Abstract
The promise of cardiac tissue engineering is in the ability to recapitulate in vitro the functional aspects of healthy heart and disease pathology as well as to design replacement muscle for clinical therapy. Parts of this promise have been realized; others have not. In a meeting of scientists in this field, five central challenges or “big questions” were articulated that, if addressed, could substantially advance the current state-of-the-art in modeling heart disease and realizing heart repair.
Heart is the first functional organ that forms in the human body. Only a few weeks into gestation, the heart starts to beat and pump blood, and continues to do so throughout lifetime. As soon as its development is complete, the capacity of the heart to regenerate after damage or disease becomes only minimal. As a result, cardiovascular disease remains the main cause of death worldwide, prompting the need for new effective approaches to heart repair. In contrast to all other options - cell cycle reentry, administration of therapeutic cells, and recruitment of endogenous cardiac and vascular progenitors - cardiac tissue engineering is focused on providing a definitive solution by growing or regenerating heart muscle and vasculature.
Both the in vitro and in vivo methods tend to recapitulate cell-cell and cell-matrix interactions and the original physical structure and physiological signaling in the heart. The ultimate goal of tissue engineering is to build functional tissues or whole organs for transplantation, but the field is in its infancy. Current efforts are focused on the creation of the individual tissues (the vasculature, valves, myocardium) in sizes that are limited by the existing tissue engineering technologies. Our meeting focused on the challenges and opportunities for growing functional myocardium, with two major translational goals: in vitro modeling of disease and cardiac grafts for transplantation.
To efficiently pump blood through the body, the myocardium provides the necessary contractile force regulated by a highly specialized electrical conduction system that responds to external stimuli. To support these functions, the tissue draws a high metabolic demand and requires comprehensive vascular support. To minimize complexity, myocardial tissue engineering has sought to develop minimally functional tissue units that are three-dimensional from the cellular perspective but thin enough to benefit from simplified methods for exchange of nutrients—most critically oxygen—and metabolites. Recent advances in cardiac tissue engineering include the generation of microtissues capable of force generation and predictable responses to cardiac drugs (1) on one end of the spectrum, and the clinical implementation of cardiac tissues engineered from progenitor cells and encapsulated in hydrogel for heart failure patients (2) on the other end of the spectrum.
Here we delineate our collective perspective on the challenges facing the in vitro modeling of myocardial disease and the generation and delivery of transplantable cardiac grafts. Our goal was to envision strategies that would most effectively advance our understanding of cardiac disease, and lead to effective and safe repair of the failing heart.
TACKLING TISSUE-ENGINEERED HEART REPAIR
Question 1: What kinds of microphysiological platforms have clinical impact?
For decades, cardiac tissue engineering has been driven by the need to repair damaged myocardium. Clinical translation in this area is becoming increasingly plausible but remains far from being a routine practice, with scale-up, vascularization and electromechanical integration still posing major challenges. A new paradigm is now emerging that is poised to accelerate therapeutic discovery: microphysiological tissue platforms for predictive drug testing and modeling of disease (3).
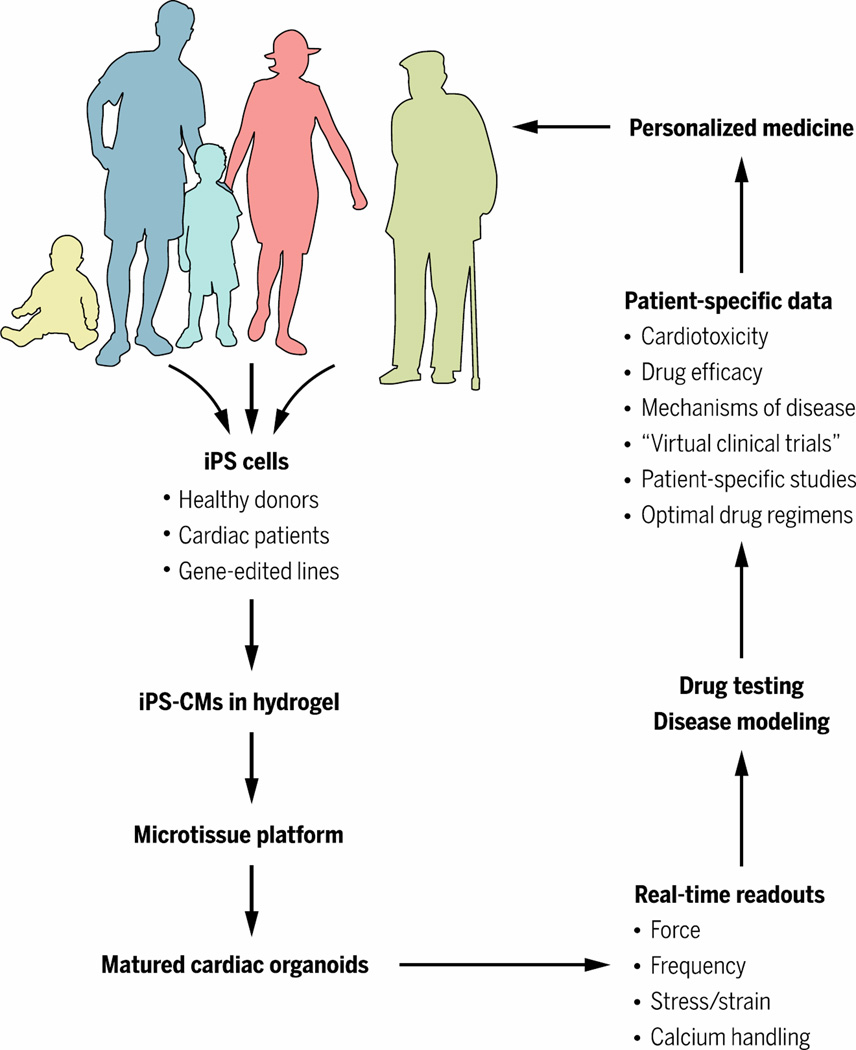
These platforms range in scale from single-cell functional assays to micro-sized human tissues connected by microfluidic vascular conduits designed to model human physiology in vitro. Although it is not possible (or even necessary) to recapitulate the entire complexity of human myocardium, these models provide a minimal set of physiological functions that are necessary to study drug efficacy, safety, and mode of action (4, 5). For example, cardiomyocytes (CM) derived from human induced pluripotent stem cells (hiPSC) and matured on engineered substrates can recapitulate adult-like sarcomere structure, contractility and responses to mechanical stimulation and agonists of sarcomere function (6). Clearly, the simplest systems are best, and it remains to be determined “how simple is complex enough” depending on the drug type, cell phenotype, and disease pathology being studied (Fig. 1).
Fig. 1. Microtissue platforms: Achieving complexity on a small scale.
Microtissue platforms, also called “organs-on-a-chip”, are likely to be transformative to drug testing, modeling of cardiac disease, and implementation of personalized medicine. The impact of these technologies likely will be translated sooner than clinical applications, because of the simpler tissue engineering and regulatory requirements.
With recent advances in gene editing, it is possible to generate isogenic human pluripotent stem cell lines for the in vitro modeling of human cardiovascular disease. Such lines can be generated by inducing disease-causing mutations into wild-type iPSCs, or by correcting such mutations in patient-specific and disease-specific iPSCs. These approaches allow linking of genetic mutations with clinical phenotypes (7–9). Success in recapitulating disease phenotypes depends on implementation of mechanical loading, a key factor associated with both the heart development and adult function. The initial strategy for mechanical stimulation - isometric loading of the tissues on static holders, was later extended to the more physiological auxotonic loading.
Despite advances, the effects of mechanical stimulation on cardiac tissue maturation have been inconsistent. An alternative strategy for cardiac maturation is electrical stimulation, which was shown to improve calcium handling and electrophysiological properties of iPSC-CMs (10) and to regulate their beating rate (11). However, the evidence that mechanical and/or electrical stimulation can lead to ultrastructural and functional hallmarks of adult heart muscle: sarcomeres with dense mitochondria, T-tubules and M-lines, and a positive force-frequency relationship (Bowditch phenomenon) is yet to be published. We anticipate that ongoing work will lead to better understanding of environmental factors for engineering adult-like human heart tissue, and enable predictive physiological studies of drugs and disease.
Question 2: Which cells should be used for cardiac regeneration?
Both the in vitro microphysiological systems and the in vivo repair of contractile myocardium are based on the availability of functional CMs derived from human stem cells. Myocardial repair will likely require restoration of all of the muscle, vascular, and stromal components of the heart tissue. In the context of cardiovascular tissue engineering, this will necessitate careful optimization of the initial cellular makeup of cardiac implants. Although CMs are responsible for electrical conduction and generation of contractile force, fibroblasts, stromal and endothelial cells all play important roles in matrix deposition, vascularization, and paracrine signaling.
An advantage of cardiac tissue engineering is in its ability to systematically vary the starting cellular composition and biomaterial scaffolds toward optimizing the function of engineered myocardium. In human tissue constructs, velocity of action potential propagation improved with increasing amounts of virtually pure CMs in the initial cell composition, whereas contractile force output was optimal for CM populations with purities of 60 to 80% (12). Overall, the presence of fibroblasts or other stromal cells in iPSC-derived cardiac tissues is found to be beneficial to CM maturation, via engagement of extracellular matrix proteins (13) and establishment of intercellular contacts, while excess of non-myocytes may compromise conduction and integration.
The presence of endothelial cells in engineered cardiac tissues promotes CM maturation in vitro and survival and integration in vivo (14). A better understanding of the optimal cellular makeup along with computer-assisted fabrication methodologies should enable generation of tissues with sophisticated architectures and functional properties approaching those of native myocardium.
Cardiac tissue function involves multiple CM phenotypes (e.g., ventricular, atrial, nodal, Purkinje). The current protocols predominantly give rise to ventricular cardiomyocytes, whereas control of retinoic acid signalling can enhance atrial specification. Going forward, the field needs to develop precise, directed protocols for cardiomyocyte subtype specification and functional maturation. Removal of spontaneously active nodal cells along with enhanced functional maturation of CMs is expected to allow engineering of electrically quiescent working myocardium, suitable for safe repair of infarcted heart. Purification of mature sinoatrial nodal cells might, in turn, permit engineering of biological pacemaker tissues (15).
The heart also contains epicardial and endocardial cells, which, although critically important for myocardial development and homeostasis, have received little attention. Of particular interest is that postnatal epicardial cells can migrate into the myocardium after injury to give rise to new coronary vessels (16). Recently derived epicardial cells from human iPSCs (17) could be used in engineered cardiac tissues to support CM proliferation and maturation in vitro and vascular integration in vivo. Biochemical or genetic (18) manipulation of host or transplanted epicardial cells might be an important strategy for facilitating electrical integration of cardiac tissue patches with the host myocardium (19). Furthermore, endocardial-like cells derived from human embryonic stem cells (hESCs) (20) may provide a progenitor population capable both of lining a chamber and of robust de novo vascular assembly.
Question 3: Is vascularization of cardiac tissue grafts a necessary complexity?
Prolonged survival and functionality of three-dimensional (3D) tissue-engineered products requires efficient supply of oxygen and nutrients, along with the removal of metabolites. Although perfusion bioreactors can transport nutrients across large tissue thicknesses and maintain cardiomyocyte viability in the absence of a vascular network, a preformed vascular structure in vitro can markedly enhance cardiomyocyte function and viability by accelerating anastomosis to the host circulation, compared to nonvascularized engineered cardiac tissues (14). Vascular cell coculture can be used in conjunction with mechanical loading to control cardiomyocyte proliferation, and the hypertrophy and architecture of engineered human myocardium (21). Endothelial cells release paracrine factors such as neuregulin (22) and nitric oxide that improve cell survival after myocardial ischemia and thus are a favorable component of engineered myocardium for therapeutic applications.
The importance of preformed vascular networks in microtissue platforms for drug screening and modeling of disease is now being actively investigated. Engineered tissues for in vitro application should in general be designed as minimally functional tissue units that enable quantitative physiological studies under normal and pathological conditions (4). Many groups have shown that the presence of endothelial cells in engineered cardiac tissues promotes CM survival and function. Beyond paracrine signalling, engineered vasculature provides a route for the exchange of nutrients and metabolites and delivery of drugs to the target tissue.
Current studies are focused on the establishment of functional microvasculature for connecting components of multi-organ microdevices, such as the vascular-liver-heart platform to examine cardiotoxicity of a drug metabolized by the liver (23). Looking forward, we envision that such platforms will use microvasculature both to support the metabolic needs of cultured tissues and to deliver drugs in disease modeling settings.
Question 4: Preclinical trials: What are reasonable expectations?
Experiments in small and large animal models have shown that tissue-engineered cardiac patches can improve recovery from myocardial injury (24–26). The contractile activity of engineered tissue is expected to contribute directly to myocardial performance, but improvements can also evolve through the release of cytokines that promote angiogenesis, activate endogenous progenitor cells (26, 27), or stimulate paracrine pathways (Fig. 2).
Fig. 2. Tissue-engineered heart repair.
Major progress is being made in translational studies of various types of engineered cardiac patches for implantation. An IGF-1–loaded fibrin patch markedly enhances the effects of iPSC-derived cells in a swine model of ischemia-reperfusion. Modified from (33), with permission.
The damage induced by acute infarct is exacerbated by chronic volume overload as the left ventricular (LV) chamber dilates, overstretches the peri-infarct myocytes, and activates detrimental apoptotic signaling pathways. LV dilatation usually is accompanied by hypertrophy and metabolic abnormalities, both of which could be alleviated at least in part by cardiac cell and cardiac patch therapy. Cells and cytokines that mediate cardiomyocyte-noncardiomyocyte communication are critical for the beneficial paracrine activity induced by implanted cardiac tissue patches.
One of the most prominent safety concerns associated with tissue-based myocardial therapies is the risk of arrhythmia. Studies with injected cells suggest that this risk is related to the size of the remuscularized region of treated hearts. Injected cardiomyocytes derived from hESCs have not been associated with arrhythmia in rodents, but when the same dose of cells (cells/per kg body weight) was scaled up for delivery to macaques, all four of the cell-treated animals experienced periods of premature ventricular contractions and/or tachycardia (24, 28). This discrepancy between observations in small and large animals may have occurred because of the physically larger grafts in the non-human primate model, which contain millimeters to centimeters of new myocardium that may alter electrical propagation. Conversely, the arrhythmias might have been unmasked by the slower heart rates in this model (~120 beats/min) compared to guinea pigs, rats and mice (250, 400 and 600 beats/min, respectively). Of note, human heart tissue patches do not electrically integrate with the underlying rodent myocardium after transplantation and beat at an independent, typically slower, rate (19). This means that the current epicardial patches are unlikely to cause arrhythmias or to contribute to coordinated systolic function. Clinical trials of engineered cardiac tissue patches should address ways the remuscularized regions of treated hearts can be synchronized to contract in concert with the native myocardium after transplantation so that the mechanical support is provided without inducing arrhythmias. We have yet to understand how to establish a functional host-graft interface in xenograft models, through experiments in large animal models.
Question 5: How will cardiac regeneration be implemented clinically?
Given the relatively new entry of tissue engineering into cardiovascular sciences, there has been little therapeutic application of engineered cardiac tissues. The most recent trial involves epicardial delivery of a fibrin patch loaded with cardiac progenitor cells derived from human embryonic stem cells in heart failure patients (2). A key challenge for translating engineered tissues to the clinic is the need for a pre-fabricated vascularized network that can be directly connected to the circulatory system of the host in order to protect the survival of cells subjected to ischemia. An alternate option, possibly easier to implement, could be to functionalize the construct with peptides that mobilize circulating angiogenic cells and thereby contribute to the vascularization of embedded cells.
After vascularization, the electromechanical integration of graft and host is critically important, and efforts to reduce isolation of the graft by scar tissue would be highly beneficial. Additional concerns include the regulatory hurdles, high costs of developing autologous products, and the risks of immunogenic rejection when transplanting allogeneic products. Finally, the kinetics of degradation of implanted materials and the possible toxicity of degradation products must be addressed.
To this end, a number of labs are beginning to use innovative tools such as synthetic biodegradable scaffolds and 3D bioprinting. Until microvascularization can be achieved, a number of acellular tissue-engineered products, such as epicardial patches (29) or injectable biomatrix, have shown promise as delivery vehicles for drugs or as biomechanical support to modulate cardiac remodeling, respectively. The unsuccessful AUGMENT-HF trial, in which intramyocardial injections of an alginate gel in heart-failure patients failed to improve left ventricular function (30), raised doubts as to whether such materials can be used for stand-alone treatment.
An attractive option could be the use of an acellular scaffold functionalized with biologics that foster endogenous repair by activating appropriate signaling pathways in a time-controlled fashion. The development of acellular, rather than cellular, heart repair products could have a faster path to clinical use, given the long history of synthetic vascular grafts – such as acellular porcine and cadaveric heart valves, and the recent introduction of biodegradable coronary stents (31). The implementation of cell-based products will take more time to develop because of the need for vascularization and the lack of a well-forged regulatory pathway. Recent clinical trials to inject hESC- and hiPSC-derived retinal epithelial cells into patients for the treatment of age-related macular degeneration and Stargardt’s disease may provide some early regulatory guidance for pluripotent stem cell–derived products (32).
In addition, researchers must consider the specific disease indications that might benefit from an engineered product and the mode of implementation for each indication. Most research to date has focused on the treatment of heart failure resulting from myocardial infarction - the main driver of morbidity and mortality in Western society. The creation of perfusable human tissue that can survive transplantation will also enable researchers to address more chronic myocardial diseases such as post-infarct and nonischemic heart failure.
THE FUTURE OF HEART REPAIR
New ideas on how and under which exact conditions we might effectively model heart disease in vitro and provide heart repair in vivo are emerging at the boundaries of stem cell science, bioengineering, and clinical disciplines. It is now clear that cardiomyocyte health is intimately connected with the health of other cardiac cell types. Most prominent is the interaction between cardiomyocytes and cardiac fibroblasts, which appear to provide the extracellular matrix necessary for proper mechanical anchorage and support of cell-cell interactions. Identification of links between focal adhesions, costameres, and intercalated discs with the signaling pathways involved in cardiomyocyte maturation could reduce our dependence on co-culture if the appropriate cues could be supplied exogenously.
Endothelial cells in co-culture with cardiomyocytes have shown benefits in the context of paracrine signaling, but also as functional lining of newly formed blood vessels. Vascularization is not essential for in vitro model systems or even for a thin cardiac patch for implantation. Still, vascularizing even these small tissue constructs might have advantages for maturation of cardiomyocytes and physiological delivery of nutrients and drugs. The transition to thick tissues and ultimately an intact heart graft will, however, require vasculature, either by tissue-engineered design or facilitated ingrowth from the host. This is an area in which tissue engineers excel. In fact, any advance in vascularization, either by controlled delivery of angiogenic agents or by 3D printing or microfabrication of vessel structures, will aid not only the cardiac field, but also nearly every other effort to engineer tissues (Fig. 3).
Fig. 3. A map of heart repair.
Among variety of tissue-engineering systems currently under investigation, the best options for clinical translation are found in a Venn diagram between the biological complexity, feasibility, and safety and efficacy for the patient.
An unforeseen issue with studies in large animals is the level of similarity between the engineered and the recipient tissues. Interestingly, the U.S. Food and Drug Administration prefers the testing of tissue engineered products as xenograft, whereas European regulatory agencies prefer testing in homologous (auto- or allograft) large animal models. Clearly, both approaches have their pros and cons and only continued testing will show which of them is more predictive of clinical function.
How will clinical implementation take shape? The clinical experience will continue to evolve as far as possible using cell-free approaches. Should these approaches come up short in providing functional advantages, cell-based products will be implemented. Impediments to their success will certainly include costs, but also clinical indications. To date, tremendous emphasis has been placed on post-infarction repair, but other indications could present additional starting points. For example, nonischemic dilated cardiomyopathy is a devastating and prevalent failure of the heart, but avoids the complexities of scar formation and intricacies of timing of the tissue replacement. Finally, consideration should be given to the route of delivery of engineered cardiac tissues. The majority of the field is developing a patch to be placed on the epicardial surface of the heart that requires open chest surgery. A future challenge will be to adapt the application of tissue engineered products for endocardial delivery. This will in turn necessitate interactions among cardiovascular interventionalists, surgeons, stem cell scientists, and tissue engineers to inform each other about the needs, requirements, and actionable opportunities.
Acknowledgments
This Perspective is a product of discussions at the Cardiovascular Tissue Engineering Workshop, held at Stanford Cardiovascular Institute in May 2015. Special thanks go to D. Buxton and M. Lundberg (both from NIH) and M. Terrin (University of Maryland) for their leadership in organizing the workshop, and to F. Yang (Stanford University), S. Palecek (University of Wisconsin), L. Niklason (Yale University), N. Melosh (Stanford University), M. Tiburcy (University Medical Center Göttingen) and O. Abilez (Stanford University) for their contributions to the discussion. Funding: The authors gratefully acknowledge support of the symposium by the NIH Progenitor Cell Biology Consortium (grant HL099997).
Footnotes
Competing interests: The authors declare that they have no competing interests related to the contents of this article.
References
- 1.Coulombe KL, Bajpai VK, Andreadis ST, Murry CE. Heart regeneration with engineered myocardial tissue. Annu Rev Biomed Eng. 2014;16:1–28. doi: 10.1146/annurev-bioeng-071812-152344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 4.Cimetta E, Godier-Furnemont A, Vunjak-Novakovic G. Bioengineering heart tissue for in vitro testing. Curr Opin Biotechnol. 2013;24:926–932. doi: 10.1016/j.copbio.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE. Human iPSC-based cardiac microphysiological system for drug screening applications. Scientific reports. 2015;5:8883. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A. 2015;112:12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Science translational medicine. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science. 2015;349:982–986. doi: 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eng G LB, Protas L, Gagliardi M, Browh K, Kass RS, Keller G, Robinson RB, Vunjak-Novakovic G. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nature Communications. 2015 doi: 10.1038/ncomms10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung JP, Hu D, Domian IJ, Ogle BM. An integrated statistical model for enhanced murine cardiomyocyte differentiation via optimized engagement of 3D extracellular matrices. Scientific reports. 2015;5:18705. doi: 10.1038/srep18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen MR, Robinson RB, Brink PR, Cohen IS. The road to biological pacing. Nature reviews. Cardiology. 2011;8:656–666. doi: 10.1038/nrcardio.2011.120. [DOI] [PubMed] [Google Scholar]
- 16.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 17.Witty AD, Mihic A, Tam RY, Fisher SA, Mikryukov A, Shoichet MS, Li RK, Kattman SJ, Keller G. Generation of the epicardial lineage from human pluripotent stem cells. Nat Biotechnol. 2014;32:1026–1035. doi: 10.1038/nbt.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkton RD, Bursac N. Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies. Nat Commun. 2011;2:300. doi: 10.1038/ncomms1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerbin KA, Yang X, Murry CE, Coulombe KL. Enhanced Electrical Integration of Engineered Human Myocardium via Intramyocardial versus Epicardial Delivery in Infarcted Rat Hearts. PLoS One. 2015;10:e0131446. doi: 10.1371/journal.pone.0131446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palpant NJ, Pabon L, Roberts M, Hadland B, Jones D, Jones C, Moon RT, Ruzzo WL, Bernstein I, Zheng Y, Murry CE. Inhibition of beta-catenin signaling respecifies anterior-like endothelium into beating human cardiomyocytes. Development. 2015;142:3198–3209. doi: 10.1242/dev.117010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedhli N, Huang Q, Kalinowski A, Palmeri M, Hu X, Russell RR, Russell KS. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123:2254–2262. doi: 10.1161/CIRCULATIONAHA.110.991125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 24.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Q, Ye L, Zhang P, Lepley M, Swingen C, Zhang L, Kaufman DS, Zhang J. Bioenergetic and functional consequences of cellular therapy: activation of endogenous cardiovascular progenitor cells. Circ Res. 2012;111:455–468. doi: 10.1161/CIRCRESAHA.112.269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, Marui A, Shimizu T, Ikeda T, Okano T, Sakata R, Yamashita JK. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–1205. doi: 10.1002/stem.1089. [DOI] [PubMed] [Google Scholar]
- 28.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anker SD, Coats AJ, Cristian G, Dragomir D, Pusineri E, Piredda M, Bettari L, Dowling R, Volterrani M, Kirwan BA, Filippatos G, Mas JL, Danchin N, Solomon SD, Lee RJ, Ahmann F, Hinson A, Sabbah HN, Mann DL. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial) Eur Heart J. 2015;36:2297–2309. doi: 10.1093/eurheartj/ehv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilgrim T, Heg D, Roffi M, Tuller D, Muller O, Vuilliomenet A, Cook S, Weilenmann D, Kaiser C, Jamshidi P, Fahrni T, Moschovitis A, Noble S, Eberli FR, Wenaweser P, Juni P, Windecker S. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet. 2014;384:2111–2122. doi: 10.1016/S0140-6736(14)61038-2. [DOI] [PubMed] [Google Scholar]
- 32.Neofytou E, O'Brien CG, Couture LA, Wu JC. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125:2551–2557. doi: 10.1172/JCI80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serpooshan V, Wu SM. Patching up broken hearts: cardiac cell therapy gets a bioengineered boost. Cell Stem Cell. 2014;15:671–673. doi: 10.1016/j.stem.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]