Abstract
In the present study we demonstrate for the first time that aging increases the levels of ubiquitinated protein in the spleen, and that dietary restriction (DR) significantly reduces these age-related increases in ubiquitinated protein. Sumoylated protein, proteasome subunits, and a protein essential for proteasome biogenesis (POMP1) were also increased with age in the spleen but were not significantly affected by DR. Chymotrypsin-like proteasome activity was elevated in the aged spleen, and was not significantly altered by DR. Together, these data demonstrate for the first time the multiple effects of aging and DR on ubiquitination, sumoylation, and the proteasome in the spleen.
Keywords: Aging, POMP1, Proteasome, Spleen, Sumo, Ubiquitin
1. Introduction
Aging is known to have effects on multiple cellular processes, including the modulation of steady state protein dynamics. For example, the processes of protein synthesis and protein degradation appear to be deleteriously effected by aging in a variety of tissues [1,2]. Numerous lines of evidence suggest that alterations in the proteome are not only present during aging, but likely play a direct role in promoting aging and age-related disease in a variety of tissues [1-6].
The ubiquitin proteasome system is a principle mediator of intracellular protein degradation. The proteasome is composed of multiple subunits, including subunits of the 20S core proteasome, as well as proteins which comprise the cap-like proteins of the 26S proteasome [7]. Generation of proteasome complexes requires a variety of cofactors including the protein proteoassemblin, also known as proteasome maturation protein (POMP1), which facilitate proteasome complex formation [8-10]. The ubiquitination and sumoylation of proteins is a mechanism by which selectively proteins are targeted for degradation, with such specificity believed to be necessary for maintenance of cellular homeostasis [11,12].
At present dietary restriction (DR) is the only known intervention to consistently and reliably increase mammalian lifespan. In addition to mediating beneficial effects on lifespan, DR is known to ameliorate the effects of aging on a variety of organ systems including hepatic, cardiovascular, immune, and central nervous system [13-17]. At present the cellular and biochemical basis for each of these beneficial effects of DR remains unclear.
The spleen plays an important role in the immune system, in particular serving as an important site for the initiation of adaptive immune responses [18]. Both aging and DR are known to affect the function of the spleen [17-23], although the effects of aging and DR on the ubiquitin-proteasome pathway in the spleen have not yet been elucidated. In the current study we examined the effects of both aging and DR on the expression of ubiquitin-proteasome related proteins in the spleen, as well as an analysis of different proteasome activities. Together, these data demonstrate that aging and DR modulate the levels of multiple components of the ubiquitin proteasome system including the levels of proteasome subunits, ubiquitinated protein levels, and sumoylated protein levels. Additionally, we observed that aging selectively modulated proteasome peptidase activities. The implications of each of these observations to aging and dietary restriction are discussed.
2. Materials and Methods
2.1. Materials
In this study, the POMP1/Ump1 (proteasome maturation protein) antibody (PW9715), 20S proteasome subunits α1, 2, 3, 5, 6 & 7 (C2, C3, C8, C9, iota and zeta) antibody (PW8195) and 20S proteasome ‘core’ (β) subunits antibody (PW8155) were purchase from Biomol International, LP (Plymouth Meeting, PA, USA). Ub (P4D1) antibody (sc-8017), SUMO-1 (D-11) antibody (sc-5308), SUMO-2/3 (N-18) antibody (sc-26969) were purchased from Santa Cruz Biotech, Inc. (Santa Cruz, CA, USA). Proteasome Activator Subunit 4 antibody (ab5620) was purchased from Abcam (Cambridge, MA, USA). The secondary antibodies peroxidase conjugated goat anti-rabbit IgG (H+L) (111-035-003) and peroxidase conjugated goat anti-mouse IgG (H+L) (115-035-003) were purchase from Jackson ImmunoResearch Lab, Inc. (West Grove, PA, USA). Protease Inhibitor Cocktail (P2714-1BTL) and all other reagents were obtained from Sigma (St. Louis, MO, USA).
2.2. Animal studies
Male Helicobacter-free F344/Brown Norway (F344 × BN F1) rats were obtained from the NIA Dietary Restriction (DR) colony. The spleens were manually dissected following euthanatization via CO2 overdose and subsequent decapitation. The rats in this study consisted of 6 three month-old ad libitum (AL), 6 twenty-five month old AL, and 6 twenty five month old DR rodents. These rodents were all utilized as outlined in IACUC approved protocols.
2.3. Western blot analysis
Western blot analysis was conducted to measure levels of POMP1, 20S proteasome α and β subunits, and measure changes in protein ubiquitination and sumoylation cause by aging and DR in rat spleen. Rat spleens were homogenized in the presence of protease inhibitor cocktail, 15 μg of each spleen protein sample was separated by SDS-PAGE (BioRad, 7.5% or 10-20% precast gel) and transferred to nitrocellulose membrane (Whatman Schleicher & Schuell Protran Nitrocellulose Transfer Membrane, Dassel, Germany). Membranes were then successively exposed to different primary and secondary antibodies before being developed using Pierce ECL Western Blotting Substrate (Pierce, Rockford, IL). The resulting bands were digitalized and quantified using NIH ImageJ software.
2.4. Analysis of proteasome activities
Proteasome activity was analyzed as described previously by our laboratory [24,25]. Briefly, spleen lysates were incubated with peptides specific for the individual proteasome peptidase activities (chymotrypsin-like, trypsin-like, post-glutamyl peptidase activities) and after two hours the cleavage of the peptides measured and reported as arbitrary fluorescence units. In each analysis several lysates were incubated with the proteasome inhibitor MG132 (5 μM) for 30 minutes prior to the addition of substrates, to allow for determination of background values.
2.5. Statistical Analysis
Statistical significance was determined using a t test, with a minimum p value of < 0.05 required for significance.
3. Results
3.1 Effects of aging and DR on proteasome activity and expression of proteasome components
Proteasome activity was determined in the lysates of spleens from male 3 month-old AL, 25 month-old AL, and 25 month-old DR rodents from the NIA rodent colony. In these studies we observed that there were no effects of aging or DR on the trypsin-like or post-glutamyl peptidase activities of the proteasome in the spleen (Table 1). Interestingly, aging was observed to significantly increase the chymotrypsin-like activity of the proteasome in the spleen (Table 1), with DR having no effect age-related increases in chymotrypsin-like activity of the proteasome. In order to begin to understand the effects of aging and DR on the expression of 20S proteasome subunits we conducted Western blot analysis on spleens obtained from the 3 month-old AL, 25 month-old AL, and 25 month-old DR rats. Analysis of 20S beta subunits (Fig. 1A) revealed no significant alteration in proteasome subunit expression, while analysis of 20S alpha subunits revealed a significant elevation in proteasome subunit expression (~3 fold) in both aged AL and DR rodents (Fig. 1B).
Table 1.
Age-Related Changes in Proteasome Activities in the Spleen
| 3 Month | 25 Month | 25 Month | |
|---|---|---|---|
| AL | AL | DR | |
| Chymotrypsin | 107 ±34 | 225 ±60* | 236± 54* |
| Trypsin | 72 ±14 | 89±11 | 101 ±19 |
| Post-Glutamyl Peptidase | 251 ±25 | 263 ±23 | 297 ±27 |
All proteasome activities are reported as the mean and standard deviation for the arbitrary fluorescence units following a 2 hour analysis of proteasome peptidase activities in the different spleen lysates Each of the values have had the background fluorescence subtracted, by incubating additional spleen lysates with the proteasome inhibitor MG132 (5 μM) for 30 minutes prior to the addition of individual fluorogenic substrate. See methods for details. AL= ad libitum, DR = dietary restriction.
p < 0.05 compared to 3 month old AL spleen
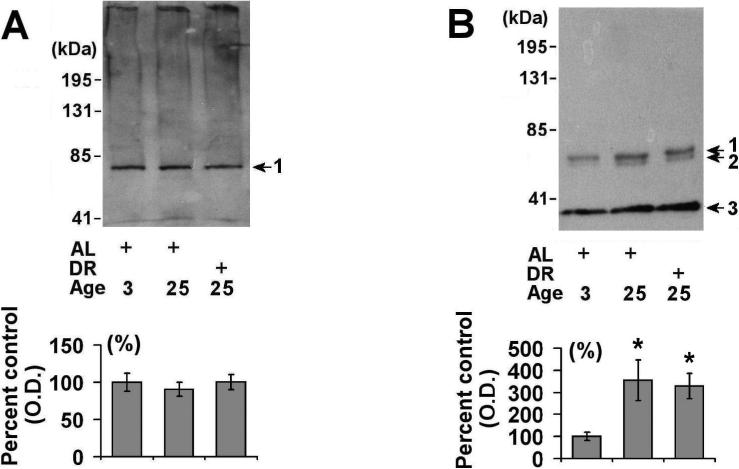
Figure 1. Effects of aging and dietary restriction on the levels of 20S proteasome components in the spleen.
The levels of 20S beta (A) and 20S alpha (B) proteasome subunits were analyzed in the spleen of 3 month-old AL, 25 month-old AL, and 25 month-old DR rodents. A representative blot for each analyses is provided, with the molecular weight markers provided on left hand margin of each blot. The graphs represent the mean and S.E.M. of the optical density of the immunoreactivity from 6 animals in each experimental group. *p < 0.05 compared to 3 month old AL animals.
We next conducted Western blot analysis of POMP1, a protein necessary for 20S proteasome biogenesis [8-10], and observed that aged AL rodents had significantly elevated (~50% elevated) levels of POMP1 as compared to 3 month-old rodents (Fig. 2). DR did not significantly decrease POMP1 levels, though a trend toward lower POMP1 levels was observed in the DR spleen relative to age-matched AL rodents (Fig. 2). Interestingly, POMP1 immunoreactivity was never observed in the 17 kDa range in any of our analyses (which would be predicted based on the molecular weight of POMP1), but instead was evident as a higher molecular weight complexes, consistent with previous studies which describe POMP1 as preferentially forming higher molecular weight complexes in HEK293 cells [26].
Figure 2. Effects of aging and dietary restriction on the levels of POMP-1 in the spleen.
The level of POMP-1 was analyzed in the spleen of 3 month-old AL, 25 month-old AL, and 25 month-old DR rodents. A representative blot for the analyses is provided, with the molecular weight markers provided on left hand margin of the representative blot. The graph represent the mean and S.E.M. of the optical density of the immunoreactivity from 6 animals in each experimental group. *p < 0.05 compared to 3 month old AL animals.
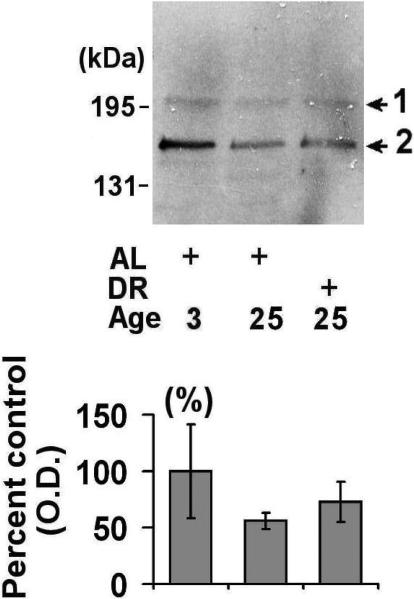
Lastly, we sought to determine the effect of aging and DR on the expression of 26S proteasome components. In these studies we focused on the expression of PA200, a component of the 26S proteasome cap-like structures [27]. Western blot analysis revealed that there was tremendous variability in the levels of PA200 in young rodents, with a trend towards decreased levels of PA200 in the spleens of aged AL and DR rodents (Fig. 3).
Figure 3. Effects of aging and dietary restriction on the levels of PA200 in the spleen.
The level of PA200 was analyzed in the spleen of 3 month-old AL, 25 month-old AL, and 25 month-old DR rodents. A representative blot for the analyses is provided, with the molecular weight markers provided on left hand margin of the representative blot. The graph represent the mean and S.E.M. of the optical density of the immunoreactivity from 6 animals in each experimental group.
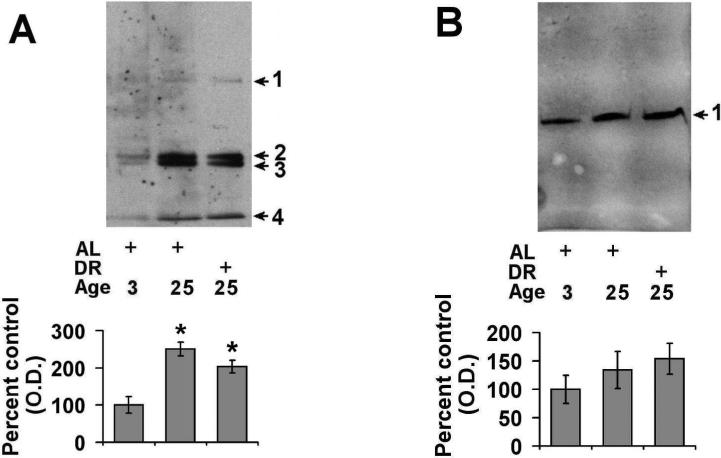
3.2. Effects of aging and DR on the levels of ubiquitinated and sumoylated proteins
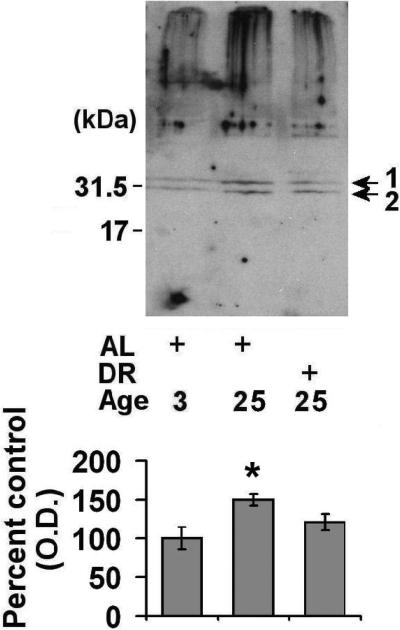
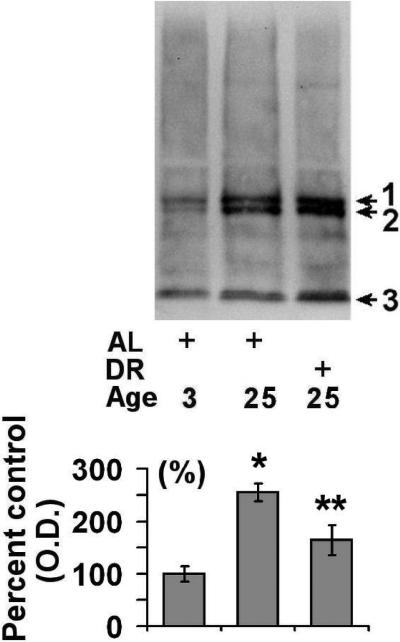
In order to understand how the observed alterations in 20S and 26S proteasome expression relates to the levels of ubiquitinated protein in the spleen, we conducted additional Western blot analyses. We observed that there was a significant elevation in the levels of ubiquitinated protein in the aged AL animals (~2.7 fold) as compared to young rodents (Fig. 4). DR was observed to significantly attenuate the age-related increase in ubiquitinated protein levels (Fig. 4). Similar to ubiquitin alterations, aging was observed to elevate the levels of sumoylation by sumo-1 in the spleen (Fig. 5A), although DR was not observed to significantly decrease the levels of proteins modified by sumo-1 (p = 0.08) (Fig. 5A). Analysis of sumo-2/3 levels revealed that there was a trend toward elevations in sumo-2/3 levels in both aged AL and aged DR rodents as compared to young rodents (Fig 5B).
Figure 4. Effects of aging and dietary restriction on the levels of ubiquitinated protein in the spleen.
The level of ubiquitinated protein was analyzed in the spleen of 3 month-old AL, 25 month-old AL, and 25 month-old DR rodents. A representative blot for the analyses is provided. The graph represent the mean and S.E.M. of the optical density of the immunoreactivity from 6 animals in each experimental group. *p < 0.05 compared to 3 month old AL animals; **p < 0.05 compared to 24 month old AL animals
Figure 5. Effects of aging and dietary restriction on the levels of sumolated protein in the spleen.
The level of sumo-1 (A) and sumo-2/3 (B) immunoreactivity were analyzed in the spleen of 3 month-old AL, 25 month-old AL, and 25 month-old DR rodents. A representative blot for the analyses is provided. The graphs represent the mean and S.E.M. of the optical density of the immunoreactivity from 6 animals in each experimental group. *p < 0.05 compared to 3 month old AL animals
4. Discussion
Previous studies from our laboratory and others have demonstrated that during aging most tissues, including most rodent tissues analyzed thus far, exhibit a progressive decline in multiple proteasome peptidase activities [1-4]. The current study for the first time demonstrates that unlike most tissues the aging rodent spleen does not exhibit a decline in proteasome peptidase activities, and actually has an elevation in chymotrypsin-like proteasome activity with age (Table 1). Interestingly, DR did not have any significant effect on the age-related changes in proteasome peptidase activities, even though DR has been reported to ameliorate age-related declines in proteasome function in some tissues [6].
Our studies demonstrate that aging and DR have effects on the expression levels of multiple proteasome components. Significant elevations in 20S alpha subunits and POMP1 were observed in the aged spleen relative to young rodents. In contrast, no significant alterations in 20S beta subunits was detected in the aged spleen, with the levels of PA200 trending towards being decreased in the aged spleen. Taken together, these data suggest that the effects of aging on proteasome subunit expression in the spleen are subunit specific. Recent studies have demonstrated that in viable cells proteasome subunit expression can be increased in response to multiple stressors including oxidative stress and inflammatory stimuli [28, 29]. Presumably, the elevations in proteasome subunit expression following these stressors serves a direct role in trying to preserve proteasome-mediated protein degradation, and thereby contribute to cellular homeostasis. Such stressors may potentially contribute to the changes in proteasome subunit expression observed in the present study, since both oxidative stress and increased inflammation are believed to contribute to the effects of aging on the different tissues. It is also likely that the elevation in chymotrypsin-like activity observed in the aging spleen is mediated in part by an elevations in the amount of proteasome complexes present in the aging spleen.
The significant elevation in both 20S alpha and POMP1 in the aged spleen are consistent with increased levels of 20S proteasome biogenesis potentially occurring during the aging of the spleen. Such an elevation could perhaps be a response to help the spleen increase proteasome activity in the face of elevated levels of stressors during aging, and thereby promote cellular homeostasis in the spleen. It is important to note that the Western blot analysis in the current study, while providing a useful assessment of proteasome subunit levels, does not allow for the determination of alterations in the amount or composition of proteasome complexes, both of which are important factors in regulating overall proteasome activity. In particular, our present findings cannot distinguish between the amount of proteasome subunits existing as free subunits and proteasome subunits present in functional 20S or 26S proteasome complexes. Studies are currently underway to determine the effects of aging on the amount, composition, and specific activity of purified 20S and 26S proteasome complexes in the spleen of aging and DR rodents.
In the present study we demonstrate for the first time that aging increases the levels of ubiquitinated protein in the spleen, and that DR significantly reduces the age-related increases in ubiquitinated protein in the spleen. The elevation in ubiquitinated protein levels during aging presumably is the result of elevated levels of aberrant proteins (damaged, misfolded), and not likely due to an impaired ability to degrade proteins (since proteasome activity is actually elevated in the aging spleen). Because DR does not alter the levels of 20S proteasome subunits or POMP1 relative to what is observed in aging AL rodents, and does not alter age related changes in proteasome activities, our data suggest that the reduced levels of ubiquitinated protein in the spleen following DR are not likely mediated by an increase in proteasome expression. It is likely that DR suppresses the levels of ubiquitinated protein in the spleen via reducing the rate of aberrant protein formation, and therefore makes the spleen a particularly interesting target to study in future DR related studies.
This study for the first time demonstrates that the amount of sumoylated protein increases with age in the spleen, and that in contrast to DR mediated effects on the levels of ubiquitinated protein, age-related increases in sumoylation are not significantly affected by DR. Because sumoylation is known to have effects on protein function/localization these data suggests a new role for sumoylation potentially contributing to altered protein function in the aging spleen. It is interesting to note that in immunoprecipitation experimentation we observed that at least 1 protein band in the anti-ubiquitin precipitate exhibited cross-reactivity with our anti-sumo1 antibody (data not shown). Taken together, these data suggest that while ubiquitination and sumoylation are highly related post-translation modifications, and both are clearly involved in regulating protein turnover [11,12], they do not appear to be equally affected by DR in the spleen.
The spleen is the second largest immune organ of the body, with the ratio of spleen weight to body weight known to not be significantly altered in response to aging [18]. What is known to be affected by aging is the composition of the cell types within the aging spleen, with lymphocyte number reduced significantly in the aging spleen, which is accompanied by a concomitant increase in reticular cells and macrophages [18]. DR is known to promote selective declines in specific lymphocyte populations [30]. Our data suggest that changes in ubiquitination, sumoylation, proteasome expression, and proteasome function may result from these changes in the cellular makeup of the spleen during aging and in response to DR. Identifying the specific cells in the spleen which have the most dramatic alterations in proteasome levels, proteasome function, ubiquitination, and sumoylation during aging and in response to DR will be crucial to understanding the ultimate contribution of the observed proteome changes to the function of the spleen. Regardless, our data demonstrate that both aging and DR promote multiple alterations in the proteome of the spleen, and raise the possibility that these specific modifications may ultimately contribute to the effects of aging and DR on the function of the spleen.
Acknowledgments
This work was supported by grants from the National Institute of Aging (AG0257701, AG029885).
Abbreviations
- AL
ad libitum
- DR
dietary restriction
- NIA
National Institute of Aging
- POMP
proteasome maturation protein
- Sumo
small ubiquitin-like modifier
- Ub
ubiquitin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rattan SI. Synthesis, modifications, and turnover of proteins during aging. Exp Gerontol. 1996;31:33–47. doi: 10.1016/0531-5565(95)02022-5. [DOI] [PubMed] [Google Scholar]
- 2.Szweda PA, Camouse M, Lundberg KC, Oberley TD, Szweda LI. Aging, lipofuscin formation, and free radical-mediated inhibition of cellular proteolytic systems. Ageing Res Rev. 2003;2:383–405. doi: 10.1016/s1568-1637(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 3.Ding Q, Dimayuga ED, Keller JN. Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid Redox Signal. 2006;8:163–172. doi: 10.1089/ars.2006.8.163. [DOI] [PubMed] [Google Scholar]
- 4.Grune T. Oxidative stress, aging and the proteasomal system. Biogerontology. 2000;1:31–40. doi: 10.1023/a:1010037908060. [DOI] [PubMed] [Google Scholar]
- 5.Torres C, Lewis L, Christofalo VJ. Proteasome inhibitors shorten replicative life span and induce a senescent-like phenotype of human fibroblasts. J Cell Physiol. 2006;207:845–853. doi: 10.1002/jcp.20630. [DOI] [PubMed] [Google Scholar]
- 6.Merker K, Stolzing A, Grune T. Proteolysis, caloric restriction and aging. Mech Ageing Dev. 2001;122:595–615. doi: 10.1016/s0047-6374(01)00219-6. [DOI] [PubMed] [Google Scholar]
- 7.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 8.Ramos PC, Hockendorff J, Johnson ES, Varshavsky A, Dohmen RJ. Ump1p is required for proper maturation of the 20S proteasome and becomes its substrate upon completion of the assembly. Cell. 1998;92:489–499. doi: 10.1016/s0092-8674(00)80942-3. [DOI] [PubMed] [Google Scholar]
- 9.Burri L, Hockendorff J, Boehm U, Klamp T, Dohmen RJ, Levy F. Identification and characterization of a mammalian protein interacting with 20S proteasome precursors. Proc. Natl. Acad. Sci. USA. 2000;97:10348–10353. doi: 10.1073/pnas.190268597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruger E, Kloetzel PM, Enenkel C. 20S proteasome biogenesis. Biochimie. 2001;83:289–293. doi: 10.1016/s0300-9084(01)01241-x. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann J, Lerman LO, Lerman A. Ubiquitin and ubiquitin-like proteins in protein regulation. Circ. Res. 2007;100:1276–1291. doi: 10.1161/01.RES.0000264500.11888.f0. [DOI] [PubMed] [Google Scholar]
- 12.Melchior F. SUMO--nonclassical ubiquitin. Annu. Rev. Cell. Dev. Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- 13.Yu BP. Aging and oxidative stress: modulation by dietary restriction. Free Radic. Biol. Med. 1996;21:651–668. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]
- 14.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Mattson MP. Gene–diet interactions in brain aging and neurodegenerative disorders. Ann. Intern. Med. 2003;139:441–444. doi: 10.7326/0003-4819-139-5_part_2-200309021-00012. [DOI] [PubMed] [Google Scholar]
- 17.Pahlavani MA. Influence of caloric restriction on aging immune system. J. Nutr. Health Aging. 2004;8:38–47. [PubMed] [Google Scholar]
- 18.Cesta MF. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006;34:455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J. Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 20.Provinciali M, Smorlesi A. Immunoprevention and immunotherapy of cancer in aging. Cancer Immunol. Immunother. 2005;54:93–106. doi: 10.1007/s00262-004-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linton P, Thoman ML. T cell senescence. Front. Biosci. 2001;6:D248–D261. doi: 10.2741/linton. [DOI] [PubMed] [Google Scholar]
- 22.Hosea HJ, Rector ES, Taylor CG. Dietary repletion can replenish reduced T cell subset numbers and lymphoid organ weight in zinc-deficient and energy-restricted rats. Br. J. Nutr. 2004;91:741–747. doi: 10.1079/BJN20041104. [DOI] [PubMed] [Google Scholar]
- 23.Aidoo A, Mittelstaedt RA, Bishop ME, Lyn-Cook LE, Chen YJ, Duffy P, Heflich RH. Effect of caloric restriction on Hprt lymphocyte mutation in aging rats. Mutat. Res. 2003;527:57–66. doi: 10.1016/s0027-5107(03)00072-1. [DOI] [PubMed] [Google Scholar]
- 24.Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech. Ageing Dev. 2000;113:61–70. doi: 10.1016/s0047-6374(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 25.Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer's disease. J. Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoefer MM, Boneberg EM, Grotegut S, Kusch J, Illges H. Possible tetramerisation of the proteasome maturation factor POMP/proteassemblin/hUmp1 and its subcellular localisation. Int J Biol Macromol. 2006;38:259–267. doi: 10.1016/j.ijbiomac.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Ustrell V, Hoffman L, Pratt G, Rechsteiner M. PA200, a nuclear proteasome activator involved in DNA repair. EMBO J. 2002;21:3516–3525. doi: 10.1093/emboj/cdf333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Q, Reinacker K, Dimayuga E, Nukala V, Drake J, Butterfield DA, Dunn JC, Martin S, Bruce-Keller AJ, Keller JN. Role of the proteasome in protein oxidation and neural viability following low-level oxidative stress. FEBS Lett. 2003;546:228–232. doi: 10.1016/s0014-5793(03)00582-9. [DOI] [PubMed] [Google Scholar]
- 29.Akiyama K, Kagawa S, Tamura T, Shimbara N, Takashina M, Kristensen P, Hendil KB, Tanaka K, Ichihara A. Replacement of proteasome subunits X and Y by LMP7 and LMP2 induced by interferon-gamma for acquirement of the functional diversity responsible for antigen processing. FEBS Lett. 1994;343:85–88. doi: 10.1016/0014-5793(94)80612-8. [DOI] [PubMed] [Google Scholar]
- 30.Ha CL, Wong SS, Gray MM, Watt J, Hillyer LM, Woodward BD. Overabundance of CD45RA(+) (quiescent-phenotype) cells within the involuted CD4(+) T-cell population follows initiation of immune depression in energy-deficient weanling mice and reflects involution exclusive to the CD45RA (–) subset. J. Nutr. 2001;131:1812–1818. doi: 10.1093/jn/131.6.1812. [DOI] [PubMed] [Google Scholar]