, and the TAG concentration in LDs was measured using a commercial TAG quantification kit. The loss or overproduction of MS_2695/Rv2744c did not impact the amount of TAG detected within purified LDs (Fig. 5D). Thus, while MS_2695 or Rv2744c is not required to incorporate TAGs into LDs, MS_2695 or Rv2744c is involved in the regulation of aspects of LD homeostasis, including the number and size of LDs.
Rv2744c regulates survival of M. tuberculosis during NRP.
LD production is induced in M. tuberculosis under conditions that also promote the establishment of NRP (56). Furthermore, it has been shown that M. tuberculosis mobilizes TAGs contained within LDs as an energy source during NRP (58–61). To determine whether Rv2744c regulated the survival of M. tuberculosis during NRP, wild-type H37Rv, the ΔRv2744c mutant, and the ΔRv2744c variant overproducing Rv2744c were assessed using the rapid anaerobic dormancy (RAD) model (39). In this in vitro culture model of latency, bacteria are grown in tightly sealed tubes containing stir bars to self-deplete oxygen with culture medium over time. Once oxygen levels fall below a minimum level, strains enter into NRP that can be maintained for at least several weeks with no appreciable drop in viability (39). Oxygen depletion in this model is monitored by a color loss of methylene blue (an oxygen-sensitive indicator dye) added to the growth medium. To enhance LD production, the nutrient-rich Dubos medium normally used in the RAD model was replaced with the nutrient-limiting Sauton's medium. While survival between wild-type M. tuberculosis, the ΔRv2744c mutant, and the ΔRv2744c mutant overproducing Rv2744c was similar over the first 9 days of the experiment (Fig. 6A), a reduction in viability was observed with the ΔRv2744c mutant and the ΔRv2744c mutant overproducing Rv2744c at later time points relative to the wild-type control (Fig. 6A). This drop in viability was greater with the ΔRv2744c mutant overproducing Rv2744c than that with the ΔRv2744c mutant alone, indicating that both loss and overproduction of Rv2744c alter M. tuberculosis survival during NRP. Furthermore, the observed drop in viability occurred after the methylene blue indicator in the medium became colorless around day 9 (data not shown), indicating that the cultures had entered into NRP. To determine whether the observed viability loss was dependent on strains being in NRP, M. tuberculosis cultures were grown for 1 week in Sauton's medium to induce LD production, washed, and then incubated in PBS to induce nutrient starvation. Under these conditions, bacteria stop replicating but do not enter into NRP. As can be observed, there was no difference in survival between any of the M. tuberculosis derivatives tested over the 60-day time course examined (Fig. 6B). Thus, Rv2744c regulates the survival of M. tuberculosis under conditions of NRP.
FIG 6.
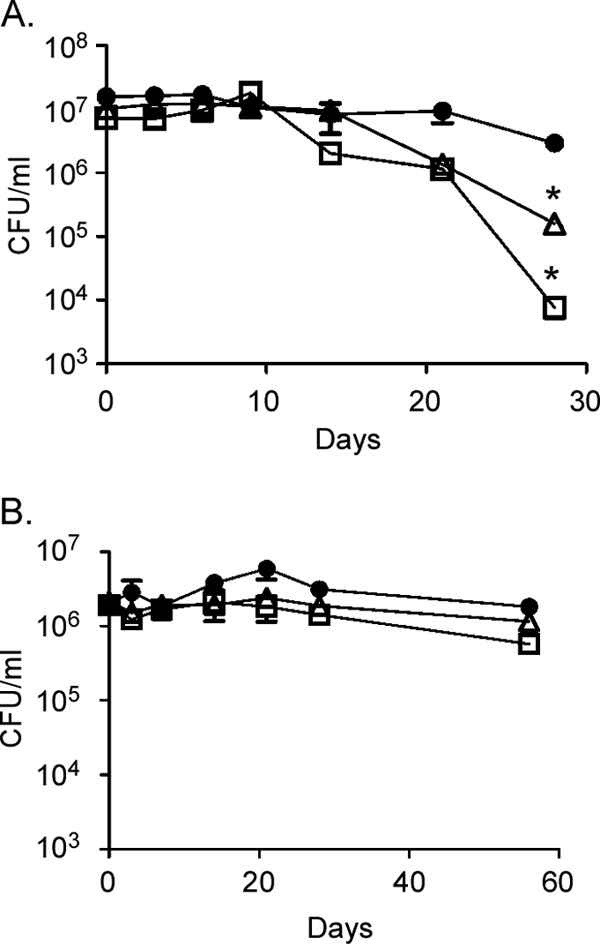
Rv2744c regulates M. tuberculosis survival under conditions of NRP. Growth and survival of M. tuberculosis H37Rv (●), ΔRv2744c (△), and ΔRv2744c/pTZ1227 (□) strains were examined using a modified version of the rapid anaerobic dormancy (RAD) model (A) or a PBS starvation assay (B). The data represent the mean survival of bacteria from triplicate cultures ± the standard error of the mean (SEM) (*, P < 0.05).
DISCUSSION
PspA is a key component of the phage shock protein (Psp) system present in many bacteria. This system is thought to mediate resistance to cell envelope stress by stabilizing the plasma membrane and by preventing the loss of proton motive force. PspA family members possess a number of evolutionarily conserved characteristics, including (i) a largely α-helical secondary structure, (ii) the ability to homo-oligomerize and form structured complexes of high molecular weight, and (iii) association with lipids. In this study, the structural and functional attributes of Rv2744c were characterized to determine whether this protein represented an ortholog of PspA in M. tuberculosis. While Rv2744c possessed characteristics consistent with its designation as a PspA family member, Rv2744c did not function to regulate resistance to cell envelope stress. Rather, Rv2744c localized to the surface of LDs, where it regulated LD number, size, and survival of M. tuberculosis during NRP. These findings indicate that Rv2744c represents a novel PspA family member whose function includes the regulation of LD homeostasis and M. tuberculosis survival under conditions of NRP.
Rv2744c shares limited primary amino acid sequence homology with E. coli PspA and PspA-like proteins from other organisms. Rv2744c aligns to a unique clade of atypical PspA-like proteins that are found predominantly in members of the Actinobacteria. This clade is evolutionarily distant from the archetypical clade containing E. coli PspA, as well as the clade containing A. thaliana Vipp1, a PspA family member whose function includes membrane maintenance and thylakoid biogenesis in Cyanobacteria and plants. In contrast to the minimal primary amino acid sequence homology, bioinformatics predicts that Rv2744c possesses secondary and tertiary structures that are highly conserved with other PspA family members. Rv2744c is largely α-helical, and our Rosetta models predict that it may form five distinct helical domains organized in a fashion similar to those in E. coli PspA. Interestingly, Rv2744c contains a 48-amino-acid C-terminal extension that is absent in proteins from the PspA clade but that is present in PspA members from the Vipp1 clade. While the exact role of this extension in Rv2744c function remains unknown, it has been proposed that the C-terminal extension present in Vipp1 confers specificity and allows this protein to carry out specialized functions related to its role in oxygenic photosynthesis (47). Rv2744c also possesses physical characteristics that are conserved with E. coli PspA and other PspA family members. Rv2744c self-associates and forms homo-oligomeric complexes of high molecular weight. While the molecular weight of Rv2744c could not be resolved using size exclusion chromatography, sucrose gradient ultracentrifugation studies indicate that Rv2744c forms homo-oligomeric complexes between 650 kDa and 2,000 kDa. When imaged by negative-stain electron microscopy, Rv2744c assumed a spheroid-like structure and elongated filamentous-like superstructures that likely consist of stacked spheroidal subunits. These structural characteristics are consistent with those observed with other PspA proteins (42, 47, 51) and indicate that Rv2744c is a bona fide ortholog of PspA.
Rv2744c is located within an operon (Rv2745c-Rv2744c-Rv2743c-Rv2742c) whose organization and transcriptional arrangement are highly conserved in members of the M. tuberculosis complex (24). However, nontuberculous Mycobacterium species, including M. smegmatis, carry a smaller version of this locus that includes homologs of Rv2745c-Rv2744c-Rv2743c but that lacks Rv2742c (24). While the significance of this difference is unclear, the presence of Rv2742c in human TB-causing species indicates that inclusion of this gene may confer additional capabilities to this system that are associated with virulence and/or disease elicitation. Rv2745c encodes the ClgR transcription factor, which is an ortholog of the Clp protease gene regulator (23, 62). ClgR regulates the expression of its own operon and various other determinants (23), including the ClpC1 and ClpP2 proteases responsible for degradation of the SigE anti-sigma factor, RseA (21). Interestingly, clgR expression is induced by a variety of different stress conditions, including redox stress, cell envelope stress, stress induced following heat shock, and acid stress (62–67). Furthermore, clgR is upregulated under conditions of low oxygen tension and following reaeration from hypoxia-induced dormancy (62). However, in contrast to the well-characterized nature of ClgR, virtually nothing is known about the function of the other two determinants present in this operon, Rv2743c and Rv2742c. Both are conserved alanine-rich proteins, and Rv2743c is predicted to possess a transmembrane region, which suggests that this protein interacts with the plasma membrane.
Recently, it has been proposed that genes contained within the Rv2745c-Rv2744c-Rv2743c-Rv2742c operon may encode a minimal functional unit of the Psp system, helping to regulate plasma membrane homeostasis in M. tuberculosis under conditions of cell envelope stress (24). In this scenario, ClgR would function in a role analogous to PspF, Rv2744c to PspA, Rv2743c to PspB, and Rv2742c to PspC. However, our studies characterizing Rv2744c and its ortholog in M. smegmatis, MS_2695, suggest that this protein may function in a role that is independent of or in addition to its proposed role in plasma membrane homeostasis. For example, although the expression of both MS_2695 and Rv2744c is upregulated in response to the cell envelope perturbant SDS, neither M. smegmatis nor M. tuberculosis derivatives deleted in these determinants exhibited increased sensitivity to SDS or other stressors compared to their wild-type counterparts. This contrasts the SDS-sensitive phenotype seen with clgR and Rv2743c mutants of M. tuberculosis (24). In addition, although Rv2744c has been shown to immunoprecipitate or pull down ClgR (a cytoplasmic protein) and Rv2743c (a predicted membrane protein) when coproduced in a surrogate E. coli host (24), we failed to observe Rv2744c interaction with either ClgR or Rv2743c in E. coli in vivo when assayed using a bacterial adenylate two-hybrid assay (data not shown). While the reason(s) underlying this discrepancy is currently unclear, it is possible that the association of Rv2744c with ClgR and Rv2743c occurs indirectly through another protein. Alternatively, it is possible that the interaction between Rv2744c and ClgR or between Rv2744c and Rv2743c is not stable enough to promote an association between the adenylate cyclase subunits used in the BACTH assay.
Regardless of the potential protein-interacting partners of Rv2744c, the loss or overproduction of MS_2695/Rv2744c alters the number and size distribution of LDs produced in M. smegmatis and negatively impacts the survival of M. tuberculosis following the establishment of NRP. Interestingly, overproduction of Rv2744c exacerbates the phenotypes observed in both M. smegmatis and M. tuberculosis; overproduction of Rv2744c increases the size of LDs produced over that seen in the wild-type parent and also results in a greater survival defect during NRP than that seen in the uncomplemented ΔRv2744c mutant in M. tuberculosis. This phenotype is due to the production of Rv2744c at levels much higher than those normally seen in the wild-type parent, as introduction of the TetR repressor into the Rv2744c overexpression strain reduces Rv2744c production to wild-type levels and correspondingly restores the LD distribution pattern to one resembling that of the wild-type parent. While the exact role of Rv2744c remains unknown, we speculate that Rv2744c may function to stabilize the phospholipid monolayer surrounding the TAG core. As such, dysregulation of Rv2744c levels would be expected to not only impact the size and number of LDs produced but also regulate accessibility to TAG contained within LDs needed for use as an energy source during NRP. Previously, we reported localization of Rv2744c to the plasma membrane/cell wall compartment (31). However, the cell lysate utilized for those studies was generated by bead-beating, which would mechanically disrupt the integrity of LDs. In the current study, bacteria were lysed using a French press and the resulting cell lysates fractionated in the presence of a low concentration of sucrose, which allows for efficient separation of intact LDs from cytoplasmic and membrane proteins. In conclusion, our results suggest that Rv2744c may not be involved in plasma membrane homeostasis but rather may carry out a unique function among PspA family members in Mycobacterium that includes regulation of the homeostasis of LDs.
The production of LDs is a common characteristic of most eukaryotes (reviewed in reference 68). Of note, “foamy” macrophages, a predominant cell type comprising the M. tuberculosis granuloma, contain a high abundance of LDs containing TAGs, and M. tuberculosis is often seen within or in close proximity to these cells (69–71). TAG-containing LDs are also produced by a small number of prokaryotes, primarily those from the Actinobacteria family, to which Mycobacterium belongs (reviewed in reference 41). In vitro, Mycobacterium spp. induce LD production when grown under a variety of stressful conditions, including those thought to be present within the granuloma (i.e., low oxygen tension, low nutrient availability, nitric oxide exposure, etc.) (41, 58, 72). Furthermore, pathogenic Mycobacterium species usurp and hydrolyze TAG from host LDs within foamy macrophages, incorporating the resulting fatty acid products into its own LDs for use as an energy source during NRP (58, 69, 71). Consistent with this observation, Mycobacterium bovis BCG strains that are inhibited in their ability to mediate TAG hydrolysis are attenuated for survival in vitro during and following resuscitation from NRP (60, 61). Similarly, an M. tuberculosis mutant deleted in triacylglycerol-1 synthase (Δtgs1), an enzyme used to make TAGs, is compromised in its ability to accumulate intracellular TAGs and is unable to enter into NRP in an in vitro human TB granuloma model (73). Finally, an M. tuberculosis mutant deleted in lipase lipY, which is required for TAG hydrolysis, is compromised in its ability to mobilize stored TAGs and is unable to reactivate from NRP in the granuloma model system (73). Thus, LDs represent an important energy reservoir utilized by M. tuberculosis for survival during and resuscitation from NRP.
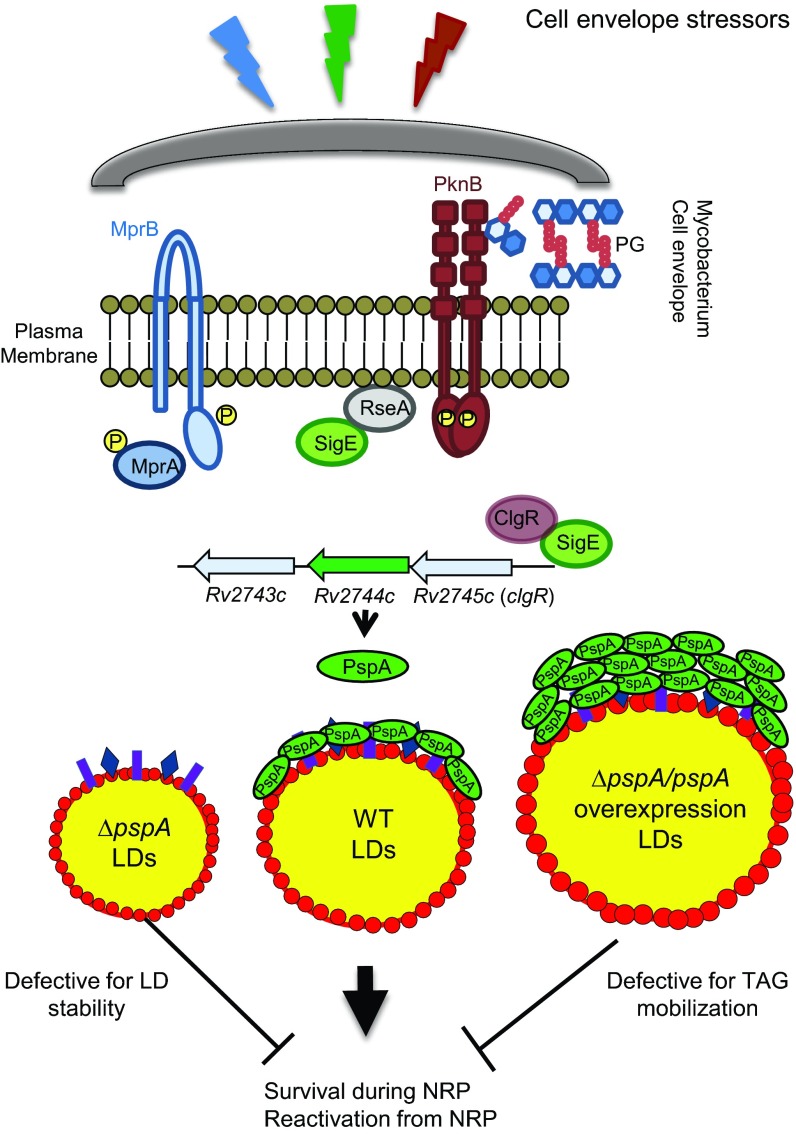
Based on the data presented here, we propose the following working model whereby Rv2744c/MS_2695 represents a unique ortholog of PspA that associates with and regulates LD homeostasis in Mycobacterium species under conditions of stress (Fig. 7). Within the granuloma, M. tuberculosis is exposed to a variety of stressors that are capable of (i) activating regulatory components of the cell envelope stress response network, including MprAB, SigE, and PknB, and (ii) leading to the production of LDs containing TAG for use as an energy source during NRP and/or reactivation from NRP. Activation of the MprAB, SigE, and PknB signaling networks following exposure to cell envelope stressors leads to upregulation of the locus that encodes Rv2744c/MS_2695, herein called pspA. Once produced, PspA localizes to LDs, where the protein homo-oligomerizes on the LD surface to help stabilize the phospholipid monolayer and regulate the mobilization of TAG for use as an energy source during periods of NRP.
FIG 7.
Model of Rv2744c/MS_2695 function in LD homeostasis. Within the granuloma, M. tuberculosis persists within alveolar macrophages, where the bacterium is exposed to external stimuli and stressors that activate components of the cell envelope stress response network, including the two-component system MprAB, extracytoplasmic function sigma factor SigE, and serine-threonine protein kinase PknB. These signaling pathways are integrated at the locus that contains Rv2744c, encoding an ortholog of the phage shock protein, PspA. Following production, Rv2744c (PspA) localizes to the surface of LDs that are also produced by M. tuberculosis under various conditions of stress. We hypothesize that PspA functions to help stabilize the phospholipid monolayer of LDs so that the TAG contained within these quasiorganelles can be used to maintain energy production under conditions of nonreplicating persistence (NRP) and/or during the reactivation from NRP. Loss of PspA leads to unstable LDs and a corresponding loss in TAG availability for mobilization under conditions of stress. In contrast, overproduction of PspA on the LD surface leads to occluded LDs in which TAG is unable to be accessed and mobilized for use in energy-generating pathways. PG, peptidoglycan. RseA is the anti-sigma factor for SigE, which is phosphorylated by PknB following activation and degraded, leading to the release of SigE from the plasma membrane; red circles encompassing LDs represent the phospholipid monolayer; proteins comprising the LD proteome are designated with colored rectangles and diamonds. PspA is denoted in green. While different-size LDs are depicted, they contain similar TAG concentrations.
In conclusion, Rv2744c encodes a unique ortholog of PspA in M. tuberculosis. While Rv2744c possesses structural and physical characteristics consistent with its designation as a PspA family member, the function of this protein is unique and differs from the archetypical role this protein plays in mediating resistance to cell envelope stressors. As Rv2744c dysregulation leads to defects in M. tuberculosis survival during NRP, this protein may represent a novel determinant that can be therapeutically targeted to abrogate the survival of the tubercle bacillus within the host during latent infections.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Sabine Ehrt for providing pSE100 and pMC1s and to Eric Rubin for providing plasmids expressing GFP variants. We also acknowledge Clive Wells and the MCW Facility for Electron Microscopy for imaging of Mycobacterium strains and purified Rv2744c.
This work was supported by a grant from the Potts Memorial Foundation to T.C.Z.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01001-15.
REFERENCES
- 1.World Health Organization. 2014. Global tuberculosis report. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf. [Google Scholar]
- 2.Gomez JE, McKinney JD. 2004. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.McKinney JD, Höner zu Bentrup K, Muñoz-Elías EJ, Miczak A, Chen B, Chan W-T, Swenson D, Sacchettini JC, Jacobs WR Jr, Russell DG. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 4.Russell DG, Cardona P-J, Kim M-J, Allain S, Altare F. 2009. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol 10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64:2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wayne LG, Sohaskey CD. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol 55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698–702. doi: 10.1001/jama.1994.03510330076038. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues LC, Diwan VK, Wheeler JG. 1993. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y. 2004. Persistent and dormant bacilli and latent tuberculosis. Frontier Biosci 9:1136–1156. doi: 10.2741/1291. [DOI] [PubMed] [Google Scholar]
- 10.Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F. 2012. The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin Dev Immunol 2012:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyka W. 1974. Studies on the effect of starvation on mycobacteria. Infect Immun 9:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Butcher PD, Mangan JA, Rajandream M-A, Coates ARM. 1999. Regulation of hmp gene transcription in Mycobacterium tuberculosis: effects of oxygen limitation and nitrosative and oxidative stress. J Bacteriol 181:3486–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivas-Santiago B, Hernandez-Pando R, Carranza C, Juarez E, Contreras JL, Aguilar-Leon D, Torres M, Sada E. 2008. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun 76:935–941. doi: 10.1128/IAI.01218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturgill-Koszycki S, Schlesinger P, Chakraborty P, Haddix P, Collins H, Fok A, Allen R, Gluck S, Heuser J, Russell D. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 15.Bretl DJ, Demetriadou C, Zahrt TC. 2011. Adaptation to environmental stimuli within the host: two-component signal transduction systems of Mycobacterium tuberculosis. Microbiol Mol Biol Rev 75:566–582. doi: 10.1128/MMBR.05004-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bretl DJ, Zahrt TC. 2013. Regulation of envelope stress responses by Mycobacterium tuberculosis. In Vasil ML, Darwin AJ (ed), Regulation of bacterial virulence. ASM Press, Washington, DC. [Google Scholar]
- 17.Bretl DJ, Bigley TM, Terhune SS, Zahrt TC. 2014. The MprB extracytoplasmic domain negatively regulates activation of the Mycobacterium tuberculosis MprAB two-component system. J Bacteriol 196:391–406. doi: 10.1128/JB.01064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H, Hovey R, Kane J, Singh V, Zahrt TC. 2006. MprAB is a stress-responsive two-component system that directly regulates expression of sigma factors SigB and SigE in Mycobacterium tuberculosis. J Bacteriol 188:2134–2143. doi: 10.1128/JB.188.6.2134-2143.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontán PA, Aris V, Alvarez ME, Ghanny S, Cheng J, Soteropoulos P, Trevani A, Pine R, Smith I. 2008. Mycobacterium tuberculosis sigma factor E regulon modulates the host inflammatory response. J Infect Dis 198:877–885. doi: 10.1086/591098. [DOI] [PubMed] [Google Scholar]
- 20.Manganelli R, Voskuil MI, Schoolnik GK, Smith I. 2001. The Mycobacterium tuberculosis ECF sigma factor σE: role in global gene expression and survival in macrophages. Mol Microbiol 41:423–437. doi: 10.1046/j.1365-2958.2001.02525.x. [DOI] [PubMed] [Google Scholar]
- 21.Barik S, Sureka K, Mukherjee P, Basu J, Kundu M. 2010. RseA, the SigE specific anti-sigma factor of Mycobacterium tuberculosis, is inactivated by phosphorylation-dependent ClpC1P2 proteolysis. Mol Microbiol 75:592–606. [DOI] [PubMed] [Google Scholar]
- 22.Chawla Y, Upadhyay SK, Khan S, Nagarajan SN, Forti F, Nandicoori VK. 2014. Protein kinase B (PknB) of Mycobacterium tuberculosis is essential for growth of the pathogen in vitro as well as for survival within the host. J Biol Chem 289:13858–13875. doi: 10.1074/jbc.M114.563536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estorninho M, Smith H, Thole J, Harders-Westerveen J, Kierzek A, Butler RE, Neyrolles O, Stewart GR. 2010. ClgR regulation of chaperone and protease systems is essential for Mycobacterium tuberculosis parasitism of the macrophage. Microbiology 156:3445–3455. doi: 10.1099/mic.0.042275-0. [DOI] [PubMed] [Google Scholar]
- 24.Datta P, Ravi J, Guerrini V, Chauhan R, Neiditch MB, Shell SS, Fortune SM, Hancioglu B, Igoshin OA, Gennaro ML. 2015. The Psp system of Mycobacterium tuberculosis integrates envelope stress-sensing and envelope-preserving functions. Mol Microbiol 97:408–422. doi: 10.1111/mmi.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joly N, Engl C, Jovanovic G, Huvet M, Toni T, Sheng X, Stumpf MPH, Buck M. 2010. Managing membrane stress: the phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiol Rev 34:797–827. doi: 10.1111/j.1574-6976.2010.00240.x. [DOI] [PubMed] [Google Scholar]
- 26.Darwin AJ. 2005. The phage-shock-protein response. Mol Microbiol 57:621–628. doi: 10.1111/j.1365-2958.2005.04694.x. [DOI] [PubMed] [Google Scholar]
- 27.Ding Y, Yang L, Zhang S, Wang Y, Du Y, Pu J, Peng G, Chen Y, Zhang H, Yu J, Hang H, Wu P, Yang F, Yang H, Steinbüchel A, Liu P. 2012. Identification of the major functional proteins of prokaryotic lipid droplets. J Lipid Res 53:399–411. doi: 10.1194/jlr.M021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dworkin J, Jovanovic G, Model P. 2000. The PspA protein of Escherichia coli is a negative regulator of σ54-dependent transcription. J Bacteriol 182:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi R, Suzuki T, Yoshida M. 2007. Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol Microbiol 66:100–109. doi: 10.1111/j.1365-2958.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- 30.Camus J-C, Pryor MJ, Médigue C, Cole ST. 2002. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 148:2967–2973. doi: 10.1099/00221287-148-10-2967. [DOI] [PubMed] [Google Scholar]
- 31.White MJ, Savaryn JP, Bretl DJ, He H, Penoske RM, Terhune SS, Zahrt TC. 2011. The HtrA-like serine protease PepD interacts with and modulates the Mycobacterium tuberculosis 35-kDa antigen outer envelope protein. PLoS One 6:e18175. doi: 10.1371/journal.pone.0018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He H, Bretl DJ, Penoske RM, Anderson DM, Zahrt TC. 2011. Components of the Rv0081-Rv0088 locus, which encodes a predicted formate hydrogenlyase complex, are coregulated by Rv0081, MprA, and DosR in Mycobacterium tuberculosis. J Bacteriol 193:5105–5118. doi: 10.1128/JB.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardarov S, Bardarov S Jr, Pavelka MS Jr, Sambandamurthy V, Larsen M, Tufariello J, Chan J, Hatfull G, Jacobs WR Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M smegmatis. Microbiology 148:3007–3017. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs WR Jr, Kalpana GV, Cirillo JD, Pascopella L, Snapper SB, Udani RA, Jones W, Barletta RG, Bloom BR. 1991. Genetic systems for mycobacteria. Methods Enzymol 204:537–555. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J, Russell DW. 1989. Molecular cloning: a laboratory manual, vol 3 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 36.Fisher MA, Plikaytis BB, Shinnick TM. 2002. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol 184:4025–4032. doi: 10.1128/JB.184.14.4025-4032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo XV, Monteleone M, Klotzsche M, Kamionka A, Hillen W, Braunstein M, Ehrt S, Schnappinger D. 2007. Silencing essential protein secretion in Mycobacterium smegmatis by using tetracycline repressors. J Bacteriol 189:4614–4623. doi: 10.1128/JB.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, Voskuil MI. 2010. The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol 192:1662–1670. doi: 10.1128/JB.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding Y, Zhang S, Yang L, Na H, Zhang P, Zhang H, Wang Y, Chen Y, Yu J, Huo C, Xu S, Garaiova M, Cong Y, Liu P. 2013. Isolating lipid droplets from multiple species. Nat Protoc 8:43–51. [DOI] [PubMed] [Google Scholar]
- 41.Garton NJ, Christensen H, Minnikin DE, Adegbola RA, Barer MR. 2002. Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiology 148:2951–2958. doi: 10.1099/00221287-148-10-2951. [DOI] [PubMed] [Google Scholar]
- 42.Hankamer BD, Elderkin SL, Buck M, Nield J. 2004. Organization of the AAA(+) adaptor protein PspA is an oligomeric ring. J Biol Chem 279:8862–8866. doi: 10.1074/jbc.M307889200. [DOI] [PubMed] [Google Scholar]
- 43.Maxson ME, Darwin AJ. 2006. Multiple promoters control expression of the Yersinia enterocolitica phage-shock-protein A (pspA) operon. Microbiology 152:1001–1010. doi: 10.1099/mic.0.28714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vrancken K, De Keersmaeker S, Geukens N, Lammertyn E, Anné J, Van Mellaert L. 2007. pspA overexpression in Streptomyces lividans improves both Sec- and Tat-dependent protein secretion. Appl Microbiol Biotechnol 73:1150–1157. [DOI] [PubMed] [Google Scholar]
- 45.Vrancken K, Van Mellaert L, Anné J. 2008. Characterization of the Streptomyces lividans PspA response. J Bacteriol 190:3475–3481. doi: 10.1128/JB.01966-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westphal S, Heins L, Soll J, Vothknecht UC. 2001. Vipp1 deletion mutant of Synechocystis: a connection between bacterial phage shock and thylakoid biogenesis? Proc Natl Acad Sci U S A 98:4243–4248. doi: 10.1073/pnas.061501198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao F, Wang W, Zhang W, Liu C. 2015. α-helical domains affecting the oligomerization of Vipp1 and its interaction with Hsp70/DnaK in Chlamydomonas. Biochemistry 54:4877–4889. doi: 10.1021/acs.biochem.5b00050. [DOI] [PubMed] [Google Scholar]
- 48.Buchan DWA, Minneci F, Nugent TCO, Bryson K, Jones DT. 2013. Scalable Web services for the PSIPRED protein analysis workbench. Nucleic Acids Res 41:W340–W348. doi: 10.1093/nar/gkt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhury S, Lyskov S, Gray JJ. 2010. PyRosetta: a script-based interface for implementing molecular modeling algorithms using Rosetta. Bioinformatics 26:689–691. doi: 10.1093/bioinformatics/btq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuhrmann E, Bultema JB, Kahmann U, Rupprecht E, Boekema EJ, Schneider D. 2009. The vesicle-inducing protein 1 from Synechocystis sp. PCC 6803 organizes into diverse higher-ordered ring structures. Mol Biol Cell 20:4620–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Southern SJ, Male A, Milne T, Sarkar-Tyson M, Tavassoli A, Oyston PCF. 2015. Evaluating the role of phage-shock protein A in Burkholderia pseudomallei. Microbiology 161:2192–2203. doi: 10.1099/mic.0.000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Standar K, Mehner D, Osadnik H, Berthelmann F, Hause G, Lünsdorf H, Brüser T. 2008. PspA can form large scaffolds in Escherichia coli. FEBS Lett 582:3585–3589. doi: 10.1016/j.febslet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Brissette JL, Russel M, Weiner L, Model P. 1990. Phage shock protein, a stress protein of Escherichia coli. Proc Natl Acad Sci U S A 87:862–866. doi: 10.1073/pnas.87.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L, Ding Y, Chen Y, Zhang S, Huo C, Wang Y, Yu J, Zhang P, Na H, Zhang H, Ma Y, Liu P. 2012. The proteomics of lipid droplets: structure, dynamics, and functions of the organelle conserved from bacteria to humans. J Lipid Res 53:1245–1253. doi: 10.1194/jlr.R024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Etienne G, Laval F, Villeneuve C, Dinadayala P, Abouwarda A, Zerbib D, Galamba A, Daffé M. 2005. The cell envelope structure and properties of Mycobacterium smegmatis mc2155: is there a clue for the unique transformability of the strain? Microbiology 151:2075–2086. doi: 10.1099/mic.0.27869-0. [DOI] [PubMed] [Google Scholar]
- 56.Garton NJ, Waddell SJ, Sherratt AL, Lee S-M, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS, Butcher PD, Barer MR. 2008. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med 5:e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenspan P, Mayer EP, Fowler SD. 1985. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE. 2004. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol 186:5017–5030. doi: 10.1128/JB.186.15.5017-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE. 2011. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog 7:e1002093. doi: 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Low KL, Rao PSS, Shui G, Bendt AK, Pethe K, Dick T, Wenk MR. 2009. Triacylglycerol utilization is required for regrowth of in vitro hypoxic nonreplicating Mycobacterium bovis bacillus Calmette-Guérin. J Bacteriol 191:5037–5043. doi: 10.1128/JB.00530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Low KL, Shui G, Natter K, Yeo WK, Kohlwein SD, Dick T, Rao SPS, Wenk MR. 2010. Lipid droplet-associated proteins are involved in the biosynthesis and hydrolysis of triacylglycerol in Mycobacterium bovis bacillus Calmette-Guérin. J Biol Chem 285:21662–21670. doi: 10.1074/jbc.M110.135731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherrid AM, Rustad TR, Cangelosi Ga Sherman DR. 2010. Characterization of a Clp protease gene regulator and the reaeration response in Mycobacterium tuberculosis. PLoS One 5:e11622. doi: 10.1371/journal.pone.0011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dutta NK, Mehra S, Kaushal D. 2010. A Mycobacterium tuberculosis sigma factor network responds to cell-envelope damage by the promising anti-mycobacterial thioridazine. PLoS One 5:e10069. doi: 10.1371/journal.pone.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGillivray A, Golden NA, Gautam US, Mehra S, Kaushal D. 2014. The Mycobacterium tuberculosis Rv2745c plays an important role in responding to redox stress. PLoS One 9:e93604. doi: 10.1371/journal.pone.0093604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehra S, Dutta NK, Mollenkopf H-J, Kaushal D. 2010. Mycobacterium tuberculosis MT2816 encodes a key stress-response regulator. J Infect Dis 202:943–953. doi: 10.1086/654820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Personne Y, Brown AC, Schuessler DL, Parish T. 2013. Mycobacterium tuberculosis ClpP proteases are co-transcribed but exhibit different substrate specificities. PLoS One 8:e60228. doi: 10.1371/journal.pone.0060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Provvedi R, Boldrin F, Falciani F, Palù G, Manganelli R. 2009. Global transcriptional response to vancomycin in Mycobacterium tuberculosis. Microbiology 155:1093–1102. doi: 10.1099/mic.0.024802-0. [DOI] [PubMed] [Google Scholar]
- 68.Murphy DJ. 2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40:325–438. doi: 10.1016/S0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 69.Caire-Brändli I, Papadopoulos A, Malaga W, Marais D, Canaan S, Thilo L, de Chastellier C. 2014. Reversible lipid accumulation and associated division arrest of Mycobacterium avium in lipoprotein-induced foamy macrophages may resemble key events during latency and reactivation of tuberculosis. Infect Immun 82:476–490. doi: 10.1128/IAI.01196-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunter RL, Jagannath C, Actor JK. 2007. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis (Edinb) 87:267–278. doi: 10.1016/j.tube.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, Daffé M, Emile J-F, Marchou B, Cardona P-J, de Chastellier C, Altare F. 2008. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog 4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deb C, Lee C-M, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, Pawar S, Rogers L, Kolattukudy PE. 2009. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One 4:e6077. doi: 10.1371/journal.pone.0006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kapoor N, Pawar S, Sirakova TD, Deb C, Warren WL, Kolattukudy PE. 2013. Human granuloma in vitro model, for TB dormancy and resuscitation. PLoS One 8:e53657. doi: 10.1371/journal.pone.0053657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.