SUMMARY
Current treatments for traumatic brain injury (TBI) have not focused on improving microvascular perfusion. Drag-reducing polymers (DRP), linear, long-chain, blood soluble non-toxic macromolecules, may offer a new approach to improving cerebral perfusion by primary alteration of the fluid dynamic properties of blood. Nanomolar concentrations of DRP have been shown to improve hemodynamics in animal models of ischemic myocardium and limb, but have not yet been studied in the brain. Recently, we demonstrated that that DRP improved microvascular perfusion and tissue oxygenation in a normal rat brain. We hypothesized that DRP could restore microvascular perfusion in hypertensive brain after TBI. Using the in-vivo 2-photon laser scanning microscopy we examined the effect of DRP on microvascular blood flow and tissue oxygenation in hypertensive rat brains with and without TBI. DRP enhanced and restored capillary flow, decreased microvascular shunt flow and, as a result, reduced tissue hypoxia in both un-traumatized and traumatized rat brains at high ICP. Our study suggests that DRP could be an effective treatment for improving microvascular flow in brain ischemia caused by high ICP after TBI.
Keywords: drug reducing polymer (DRP), polyethylene oxide (PEO), traumatic brain injury (TBI), intracranial pressure (ICP), cerebral blood flow (CBF), capillaries, microvascular shunts (MVS), NADH, hypoxia, ischemia, rats
INTRODUCTION
Ischemia is a secondary injury which frequently occurs after traumatic brain injury (TBI). Oxygen and nutrient deprivation ultimately leads to permanent cell death. Currently, none of the treatments for TBI have focused on restoring or improving microvascular perfusion after TBI. Drag-reducing polymers (DRP), linear, long-chain, blood soluble non-toxic macromolecules, may offer a new approach to improving cerebral perfusion by primary alteration of the fluid dynamic properties of blood. DRP have been shown to improve hemodynamics and survival in animal models of ischemic myocardium [1–3], ischemic limb [4] and hemorrhagic shock [5, 6]. However, despite their promising therapeutic potential, DRP have not yet been studied in the brain. In a single observational qualitative study in rabbits, intravenous injection of DRP restored brain circulation after global ischemia caused by permanent occlusion of carotid and vertebral arteries [7].
The increased intracranial pressure (ICP) after TBI is, among other detrimental consequences, restricting blood supply to the tissue i.e. causing ischemia. In previous studies we showed that high ICP compromised capillary flow leading to the transition of the blood flow to non-nutritive microvascular shunts (MVS) in both, un-traumatized [8] and traumatized [9] brains. This transition was accompanied by tissue hypoxia, brain edema and blood brain barrier (BBB) damage [8, 9].
In the present study we examined the effects of intravenous DRP on the non-traumatized and traumatized rat brain at high ICP by in vivo two-photon laser scanning microscopy (2PLSM).
MATERIAL AND METHODS
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center and done in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Experimental paradigm
Two models were employed in this study: 1) intracranial hypertension where ICP was increased from normal 10 mmHg to 40 mmHg by vertical positioning of an artificial cerebrospinal fluid reservoir connected to the cisterna magna; and 2) TBI resulting in an increase in ICP by fluid percussion using a custom built Pneumatic Impactor connected to a transducer filled with ACSF and glued over craniotomy above left parietal cortex for transmission pressure on to the brain (1.5 ATA, 100 ms pulse duration). Using in-vivo 2-photon laser scanning microscopy (2PLSM) through a cranial window over the parietal cortex (peri-contusion area in TBI) we measured microvascular red blood cells flow velocity visualized by serum labeled with tetramethylrhodamine dextran and NADH autofluorescence for tissue oxygenation. Arterial pressure; blood gases, electrolytes, hematocrit and pH; rectal and cranial temperatures were monitored and maintained within normal values throughout the studies. All measurements were done at Baseline and after ICP increase or after trauma induction time points. DRP (1 µg/ml blood) was injected i.v. after increase of ICP (5 rats) or after TBI (5 rats). For TBI control, additional 5 animals were injected with vehicle (normal saline).
Surgery
Most of the procedures used in this study have been previously described [8, 10]. Briefly, acclimated Sprague-Dawley male rats (Harlan Laboratories, Indianapolis, IN) weighing between 300–350 g were intubated and mechanically ventilated on 2% isoflurane / 30% oxygen / 70% nitrous oxide. Rectal and temporal muscle probes were inserted. Femoral venous and arterial catheters were inserted for injections, arterial pressure monitoring and blood sampling. A catheter was inserted into cisterna magna for ICP monitoring and manipulation. For imaging and TBI, a craniotomy 5 mm in diameter was made over the left parietal cortex, filled with 2% agarose/saline and sealed with cover glass.
Microscopy
An Olympus BX51WI upright microscope and water-immersion LUMPlan FL/IR 20×/0.50W objective were used. Excitation (740 nm) was provided by a Prairie View Ultima multiphoton laser scan unit powered by a Millennia Prime 10 W diode laser source pumping a Tsunami Ti: sapphire laser (Spectra-Physics, Mountain View, CA). Blood plasma was labeled by i.v. injection of tetramethylrhodamine isothiocyanate dextran (155 kDa) in physiological saline (5% wt/vol). All microvessels in an imaging volume (500×500×300 µm) were scanned at each study point, measuring the diameter and blood flow velocity in each vessel (3–20 µm Ø). Tetramethylrhodamine fluorescence was band pass filtered at 560–600 nm, NADH autofluorescence was band pass filtered at 425–475 nm. Imaging data processing and analysis were done using Fiji image processing package [11].
Statistical analyses
Statistical analyses were done by Student’s t-test or Kolmogorov-Smirnov test where appropriate. Differences between groups were determined using two-way analysis of variance (ANOVA) for multiple comparisons and post hoc testing using the Mann–Whitney U-test. Statistical significance level was set at P<0.05. Data are presented as mean ± SEM.
RESULTS
Intracranial hypertension
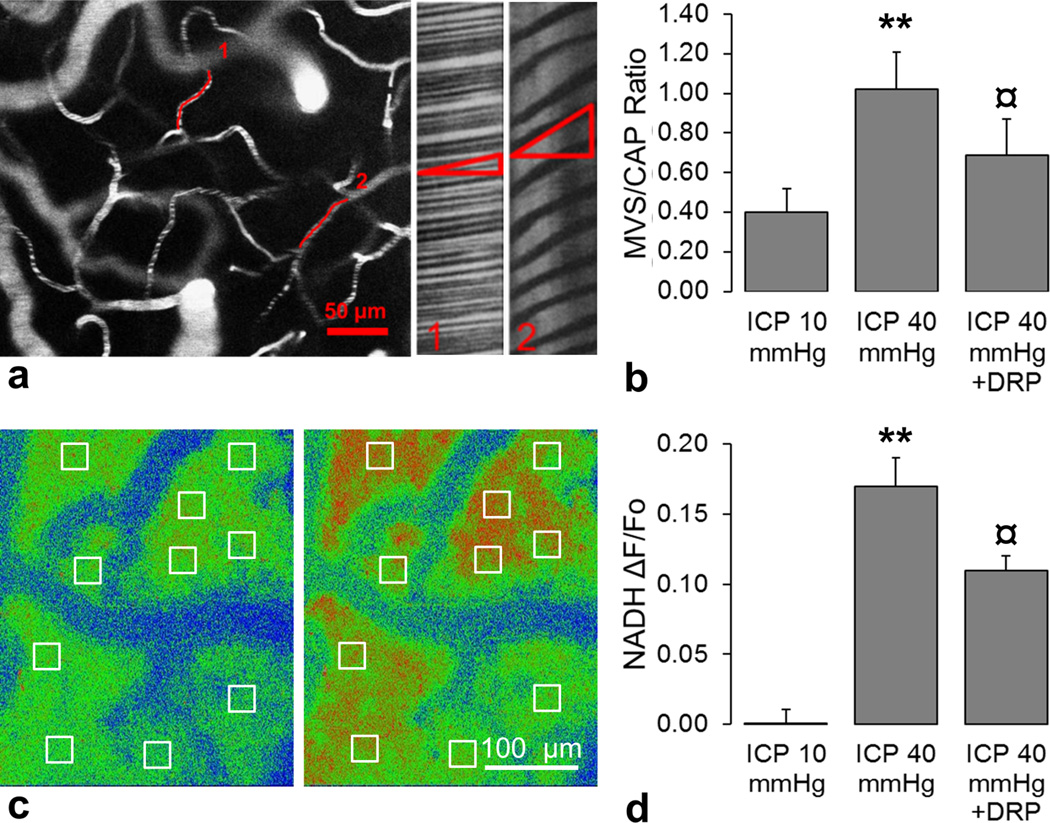
At normal ICP of 10mmHg, microvascular RBC flow velocity in microvessels ranged from 0.12 to 4.05 mm/sec with normal frequency distribution, as was measured in an imaging volume of (500×500×300 µm) by line scans in each microvessel ranging from 3 to 20 µm in diameter (Figure 1 a). An ICP increase to 40 mmHg caused redistribution of microvascular flow; capillary flow (diameter of 3–8 µm and velocities <1mm/sec) was compromised which led to transition of flow to MVS (diameter of 8–20 µm and velocities >1 mm/sec) as reflected by the increase of capillary/MVS ratio (CAP/MVS) to 1.02 ± 0.19 compared from a baseline MVS/CAP ratio of 0.42 ± 0.12 at an ICP of 10 mmHg (Figure 1 b, P<0.01). DRP enhanced capillary flow and reduced MVS flow as indicated by the decrease in the MVS/CAP ratio to 0.69 ± 0.18 (Figure 1 b, P<0.05) compared to ICP of 40 mmHg before DRP injection.
Figure 1.
a) (left) A representative in-vivo 2-photon laser scanning microscopy micrograph showing a region from which microvascular flow was recorded. (right) Line-scan data of red blood cell flow velocities in two microvessels shown on the left. A line scan through a microvessel leads to a sequence of alternating bright and dark pixels corresponding to labeled plasma and unlabeled RBC. The result is diagonal bands in a space–time image as illustrated. The slope of the stripes inversely reflects RBC velocity; second microvessel has lower RBC flow velocity than the first. b) Changes in microvascular shunt/capillary flow (MVS/CAP) ratio showing that DRP attenuated MVS flow, which is elevated at high ICP of 40 mmHg. c) Representative in-vivo 2-photon laser scanning microscopy micrographs with regions of interest (ROI) of NADH autofluorescence show an increase in tissue hypoxia after ICP elevation to 40 mmHg (right) compared to baseline ICP of 10 mmHg (left) mmHg. d) Graph shows that DRP reduced tissue hypoxia caused by ICP elevation to 40 mmHg as reflected by an increase in NADH. Data a presented as a ratio ΔF/Fo, where Fo is NADH at ICP = 10 mmHg. All data are presented as Mean ± SEM, n = 5, ** P<0.01 compared to baseline ICP of 10 mmHg, ¤ P<0.05 compared to ICP of 40 mmHg.
The increase of ICP to 40 mmHg caused a significant increase in NADH autofluorescence (ΔF/Fo[ICP = 10 mmHg] = 0.16 ± 0.02, Figure 1 d, P<0.01). NADH is a sensitive indicator of tissue hypoxia; reduced (NADH) is fluorescent whereas the oxidized form (NAD+) is not, therefore increased fluorescence reflects accumulation of NADH which occurs due to reduced tissue oxygenation (Figure 1 c) [12, 13]. DRP decreased NADH autofluorescence indicating improved tissue oxygenation related to enhanced microvascular perfusion (ΔF/Fo[ICP = 10 mmHg] = 0.11 ± 0.01, Figure 1 d, P<0.05) compared to ICP of 40 mmHg before injection.
Traumatic brain injury with intracranial hypertension
Fluid percussion injury in the saline treated group resulted in a sustained increase in intracranial pressure to 30.8±4.7 mmHg from the pre-injury level of 10.3±3.6 mmHg (n=5, P<0.01). In DRP treated group, the ICP raised only to 26.9±6.5 mmHg from the pre-injury level 10.5±4.1 mmHg (n=5, P<0.05), however the difference between saline and DRP treated groups was not statistically significant (P=0.18). Arterial pressure in both groups was unaltered.
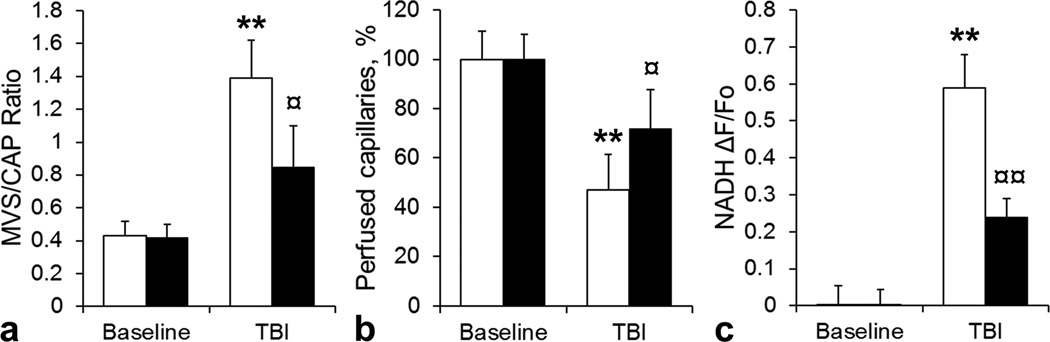
In a control group, the rise in ICP was associated with an increase in the MVS/CAP ratio from 0.43 ± 0.09 before injury to 1.39 ± 0.23 after injury, respectively (Figure 2 a, P<0.01). In DRP treated group, the MVS/CAP increased from 0.42 ± 0.08 before injury to 0.85 ± 0.25 after injury (P<0.05), and was significantly lower than in control group (Figure 2 b, P<0.05). Therefore, DRP attenuated pathological MVS flow and enhanced capillary flow.
Figure 2.
a) Bar graph showing that post-traumatic increase of microvascular (MVS) flow was less in DRP group than in saline-control as reflected by MVS/capillary (CAP) ratio. ■ – DRP treated group, □ – saline treated control group. b) Bar graph showing that after TBI fewer capillaries became collapsed in DRP treated group than in saline-control group. c) Bar graph showing that DRP treated animals had less cortical tissue hypoxia than saline –control animals as reflected by NADH autofluorescence. Data a presented as ΔF/Fo, where Fo is pre-TBI baseline. All data are presented as Mean ± SEM, n = 5 per group, ** P<0.01 compared to baseline ICP of 10 mmHg, ¤ P<0.05 compared to ICP of 40 mmHg.
Traumatic brain injury compromised capillary perfusion. In peri-contusion area of a saline treated brain the percent of perfused capillaries decreased to 47.3 ± 14.4% compared to a baseline (Figure 2 b, P<0.01). In DRP treated brain, the amount of capillaries with collapsed perfusion was reduced to only 72.1 ± 15.84% compared to a baseline (Figure 2 b, P<0.05). This was significantly less than in control saline treated group (P<0.05)
Post-traumatic microvascular flow impairment in saline treated group led to tissue hypoxia, reflected by NADH accumulation (ΔF/Fo[pre-injury] = 0.59 ± 0.09, Figure 1 c, P<0.01) compared to a baseline. Improved microvascular flow in DRP treated group mitigated tissue hypoxia; NADH autofluorescence increased only to 0.24 ± 0.05 (Figure 1 c, P<0.05 compared to a baseline and P<0.05 compared to saline treated group).
DISCUSSION
The intravascular mechanisms of DRP action are not completely understood. These long, molecules of DRPs, dissolved in blood plasma are thought to provide a “liquid scaffold” reducing pressure loss in small arteries and arterioles by organizing blood flow and suppressing flow separations and vortices at vascular branch points [5, 14–18]. In addition, DRP reduce “plasma skimming” at vessel bifurcations, which increases red blood cells (RBC) flow in capillaries [14, 16]. The increase in the precapillary pressure promoting an increase in the density of functioning capillaries and the elimination of capillary stasis caused by ischemia or other pathological conditions [14]. The net effect is improved microcirculation and increased red blood cells (RBC) traffic in microvessels [16–18].
We previously reported that in a healthy rat brain DRP increased near-wall blood flow velocity in arterioles and reduced plasma skimming at bifurcations leading to increased blood volume perfused through the vessel resulting in an increase in number of RBCs entering capillaries [19]. This led to enhanced capillary perfusion and increased tissue oxygenation. In a present study we showed that DRP reduced pathologically elevated non-nutritive microvascular shunt flow and partially restored perfusion in collapsed capillaries resulting in reduced tissue hypoxia in un-traumatized and traumatized rat brains at high ICP. The effect of decrease of ICP by DRP is not clear, but could be connected with decrease of pathological MVS flow and enhancement of capillary perfusion. In summary, our studies demonstrated that DRP could provide a novel hemorheologic approach for the treatment of brain ischemia caused by blood flow restriction in traumatized brain based on primary modulation of the flow properties of blood. The long-term effects of DRP treatment on neurological outcome after TBI are currently under investigation.
Acknowledgments
This work was supported by American Heart Association 14GRNT20380496 and National Institutes for Health 8P30GM103400. Pneumatic Percussion Device was custom made at UNM Physics and Astronomy Department Machine Shop by John DeMoss, Anthony Gravagne and John Behrendt.
Footnotes
CONFLICT OF INTEREST STATEMENT We declare that we have no conflict of interest.
REFERENCES
- 1.Pacella JJ, Kameneva MV, Csikari M, Lu E, Villanueva FS. A novel hydrodynamic approach to the treatment of coronary artery disease. European heart journal. 2006;27:2362–2369. doi: 10.1093/eurheartj/ehl165. [DOI] [PubMed] [Google Scholar]
- 2.Pacella JJ, Kameneva MV, Villanueva FS. Drag reducing polymers improve coronary flow reserve through modulation of capillary resistance. Biorheology. 2009;46:365–378. doi: 10.3233/BIR-2009-0548. [DOI] [PubMed] [Google Scholar]
- 3.Sakai T, Repko BM, Griffith BP, Waters JH, Kameneva MV. I.V. infusion of a drag-reducing polymer extracted from aloe vera prolonged survival time in a rat model of acute myocardial ischaemia. Br J Anaesth. 2007;98:23–28. doi: 10.1093/bja/ael307. [DOI] [PubMed] [Google Scholar]
- 4.Hu F, Zha D, Du R, Chen X, Zhou B, Xiu J, Bin J, Liu Y. Improvement of the microcirculation in the acute ischemic rat limb during intravenous infusion of drag-reducing polymers. Biorheology. 2011;48:149–159. doi: 10.3233/BIR-2011-0592. [DOI] [PubMed] [Google Scholar]
- 5.Kameneva MV, Wu ZJ, Uraysh A, Repko B, Litwak KN, Billiar TR, Fink MP, Simmons RL, Griffith BP, Borovetz HS. Blood soluble drag-reducing polymers prevent lethality from hemorrhagic shock in acute animal experiments. Biorheology. 2004;41:53–64. [PubMed] [Google Scholar]
- 6.McCloskey CA, Kameneva MV, Uryash A, Gallo DJ, Billiar TR. Tissue hypoxia activates JNK in the liver during hemorrhagic shock. Shock. 2004;22:380–386. doi: 10.1097/01.shk.0000140660.78744.bf. doi: 00024382-200410000-00014 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Gannushkina IV, Grigorian SS, Kameneva MV, Shakhnazarov AA. Possibility of restoring the cerebral blood flow in cerebral ischemia by injecting special polymers into the blood. Patol Fiziol Eksp Ter. 1982:58–59. [PubMed] [Google Scholar]
- 8.Bragin DE, Bush RC, Muller WS, Nemoto EM. High intracranial pressure effects on cerebral cortical microvascular flow in rats. J Neurotrauma. 2011;28:775–785. doi: 10.1089/neu.2010.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bragin DE, Statom G, Nemoto EM. Microvascular Shunt Flow after Traumatic Brain Injury with Intracranial Hypertension in Rats. Journal of Neurotrauma. 2012;29:A-22. [Google Scholar]
- 10.Bragin DE, Bush RC, Nemoto EM. Effect of cerebral perfusion pressure on cerebral cortical microvascular shunting at high intracranial pressure in rats. Stroke. 2013;44:177–181. doi: 10.1161/STROKEAHA.112.668293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- 13.Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. doi:nn1902 [pii] 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- 14.Kameneva MV. Microrheological effects of drag-reducing polymers in vitro and in vivo. International Journal of Engineering Science. 2012;59:168–183. doi: http://dx.doi.org/10.1016/j.ijengsci.2012.03.014. [Google Scholar]
- 15.Kameneva MV, Poliakova MS, Gvozdkova IA. The nature of the effect of polymers reducing hydrodynamic resistance on blood circulation. Doklady Akademii nauk SSSR. 1988;298:1253–1256. [PubMed] [Google Scholar]
- 16.Marhefka JN, Zhao R, Wu ZJ, Velankar SS, Antaki JF, Kameneva MV. Drag reducing polymers improve tissue perfusion via modification of the RBC traffic in microvessels. Biorheology. 2009;46:281–292. doi: 10.3233/BIR-2009-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacella JJ, Kameneva MV, Brands J, Lipowsky HH, Vink H, Lavery LL, Villanueva FS. Modulation of pre-capillary arteriolar pressure with drag-reducing polymers: a novel method for enhancing microvascular perfusion. Microcirculation. 2012;19:580–585. doi: 10.1111/j.1549-8719.2012.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao R, Marhefka JN, Antaki JF, Kameneva MV. Drag-reducing polymers diminish near-wall concentration of platelets in microchannel blood flow. Biorheology. 2010;47:193–203. doi: 10.3233/BIR-2010-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bragin DE, Thompson S, Statom G, Kameneva MV, Nemoto EM. Drag-reducing polymer improves microvascular flow and tissue oxygenation in the normal and traumatized rat brain. Journal of Neurotrauma; Abstracts from the 31st Annual National Neurotrauma Symposium; Nashville, Tennessee. 2013. p. C165. [Google Scholar]