Abstract
Recent clinical and laboratory evidences suggest that high fat diet (HFD) induced obesity and its associated metabolic syndrome conditions promotes neuropathology in aging and age-related neurological disorders. However, the effects of high fat diet on brain pathology are poorly understood, and the effective strategies to overcome these effects remain elusive. In the current study, we examined the effects of HFD on brain pathology and further evaluated whether donepezil, an AChE inhibitor with neuroprotective functions, could suppress the ongoing HFD induced pathological changes in the brain.
Our data demonstrates that HFD induced obesity results in increased neuroinflammation and increased AChE activity in the brain when compared with the mice fed on low fat diet (LFD). HFD administration to mice activated mTOR pathway resulting in increased phosphorylation of mTORser2448, AKTthr308 and S6K proteins involved in the signaling. Interestingly, donepezil administration with HFD suppressed HFD induced increases in AChE activity, and partially reversed HFD effects on microglial reactivity and the levels of mTOR signaling proteins in the brain when compared to the mice on LFD alone. However, gross levels of synaptic proteins were not altered in the brain tissues of mice fed either diet with or without donepezil. In conclusion, these results present a new insight in to the detrimental effects of HFD on brain via microglial activation and involvement of mTOR pathway, and further demonstrates the possible therapeutic role for donepezil in ameliorating the early effects of HFD that could help preserve the brain function in metabolic syndrome conditions.
Keywords: Brain, aging, high fat diet, obesity, neurodegenerative disorders, mTOR pathway, donepezil, neuroinflammation
1. Introduction
Obesity is associated with co-morbidities such as type 2 diabetes, cardiovascular complications and cancer [1-4]. Specifically, chronic consumption of a high-fat diet (HFD) was shown to result in adipose tissue infiltration and adipose inflammation leading to the development of insulin resistance and metabolic syndrome in rodents and humans[5-8]. Recent clinical and experimental evidences suggest that obesity is the greatest risk factor for the development of dementia related neurodegenerative disorders [9]. Studies using mouse models of aging and neurodegenerative disease have demonstrated that HFD induced obesity results in increased neuroinflammation [10-12] and oxidative stress; decreased cognitive function [10, 13, 14] and decreased levels of anti-oxidant enzymes [10] in the brain tissues. Further, clinical as well as animal studies have also demonstrated impaired BBB integrity[15], hippocampal inflammation and altered synaptic plasticity [13, 16] following high fat or high energy diet consumption. Collectively, these data indicate that obesity is a significant contributor to neuropathology observed with the consumption of high-fat diet.
The mammalian target of rapamycin (mTOR) is a protein serine/ threonine kinase that plays main role in controlling protein homeostasis as well as synaptic functions such as memory and learning [17-19] processes. Hyper-activation of mTOR following high energy food intake has been shown to be associated with increased systemic inflammation, and result in insulin resistance through the phosphorylation of p70S6K [20] in animal models. Further, pathological hyper-activation of mTORC1, specifically the levels of p-mTOR and its downstream targets, including p-p70S6K, have been reported to be higher in the brains of Alzheimer’s Disease (AD) clinical samples and mouse models [21-28] of neurodegenerative disease. These studies suggest an association between obesity and activation of mTOR pathway to neuroinflammation. Donepezil, an acetylcholinesterase inhibitor, is one of the approved therapies for treating Alzheimer’s disease (AD), that has been shown to improve the behavioral outcomes and cognitive function associated with AD [29, 30]. Previous studies have reported that donepezil acts by inhibiting cholinesterase activity, thus increasing the acetylcholine (ACh) levels for cholinergic transmission [30]. Further, studies demonstrated that donepezil suppresses the neuroinflammation associated with neurological disorders such as AD [29] ; and exerts anti-inflammatory [31], antioxidant [32] and cytoprotective effects against LPS [32] amyloid beta [33] and glutamate [34] induced toxicity in the brain. Despite these studies suggesting the role for donepezil in exerting anti-inflammatory effects in the brain, still the mechanism of action of AChEI’s such as donepezil in suppressing the neurodegeneration progression is not clear [30, 35]. Further, the potential role for donepezil in suppressing the early events of high-fat-diet (HFD)-induced obesity on brain pathology has not yet been investigated.
In the present study, BL/6 mice were administered with either low fat diet (LFD) or high fat diet (HFD) with or without donepezil for 8 weeks, and the short-term (early) effects of diet and donepezil on neuroinflammation, mTOR pathway, and synaptic plasticity in the brain were analyzed by both biochemical and immunohistochemical analysis. For the first time, our studies have demonstrated an increase in neuroinflammation as well as activation of mTOR signaling pathway following HFD administration to the mice with donepezil reversing these effects induced by HFD on the brain. Our data suggest that donepezil, a potential therapeutic agent, could reduce the detrimental effects of HFD on brain probably through its effects on neuroinflammation thereby preventing the inflammatory related processes such as hyper-activation of mTOR signaling pathway in the brain.
2. Materials and methods
2.1 Materials
High fat (D12492) and low fat diet (D12450B) used in this study were purchased from research diets, New Brunswick, NJ, USA. Antibodies against β-actin were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Antibodies against p-Akt (Thr308), p-p70 S6 Kinase (Thr389), p70 S6 Kinase, mTOR (7C10), P-mTOR (Ser2448), p-synapsin & total Synapsin were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against PA1-741 (Synapse Associated Protein 97) were purchased from Pierce (thermo scientific), Rockland, IL, and USA. Acetyl choline esterase enzyme activity kit, Amplite™ Colorimetric, was purchased from AAT Bioquest, Inc. Antibodies against collagen IV and GFAP were obtained from Abcam, Cambridge, MA, USA. Antibodies against IBA-1 were purchased from Wako, Osaka, Japan. Biotinylated secondary antibodies, DAB peroxidase substrate kit, VIP peroxidase substrate kit, horseradish peroxidase conjugated secondary antibodies, normal goat serum, normal horse serum, were purchased from vector laboratories (Burlingame, CA, USA). All immunoblotting reagents were purchased from Bio-Rad Laboratories (Hercules, CA, USA). All other items and chemicals including 10% formalin solution, phosphate buffered saline were purchased from Sigma-Aldrich chemical company.
2.2. Animal studies and donepezil treatment
Three month old male C57BL/6 mice were purchased from Charles River Laboratories and allowed to acclimatize to the laboratory conditions for a week. Mice were housed in standard caging with a 12:12 light and dark cycle. Mice were randomly divided and fed with either high fat diet or low fat diet for 2 months (8 weeks). The high fat diet (HFD) provided 60% kcalories from fat, while the low fat diet (LFD) provided 10% kcalories from fat. The fat sources were soybean oil and lard. Another 20% kcalories were provided from the protein source. Both diets contained the necessary mineral and vitamin mixes. Half of the mice from each diet group (high fat or low fat) were given water and another half of the mice were given the drug, donepezil, in water at a concentration of 66μg/ml. Donepezil was prepared in water and filter sterilized before it was given to mice in regular water bottles. Total body fat and muscle content were measured using nuclear magnetic resonance (NMR) spectroscopy (Minispec, Brucker Optics, Billerica MA). Glucose tolerance test (i.p-GTT) was performed just before the sacrifice of mice. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Pennington Biomedical Research Center, Baton rouge, USA.
2.3. Immunohistochemical analysis of brain tissues
Mouse brains were perfused in PBS, fixed in 10% formalin solution for 48 hours. Then the brain tissues were processed, embedded in paraffin and sectioned at 5μm. Immunohistochemical staining was performed on sections with different antibodies as described earlier [36]. Briefly, tissue sections were rehydrated by passing through the series of xylene and alcohol (100%, 95%, 70%, and 50%) washes. After that tissue sections were treated for heatmediated antigen retrieval and then the slides were blocked in 10% serum followed by incubation with primary and secondary antibody in 3% serum. Later, the slides were washed, and developed using DAB peroxidase substrate (Vector labs). For double labeling, the slides were re-blocked again in 10% serum and incubated overnight with another primary antibody in 3% serum and then developed using VIP peroxidase substrate (vector labs) after secondary antibody incubation. For secondary antibody negative control, brain tissue sections were incubated with only secondary antibody (either mouse or rabbit) without any incubation in primary antibody. After staining, the slides were cover slipped and then the images were obtained using a Hamamatsu NanoZoomer Digital Slide Scanning System (Hamamatsu City, Japan) at 20X magnification.
2.4. Western blotting analysis
Protein lysates from mouse brain tissues were made in RIPA buffer using tissue homogenizer and the amount of protein was estimated using pierce BCA reagent. Protein samples were analyzed by SDS-PAGE, transferred onto the nitrocellulose membrane, blocked in 5% milk and incubated with different primary antibodies in 5% milk followed by the incubation in secondary antibody solution. The blots were developed using ECL substrate. Beta actin was used as loading control. The relative changes in the protein expression were represented as percent control.
2.5. RT-PCR analysis
RT-PCR experiments were performed as described previously by our laboratory [36]. Total RNA from brain tissues was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. The corresponding cDNA was made using M-MuLV transcriptase (New England Biolabs, Ipswich, MA). For quantitative real-time PCR (qPCR) analysis, aliquots of cDNA were subjected to qPCR in 20 μl of Brilliant II QPCR & QRT-PCR Reagents (Agilent Technologies, Santa Clara, CA), 1 × primers and TaqMan probe (6-FAM/ZEN/IBFQ mode). Primers and probes for TNF-α and IL-1 and GAPDH were ordered through the PrimeTime Pre-designed qPCR Assays system of Integrated DNA Technologies (IDT, Coralville, IA). ΔΔCt method was used for data analysis.
2.6 Data analysis
All western blot results and quantitative PCR data were analyzed using ANOVA (Bonferroni posthoc comparisons) to determine the effects of diet and donepezil on low fat and high fat diet groups. All western blot data were normalized to beta actin and expressed as relative levels taking low fat diet (with water) group as the control group. Mice body weight, body fat, water intake, food intake, glucose tolerance levels and AChE levels were analyzed using t-tests to determine the differences between high fat diet and low fat diet groups. For statistical analysis on IHC images, Images (20X) from three different regions of cortex were analyzed for the reactivity to antibody and the differences between the groups were reported using student t-test. All statistical significance is reported when p≤0.05.
3. Results
3.1. Donepezil treatment did not affect food or water intake and weight gain in the mice
Three month old C57BL/6 mice were administered LFD or HFD with or without donepezil in water. Donepezil in water was prepared as described in methods. High fat diet (HFD) administration resulted in the increased body weight, body fat and delayed disposal of a glucose load (Fig1A-D.) in mice indicating the development of glucose intolerance in the mice. Glucose disposal levels were measured by i.p GTT (Glucose Tolerance Test) through intraperitoneal administration of glucose. However, administration of donepezil with either low fat or high fat diet did not significantly influence the weight gain, muscle and fat content, or disposal of a glucose load in the mice (Fig 1A-D). Further, donepezil administration with either diet did not affect food and water intake in the mice (Fig 2A&B).
Fig.1. Effects of Diet and Donepezil on body weight and body fat of mice.
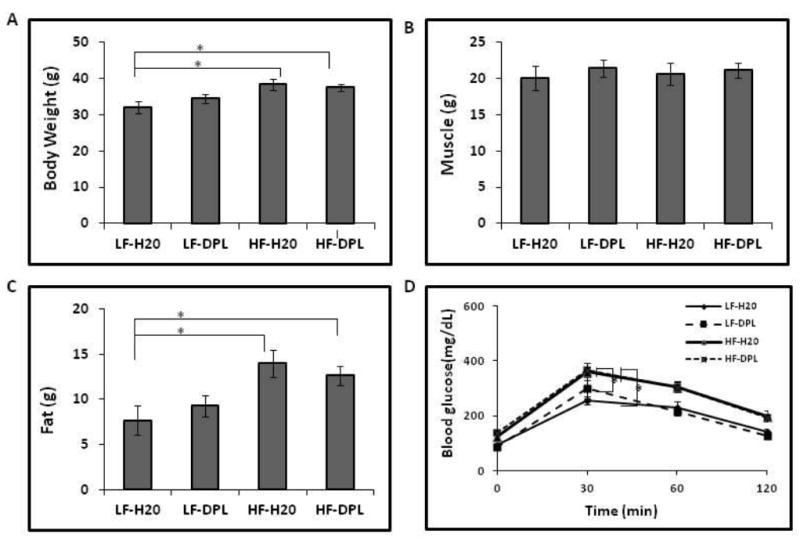
Mice were treated with high fat/ low fat diet without or with donepezil for 8 weeks and the effect of diet and donepezil on (A) body weight (B) body muscle content (C) body fat content were analyzed by NMR (D) Fasting blood glucose levels were analyzed using intraperitonial glucose tolerance test (i.p-GTT). Significant differences in body weight, body fat and glucose tolerance levels were observed between LF-H20 & LF-DPL vs HF-H20 & HF-DPL groups (p<0.05). η=7. Mice group on low fat diet and water was taken as control. *p<0.05, when compared with control group. η=7. HF: high fat diet; LF: low fat diet; DPL: Donepezil.
Fig.2. High fat diet and donepezil doesn’t alter the water and food intake of mice.
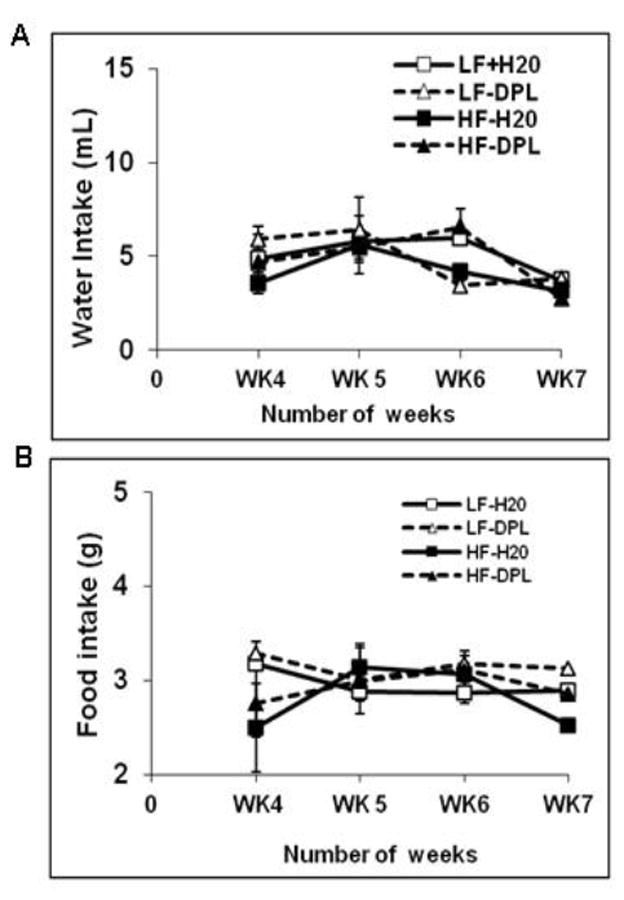
Mice were treated with high fat/ low fat diet without or with donepezil for 8 weeks and the effect of diet and donepezil on (A) water intake (B) food intake were analyzed. No differences in food intake or water intake were observed between the groups. η=7. HF: high fat diet; LF: low fat diet; DPL: donepezil.
3.2. Effects of high fat diet (HFD) and donepezil on AChE enzyme activity levels in the brain
In order to know whether HFD and donepezil have any effects on acetylcholine esterase (AChE) enzyme activity in the brain, which is generally associated with decreased acetylcholine levels and cognitive decline in age-related neurodegenerative disorders, we analyzed brain lysates for in-vitro acetylcholine esterase enzyme (AchE) activity. In our analysis, mice administered with HFD showed significant increases in the AChE levels in the brain tissues. Further, compared with the mice on high fat diet alone, mice administered with high fat diet and donepezil showed significant decreases in the levels of AChE enzyme activity (Fig.3) suggesting the role of donepezil in reversing the effects of HFD on brain. However, donepezil administration with LFD did not significantly alter the AchE activity levels in the brain tissues of the mice when compared with the mice on LFD plus water (Fig.3). Together, these results indicate a role for donepezil in suppressing the HFD induced effects on acetylcholine enzyme activity in the brain.
Fig.3. Diet and Donepezil modulate AcetylCholine Esterase (AChE) enzyme activity levels in the brain.
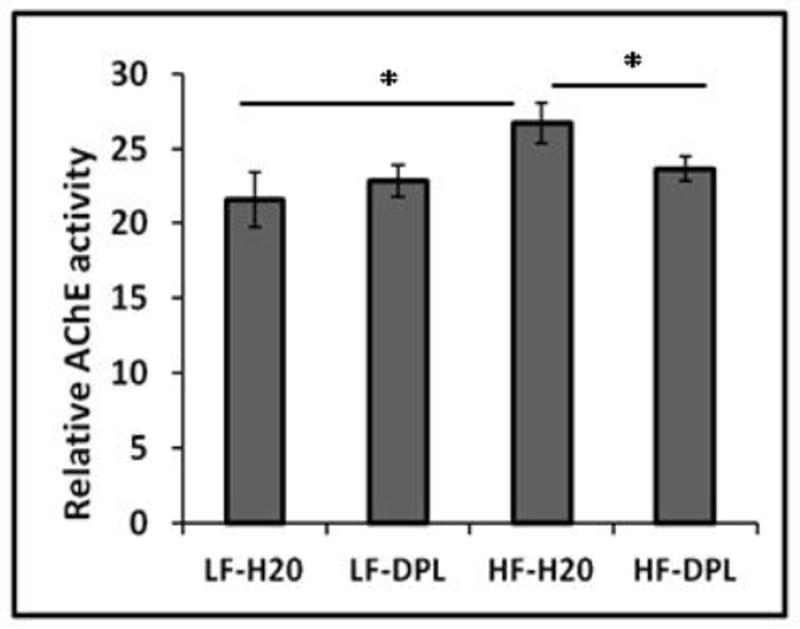
Mice were treated with high fat/ low fat diet without or with donepezil for 8 weeks starting from 3 months of age and the effect of diet and donepezil on acetylcholine esterase activity was measured using in vitro activity assay(η=5). Mice group on low fat diet and water were taken as control. *p<0.05, when compared with control group (LFD).
3.3. Effects of HFD and donepezil on neuroinflammation in the brain
To know the effects of donepezil on HFD induced neuroinflammation in the brain, we analyzed the brain tissues for microglial and astrocyte activation using immunohistochemical and western blotting methods. Glial fibrillary acidic protein (GFAP) and IBA1 (a calcium binding protein specifically expressed in microglia and macrophages) were used as markers to evaluate the astrocyte and microglial reactivity and their expression in the brain respectively. Western blot analysis did not show significant alterations in the gross levels of IBA1 or GFAP in the brain tissues of mice administered with either diet or diet with donepezil (Fig.4A), though the levels of these proteins were higher in HFD administered brains. However, double immunohistochemical staining of cortical brain sections from the mice on high fat diet showed increases in IBA1 (plus collagen) and GFAP (collagen) staining which is consistent with the increased microglial and astrocyte reactivity respectively (Fig.4B&C). While the mice administered with donepezil and HFD did not show increases in microglial activation as compared to the mice on LFD demonstrating the role for donepezil in suppressing the HFD induced effects on neuroinflammation (Fig.4B&C). Brain tissue sections that were incubated with only secondary antibody (mouse and rabbit) were used as negative control, which did not show any background staining (Fig.4B). Further, diet and donepezil did not significantly affect the gene expression levels of inflammatory markers such as IL1 and TNF alpha in the brain tissues of mice (Fig 5) analyzed by quantitative PCR analysis. These observed increases in IBA1 levels in the brain with IHC staining in HFD group and reversal of HFD effects by donepezil indicates localized alterations in microglial reactivity.
Fig.4. Donepezil partially suppresses HFD induced microglial and astrocyte activation in the brain.
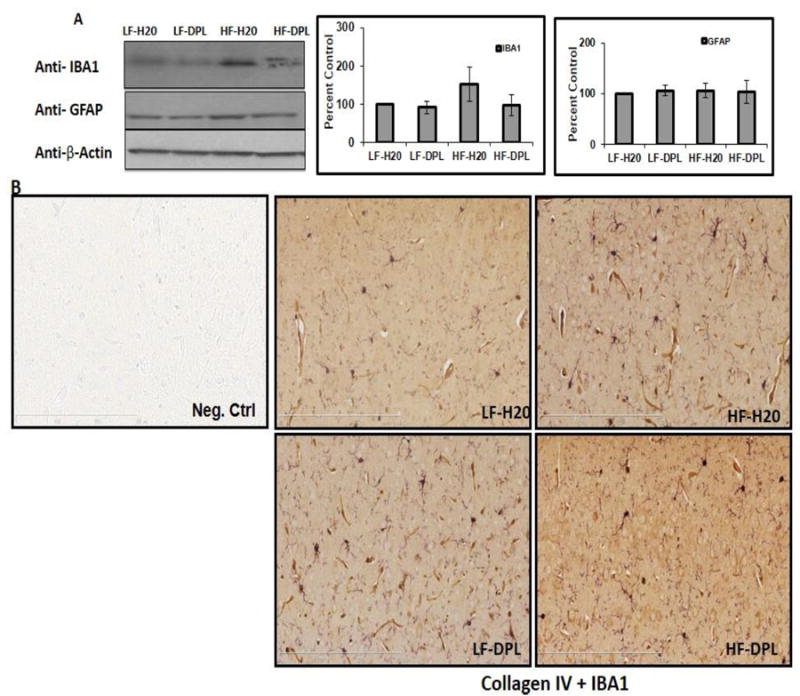
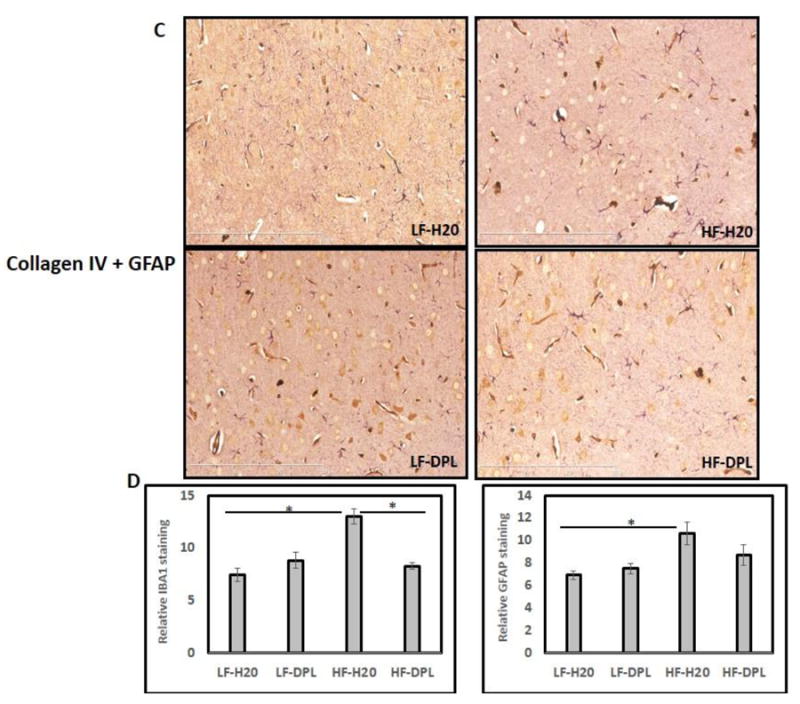
Levels of the GFAP and IBA1 were analyzed using (A) Western blotting (η=5) as well as immunohistochemical staining (B) IBA1 plus Collagen IV (C) GFAP plus Collagen IV. (η=4). Mice group on low fat diet and water were taken as control. (D) Statistical analysis of IBA1 and GFAP reactivity (number) in IHC (20X) images (η=3). HF: high fat diet; LF: low fat diet; DPL: donepezil.
Fig.5. Effects of high fat diet and donepezil on cytokine levels in the brain.
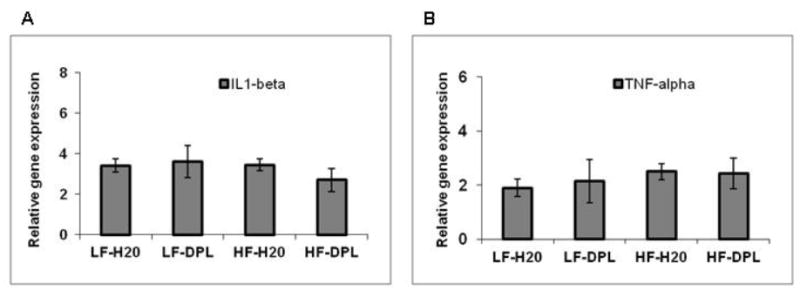
Levels of gene expression were analyzed using Q-PCR (A) IL-1 (B) TNF-alpha. Mice group on low fat diet and water were taken as control. HF: high fat diet; LF: low fat diet; DPL: donepezil. η=4.
3.4. Effects of diet and donepezil on mTOR activation and mTOR signaling protein levels in the brain
In the next set of experiments, we did an analysis to see whether HFD and donepezil have any effects on the components involved in mTOR signaling pathway. Immunohistochemical staining showed increased p-mTORser2448 to mTOR ratio levels in the brain (Fig.6) tissues of mice administered with HFD. However, western blot analysis did not show significant alterations in the gross levels of p-mTOR in the brain tissues of mice administered with either diet or diet with donepezil (Fig.6A), though the levels were higher in HFD administered brains indicating only the localized changes in p-mTOR levels as observed with IHC analysis. Further, with western blotting as well as immunohistochemical analysis, we observed increases in mTOR signaling downstream and upstream proteins (the indicators of mTOR activation) such as phospho-p70S6K and phospho-AKTthr308 (Fig7, 8A&B) in the brain tissues following HFD administration to the mice. Further, donepezil administration with LFD diet to mice did not significantly alter p-mTOR, phospho-AKTthr308 or p-S6K levels when compared to the mice on LFD alone (Fig 6-8). Brain tissue sections that were incubated with only secondary antibody (mouse and rabbit) were used as negative control, which did not show any background staining (Fig 6B and 8B). Taken together, these results suggest a role for donepezil in partially reversing the HFD induced effects on mTOR pathway in the brain tissues.
Fig 6. Effects of diet and donepezil on p-mTORser2448 levels in the brain.
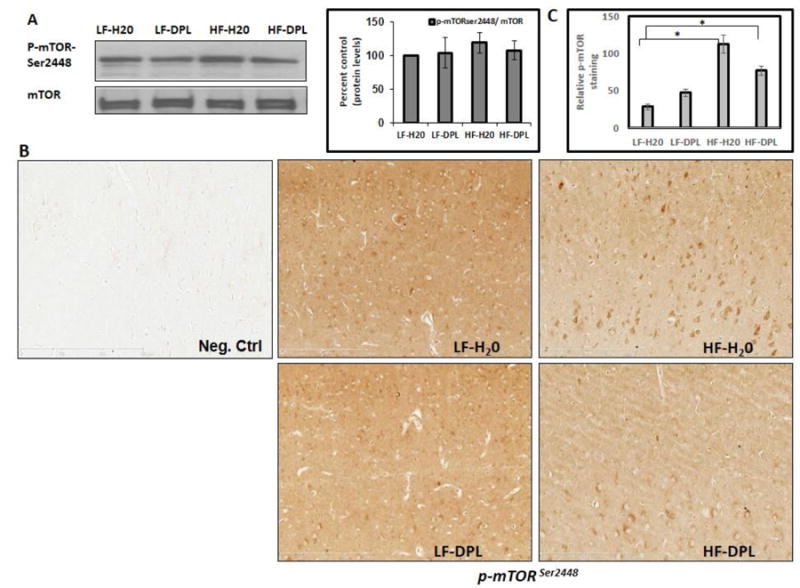
Levels of the mTOR were analyzed by (A) western blotting (η=5) as well as (B) Immunohistochemical staining (η=4). Mice group on low fat diet and water were taken as control. (D) Statistical analysis of pmTOR staining in IHC (20X) images (η=4). HF: high fat diet; LF: low fat diet; DPL: donepezil.
Fig.7. Effects of diet and donepezil on phospho-S6K levels in the brain.
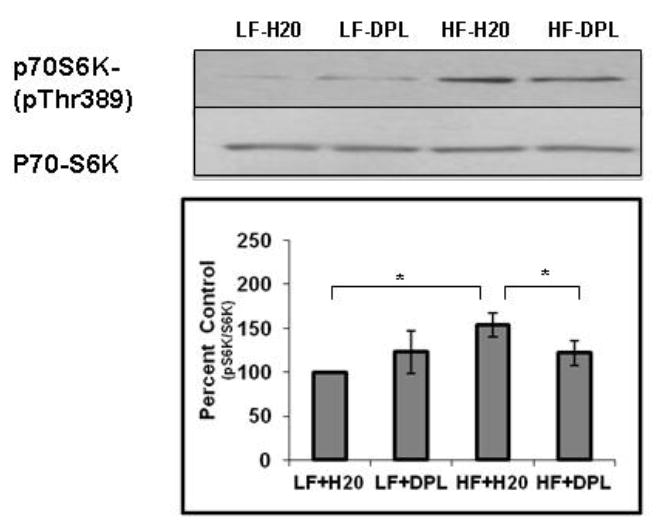
Levels of p-S6K and total S6K were analyzed using western blotting (η=5). Mice group on low fat diet and water were taken as control. *p<0.05, when compared with control group or HFD plus DPL. HF: high fat diet; LF: low fat diet; DPL: donepezil.
Fig.8. Effects of diet and donepezil on phospho-Aktthr308 levels in the brain.
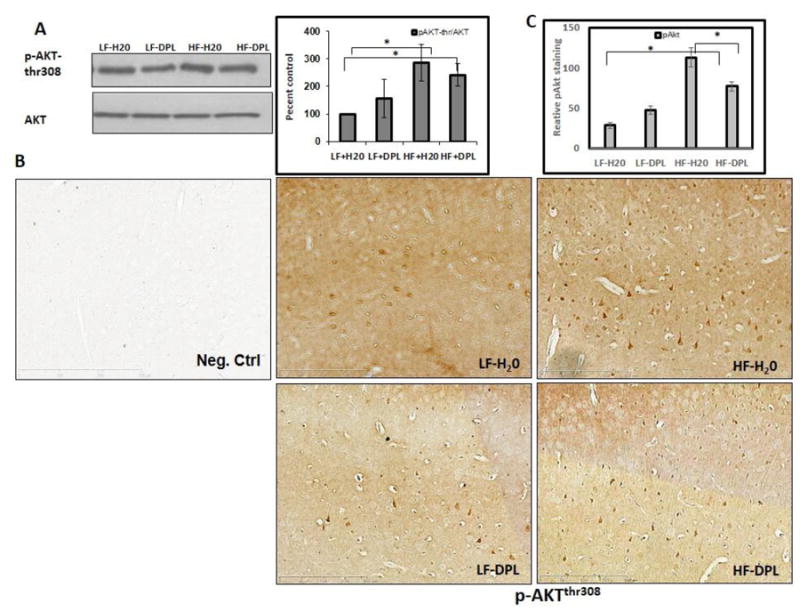
(A) Akt levels were analyzed using western blotting (η=5) as well as (B) immunohistochemical staining (η=4). Mice group on low fat diet and water were taken as control. . (D) Statistical analysis of pmTOR staining in IHC (20X) images (η=4) *p<0.05, when compared with control group. HF: high fat diet; LF: low fat diet; DPL: donepezil.
3.5. Effects of diet and donepezil on synaptic density, synaptic protein levels in the brain
We next performed the analysis for levels of synaptic proteins, synapsin (total and phosphorylated forms of the pre-synaptic protein synapsin1) and synapse associated protein 97 (SAP97), as a measure for synaptic density, using western blotting. The expression levels of these proteins have been used in previous studies to evaluate synaptic density [37, 38] in the brain tissues. Our analysis with total brain lysates did not show gross alterations in the synaptic proteins with either HFD or HFD plus donepezil administration(Fig.9). However, the mice on LFD plus DPL showed significant increases in SAP levels compared to the mice on LFD alone (Fig.9).
Fig.9. Effects of high fat diet and donepezil on the levels of synaptic proteins in the brain.
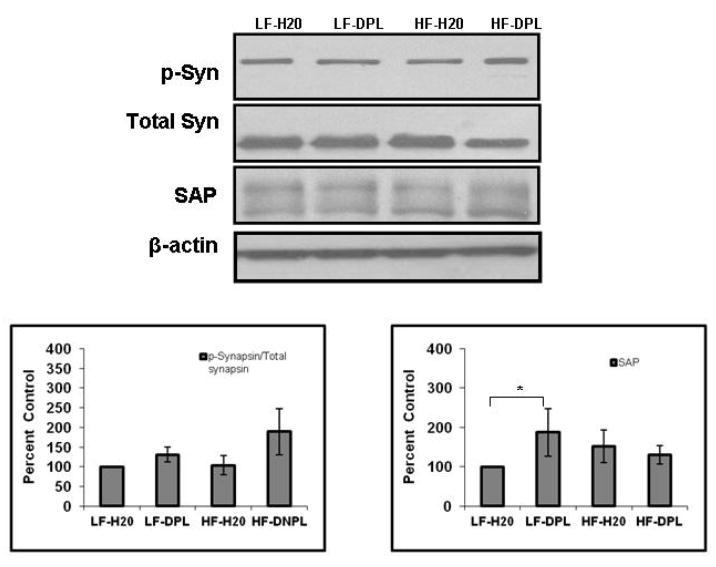
Levels of synaptic proteins, synapsin, p-Synapsin and synapse associated protein 97 (SAP97), protein were analyzed using western blotting (η=5). Mice group on low fat diet and water were taken as control. *p<0.05, when compared with control group. HF: high fat diet; LF: low fat diet; DPL: donepezil.
4. Discussion
Our current study demonstrated that HFD induces neuroinflammation through microglial and astrocyte activation and also modulates mTOR signaling pathway in the brain. Combined administration of HFD with donepezil to mice partially suppressed the effects of HFD on neuroinflammation and altered levels of mTOR signaling pathway proteins in the brain.
Neuroinflammatory processes are reported as the early events observed prior to the neuropathology in the brain and reactive gliosis has been demonstrated to contribute to neurodegeneration and memory impairment in neurodegenerative disease progression [39, 40]. Our results with immunohistochemical analysis of brain tissues following the administration of mice with HFD for 8 weeks showed an increase in localized microglial and astrcoyte activation in the cortical region of the brain. These results substantiate the previous studies from our laboratory and as well others which reported increased neuroinflammation in wild type mice as well in a mouse model of neurodegenerative disease following the administration of HFD [10, 14, 40, 41] . However, western blotting analysis of whole brain lysates following HFD administration to mice did not show significant alterations in the total levels of proteins involved in neuroinflammation indicating only localized changes in the brain.
Because of the role of HFD in promoting neuropathology and its strong association with the development of age-related neurodegenerative disease, in our current study, we used donepezil (an AChE inhibitor) to determine its capacity to suppress HFD induced effects on brain pathology. The concentrations of donepezil used in the study were close to the doses used by others in the previous reports [29, 42]. HFD administration to mice resulted in significant increases in AChE enzyme levels, but donepezil administration with HFD did not significantly increase AChE levels in the brain tissues of mice when compared to the mice on LFD alone. Further, our results showed that donepezil administration suppresses the HFD induced neuroinflammation where decrease in microglial activation was observed in the cortical sections of the brain (Fig.4). These are the first studies to demonstrate that donepezil is capable of protecting brain partially from the early events of HFD induced neuroinflammatory effects which are likely contribute to the development of neuropathology in aging and age-related neurodegenerative diseases.
Previous studies with saturated fatty acids (SFA) in mice have shown to induce brain mediobasal hypothalamus (MBH) inflammation but did not affect the increases in systemic cytokine levels of TNF-α, MCP-1, or interleukin-6 (IL-6), indicating that the effects of SFA in the MBH were not due to systemic inflammation [43] but localized changes in the brain. Further, in some studies a rise in macrophage-specific gene expression was observed from 8 weeks of HFD that reached peak levels by 20 weeks of study [44] with additional studies suggesting that diets influence inflammatory cytokine expression in obese tissues which is only modest or local when compared with immune response or other stimuli [8]. These studies together with our observations showing no alterations in the cytokine levels (Fig.5) indicate that short term feeding of mice with HFD in the current study may have induced only localized changes in the brain.
Further in our study, when compared to the mice on LFD, short-term administration of HFD activated the mammalian target of rapamycin (mTOR) pathway through increased phosphorylation of p-mTORser2448 in the brain tissues of mice (Fig.5). Accordingly, increases in p-p70S6K and p-AKTthr308 levels were observed in the brain tissues which are the indicators of mTOR activation [45] following the HFD administration to the mice. These results are consistent with the reports demonstrating the mTOR dysregulation upon nutrient overload following the high energy food intake or in the obese setting conditions [46] in different tissues. Surprisingly, when compared with mice fed on HFD alone, donepezil administration with HFD restored the levels of mTOR signaling proteins in the brain tissues of mice. Accordingly, we also observed a decrease in the phosphorylation of mTORser2448 and its target proteins AKTthr308 and S6K in the brain tissues of mice administered with HFD and donepezil suggesting the role of donepezil in reversing the HFD effects on mTOR pathway.
Earlier studies have demonstrated that mTOR activation is associated with pro-inflammatory activation of microglial cells in response to pro-inflammatory stimuli [47]. Up-regulation of p-mTOR and p70S6K have been observed with microglial activation following brain injury [48] signifying the role of microglia in initiating and maintaining the brain inflammation. In addition, studies with obese subjects indicated correlation between increased gene expression levels of mTOR and inflammatory markers [49]. Furthermore, high body adiposity induces an inflammatory and proliferative microenvironment in rat kidneys potentially via activation of the STAT3 and mTOR signaling pathways with further studies demonstrating reduced inflammation with inhibition of mTOR [50, 51]. All these studies suggest a positive association between inflammation, obesity and mTOR. In our study, compared to mice on HFD alone, the observed decreases in microglial activation and restored levels of mTOR signaling proteins in brain tissues following the administration of mice with HFD and donepezil suggests that donepezil partially reverses the effects of high fat diet on brain probably through its well characterized role in the suppression of [34, 52-54] microglial reactivity there by restoring the alterations in mTOR signaling protein levels. These observations are further supported by the previous studies where donepezil was reported to suppress JNK activation, a signaling event that is associated with increased mTOR signaling and inflammation[19], in the brain tissues of mice with neurodegenerative disease [54]. Altogether, our studies combined with the previous studies suggest a role for donepezil in suppressing the activation of inflammatory related processes such as mTOR pathway in the brain tissues.
Balanced mTOR activity is required for the proper functioning of brain [17] but the activation or inhibition of mTOR pathway was shown to alter the synaptic function and impair learning and memory processes [55]. Interestingly, in the present study, following the administration of HFD to the mice, we observed an increase in mTOR signaling pathway proteins but found no alterations in synaptic proteins such as p-synapsin and synaptic associated protein (SAP) which are analyzed by western blotting using total brain lysates (Fig.9). However, the current study is focused and limited to investigate the early effects of HFD on brain pathology, and it is possible that short-term (8 weeks) HFD administration to the mice may not have induced synaptic alterations in contrary to the previous studies [16] which have administered HFD for more than 8 weeks. Moreover, as suggested by previous studies, [17, 56] activation of mTORC1 signaling along with mTORC1 independent downstream signaling proteins may be required to mediate changes in synaptic plasticity, which may not have been induced with short term HFD administration in our study, that could also explain the absence of observable alterations in synaptic proteins with HFD in our study with mTOR activation. In addition, mTOR pathway is well known in the regulation of autophagy [57], particularly decreased autophagy function [27] is reported with mTOR pathway activation [22] in neurodenegerative disease. These results together with our studies also suggest that mTOR activation in the conditions of obesity could cause autophagic dysfunction leading to the buildup of proteins and progression of neuropathology in the brain.
Taken together, our data highlight the modulation of mTOR pathway and microglial reactivity by high fat diet and donepezil. Additionally these studies demonstrate the role for donepezil in partially suppressing the deleterious effects of HFD on mTOR activation likely through its anti-inflammatory properties in the brain. However, further investigation is worthy to detail the mechanism of action of donepezil on mTOR pathway in the brain.
Highlights.
This is the first study demonstrating the effects of short-term administration of HFD and donepezil on neuroinflammation and mTOR signaling pathway in the brain.
This study demonstrates the role of donepezil in suppressing the HFD induced effects on neuroinflammation.
This study suggests that donepezil restores the levels of mTOR pathway proteins in the mice fed on HFD likely through its suppressing effects on microglial activation.
Acknowledgments
This work used the facilities of the Cell Biology and Bioimaging Core which are supported in part by COBRE (NIH 2P20-RR021945) and NORC (NIH 2P30-DK072476) center grants from the National Institutes of Health, the Pennington Animal Metabolism and Behavior Core. The work was supported by funds from the NIH and Hibernia National Bank/Edward G Schleider Chair to JNK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue BJOG : an international journal of obstetrics and gynaecology. 2006;113:1141–1147. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee CH, Woo YC, Wang Y, Yeung CY, Xu A, Lam KS. Obesity, adipokines and cancer: an update. Clinical endocrinology. 2015;83:147–156. doi: 10.1111/cen.12667. [DOI] [PubMed] [Google Scholar]
- 3.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 4.Walker GE, Marzullo P, Ricotti R, Bona G, Prodam F. The pathophysiology of abdominal adipose tissue depots in health and disease. Hormone molecular biology and clinical investigation. 2014;19:57–74. doi: 10.1515/hmbci-2014-0023. [DOI] [PubMed] [Google Scholar]
- 5.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging. 2012;4:98–115. doi: 10.18632/aging.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 9.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman LR, Zhang L, Nair A, Dasuri K, Francis J, Fernandez-Kim SO, Bruce-Keller AJ, Keller JN. Obesity increases cerebrocortical reactive oxygen species and impairs brain function. Free radical biology & medicine. 2013;56:226–233. doi: 10.1016/j.freeradbiomed.2012.08.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepping JK, Freeman LR, Gupta S, Keller JN, Bruce-Keller AJ. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. American journal of physiology. Endocrinology and metabolism. 2013;304:E392–404. doi: 10.1152/ajpendo.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. The Journal of clinical investigation. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, Zheng W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–122. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutritional neuroscience. 2014;17:241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman LR, Granholm AC. Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:643–653. doi: 10.1038/jcbfm.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learning & memory. 2013;20:518–530. doi: 10.1101/lm.027664.112. [DOI] [PubMed] [Google Scholar]
- 18.Laplante M, Sabatini DM. mTOR signaling at a glance. Journal of cell science. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation? Cell cycle. 2011;10:3447–3451. doi: 10.4161/cc.10.20.17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal IG, Winblad B, Pei JJ. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. The American journal of pathology. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. The Journal of biological chemistry. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends in neurosciences. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. The FEBS journal. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- 25.Orr ME, Salinas A, Buffenstein R, Oddo S. Mammalian target of rapamycin hyperactivity mediates the detrimental effects of a high sucrose diet on Alzheimer’s disease pathology. Neurobiology of aging. 2014;35:1233–1242. doi: 10.1016/j.neurobiolaging.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. Journal of neurochemistry. 2015;133:739–749. doi: 10.1111/jnc.13037. [DOI] [PubMed] [Google Scholar]
- 27.Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiology of disease. 2015 doi: 10.1016/j.nbd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Tang Z, Ioja E, Bereczki E, Hultenby K, Li C, Guan Z, Winblad B, Pei JJ. mTor mediates tau localization and secretion: Implication for Alzheimer's disease. Biochimica et biophysica acta. 2015;1853:1646–1657. doi: 10.1016/j.bbamcr.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E, Donepezil MSIG. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology. 2001;57:613–620. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- 30.Giacobini E. Cholinesterase inhibitors stabilize Alzheimer’s disease, Annals of the New York. Academy of Sciences. 2000;920:321–327. doi: 10.1111/j.1749-6632.2000.tb06942.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim HG, Moon M, Choi JG, Park G, Kim AJ, Hur J, Lee KT, Oh MS. Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo. Neurotoxicology. 2014;40:23–32. doi: 10.1016/j.neuro.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Saxena G, Singh SP, Agrawal R, Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. European journal of pharmacology. 2008;581:283–289. doi: 10.1016/j.ejphar.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Meunier J, Ieni J, Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. British journal of pharmacology. 2006;149:998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, Akaike A. Acetylcholinesterase inhibitors used in treatment of Alzheimer’s disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology. 2006;51:474–486. doi: 10.1016/j.neuropharm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs & aging. 2004;21:453–478. doi: 10.2165/00002512-200421070-00004. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Dasuri K, Fernandez-Kim SO, Bruce-Keller AJ, Freeman LR, Pepping JK, Beckett TL, Murphy MP, Keller JN. Prolonged diet induced obesity has minimal effects towards brain pathology in mouse model of cerebral amyloid angiopathy: implications for studying obesity-brain interactions in mice. Biochimica et biophysica acta. 2013;1832:1456–1462. doi: 10.1016/j.bbadis.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S, Knight AG, Losso BY, Ingram DK, Keller JN, Bruce-Keller AJ. Brain injury caused by HIV protease inhibitors: role of lipodystrophy and insulin resistance. Antiviral research. 2012;95:19–29. doi: 10.1016/j.antiviral.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, Murphy MP, Van Eldik LJ, St Clair D, Keller JN. Cognitive impairment in humanized APPxPS1 mice is linked to Abeta(1-42) and NOX activation. Neurobiology of disease. 2011;44:317–326. doi: 10.1016/j.nbd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PloS one. 2012;7:e30378. doi: 10.1371/journal.pone.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. The Journal of comparative neurology. 2013;521:1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartolini M, Bertucci C, Cavrini V, Andrisano V. beta-Amyloid aggregation induced by human acetylcholinesterase: inhibition studies. Biochemical pharmacology. 2003;65:407–416. doi: 10.1016/s0006-2952(02)01514-9. [DOI] [PubMed] [Google Scholar]
- 43.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell reports. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 45.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. The Journal of biological chemistry. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 46.Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dello Russo C, Lisi L, Tringali G, Navarra P. Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochemical pharmacology. 2009;78:1242–1251. doi: 10.1016/j.bcp.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 48.Park J, Zhang J, Qiu J, Zhu X, Degterev A, Lo EH, Whalen MJ. Combination therapy targeting Akt and mammalian target of rapamycin improves functional outcome after controlled cortical impact in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:330–340. doi: 10.1038/jcbfm.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Andrada P, Rotellar F, Valenti V, Moncada R, Marti P, Silva C, Salvador J, Fruhbeck G. Expression of S6K1 in human visceral adipose tissue is upregulated in obesity and related to insulin resistance and inflammation. Acta diabetologica. 2015;52:257–266. doi: 10.1007/s00592-014-0632-9. [DOI] [PubMed] [Google Scholar]
- 50.Stemmer K, Perez-Tilve D, Ananthakrishnan G, Bort A, Seeley RJ, Tschop MH, Dietrich DR, Pfluger PT. High-fat-diet-induced obesity causes an inflammatory and tumor-promoting microenvironment in the rat kidney. Disease models & mechanisms. 2012;5:627–635. doi: 10.1242/dmm.009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weichhart T, Haidinger M, Katholnig K, Kopecky C, Poglitsch M, Lassnig C, Rosner M, Zlabinger GJ, Hengstschlager M, Muller M, Horl WH, Saemann MD. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–4283. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 52.Dong H, Yuede CM, Coughlan CA, Murphy KM, Csernansky JG. Effects of donepezil on amyloid-beta and synapse density in the Tg2576 mouse model of Alzheimer’s disease. Brain research. 2009;1303:169–178. doi: 10.1016/j.brainres.2009.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takada Y, Yonezawa A, Kume T, Katsuki H, Kaneko S, Sugimoto H, Akaike A. Nicotinic acetylcholine receptor-mediated neuroprotection by donepezil against glutamate neurotoxicity in rat cortical neurons. The Journal of pharmacology and experimental therapeutics. 2003;306:772–777. doi: 10.1124/jpet.103.050104. [DOI] [PubMed] [Google Scholar]
- 54.Yoshiyama Y, Kojima A, Ishikawa C, Arai K. Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss, and neurodegeneration in a tauopathy mouse model. Journal of Alzheimer’s disease : JAD. 2010;22:295–306. doi: 10.3233/JAD-2010-100681. [DOI] [PubMed] [Google Scholar]
- 55.Gkogkas CG, Sonenberg N. Translational control and autism-like behaviors. Cellular logistics. 2013;3:e24551. doi: 10.4161/cl.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weatherill DB, Dyer J, Sossin WS. Ribosomal protein S6 kinase is a critical downstream effector of the target of rapamycin complex 1 for long-term facilitation in Aplysia. The Journal of biological chemistry. 2010;285:12255–12267. doi: 10.1074/jbc.M109.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS letters. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


