Abstract
Atherosclerotic coronary artery disease remains a leading cause of worldwide morbidity and mortality. Invasive angiography currently remains the gold standard method of diagnosing and treating coronary disease; however, more sophisticated adjunctive interventional technologies have been developed to combat the inter and intra-observer variability frequently encountered in the assessment of lesion severity. Intravascular imaging now plays a key role in optimising percutaneous coronary interventions and provides invaluable information as part of the interventional cardiologist’s diagnostic arsenal. The principles, technical aspects and uses of two modalities of intracoronary imaging, intravascular ultrasound and optical coherence tomography, are discussed. We additionally provide examples of cases where the adjunctive intracoronary imaging was superior to angiography alone in successfully identifying and treating acute coronary syndromes.
Keywords: Acute coronary syndromes, etiology, cardiology, risk factors, atherosclerosis, cardiology, coronary imaging: angiography / ultrasound/ Doppler / CC, diagnostic testing, cardiology
Introduction
Cardiovascular disease remains the principal non-communicable cause of death worldwide.1 The predominant underlying aetiology is coronary artery disease due to the process of atherosclerosis within medium and large-sized vessels. It results in connective tissue proliferation and lipid accumulation with the vascular intima leading to plaque formation and subsequent plaque thrombosis, resulting from either denuded endothelium or deep plaque rupture.2
There have been major advances in the diagnosis and treatment of coronary artery disease over the past several decades with invasive angiography remaining the gold standard for diagnosis. However, since its inception over four decades ago, numerous adjunctive techniques have been developed to improve its diagnostic accuracy.3 In general, angiography overestimates lumen dimensions.4 Its limitations include the fact that it only provides a two-dimensional view of a three-dimensional structure and, consequently, is poor at accurately estimating plaque volume, morphology or lesion severity. Considerable remodelling of the vessel wall and lumen shape occurs in the natural history of the atherosclerotic process and this cannot be accounted for when assessing coronary artery stenoses with angiography only, thereby introducing errors.5,6 In addition, there is also considerable intra- and inter-observer variability in interpretation of stenosis severity during left heart catheterisation.7
Recent advances in the invasive management of coronary artery disease including the use of rotational atherectomy, intracoronary brachytherapy and the introduction of bioabsorbable coronary stents, demand precision and more accurate assessment of the culprit lesion and surrounding vessel than can be met by simple diagnostic angiography alone. Even though success rates and long-term outcomes from percutaneous coronary procedures have significantly improved with time, the procedure is not without its inherent risks such as re-stenosis and stent thrombosis.8
Several adjunctive technologies that are currently in use in the cardiac catheterisation laboratory evaluate either the physiological significance (determining pressure wire indices to calculate fractional flow reserve) or anatomically visualise a coronary atherosclerotic lesion. The latter strategy employs invasive imaging techniques such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT). These newer technologies are of particular value in assessing intermediate coronary artery lesions (lesion with a stenosis diameter of between 40% and 70%), left main stem disease, bifurcation disease, ostial lesions and coronary bypass conduit disease.
The aim of this review is to provide an overview of the principles and current uses of IVUS and OCT. We additionally provide examples of cases where the adjunctive intracoronary imaging was superior to angiography alone in successfully identifying and treating acute coronary syndromes.
Intravascular ultrasound
Intravascular ultrasound (IVUS) is a catheter-based imaging technique, developed over 20 years ago by Yock, Hodgson and colleagues in 1989.9,10 It uses reflected sound waves to provide a cross-sectional tomographic view of the vessel lumen and the entire wall, including the full thickness of any present plaque in vivo.11
It is now widely used as an adjunct to conventional coronary angiography and percutaneous coronary intervention procedures. IVUS enables the operator to directly visualise the full 360° circumference and take accurate measurements by direct planimetry of the vessel lumen and atherosclerotic plaque. By doing so, it has been shown to detect atherosclerotic abnormalities in angiographically normal arteries. This is of relevance because previous studies have suggested that apparently minimal to moderate plaque lesions on angiography may be most likely to rupture and cause acute coronary syndromes.12–14 The literature, however, reports a number of conflicting theories regarding the severity of culprit lesions, particularly in the context of ST-segment elevation myocardial infarction prior to the index presentation.15–17 Invasive angiography alone is insensitive to plaque size and atherosclerotic burden, and it is now recognised that IVUS allows accurate depiction of positive remodelling, whereby luminal area has been maintained despite heavy plaque burden, which may itself increase the risk for acute coronary presentations.17
When used to assess the atherosclerotic burden, IVUS has a higher resolution than non-invasive methods such as multi-detector computed tomography or cardiac magnetic resonance imaging.18 Complex vessel areas such as angiographically foreshortened segments, ostial disease, diffusely diseased areas, bifurcation stenosis and long lesions can be more accurately assessed. Therefore, IVUS is not only used during elective percutaneous procedures but also in the setting of acute coronary syndromes.19,20
IVUS is able to assess vessel wall composition and identify any abnormalities such as inadequate stent apposition or expansion, as well as the presence of dissections following PCI, thus guiding the operator to optimise intervention.21–23 The distribution and structure of the atheromatous plaque are important factors affecting the success of PCI, brachytherapy as well as atherectomy24 and by facilitating more precise plaque assessment, IVUS has become increasingly invaluable to the interventional cardiologist.25,26
In the era of drug eluting stents, IVUS-guided PCI has been shown to reduce major adverse cardiovascular events (MACE) when compared with angiographically guided PCI.27 This IVUS-guided PCI associated MACE reduction has also been found with implantation of newer generation stents. In their study, Hong demonstrated at 1-year follow-up among patients requiring long coronary stents, IVUS-guided everolimus-eluting stent implantation was associated with a composite reduction in MACE, although this was largely driven by a reduction in target lesion revascularisation.28 This was further supported by a recent meta-analysis corroborating the reduction in ischaemia-driven target lesion revascularisation, as well as lower cardiovascular mortality and stent thrombosis rates with an IVUS-guided approach.29
The procedure itself is technically simple to perform and is associated with a low complication risk. A multi-centre survey of over 2000 IVUS cases showed only an association with (but not necessarily the direct cause of) minor acute clinical risks. The most common of these was spasm in 2.9% of all the IVUS studies. Other acute procedural and major complications (including vessel occlusion, dissection and thrombosis resulting in myocardial infarction, CABG or worse) occurred in 0.3% and 0.1% of patients, respectively.11
The device consists of a miniaturised ultrasound transducer (either a mechanically rotated single transducer system device or multi-element electronic array system), mounted on the tip of a catheter and attached to a console, which reconstructs the image. The transducer is oriented so that the ultrasonic beam produced is aimed parallel to the long axis of the catheter.19 Typically high ultrasound frequencies around 20–50 MHz are used to achieve greater radial resolution at the expense of penetration depth.
Heparin and intracoronary nitroglycerin are administered to reduce the risk of coronary vasospasm prior to target coronary artery sub-selective cannulation. The IVUS catheter is then advanced into the target vessel and the transducer positioned beyond the target segment. A motorised drive unit with an automatic pullback system progressively withdraws the transducer at a constant speed, usually at 0.5 mm/s, although this can be done manually. IVUS measurements are made at every 1 mm distance of pullback. Three layers of the coronary arterial wall are identified on gray-scale IVUS: the intima layer, the muscular media layer, which appears as a dark band, and the adventitia layer. Calcified plaque appears brightly echo-reflective and creates a dense shadow, as well as reverberation markers appearing radially at spaced intervals from the calcified segment.30 Fibrotic tissue gives a bright, but less intense appearance and fatty plaque tissue is less bright than the adventitia layer. Using electronic callipers and computerised planimetry, quantitative measurements are made at the narrowest cross-section to determine the minimal luminal area (MLA) and diameter, and at a reference ‘normal’ segment (within 10 mm proximal or distal to the culprit lesion). This allows calculation of percentage stenosis, plaque area and plaque burden.
Virtual histology IVUS (VH-IVUS) is an iteration of the standard gray-scale version. It uses an automated algorithm to process the reflected ultrasound backscatter signal, further distinguishing plaque components such as fibrous tissue, fibrofatty tissue, necrotic lipid core and dense calcium.31 Atherosclerotic plaque rupture is now accepted as the main cause of acute coronary events. These vulnerable plaques are characterised by a thin fibrous cap (less than 65 µm), large lipid pool with increased macrophage activity and play a key role in the atherosclerotic process. Detection of these vulnerable thin caps, as well as additional features of plaque instability such as cholesterol crystals and neovascularisation, are well below the axial resolution of VH-IVUS.32 In order to address these concerns, modified criteria have been implemented for VH-IVUS identification of vulnerable thin-capped fibroatheroma (TCFA); a focal necrotic core (>10% of total plaque area) without overlying fibrous tissue in the presence of percent atheroma volume >40%.33 A number of prospective studies have now shown associations between VH-IVUS derived high risk plaque morphology, such as the presence of VH-IVUS TCFA, and future MACE confirming the biological and to some extent, prognostic importance of VH-IVUS in identifying plaque composition.34–36 Indeed, plaque modification with statin therapy has recently been evaluated with VH-IVUS by Park et al. In their prospective single-centre study, rosuvastatin was associated with a reduction in proportionate necrotic core volume, total plaque volume and VH-IVUS defined TCFA at 1-year follow-up in non-culprit lesions. Further studies are warranted to assess whether the plaque modification observed translates to outcome improvement.37
Although an accurate anatomical assessment can be made, IVUS does not provide physiological information on which revascularisation decisions frequently depend.
Fractional flow reserve (FFR) is currently the invasive modality of choice in assessing the haemodynamic significance of stenoses. A haemodynamically insignificant lesion assessed by FFR correlates well with an insignificant lesion detected by IVUS. However, predicting abnormal FFR with IVUS assessment proves more challenging due to various factors other than percent area stenosis influencing the functional effects of a lesion.38 Previous studies have demonstrated moderate correlation at best between MLA cut-off values and FFR confirmed ischaemic lesions.38,39 An international, prospective, multi-centre registry concurred with this premise and identified an optimal threshold for correlation with an ischaemic FFR (<0.8) of MLA below 3.07 mm2 in non-left-main stem lesions (64% sensitivity, 64.9% specificity). FFR correlated with plaque burden but not VH-IVUS-derived plaque composition. The authors conceded that despite moderate correlation and a high ischaemic negative predictive value for lesions with MLA >3.07mm2, accuracy could be improved when using a reference vessel-specific analysis. This led to the conclusion that IVUS has a limited role in the functional assessment of intermediate stenosis to accurately identify ischaemia-inducible lesions and ascertain the indication for revascularization, but remains a valuable and established tool to guide percutaneous coronary intervention.40
Optical coherence tomography
Optical coherence tomography (OCT) is a more novel modality in comparison to IVUS. It was first developed for cross-sectional retinal imaging in the 1990 s but over the last two decades its application has rapidly expanded to other fields and is now extensively used in interventional cardiology.41 It provides the highest resolution of all currently used invasive imaging modalities (5 to 20 µm) resulting in high quality cross-sectional tomographic images of the coronary architecture.42
Ultrasound is replaced by infra-red light emitted from a rotating fibre-optic system. This is then reflected or back-scattered from internal structures within tissue and analysed by interferometric techniques. The OCT image is produced from the backscattered light from the vessel wall and the echo time-delay signal. It provides a detailed image of around ten times greater resolution than that achieved with IVUS due to the shorter wavelength of the imaging light (∼1300 nm) compared with ultrasound.43 This is at the expense of a reduced tissue penetration depth of 1–3 mm (compared with 4 to 8 mm with IVUS) with the exception of calcified lesions.44
Two main technologies exist to create OCT images: the time-domain (TD-OCT) or frequency domain (Fourier or FD-OCT) technique. The latter is preferred due to faster image acquisition rates and a better signal-to-noise ratio. It uses a fixed mirror with a variable frequency light source allowing simultaneous detection of reflections.45 The older TD-OCT uses a moving mirror as its reference arm and a broadband light source. Because of its slow acquisition speed, it is necessary to occlude the target proximal coronary artery. An over-the-wire low-pressure occlusion balloon catheter with distal flush ports is used to infuse saline or Ringer’s Lactate at a rate of approximately 0.5 mL/s for a maximum of 30 s in total in order to selectively displace blood during the image acquisition. Any amount of residual red blood cells will otherwise cause significant signal attenuation. Unfortunately, this action increases the risk of coronary damage and myocardial ischaemia. In the newer FD-OCT system, the higher acquisition frame rate and accelerated pullback speed permits the use of a single, high rate injection of a bolus of contrast to produce a blood-free environment, thereby eliminating the need for balloon occlusion46,47 and its potential ischaemic complications.
TCFA are the most rupture-prone lesions and, consequently, remain a predictor of major adverse cardiovascular events.48,49 Studies to quantify fibrous cap thickness have shown that the incidence of TCFA is highest in acute myocardial infarction, intermediate in acute coronary syndromes and lowest in chronic stable angina. OCT with its superior resolution capacity is the only current imaging tool than can accurately visualise and quantify the thin fibrous cap.50 Correlating with histopathology, the sensitivity and specificity of OCT are 92% and 94% respectively for identifying lipid-rich plaque, 95% and 100% for fibro-calcific plaque and 87% and 97% respectively for fibrous plaque.42
OCT is also able to quantify the activated macrophage content beneath the plaque fibrous cap. Macrophages with their high lipid content produce strong optical signals detectable by OCT, which is relevant as increased macrophage density is thought to contribute to the instability of vulnerable plaques.51–53 Due to its limited tissue penetration, however, OCT is unable to assess in depth total plaque volume or vascular remodelling. Current OCT systems are not suitable to reliably assess tissue at depths beyond 2 mm.54 In contrast to ultrasound, however, light penetrates calcium and OCT is able to depict calcific nodules with well-defined boundaries with high sensitivity and specificity.55 OCT has also demonstrated superiority in assessing culprit lesions, particularly identifying thrombus, plaque erosion and rupture when compared to IVUS or angioscopy.56 Earlier papers have also alluded to OCT outperforming IVUS in detecting spontaneous and PCI-induced dissection, tissue prolapse and incomplete stent apposition, all of which have been implicated in acute as well as late stent thrombosis.57,58 In addition, limited outcome data exists to show OCT-guided PCI as superior to angiography alone.59 This unrivalled endovascular resolution has allowed assessment of the vascular response at individual stent strut level following device deployment. Hence, numerous in vivo OCT studies have exploited its capability to study factors related to the prognosis of stent-implanted lesions, including neointimal hyperplasia, stent strut coverage, stent malapposition and in-stent neoatherosclerosis.60–62 With the emergence of bioabsorbable vascular scaffolds (BVS) and polymer-free drug eluting stents, OCT has also been the primary imaging modality to follow-up recruited cohorts assessing stent strut resorption/ coverage, as well as optimisation at the time of implantation.63,64
Both IVUS and OCT therefore provide useful and differential information to diagnose, plan and evaluate PCI. When enhanced visualisation of plaque characteristics and microstructure are required such as detection of intimal tears, thrombus, stent malapposition and intimal hyperplasia, OCT provides a more superior modality given its greater spatial resolution. Despite limitations in resolution, IVUS provides valuable information regarding plaque area, volume, morphology and vessel size. Unlike OCT, ostial disease can be imaged adequately as blood expulsion with contrast from the field of view is not necessary. Its superior depth of penetration also allows greater intracoronary visualisation of larger diameter and ectatic vessels.
A summary of the main differences between FD-OCT and IVUS are provided in Table 1, and the tissue characteristics with OCT appearance are outlined in Table 2.
Table 1.
Major differences between OCT and IVUS.
| OCT | IVUS | |
|---|---|---|
| Tissue penetration | 1–2 mm | 6–10 mm |
| Technology | Near infra-red | Ultrasound |
| Imaging speed (pull back) | 20 mm/s | 1 mm/s |
| Resolution: Axial | 15 µm | 100–200 µm |
| Transverse | 20–40 µm | 200–300 µm |
| Catheter size | 3.2Fr | 3.5Fr |
| Blood removal with contrast | Yes | No |
OCT: optical coherence tomography; IVUS: intravascular ultrasound.
Table 2.
Tissue characteristics observed with OCT.
| Tissue characteristics | OCT characteristics |
|---|---|
| Fibrous | Homogenous High reflectivity Low attenuation |
| Lipid | Diffuse edges High reflectivity High attenuation |
| Calcific | Sharp well-defined edges Low reflectivity (compared to IVUS) Low attenuation |
| Red thrombus | Mass protruding into vessel lumen Medium reflectivity High attenuation |
| White thrombus | Luminal protrusion Medium reflectivity Low attenuation |
| Stents: Metallic | High reflectivity High attenuation |
| Bioresorbable | Low reflectivity (if residual polymer present) Low attenuation |
OCT: optical coherence tomography.
Case studies
IVUS-guided pci following acute coronary syndrome of an angiographically unobstructed coronary artery
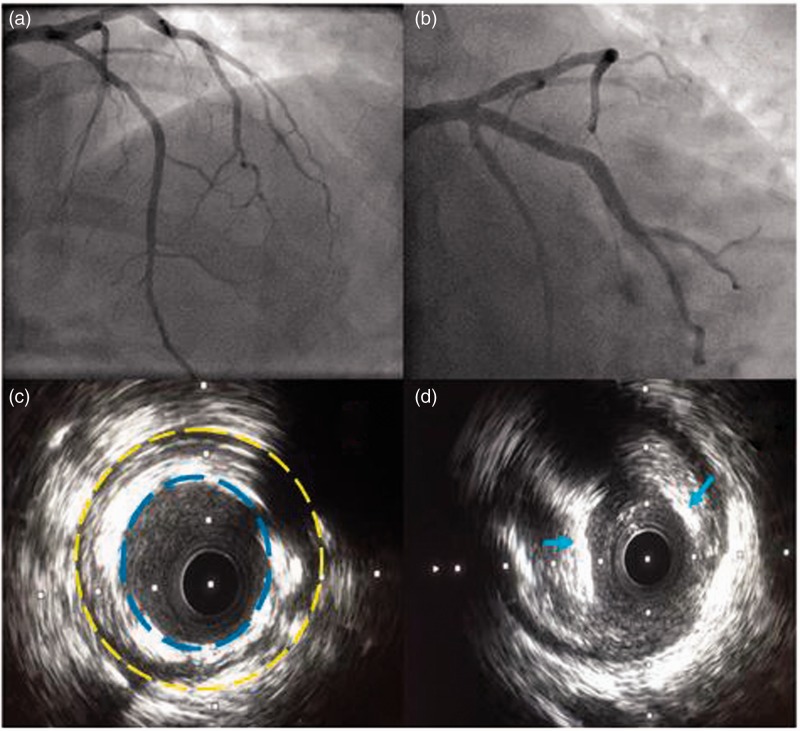
A 67-year-old Asian female, with a background of primary percutaneous coronary intervention (PPCI) to the left anterior descending artery (LAD) in 2012, was admitted to our unit with cardiac chest pain, elevated troponin I titres and anterior T-wave inversion on electrocardiography (ECG). An echocardiogram confirmed anterior regional wall motion abnormality in the context of mild left ventricular impairment. Following appropriate initiation of medical therapy for non-ST-elevation myocardial infarction (NSTEMI), coronary angiography was performed demonstrating a right dominant unobstructed coronary circulation and a patent stent in the proximal LAD (Figure 1(a) and (b)). In view of her presentation, we undertook IVUS assessment of the LAD demonstrating a grossly under-expanded stent as well as severe eccentric fibro calcific disease in the ostium (Figure 1(c) and (d)). The stent was post dilated and following predilatation of the ostium, a new drug eluting stent was deployed. The patient’s recovery was uneventful and she was discharged home.
Figure 1.
(a) Postero-anterior (PA) cranial view of the left coronary circulation demonstrating mild proximal and mid LAD disease; (b) PA caudal view demonstrating mild ostial LAD disease with a patent proximal stent; (c) IVUS revealing a grossly under-expanded proximal portion of the LAD stent. (The dashed yellow line delineates the border of the coronary vessel wall. The blue dashed line demonstrates the inner most stent struts malapposed from the vessel wall.); (d) IVUS of severe eccentric fibrocalcific ostial LAD disease. (Note the blue arrows indicating areas of calcification.).
OCT-guided pci following acute coronary syndrome of an angiographically unobstructed coronary artery
A 67-year-old hypertensive male presented to our unit with a 24-hour history of intermittent episodes of chest pain at rest. Routine investigations demonstrated T-wave inversion on the ECG, and elevated troponin I level. Coronary angiography demonstrated a right dominant unobstructed coronary circulation (Figure 2(a) and (b)). OCT assessment of the LAD showed a long segment of fibrotic disease with a minimal luminal area of 1.98 mm2 at the level of the first diagonal (Figure 2(c) and (d)). Using an average for reference vessel area of 7.29 mm2, this equated to a cross-sectional area stenosis of 73%. The lesion was stented with two drug-eluting stents. He was discharged home the following day and remains well at follow-up.
Figure 2.
(a) PA caudal view of left coronary circulation revealing no obstructive coronary disease; (b) Right anterior oblique view demonstrating mild proximal and mid LAD disease; (c) OCT of LAD revealing significant disease proximal to mid vessel, with minimal luminal area at the first diagonal bifurcation; (d) three-dimensional OCT reconstruction of LAD.
Future perspectives
IVUS technology is continually evolving. Radiofrequency back-scatter analysis converts radiofrequency signals from IVUS into colour-coded regions depending on the plaque composition thereby providing a virtual histology of the atherosclerotic lesion,65 which is now widely utilised. VH-IVUS has recently been shown to reliably identify TCFA when compared with OCT analysis of autopsied human hearts.66 Mechanical strain assessment with IVUS elastography measures the mechanical strain property of the vessel wall with studies demonstrating different mean strain values between different plaque components. Histological vulnerable plaque correlates with an elevated strain value with adjacent low strain values.67 More recently, a hypothesis generating study analysing plaque structural stress has also demonstrated a positive correlation with higher-risk underlying plaque subtypes, representing a potential future application to aid with predicting plaque rupture.68 Hybrid imaging combining IVUS technology with near infra-red spectroscopy (NIRS) accurately characterises lipid-rich plaques within coronary arteries. Despite data reporting NIRS derived lipid core burden index being no different between acute coronary and stable angina patients,69 ST elevation MI patients have been identified by NIRS as having large lipid-rich plaques at the culprit site.70 Ongoing prospective studies will investigate whether this combined dual probe catheter-based approach translates to an accurate prediction of MACE.
In the realm of OCT, research studies assessing stent edge and strut detection capabilities, qualitative and quantitative strut-level analysis, improved tissue characterisation with texture analysis and polarisation (making use of random scattering found in some tissues and reproducible birefringence found in highly organized collagen-rich tissues) are either in progress or are being proposed. An important area of continual research assesses the impact and efficacy of proposed anti-platelet and anti-atherosclerotic therapy in the management of coronary artery disease.
There are several randomised open-labelled studies in progress which aim to address the current dearth of clinically applicable OCT outcome trial data. The FORZA study will compare the economic and clinical impact of OCT guidance versus FFR in the assessment of angiographically intermediate coronary lesions.71
The DOCTORS study will evaluate the impact of changes in procedural strategy resulting from the use of OCT after angioplasty and stent implantation of a lesion responsible for non-ST-elevation acute coronary syndrome.72
Novel stent technologies such as BVS rely heavily upon adequate intracoronary imaging guidance. Co-registration has demonstrated superiority of OCT over VH-IVUS in optimising PCI with BVS to reduce rates of scaffold thrombosis.73,74 The use of OCT in BVS optimisation has also been used in more complex coronary anatomy such as chronic total occlusions.75
Future developments include ultra-high resolution or micro-OCT, which will provide even higher quality images with spatial resolutions ten times that of FD-OCT, permitting sub-cellular analysis of atherosclerosis, which may be used for individualised or targeted therapy in the prevention of coronary artery disease.
Conclusion
Our cases have emphasised the value of intracoronary imaging in cases where no clear culprit lesion is identifiable on the unaided angiographic images. As demonstrated, adjunctive imaging is now recommended to ascertain the aetiology of stent failure where inadequate stent apposition or stent under-expansion could propagate stent thrombosis or re-stenosis. Their roles in research are well documented, including assessing the stability of vulnerable plaques by analysing its elastic and acoustic features with elastography and radiofrequency analysis, qualitative and quantitative stent strut analysis, hybrid imaging technologies and studying the efficacy of new anti-atherosclerotic therapies. Further research is warranted to ascertain if prognostic benefit can be gained with future clinical applications both in the cardiac catheter lab and wider cardiology practice.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
None.
References
- 1.Global status report on non-communicable diseases 2014, Geneva, Switzerland: World Health Organisation, 2015. [Google Scholar]
- 2.Davies MJ. A macro and micro view of coronary vascular insult in ischaemic heart disease. Circulation 1990; 82: II 38–46. [PubMed] [Google Scholar]
- 3.Kiyoshi H, Kazuo K, Satoshi U. Clinical utility and significance of intravascular Ultrasound and Optical Coherence Tomography in guiding percutaneous coronary interventions. Circ J 2015; 79: 24–33. [DOI] [PubMed] [Google Scholar]
- 4.Nakaramu S, Mahon D, Maheswaran B, et al. An explanation for the discrepancy between angiographic and intravascular ultrasound measurements after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 1995; 25: 633–639. [DOI] [PubMed] [Google Scholar]
- 5.Steil GM, Stiel LS, Schofer J, et al. Impact of compensatory enlargement of atherosclerotic coronary arteries on angiographic assessment of coronary artery disease. Circulation 1989; 80: 1603–1603. [DOI] [PubMed] [Google Scholar]
- 6.Thomas AC, Davies MJ, Dilly S, et al. Potential errors in estimation in coronary arterial stenosis from clinical arteriography with reference to the shape of the coronary arterial lumen. Br Heart J 1986; 55: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zir LM. Observer variability in coronary angiography. Int J Cardiol 1983; 3: 171–173. [DOI] [PubMed] [Google Scholar]
- 8.Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: The TAXUS-IV trial. Circulation 2004; 109: 1942–1947. [DOI] [PubMed] [Google Scholar]
- 9.Marco J, Fajadet J, Robert G. Intracoronary ultrasound imaging: Initial clinical trials. Circulation 1989; 80: 374–374. [Google Scholar]
- 10.Graham SP. Assessment of arterial wall morphology using intravascular ultrasound in vitro and in patients. Circulation 1989; 80: 565–565. [Google Scholar]
- 11.Hausmann D, Erbel R, Alliberi-Chemarin MJ, et al. The safety of intracoronary ultrasound: A multicenter survey of 2207 examinations. Circulation 1995; 91: 623–630. [DOI] [PubMed] [Google Scholar]
- 12.Blasini R, Neumann F, Schmitt C, et al. Comparison of angiography and intravascular ultrasound for the assessment of lumen size after coronary stent placement: Impact of dilatation pressures. Cathet Cardiovasc Diagn 1997; 42: 113–119. [DOI] [PubMed] [Google Scholar]
- 13.Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation 2001; 103: 934–940. [DOI] [PubMed] [Google Scholar]
- 14.Burke AP, Farb A, Malcom GT, et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997; 336: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 15.Manoharan G, Ntalianis A, Muller O, et al. Severity of coronary arterial stenoses responsible for acute coronary syndromes. Am J Cardiol 2009; 103: 1183–1188. [DOI] [PubMed] [Google Scholar]
- 16.Hackett D, Verwilghen J, Davies G, et al. Coronary stenoses before and after acute myocardial infarction. Am J Cardiol 1989; 63: 1517–1518. [DOI] [PubMed] [Google Scholar]
- 17.Niccoli G, Stefanini GG, Capodanno D, et al. Are the culprit lesions severely stenotic? J Am Coll Cardiol Img 2013; 6: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 18.Ilke S, Nicholls S, Tuzcu E. Recent trends in coronary intravascular ultrasound: tracking atherosclerosis, pursuit of vulnerable plaques, and beyond. J Nucl Cardiol 2006; 13: 91–96. [DOI] [PubMed] [Google Scholar]
- 19.Tobis J, Mahon D, Moriuchi M, et al. Intravascular ultrasonic imaging. Texas Heart Inst J 1990; 17: 181–189. [PMC free article] [PubMed] [Google Scholar]
- 20.Hur SH, Kang SJ, Kim YH, et al. Impact of ultrasound-guided percutaneous coronary intervention on long term clinical outcomes in a real world population. Catheter Cardiovasc Interv 2013; 81: 407–416. [DOI] [PubMed] [Google Scholar]
- 21.St. Goar FG, Pinto FJ, Alderman EL, et al. Intracoronary ultrasound in cardiac transplant recipients: In vivo evidence of ‘angiographically silent’ intimal thickening. Circulation 1992; 85: 979–987. [DOI] [PubMed] [Google Scholar]
- 22.Nissen S, Yock P. Intravascular ultrasound novel pathophysiological insights and current clinical applications. Circulation 2001; 103: 604–616. [DOI] [PubMed] [Google Scholar]
- 23.Ako J, Morino Y, Honda Y, et al. Late incomplete stent apposition after Sirolimus-Eluting stent implantation: A serial intravascular ultrasound analysis. J Am Coll Cardiol 2005; 46: 1002–1005. [DOI] [PubMed] [Google Scholar]
- 24.Anderson M, Simpson I, Katritsis D, et al. Intravascular ultrasound imaging of the coronary arteries: An in vitro evaluation of measurement of area of the lumen and atheroma characterisation. Br Heart J 1992; 68: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong MK, Mintz GS, Lee CW, et al. Paclitaxel coating reduces in-stent intimal hyperplasia in human coronary arteries: A serial volumetric intravascular ultrasound analysis from the Asian Paclitaxel-Eluting Stent Clinical Trial (ASPECT). Circulation 2003; 107: 517–520. [DOI] [PubMed] [Google Scholar]
- 26.Morino Y, Bonneau HN, Fitzgerald PJ. Vascular brachytherapy: What have we learned from intravascular ultrasound? J Invasive Cardiol 2001; 13: 409–416. [PubMed] [Google Scholar]
- 27.Jang J, Song Y, Kang W, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: A meta-analysis. J Am Coll Cardiol Intv 2014; 7: 233–243. [DOI] [PubMed] [Google Scholar]
- 28.Hong S-J, Kim B-K, Shin D-H, et al. Effect of intravascular ultrasound–guided vs angiography-guided everolimus-eluting stent implantation: The IVUS-XPL randomized clinical trial. JAMA 2015; 314: 2155–2163. [DOI] [PubMed] [Google Scholar]
- 29.Elgendy IY, Mahmoud AN, Elgendy AY, et al. Outcomes with intravascular ultrasound-guided stent implantation: A meta-analysis of randomized trials in the era of drug-eluting stents. Circ Cardiovasc Interv 2016; 9: e003700–e003700. [DOI] [PubMed] [Google Scholar]
- 30.Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound scan and coronary angiography. Circulation 1995; 91: 1959–1965. [DOI] [PubMed] [Google Scholar]
- 31.Nair A, Margolis MP, Kuban BD, et al. Automated coronary plaque characterisation with intravascular ultrasound backscatter: Ex vivo validation. EuroIntervention 2007; 3: 113–120. [PubMed] [Google Scholar]
- 32.Brown AJ, Costopoulos C, West NE, et al. Contemporary invasive imaging modalities that identify and risk-stratify coronary plaques at risk of rupture. Expert Rev Cardiovasc Ther 2015; 13: 9–13. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Granillo GA, Garcia-Garcia HM, McFadden EP, et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol 2005; 46: 2038–2042. [DOI] [PubMed] [Google Scholar]
- 34.Calvert PA, Obaid DR, O’sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: The VIVA (VH-IVUS in Vulnerable Atherosclerosis) study. JACC Cardiovasc Imag 2011; 4: 894–901. [DOI] [PubMed] [Google Scholar]
- 35.Cheng JM, Garcia-Garcia HM, de Boer SPM, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Eur Heart J 2014; 35: 639–647. [DOI] [PubMed] [Google Scholar]
- 36.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364: 226–235. [DOI] [PubMed] [Google Scholar]
- 37.Park S, Kang S, Ahn J, et al. Effect of statin treatment on modifying plaque composition: A double-blind, randomized study. J Am Coll Cardiol 2016; 67: 1772–1783. [DOI] [PubMed] [Google Scholar]
- 38.Kang SJ, Lee JY, Ahn JM, et al. Validation of intravascular ultrasound-derived parameters with fractional flow reserve for assessment of coronary stenosis severity. Circ Cardiovasc Interv 2011; 4: 65–71. [DOI] [PubMed] [Google Scholar]
- 39.Koo BK, Yang HM, Doh JH, et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. J Am Coll Cardiol Intv 2011; 4: 803–811. [DOI] [PubMed] [Google Scholar]
- 40.Waksman R, Legutko J, Singh J, et al. FIRST: Fractional flow reserve and intravascular ultrasound relationship study. J Am Coll Cardiol 2013; 61: 917–923. [DOI] [PubMed] [Google Scholar]
- 41.Zysk A, Nguyen F, Oldenburg AL, et al. Optical Coherence Tomography: A review of clinical development from bench to bedside. J Biomed Optics 2007; 12. [DOI] [PubMed] [Google Scholar]
- 42.Low AF, Tearney GJ. Optical coherence tomography. Current status and future developments. Nat Clin Pract Cardiovasc Med 2006; 3: 154–162. [DOI] [PubMed] [Google Scholar]
- 43.Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: Comparison with intravascular ultrasound. J Am Coll Cardiol 2002; 39: 604–609. [DOI] [PubMed] [Google Scholar]
- 44.Prati F, Regar E, Mintz GS, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010; 31: 401–415. [DOI] [PubMed] [Google Scholar]
- 45.Chinn SR, Swanson EA, Fujimoto JG. Optical coherence tomography using a frequency-tunable optical source. Opt Lett 1997; 22: 340–342. [DOI] [PubMed] [Google Scholar]
- 46.Tearney GJ, Waxman S, Shishkov M, et al. Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. J Am Coll Cardiol Imag 2008; 1: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barlis P, Schmitt JM. Current and future developments in intracoronary optical coherence tomography imaging. EuroIntervention 2009; 4: 529–533. [DOI] [PubMed] [Google Scholar]
- 48.Schaar JA, Muller JE, Falk E, et al. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque. Eur Heart J 2004; 25: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto Y, Okura H, Kume T, et al. Plaque characteristics of thin-cap fibroatheroma evaluated by OCT and IVUS. JACC Cardiovasc Imag 2011; 4: 638–646. [DOI] [PubMed] [Google Scholar]
- 50.Jang IK, Tearney G, MacNeill B, et al. In vivo characterisation of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 2005; 111: 1551–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tearney G, Yabushita H, Houser S, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003; 107: 113–119. [DOI] [PubMed] [Google Scholar]
- 52.MacNeill B, Bouma B, Yabushita H, et al. Intravascular optical coherence tomography: Cellular imaging. J Nucl Cardiol 2005; 12: 460–465. [DOI] [PubMed] [Google Scholar]
- 53.MacNeill BD, Jang IK, Bouma BE, et al. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol 2004; 44: 972–979. [DOI] [PubMed] [Google Scholar]
- 54.Bezerra HG, Costa MA, Guagliumi G, et al. Intracoronary optical coherence tomography: A comprehensive review. Clinical and research applications. J Am Coll Cardiol Intv 2009; 2: 1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002; 106: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 56.Kubo T, Imanishi T, Takarada S, et al. Assessment of culprit lesion morphology in acute myocardial infarction: Ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol 2007; 50: 933–939. [DOI] [PubMed] [Google Scholar]
- 57.Bouma BE. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart 2003; 89: 317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bezerra HG, Attizzani GF, Sirbu V, et al. Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention. JACC Cardiovasc Interv 2013; 6: 228–236. [DOI] [PubMed] [Google Scholar]
- 59.Prati F, Di Vito L, Biondi-Zoccai G, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: The Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 2012; 8: 823–829. [DOI] [PubMed] [Google Scholar]
- 60.Guagliumi G, Musumeci G, Sirbu V, et al. Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc Interv 2010; 3: 531–539. [DOI] [PubMed] [Google Scholar]
- 61.Kang SJ, Mintz GS, Akasaka T, et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation 2011; 123: 2954–2963. [DOI] [PubMed] [Google Scholar]
- 62.Guagliumi G, Costa MA, Sirbu V, et al. Strut coverage and late malapposition with paclitaxel-eluting stents compared with bare metal stents in acute myocardial infarction: Optical coherence tomography substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial. Circulation 2011; 123: 274–281. [DOI] [PubMed] [Google Scholar]
- 63.Mattesini A, Secco GG, Dall’Ara G, et al. ABSORB biodegradable stents versus second-generation metal stents: A comparison study of 100 complex lesions treated under OCT guidance. JACC Cardiovasc Intv 2014; 7: 741–750. [DOI] [PubMed] [Google Scholar]
- 64.Serruys PW, Ormiston JA, Onuma Y, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 2009; 373: 897–910. [DOI] [PubMed] [Google Scholar]
- 65.Sano K, Kawasaki M, Ishihara Y, et al. Assessment of vulnerable plaques using causing acute coronary syndromes using integrated backscatter intravascular ultrasound. J Am Coll Cardiol 2006; 47: 734–741. [DOI] [PubMed] [Google Scholar]
- 66.Brown AJ, Obaid DR, Costopoulos C, et al. Direct comparison of virtual-histology intravascular ultrasound and optical coherence tomography imaging for identification of thin-cap fibroatheroma. Circ Cardiovasc Imag 2015; 8: e003487–e003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaar JA, de Corte CL, Mastik F, et al. Characterising vulnerable plaque features with intravascular elastography. Circulation 2003; 108: 2636–2641. [DOI] [PubMed] [Google Scholar]
- 68.Teng Z, Brown AJ, Calvert PA, et al. Coronary plaque structural stress is associated with plaque composition and subtype and higher in acute coronary syndrome: The BEACON I (Biomechanical Evaluation of Atheromatous Coronary Arteries) study. Circ Cardiovasc Imag 2014; 7: 461–470. [DOI] [PubMed] [Google Scholar]
- 69.de Boer SPM, Brugaletta S, Garcia-Garcia HM, et al. Determinants of high cardiovascular risk in relation to plaque composition of a non-culprit coronary segment visualized by near-infrared spectroscopy in patients undergoing percutaneous coronary intervention. Eur Heart J 2014; 35: 282–289. [DOI] [PubMed] [Google Scholar]
- 70.Madder RD, Steinberg DH, Anderson RD. Multimodality direct coronary imaging with combined near-infrared spectroscopy and intravascular ultrasound: Initial US experience. Catheter Cardiovasc Interv 2013; 81: 551–557. [DOI] [PubMed] [Google Scholar]
- 71.FFR or OCT Guidance to RevasculariZe Intermediate Coronary Stenosis Using Angioplasty (FORZA), 2013. www.clinicaltrials.gov/show/NCT01824030 (accessed 29 April 2016).
- 72.Does Optical Coherence Tomography Optimise Results of Stenting (DOCTORS). 2013. www.clinicaltrials.gov/show/NCT01743274.) (accessed 29 April 2016).
- 73.Brown AJ, McCormick LM, Hoole SP, et al. Coregistered intravascular ultrasound and optical coherence tomography imaging during implantation of a bioresorbable vascular scaffold. J Am Coll Cardiol Interv 2013; 6: e41–e42. [DOI] [PubMed] [Google Scholar]
- 74.Costopoulos C, Crowson MC, Brown AJ, et al. Mid-term clinical outcomes of ABSORB bioresorbable vascular scaffold implantation in a real-world population: A single-center experience. Cardiovasc Revasc Med 2015; 16: 461–464. [DOI] [PubMed] [Google Scholar]
- 75.Vaquerizo B, Barros A, Pujadas S, et al. Bioresorbable everolimus-eluting vascular scaffold for the treatment of chronic total occlusions: CTO-ABSORB pilot study. EuroIntervention 2015; 11: 555–563. [DOI] [PubMed] [Google Scholar]