Abstract
A series of novel carbamate and carbonate dimers of melampomagnolide B (MMB) have been synthesized by reaction of the MMB-triazole carbamate synthon 6 with various terminal diamino and dihydroxy alkanes. The resulting dimeric products 7b, 7c and 7f were selected and evaluated for anticancer activity against a panel of 60 human hematological and solid tumor cell lines. The most active compounds, 7b, 7c and 7f, exhibited GI50 values in the range 250-780 nM against the majority of leukemia cell lines in the tumor cell panel. Specifically, compounds 7b and 7f exhibited potent growth inhibition against non-small cell lung cancer cell lines NCI-H522 (GI50 = 160 nM) and HOP-92 (GI50 = 170 nM), respectively. Also, compound 7f also potently inhibited the growth of melanoma cell lines LOX IMVI, MALME-3M, and UACC-62 (GI50 values = 170, 190 and 190 nM, respectively); breast cancer cell line MDA-MB-468 (GI50 = 190 nM); colon cancer cell line HCT-116 (GI50 = 190 nM); and renal cancer cell line RXF 393 (GI50 = 160 nM). Compound 7f and the simple dicarbonate dimer of MMB (8) showed anticancer activity 300-fold and 1 × 106-fold, respectively, more cytotoxic than 7f and DMAPT at a concentration of 10 μM against rat 9L-SF gliosarcoma cells. The dimeric compounds 7a-7j & 8 were also screened for antileukemic activity against M9-ENL1 acute myelogenous leukemia (AML) cells and primary AML cell specimens. These compounds exhibited two to twelve-fold more potent antileukemic activity (EC50 = 0.5-2.9 μM) against the M9-ENL1 cell line when compared to parthenolide (EC50 = 6.0 μM). The dimeric analogues were also active against the primary AML cell specimens in the nanomolar to lower micromolar range and exhibited two to ten-fold more potent antileukemic activity (EC50 = 0.86-4.2 μM) when compared to parthenolide (EC50 = 2.5-16 μM). Thus, dimer 7f exhibited promising anticancer activity against a variety of both hematological and solid human tumor cell lines, while dimer 8 was superior to 7f against 9L-SF gliosarcoma, M9-EML1 and AML cells. These two novel dimeric analogs of MMB warrant further investigation with regard to their mechanism of action, especially as it relates to the activity of dimeric forms of active monomeric molecules and the implications this may have on structure-activity relationships and drug design.
INTRODUCTION
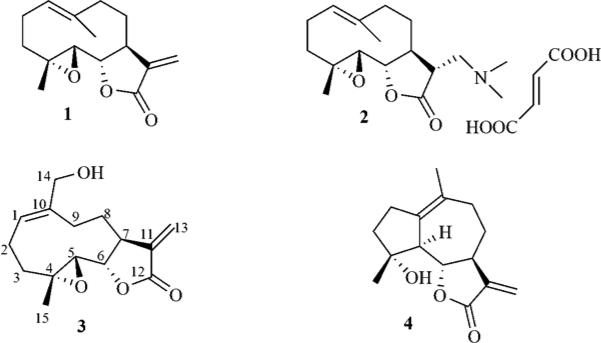
Parthenolide (PTL, 1, Fig 1), a sesquiterpene lactone isolated from the medicinal herb Feverfew (Tanacetum parthenium), has been identified as an anticancer agent that is effective against both hematological and solid tumors.1-5 PTL and its derivatives promote apoptosis by inhibiting the activity of the NF-κB transcription factor complex, thereby down-regulating anti-apoptotic genes under NF-κB control and also increasing reactive oxygen species (ROS) through inhibition of the glutathione pathway.6-11 We have previously demonstrated that PTL induces robust apoptosis of primary acute myeloid leukemia (AML) stem cells in culture.12-15 AML is a clonal malignancy of the hematopoietic system characterized by accumulation of immature cell populations in the bone marrow or peripheral blood,16 and is the most common type of leukemia in adults, but has the lowest survival rate of all leukemias.17-19
Figure 1.
The anticancer sesquiterpene lactones parthenolide (PTL) (1), dimethylaminoparthenolide (DMAPT) fumarate (2), melampomagnolide B (MMB) (3) and micheliolide (MCL) (4).
The anticancer activity of PTL is thought to be due to its pro-apoptotic action, which causes activation of p5320 and enhancement of ROS in tumor cells.21-23 Interestingly, this pro-apoptotic action of PTL is not observed in normal bone marrow stem cells,24 and we have recently demonstrated that PTL causes greater depletion of glutathione levels in CD34+ AML cells compared to normal CD34+ cells.24 Thus, PTL exhibits two selective and synergistic anticancer activities, i.e. NF-κB inhibition and induction of oxidative stress.25
Our laboratory has been successful in overcoming the poor water-solubility and bioavailability of PTL without loss of its antileukemic activity by derivatizing the molecule into several alkylamino analogues via Michael addition chemistry.3,26,27 Such analogues can then be converted into water-soluble organic salts with improved drug-like properties (e.g. dimethylaminoparthenolide, DMAPT, 2, Fig. 1).28 DMAPT fumarate (LC-1) is currently in Phase I clinical studies for evaluation as a treatment for AML and other related leukemias.28
Melampomagnolide B (MMB, 3, Fig. 1), a melampolide originally isolated from Magnolia grandiflora,29 has been identified as another antileukemic sesquiterpene with properties similar to PTL.30 MMB has been synthesized utilizing a modification of the method of Macias et al.31 via selenium oxide oxidation of the C10 methyl group of PTL, which also results in concomitant conversion of the geometry of the C1–C10 double bond from E to Z. Interestingly, from a drug design point of view, MMB is a more attractive molecule than PTL because of the presence of the primary hydroxyl group at C-14, which allows the opportunity for examining the biological activity of a wide variety of both functionally modified and conjugated analogues of MMB for improvements in potency, water solubility, bioavailability and tissue targeting. To elucidate the mechanism of action of MMB, we have prepared a C-14 biotin conjugate of the molecule, which has recently been utilized in protein pull-down experiments.30 We have also recently reported the synthesis of carbamate conjugates of MMB and their anticancer activities.32 These compounds were found to possess significantly improved anticancer activity compared to parent compounds PTL and MMB.
Another antileukemic hydroxyl-containing guaianolide sesquiterpene lactone, micheliolide (MCL, 4, Fig 1), isolated from Michelia compressa 33 and Michelia champaca 34 plant species has been reported.35 Chemical conjugates of MCL constructed via etherification or esterification at the hydroxyl group have recently been evaluated against a variety of AML cell lines, and have been shown to have comparable activity to MCL.36
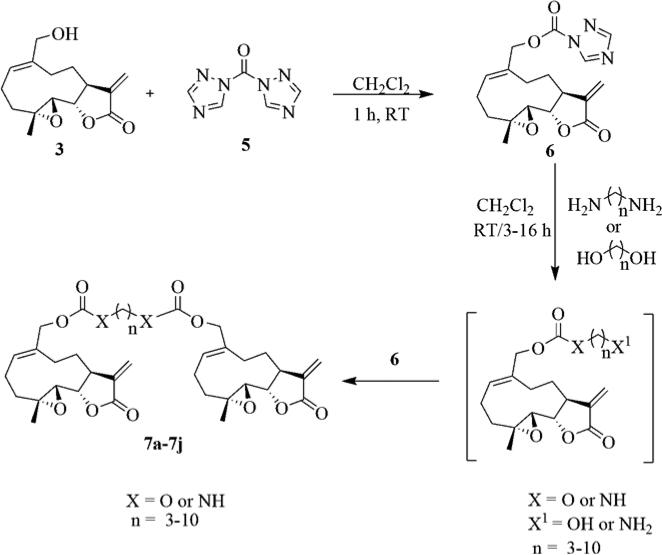
In the current study a series of novel carbamate and carbonate dimers of MMB have been synthesized by reaction of a C-14 MMB-triazole carbamate synthon with various terminal diamino- and dihydroxyalkanes (Scheme 1). The resulting dimeric products have been evaluated for anticancer activity against both hematological and solid human tumor cells, as well as against M9-ENL1 cells and primary AML cell specimens.
Scheme 1.
Synthesis of carbamate and carbonate dimers of MMB (7a-7j)
RESULTS AND DISCUSSION
Chemistry
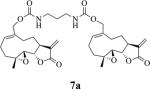
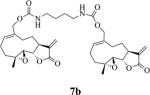
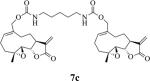
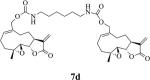
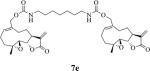
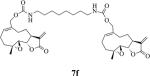
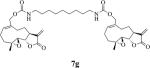
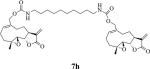
MMB (1) was synthesized from PTL via selenium dioxide oxidation using a modification of the method of Macias et al.31 The MMB-triazole carbamate synthon 6 was synthesized by reacting MMB with carbonylditriazole (5)37 in dichloromethane. Intermediate 6 was then reacted with a series of terminal diamino- and dihydroxyalkanes to obtain carbamate dimers 7a-7h and carbonate dimers 7i &7j, respectively (Scheme 1 and Table 1).
Table 1.
Structures, reaction times, yields, and melting points for the synthesis of carbamate and carbonate dimers of melampomagnolide B from intermediate 6
| Product | Yield (%)a | Time (hr) | Mp (°C) |
|---|---|---|---|
|
60 | 8 | 113-115 |
|
65 | 6 | 80-81 |
|
87 | 6 | 84-86 |
|
75 | 3 | 96-97 |
|
70 | 3 | 99-101 |
|
77 | 3 | 90-91 |
|
80 | 3 | 111-113 |
|
75 | 3 | 94-96 |
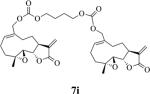
|
65 | 16 | 91-92 |
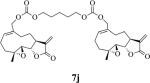
|
70 | 12 | 170-171 |
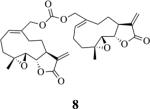
|
75 | 24 | 114-115 |
Isolated yields
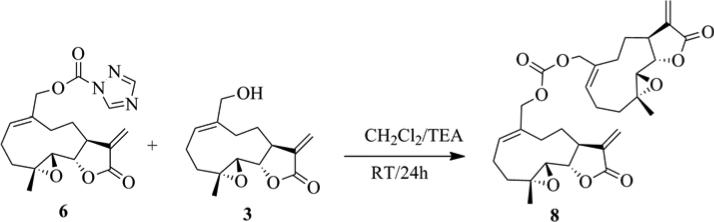
The alkane chain length in these molecules was varied from 3 to 10 methylene units. The chemical reactivity of the diaminoalkane in the above reactions was observed to be related to the number of methylene units in the molecule; i.e. the longer methylene chain diamines were more reactive than the shorter length diamines. In these reactions the diaminoalkanes were reacted with 6 in a mole ratio of 0.5:1 diamine:6. Increasing the mole ratio of the diaminoalkane to >0.5 led to the formation of C-13 Michael addition products as side products, which compromised purification of the dimeric product. The carbonate dimers of MMB were similarly synthesized by reaction of 6 with various terminal dihydroxyalkanes (Scheme 1, Table 1) in the presence of dichloromethane. The rate of formation of the carbonate dimers was found to be much slower compared to the rate of formation of the carbamate dimers. The dicarbonate MMB analog 8 was synthesized by direct reaction of 6 with MMB (Scheme 2).
Scheme 2.
Synthesis of MMB dicarbonate (8)
In vitro growth inhibition exhibited by MMB dimers against a panel of human cancer cell lines
The MMB dimers were submitted to NCI for anticancer screening. Compounds are first screened at a single concentration of 10−5 M against a panel of 60 human cancer cell lines derived from nine human cancer cell types, grouped into disease sub-panels that represent leukemia, lung, colon, central nervous system (CNS), melanoma, renal, ovary, breast, and prostate cancer cells. Compounds which show more than 60% growth inhibition in at least eight of the sixty cell lines in the panel are selected for a complete dose response study using five different concentrations (10−4 M, 10−5 M, 10−6 M, 10−7 M and 10−8 M) of drug. Based on the preliminary screening results, three carbamate dimers, 7b, 7c and 7f, were selected for five-dose testing for evaluation of their growth inhibitory properties (GI50); the results obtained are provided in Table 2.
Table 2.
Growth inhibition (GI50) and Lethal dose concentration (LC50) dataa for compounds 7c, 7d and 7f against a panel of 60 human cancer cell lines
| Panel/cell line | 7b | 7c | 7f | |||
|---|---|---|---|---|---|---|
| GI50b (μM) | LC50c (μM) | GI50b (μM) | LC50c (μM) | GI50b (μM) | LC50c (μM) | |
| Leukemia | ||||||
| CCRF-CEM | 0.27 | >100 | 0.28 | >100 | 0.25 | >100 |
| HL-60(TB) | 0.25 | >100 | 0.31 | >100 | 0.26 | >100 |
| K-562 | 0.66 | >100 | 0.56 | >100 | 0.34 | >100 |
| MOLT-4 | 1.47 | >100 | 1.48 | >100 | 0.33 | >100 |
| RPMI-8226 | 0.54 | >100 | 0.78 | >100 | 0.25 | >100 |
| SR | 0.54 | >100 | 0.38 | >100 | 0.31 | >100 |
| Non-Small Cell Lung Cancer | ||||||
| A549/ATCC | 8.02 | 58.7 | 14.9 | 71.7 | 1.70 | 7.26 |
| EKVX | 3.69 | 37.3 | ND | ND | 1.19 | 4.99 |
| HOP-62 | 3.84 | 46.1 | ND | ND | 1.53 | 7.95 |
| HOP-92 | 0.28 | 46.1 | 1.30 | 8.61 | 0.17 | 2.29 |
| NCI-H226 | 11.4 | 81.6 | 2.56 | 30.9 | 1.81 | 21.1 |
| NCI-H23 | 2.18 | 30.4 | 2.66 | 45.5 | 0.48 | 4.86 |
| NCI-H322M | 12.6 | 52.9 | 16.4 | >100 | 2.14 | 9.83 |
| NCI-H460 | 4.61 | 60.7 | 4.34 | 50.7 | 1.75 | 9.02 |
| NCI-H522 | 0.16 | 0.84 | 0.22 | 0.88 | 0.18 | 0.89 |
| Colon Cancer | ||||||
| COLO 205 | 0.46 | 5.79 | 0.52 | 7.97 | 0.30 | 3.99 |
| HCC-2998 | 2.35 | 9.73 | 6.71 | 53.9 | 1.20 | 5.28 |
| HCT-116 | 1.12 | 6.19 | 0.54 | 4.56 | 0.19 | 0.83 |
| HCT-15 | 5.61 | 56.3 | 6.18 | 49.8 | 0.34 | 4.18 |
| HT29 | 0.60 | >100 | 0.51 | 4.85 | 0.22 | 2.27 |
| KM12 | 3.18 | 43.3 | 6.53 | 54.2 | 1.29 | 7.49 |
| SW-620 | 0.58 | 5.56 | 0.43 | 5.14 | 0.23 | 3.37 |
| CNS Cancer | ||||||
| SF-268 | 3.18 | 51.0 | 3.08 | 42.3 | 0.99 | 7.24 |
| SF-295 | 13.0 | 57.7 | 11.1 | 65.2 | 1.95 | 16.2 |
| SF-539 | 1.55 | 8.84 | 1.92 | 12.7 | 0.23 | 2.26 |
| SNB-19 | ND | ND | 3.97 | 92.5 | ND | ND |
| SNB-75 | 2.19 | 66.3 | 2.20 | 33.8 | 1.25 | 9.84 |
| U251 | 2.94 | 33.3 | 3.51 | 51.5 | 0.57 | 5.13 |
| Melanoma | ||||||
| LOX IMVI | 0.51 | 5.15 | 0.46 | 5.82 | 0.17 | 0.76 |
| MALME-3M | 0.45 | 8.43 | 2.06 | 27.3 | 0.19 | 0.92 |
| M14 | 1.97 | 16.3 | 1.71 | 8.20 | 0.27 | 4.53 |
| MDA-MB-435 | 1.24 | 9.42 | 1.51 | 7.30 | 0.24 | 3.74 |
| SK-MEL-2 | 1.77 | 29.3 | 2.01 | 7.81 | 0.60 | 17.0 |
| SK-MEL-28 | 0.52 | 8.08 | 1.58 | 12.6 | 0.20 | 2.12 |
| SK-MEL-5 | 3.33 | 41.4 | 3.19 | 37.0 | 1.21 | 5.53 |
| UACC-257 | 1.68 | 22.9 | 2.07 | 42.4 | 0.84 | 8.51 |
| UACC-62 | 0.75 | 7.06 | 1.41 | 7.04 | 0.19 | 1.72 |
| Ovarian Cancer | ||||||
| OVCAR-3 | 0.69 | 4.76 | 0.76 | 5.07 | 0.33 | 3.32 |
| OVCAR-4 | 1.77 | 30.5 | 2.72 | 41.9 | 0.52 | 4.63 |
| OVCAR-5 | 1.75 | 7.79 | 1.67 | 7.47 | 0.26 | 4.04 |
| OVCAR-8 | 2.18 | 40.2 | 3.27 | 61.0 | 0.32 | 6.77 |
| NCI/ADR-RES | 37.7 | >100 | 70.0 | >100 | 3.91 | 82.0 |
| SK-OV-3 | 12.7 | 50.4 | 5.99 | 49.9 | 3.64 | 35.1 |
| Renal Cancer | ||||||
| 786-0 | 1.99 | 9.11 | 1.57 | 6.39 | 0.24 | 2.93 |
| A498 | 6.36 | 43.9 | 6.24 | 47.5 | 1.69 | 5.83 |
| ACHN | 3.44 | 39.8 | 3.05 | 35.4 | 0.25 | 2.85 |
| CAKI-1 | 10.8 | 49.1 | 16.1 | 68.1 | 0.65 | 4.79 |
| RXF 393 | 1.44 | 7.84 | 0.85 | 6.35 | 0.16 | 0.64 |
| SN12C | 2.75 | 39.5 | 2.49 | 45.5 | 0.26 | 5.49 |
| TK-10 | 1.61 | 11.3 | 1.82 | 11.8 | 0.28 | 2.95 |
| UO-31 | 7.61 | 47.7 | 8.90 | 52.3 | 0.88 | 4.90 |
| Prostate Cancer | ||||||
| PC-3 | 2.55 | 30.5 | 3.79 | 49.4 | 0.38 | 5.20 |
| DU-145 | 3.21 | 33.8 | 3.06 | 32.6 | 1.10 | 4.81 |
| Breast Cancer | ||||||
| MCF7 | 0.38 | ND | 0.39 | 6.20 | 0.22 | 4.30 |
| MDA-MB-231/ATCC | 1.53 | 6.83 | 1.47 | 6.40 | 0.20 | 1.24 |
| HS 578T | 2.54 | >100 | 2.91 | >100 | 1.17 | >100 |
| BT-549 | 1.60 | 8.23 | 1.00 | 4.96 | 0.25 | 3.57 |
| T-47D | 1.51 | >100 | 1.60 | >100 | 0.32 | >100 |
| MDA-MB-468 | 0.42 | 7.76 | 1.06 | 24.7 | 0.19 | 1.50 |
ND: Not determined
GI50 values <1 μM are bolded
GI50: 50% growth inhibition defined as concentration of drug resulting in a 50°% reduction in net protein increase compared with control cells.
LC50: 50% lethal dose concentration defined as concentration of compound that halves cell population through cell death.
Compounds 7b, 7c and 7f exhibited potent growth inhibition against both hematological and solid tumor cell lines in the human tumor cell panel. These three compounds exhibited GI50 values in the range 250-780 nM against the majority of cell lines in the leukemia sub-panel. In addition, compounds 7b and 7f exhibited potent growth inhibition against non-small cell lung cancer cell lines NCI-H522 (GI50 = 160 nM) and HOP-92 (GI50 = 170 nM), respectively. Compound 7f also potently inhibited the growth of a variety of solid tumor cell lines, including melanoma LOX IMVI, MALME-3M, and UACC-62 (GI50 values = 170, 190 and 190 nM, respectively); breast cancer MDA-MB-468 (GI50 = 190 nM); colon cancer HCT-116 (GI50 = 190 nM); and renal cancer RXF 393 (GI50 = 160 nM) cell lines. Since GI50 values for 7f were in the nanomolar range (160-999 nM) against most of the solid tumor cell lines in the 60 cell panel, and this analog exhibited GI50 values of <500 nM against more than 50% of the cancer cell lines in the panel, 7f was considered a lead compound.
Anticancer activity of 7f and 8 against rat gliosarcoma cells (9L-SF)
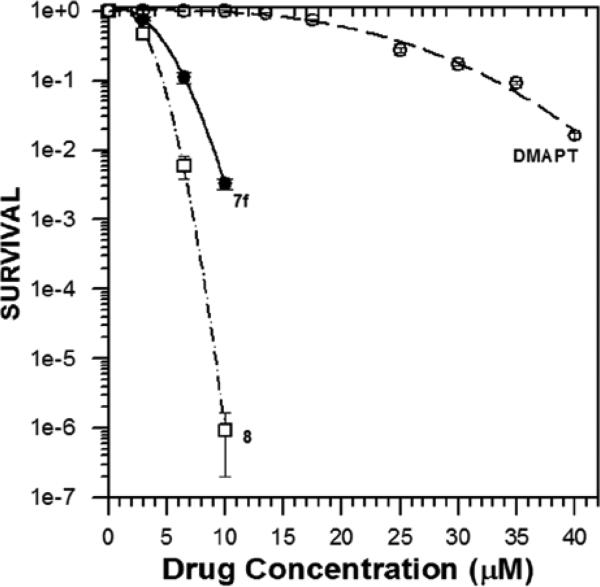
The screening of MMB dimers against the panel of 60 human cancer cell lines demonstrated that compound 7f was the most effective at inhibiting cell growth and inducing cell death. We evaluated the anticancer activity of 7f, and the simple dicarbonate ester of MMB (8) against rat gliosarcoma cells in culture. These two dimers were added to a 50% confluent culture of rat 9L-SF gliosarcoma cells (this cell line is relatively DMAPT-resistant, and was not included in the NCI 60 cancer cell panel; these cells have a high cellular content of glutathione, i.e. 53.2 ± 4.6 nmoles/mg cellular protein; Borrelli, unpublished data) and incubated for 48 h. The treatment of 9L-SF cells with compounds 7f and 8 was repeated identically, but the cells were then utilized to determine clonogenic survival utilizing the colony formation assay. Cells were also treated with the PTL analogue DMAPT to compare the cytotoxicity of compounds 7f and 8 to a drug that is currently in human Phase I clinical trials.28 Figure 2 shows that compound 8 is more cytotoxic than compound 7f and that both were markedly more cytotoxic than DMAPT. Specifically, compounds 7f and 8 were, respectively 303-fold and 1.08 × 106-fold more cytotoxic than DMAPT at a concentration of 10 μM.
Figure 2.
Dose-response curve for compounds 7f, 8 and PTL against rat gliosarcoma cells (9L-SF): Cell survival as a function of drug concentration for cells treated for 48 h with the indicated drug. Each datum point represents the mean plus standard error of the mean for three experiments. Drugs 7f and 8 were markedly more toxic than DMAPT, with drug 8 being one million-fold more toxic than DMAPT at a concentration of 10 μM.
Antileukemic activity of MMB dimers 7a-7j and 8 against M9-ENL1 and primary acute myelogenous leukemia (AML) cells
The novel carbamate and carbonate dimers 7a-7j and 8 were also evaluated for antileukemic activity against M9-ENL1 cells and primary AML cells, which were obtained with informed consent from human patients (Table 3). Five primary AML cell specimens (AML01-AML05) were utilized. Evaluations were performed after 24 hours of drug exposure using flow cytometric analysis by labeling with Annexin V and 7-aminoactinomycin D (7-AAD) to delineate apoptotic cell populations. For all experiments PTL was included as a reference control. Depending on the potency of the compound, dose-response curves were generated using over the concentration range 0.5-10 μM to determine the concentration resulting in 50% efficacy (EC50).
Table 3.
EC50 (μM) valuesa for PTL (1), and MMB derivatives 7a-7j and 8 against M9-ENL1 cells and primary AML cell specimens
| Comp | M9-ENL1 | AML01 | AML02 | AML03 | AML04 | AML01 |
|---|---|---|---|---|---|---|
| 1 | 6 | 16 | 2.5 | 5 | 8 | 7 |
| 7a | 1.0 | 2.6 | 2.3 | ND | ND | 3.7 |
| 7b | 1.5 | 2.2 | 2.0 | 7.3 | 4.2 | 2.0 |
| 7c | 0.6 | 1.6 | 0.9 | ND | 3.5 | 1.6 |
| 7d | 1.5 | 1.6 | 1.2 | 3.5 | 3.7 | 1.7 |
| 7e | 1.1 | 1.7 | 1.3 | 5.7 | 3.6 | 2.4 |
| 7f | 2.0 | 2.6 | 1.2 | 2.9 | 3.7 | 2.3 |
| 7g | 2.0 | 1.9 | 1.5 | 2.4 | 3.7 | 3.2 |
| 7h | 2.9 | 2.3 | 2.0 | 2.9 | 4.2 | 3.3 |
| 7i | 1.2 | 0.9 | ND | ND | 2.7 | 2.7 |
| 7j | 2.4 | ND | ND | 1.2 | ND | ND |
| 8 | 0.5 | 1.6 | 0.8 | ND | ND | ND |
EC50 values <1 μM are bolded, ND = not determined
Compounds 7a-7j & 8 exhibited potent antileukemic activity against M9-ENL1 cells with EC50 values in the range 0.57-2.9 μM (Figure 3A); this activity is two to twelve-fold more cytotoxic than that for parthenolide (EC50 = 6 μM). Compounds 7c and 8 were the most potent antileukemic agents examined, with EC50 values of 570 and 540 nM, respectively.
Figure 3.
Dose-response curves for PTL and MMB derivatives with AML cells. A) Dimers 7a-7j and 8 exhibited potent antileukemic activity with EC50 values in the range 0.57-2.9 μM against M9-ENL1 cells. B) Carbamate dimers were equally effective in the parthenolide-resistant primary sample AML01 relative to other primary cell samples, suggesting that these compounds may not be hindered by common mechanisms of drug resistance in relapsed AML patients.
Similarly, against primary AML specimens compounds 7a-7j & 8 exhibited two- to ten-fold more potent antileukemic activity (EC50 = 0.86-4.2 μM) when compared to PTL (EC50 = 2.5-16 μM). Carbamate dimer 7c exhibited an EC50 of 900 nM against the AML01 primary cell specimen, and the carbonate dimers 7i and 8 had EC50 values of 900 and 800 nM, respectively, against the AML02 primary cell specimen. Primary cell specimen AML01 was obtained from a patient after disease relapse, and was more resistant to PTL (EC50 = 16 μM) than any of the other four primary cell samples (EC50 [PTL] = 2.5-8.0 μM). Importantly, compounds 7a-7i all exhibited similar cytotoxicity against the AML01 cell specimen (EC50 = 0.9-2.6 μM) relative to the other four primary specimens, suggesting that these compounds may not be hindered by common mechanisms of drug resistance in relapsed AML patients (Figure 3B).
Compounds 7b-7h were also screened against normal hematopoietic progenitor cells isolated from human umbilical cord blood and exhibited significantly reduced cytotoxic effects on these cells (EC50 > 10 μM) when compared to primary AML cells.
SAR STUDIES
All the dimeric compounds (7a-7j & 8) were evaluated for antileukemic activity in dose-response experiments against the M9-ENL1 cell line. The five methylene chain length carbamate dimer (7c) exhibited an EC50 value of 600 nM, whereas the three and four methylene chain length carbamate dimers (7a &7b) exhibited slightly less cytotoxicity when compared to 7c. Increasing the methylene chain length from six to ten also did not improve anticancer activity when compared to 7c. Carbonate dimer 7i had similar activity to its carbamate linker equivalent, 7b. However, compound 7j, the corresponding carbonate dimer analogue of 7c, was about 10-fold less potent than 7c. Interestingly, the dicarbonate dimer 8 (EC50 = 500 nM) exhibited the most potent antileukemic activity in the M9-ENL1 cell assay.
CONCLUSIONS
Novel MMB carbamate and carbonate dimers have been synthesized and evaluated for their anticancer activity against a wide variety of hematological and solid human tumor cell lines. The most potent antileukemic compounds in the series from the cancer cell sub-panel of human leukemia cell lines were the carbamate dimers 7b, 7c and 7f, which exhibited GI50 values in the range 250-780 nM against the majority of leukemia cell lines in sub-panel. These compounds also exhibited GI50 values in the nanomolar to low micromolar range against a significant number of the human solid tumor cell lines in the 60 cell panel. GI50 values for 7f were in the nanomolar range (170-999 nM) against most of the solid tumor cell lines in the 60 cell panel; thus, 7f was considered a lead compound from this assay. It is interesting to note that compound 8, which was not evaluated in five dose studies against the 60 human cancer cell line panel, was found to be 3,564-fold more toxic to 9L-SF gliosarcoma cells than compound 7f (at 10 μM) in the colony formation assay.41
The carbamate and carbonate dimers 7a-7j and 8 also exhibited potent antileukemic activity against M9-ENL1 cells and a number of primary AML cell specimens, and were effective cytotoxic agents against a primary AML cell specimen that was resistant to PTL treatment.
Thus, dimer 7f exhibited promising anticancer activity against a variety of both hematological and solid human tumor cell lines, while dimer 8 was superior to 7f against 9L-SF gliosarcoma and M9-EML1 AML cells. These two novel dimeric analogs of MMB warrant further investigation with regard to their mechanism of action, especially as it relates to the activity of dimeric forms of active monomeric molecules and the implications this may have on structure-activity relationships and drug design.
EXPERIMENTAL
Melting points were recorded on a Kofler hot-stage apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Varian 400 MHz spectrometer equipped with a Linux workstation running on vNMRj software. HRMS data were obtained on an Agilent 6210 LCTOF instrument operated in multimode. All synthetic reactions were carried out at ambient temperature, and products were purified by flash column chromatography (silica gel; methanol/dichloromethane) to afford pure compounds in 65-87 % yield.
Synthetic procedure and analytical data for the triazolecarbamate conjugate of MMB (6). E-((1aR,7aS,10bS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydro-oxireno[2',3':9,10]cyclodeca[1, 2-b]furan-5-yl)methyl-1H-1,2,4-triazole-1-carboxylate (6)
To a stirred solution of MMB (50 mg, 0.18 mmol) in dichloromethane, was added carbonylditriazole (46.5 mg, 0.28 mmol) at ambient temperature, and the resulting reaction mixture was stirred for 1 h. After completion of the reaction, water was added and the mixture extracted with dichloromethane. The organic layer was washed with water (3×10 mL), dried over Na2SO4 and concentrated under reduced pressure to afford pure product 6 as a white solid (yield 93%).
1H NMR (CDCl3, 400 MHz): δ 8.83 (s, 1H), 8.06 (s, 1H), 6.26 (d, J = 3.6 Hz, 1H), 5.92 (t, J = 8.4 Hz, 1H), 5.55 (d, J = 3.2 Hz, 1H), 5.08 (d, J = 11.6 Hz, 1H), 4.90 (d, J = 12 Hz, 1H), 3.86 (t, J = 9.6 Hz, 1H), 2.91-2.83 (m, 2H), 2.56-2.17 (m, 6H), 1.84-1.72 (m, 1H), 1.55 (s, 3H), 1.14 (t, J = 12.4 Hz, 1H) ppm. 13C NMR (CDCl3, 100 MHz): δ 169.2, 154.0, 147.7, 145.8, 138.6, 134.4, 133.3, 120.6, 80.9, 71.5, 63.3, 59.9, 42.7, 36.5, 25.7, 24.3, 24.1, 18.1 ppm.
General synthetic procedure and analytical data for the dimeric carbamate and carbonate analogues of MMB (7a-7j)
To the triazole carbamate of MMB (6) (0.167 mmol) in dichloromethane (2 mL) was added the appropriate terminal diamino- or dihydroxyalkane (0.083 mmol) at ambient temperature, and the reaction mixture was stirred for 3-16 h. When the reaction was complete (monitored by silica gel TLC), water was added and the resulting aqueous mixture was extracted with dichloromethane. The organic layer was washed with water, followed by brine solution, dried over anhydrous Na2SO4, filtered, and the solvent removed to afford the crude reaction product. The crude product was purified by column chromatography (silica gel, 2% methanol in dichloromethane) to afford the appropriate carbamate or carbonate dimer as a white solid (yield: 65-87 %).
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)propane-1,3-diyldicarbamate (7a)
1H NMR (CDCl3, 400 MHz): δ 6.24 (d, J = 3.2 Hz, 1H), 5.68 (t, J = 7.2 Hz, 1H), 5.56 (d, J = 2.4 Hz, 1H), 5.12 (brs, 1H), 4.61(d, J = 12.8 Hz, 1H), 4.50 (d, J = 12.4 Hz, 1H), 3.85 (t, J = 9.2 Hz, 1H), 3.25-3.20 (m, 2H), 2.95 (t, J = 3.2 Hz, 1H), 2.88 (d, J = 9.2 Hz, 1H), 2.49-2.13 (m, 6H), 1.68-1.62 (m, 2H), 1.54 (s, 3H), 1.11 (t, J = 11.6 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.6, 156.7, 139.0, 135.5, 130.0, 120.2, 81.2, 67.3, 63.3, 60.1, 42.7, 37.5, 36.7, 30.4, 25.9, 24.8, 23.8, 18.1 ppm. HRMS (ESI) m/z calcd for C35H47N2O10 (M + H)+ 655.3225, found 655.3238.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)butane-1,4-diyldicarbamate (7b)
1H NMR (CDCl3, 400 MHz): δ 6.24 (d, J = 3.6 Hz, 1H), 5.69 (t, J = 8.4 Hz, 1H), 5.55 (d, J = 2.8 Hz, 1H), 4.85 (brs, 1H), 4.61 (d, J = 12.4 Hz, 1H), 4.50 (d, J = 12.8 Hz, 1H), 3.85 (t, J = 8.8 Hz, 1H), 3.19 (d, J = 4 Hz, 2H), 2.97 (t, J = 3.6 Hz, 1H), 2.88 (d, J = 9.6 Hz, 1H), 2.49-2.13 (m, 6H), 1.65 (t, J = 10 Hz, 1H), 1.54 (s, 3H), 1.50 (brs, 2H), 1.10 (t, J = 11.6 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.6, 156.3, 139.1, 135.5, 130.4, 120.2, 81.2, 67.3, 63.4, 60.1, 42.8, 40.7, 36.8, 27.3, 26.0, 24.9, 23.9, 18.1 ppm. HRMS (ESI) m/z calcd for C36H49N2O10 (M + H)+ 669.3382, found 669.3406.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)pentane-1,5-diyldicarbamate (7c)
1H NMR (CDCl3, 400 MHz): δ 6.24 (d, J = 2.8 Hz, 1H), 5.68 (t, J = 8.8 Hz, 1H), 5.55 (d, J = 2.4 Hz, 1H), 4.74 (brs, 1H), 4.61 (d, J = 12 Hz, 1H), 4.50 (d, J = 12.4 Hz, 1H), 3.85 (t, J = 9.2 Hz, 1H), 3.17 (d, J = 6.4 Hz, 2H), 2.96 (t, J = 3.5 Hz, 1H), 2.88 (d, J = 9.6 Hz, 1H), 2.46-2.13 (m, 6H), 1.66 (t, J = 9.6 Hz, 1H), 1.56-1.49 (m, 6H), 1.33-1.31 (m, 1H), 1.10 (t, J = 12.4 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.6, 156.3, 139.0, 135.6, 130.3, 120.2, 81.2, 67.2, 63.4, 60.1, 42.8, 40.9, 36.8, 29.7, 25.9, 24.8, 23.9, 23.8, 18.1 ppm. HRMS (ESI) m/z calcd for C37H51N2O10 (M + H)+ 683.3538, found 683.3538.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)hexane-1,6-diyldicarbamate (7d)
1H NMR (CDCl3, 400 MHz): δ 6.24 (d, J = 3.6 Hz, 1H), 5.70 (t, J = 8.4 Hz, 1H), 5.55 (d, J = 3.2 Hz, 1H), 4.73 (brs, 1H), 4.62 (d, J = 13.2 Hz, 1H), 4.49 (d, J = 12.8 Hz, 1H), 3.85 (t, J = 9.2 Hz, 1H), 3.18-3.13 (m, 2H), 2.96 (t, J = 3.6 Hz, 1H), 2.87 (d, J = 9.6 Hz, 1H), 2.46-2.13 (m, 6H), 1.66 (t, J = 10.8 Hz, 1H), 1.54 (s, 3H), 1.48 (t, J = 6.4 Hz, 2H), 1.33 (d, J = 8.0 Hz, 2H), 1.11 (t, J = 11.6 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.6, 156.3, 139.1, 135.7, 130.2, 120.2, 81.2, 67.2, 63.4, 60.1, 42.8, 41.0, 36.8, 30.0, 26.3, 26.0, 24.9, 23.9, 18.1 ppm. HRMS (ESI) m/z calcd for C38H53N2O10 (M + H)+ 697.3695, found 697.3718.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)heptane-1,7-diyldicarbamate (7e)
1H NMR (CDCl3, 400 MHz): δ 6.24 (s, 1H), 5.68 (t, J = 8 Hz, 1H), 5.54 (s, 1H), 4.73 (brs, 1H), 4.61 (d, J = 12.8 Hz, 1H), 4.50 (d, J = 12.4 Hz, 1H), 3.85 (t, J = 9.2 Hz, 1H), 3.18-3.13 (m, 2H), 2.96 (t, J = 8.8 Hz, 1H), 2.88 (d, J = 9.6 Hz, 1H), 2.49-2.14 (m, 6H), 1.65 (t, J = 11.6 Hz, 1H), 1.54 (s, 3H), 1.47 (t, J = 6.8 Hz, 2H), 1.33-1.30 (m, 3H), 1.10 (t, J = 12 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.2, 155.9, 138.8, 135.4, 129.9, 119.9, 80.9, 66.9, 63.1, 59.7, 42.4, 40.8, 36.5, 29.7, 28.6, 26.4, 25.7, 24.5, 23.6, 17.8 ppm. HRMS (ESI) m/z calcd for C39H55N2O10 (M + H)+ 711.3851, found 711.3882.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)octane-1,8-diyldicarbamate (7f)
1H NMR (CDCl3, 400 MHz): δ 6.24 (s, 1H), 5.68 (t, J = 7.2 Hz, 1H), 5.55 (s, 1H), 4.70 (brs, 1H), 4.62 (d, J = 12.4 Hz, 1H), 4.49 (d, J = 12.8 Hz, 1H), 3.85 (t, J = 9.2 Hz, 1H), 3.18-3.13 (m, 2H), 2.95 (t, J = 9.2 Hz, 1H), 2.88 (d, J = 9.2 Hz, 1H), 2.45-2.13 (m, 6H), 1.65 (t, J = 11.6 Hz, 1H), 1.54 (s, 3H), 1.47 (t, J = 6.4 Hz, 2H), 1.33-1.24 (m, 4H), 1.11(t, J = 11.6 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.5, 156.2, 139.1, 135.7, 130.2, 120.2, 81.2, 67.2, 63.4, 60.0, 42.8, 41.2, 36.8, 30.0, 29.2, 26.7, 26.0, 24.8, 23.9, 18.1 ppm. HRMS (ESI) m/z calcd for C40H57N2O10 (M + H)+ 725.4008, found 725.4025.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)nonane-1,9-diyldicarbamate (7g)
1H NMR (CDCl3, 400 MHz): δ 6.23 (s, 1H), 5.68 (t, J = 7.2 Hz, 1H), 5.54(s, 1H), 4.67 (brs, 1H), 4.62 (d, J = 12.8 Hz, 1H), 4.49 (d, J = 12.4 Hz, 1H), 3.84 (t, J = 9.2 Hz, 1H), 3.18-3.13 (m, 2H), 2.94 (t, J = 9.2 Hz, 1H), 2.87 (d, J = 9.6 Hz, 1H), 2.49-2.13 (m, 6H), 1.65 (t, J = 11.2 Hz, 1H), 1.55 (s, 3H), 1.47 (t, J = 12.4 Hz, 2H), 1.33-1.24 (m, 6 H), 1.11(t, J = 12 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.5, 156.2, 139.0, 135.7, 130.1, 120.2, 81.2, 67.2, 63.4, 60.0, 42.8, 41.2, 36.8, 30.0, 29.4, 29.2, 26.7, 26.0, 24.8, 23.9, 18.1 ppm. HRMS (ESI) m/z calcd for C41H59N2O10 (M + H)+ 739.4164, found 739.4172.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)decane-1,10-diyldicarbamate (7h)
1H NMR (CDCl3, 400 MHz): δ 6.24 (s, 1H), 5.67 (t, J = 2.8 Hz, 1H), 5. 54 (s, 1H), 4.69 (brs, 1H), 4.62 (d, J = 12.8 Hz, 1H), 4.49 (d, J = 12.4 Hz, 1H), 3.84 (t, J = 9.2 Hz, 1H), 3.18-3.13 (m, 2H), 2.95 (t, J = 9.1 Hz, 1H), 2.88 (d, J = 9.6 Hz, 1H), 2.49-2.13 (m, 6H), 1.65 (t, J = 10.8 Hz, 1H), 1.54 (s, 3H), 1.47 (t, J = 6.8 Hz, 2H), 1.34-1.24 (m, 6H), 1.11 (t, J = 12 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.5, 156.2, 139.0, 135.7, 130.2, 120.2, 81.2, 67.2, 63.4, 60.0, 42.7, 41.2, 36.8, 30.1, 29.5, 29.3, 26.8, 26.0, 24.8, 23.9, 18.1 ppm. HRMS (ESI) m/z calcd for C42H61N2O10 (M + H)+ 753.4321, found 753.4329.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)butane-1,4-diyldicarbonate (7i)
1H NMR (CDCl3, 400 MHz): δ 6.25 (d, J = 3.6 Hz, 1H), 5.74 (t, J = 7.6 Hz, 1H), 5.55 (t, J = 2.8 Hz, 1H), 4.67 (d, J = 12 Hz, 1H), 4.58 (d, J = 12.4 Hz, 1H), 4.16 (t, J = 6.8 Hz, 2H), 3.85 (t, J = 9.2 Hz, 1H), 2.90-2.81(m, 2H), 2.50-2.14 (m, 6H), 1.76-1.66 (m, 3H), 1.55 (s, 3H), 1.12 (t, J = 11.6 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.4, 155.1, 138.8, 134.5, 131.6, 120.4, 81.1, 70.4, 67.6, 63.4, 60.0, 42.7, 36.7, 25.8, 25.2, 24.5, 23.9, 18.1 ppm. HRMS (ESI) m/z calcd for C36H47O12 (M + H)+ 671.3062, found 671.3079.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl)pentane-1,5-diyldicarbonate (7j)
1H NMR (CDCl3), 400 MHz): δ 6.25 (d, J = 3.6 Hz, 1H), 5.74 (t, J = 8.4 Hz, 1H), 5.55 (d, J = 2.8 Hz, 1H), 4.67 (d, J = 12 Hz, 1H), 4.57 (d, J = 12 Hz, 1H), 4.14 (t, J = 6.8 Hz, 2H), 3.85 (t, J = 9.2 Hz, 1H), 2.90-2.81 (m, 2H), 2.50-2.14 (m, 6H), 1.73-1.66 (m, 3H ), 1.55 (s, 3H), 1.48-1.41 (m, 1H), 1.11 (t, J = 12 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.4, 155.1, 138.8, 134.5, 131.5, 120.4, 81.1, 70.3, 68.1, 63.4, 60.0, 42.7, 36.7, 28.4, 25.9, 24.5, 23.9, 22.2, 18.1 ppm. HRMS (ESI) m/z calcd for C37H49O12 (M + H)+ 685.3219, found 685.3235.
bis-E-(((1aR,7aS,10aS,10bS)-1a-Methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10] cyclodeca[1,2-b]furan-5-yl)methyl) carbonate (8)
MMB (3) (0.167 mmol) was added to the MMB-triazole carbamate (6) (0.167 mmol) in dichloromethane (2 mL) at ambient temperature and the reaction mixture was stirred for 24 h. When the reaction was complete (monitored by silica gel TLC), water was added and the resulting aqueous mixture was extracted with dichloromethane. The organic layer was washed with water, followed by brine solution, dried over anhydrous Na2SO4, filtered, and the solvent removed to afford the crude reaction product. The crude product was purified by column chromatography (silica gel, 2% methanol in dichloromethane) to afford the carbonate dimer of MMB as a white solid (yield: 75 %).
1H NMR (CDCl3, 400 MHz): δ 6.26 (d, J = 3.6 Hz, 1H), 5.75 (t, J = 8 Hz, 1H), 5.54 (d, J = 3.2 Hz, 1H), 4.67 (d, J = 12.4 Hz, 1H), 4.59 (d, J = 12.4 Hz, 1H), 3.85 (t, J = 9.20 Hz, 1H), 2.87-2.81 (m, 2H), 2.50-2.15 (m, 6H), 1.73-1.66 (m, 1H), 1.54 (s, 3H), 1.11 (t, J = 12 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ 169.4, 154.9, 138.8, 134.3, 131.9, 120.3, 81.0, 70.5, 63.3, 60.0, 42.7, 36.6, 25.7, 24.5, 23.9, 18.1 ppm. HRMS (ESI) m/z calcd for C31H39O9 (M + H)+ 555.2589, found 555.2611.
Methodology for the in vitro 60 human cancer cell screen
The methodology for the anticancer activity screening assay was carried out at the National Cancer Institute utilizing a reported literature procedure.38,39 Briefly, RPMI 1640 medium with 5% fetal bovine serum and 2 mM L-glutamine was used for growing the NCI-60 human tumor cells. Initially the tumor cells were inoculated into 96-well microtiter plates in 100 microliters of medium at plating densities starting from 5,000 to 40,000 cells per well. The range of cell numbers is dependent on the doubling time of the individual tumor cell lines. The plates were then incubated at 37 °C for 24 hours prior to addition of the submitted compounds. After 24 h, two microtiter plates of each tumor cell line were fixed in situ with TCA. The optical density reading at this point represents the cell population for each tumor cell line at the time of compound addition (ODtzero). The MMB dimers were dissolved in DMSO at 400-fold concentration to the desired final maximum test concentration, and stored at −80 °C. Then, an aliquot part of the frozen concentrate was thawed and diluted to 10-4 M concentration with medium containing 50 μg/ml gentamicin. A control sample with just DMSO was also prepared. Aliquots of 100 microliters of the different MMB dimer dilution controls were added to the appropriate microtiter wells containing 100 μl of medium, resulting in the required final drug concentrations of 10−5 M and 0 M (control). Once the MMB dimers were added, the microtiter plates were incubated for 48 hrs at 37 °C and 100 % relative humidity.
Cold TCA was used to terminate the assay for adherent cells. Cells were fixed by the addition of 50 μl of cold 50 % (w/v) TCA and further incubated for 1 hr at 4 °C. The supernatant was discarded, and the microtiter plates were splashed five times with water and air dried. Sulforhodamine B (SRB) solution (100 microliters) at 0.4 % (w/v) in 1 % CH3COOH was added to each well, and plates were incubated for another 10 minutes at room temperature. After SRB staining, free SRB was removed by washing five times with 1 % acetic acid. Bound SRB stain was successively dissolved with 10 mM trizma base, and the optical density was measured at a wavelength of 515 nm.
The growth inhibitory or cytotoxicity effects of the MMB dimers in the above cellular assay was measured by determining percentage cell growth (PG) inhibition. Optical density (OD) measurements of SRB-derived color just before exposing the cells to the test compound (ODtzero) and after 48hrs exposure to the test compound (ODtest) or the control vehicle (ODctrl) were recorded.
Colony Formation Assay with 9L-SF cells
San Francisco variant 9L, rat gliosarcoma cells (9L-SF)40 were cultured in DMEM/F12 medium containing 10% iron-supplemented calf serum and grown in a humidified 37.0 °C incubator with atmospheric air supplemented to 5% CO2. Cells were seeded into 35 mm or 60 mm culture dishes to achieve a cell density of 6.65 × 104 cells/cm2 (50% of confluency) 24 h later. The medium content in each dish was 0.313 mL/cm2 of growth surface area.
The dimeric sesquiterpene analogs 7f and 8 were dissolved in DMSO at a concentration of 12 mM and then diluted serially into complete culture medium, which kept the final DMSO concentration in the cultures below 0.1%. DMAPT was dissolved in sterile, physiological saline at a concentration of 2.93 mM and then diluted serially into culture medium. Drug medium was added to the cell cultures 24 h after the cells were seeded into the dishes and the cells were incubated with drug for 48 h. All drug treatment experiments were performed in triplicate.
The 50% confluent 9L-SF monolayers were each treated with DMAPT and dimers 7f and 8 in the concentration range of 0.3 μM to 20 μM for 48 h and testing in the colony formation assay for their ability to inhibit cell growth and induce altered cellular morphology indicative of drug-induced toxicity at the lower drug concentrations, e.g. preferably at 15 μM or lower.
Following treatment, drug-containing medium was removed from the culture dishes and cells were rinsed three times with fresh medium before the cells were trypsinized from the culture dish surface, diluted appropriately and their concentration determined using an electronic cell counter. A previously determined number of cells were seeded into 25 cm2 or 75 cm2 culture flasks to determine cell survival by means of colony formation (flasks seeded in triplicate). When the seeded cell number would result in a cell density less than 2,000 cells per cm2 the cells were placed into a flask that was seeded 24 h earlier with lethally irradiated feeder cells, at a density of 2,000 feeders per cm2.41 No feeders were used when the number of seeded experimental cells yielded a cell density in the flask greater than 2,000 cells/cm2. The seeded flasks were incubated long enough to permit formation of distinct colonies, with a longer incubation time required following drug treatments that resulted in lower survival levels. Cell survival was normalized to the plating efficiency (number of colonies divided by the number of seeded cells) exhibited by control, non-treated 9L-SF cells.
M9-ENL1 cell and primary AML cell assays
Compounds 7a-7j and 8 were screened for cytotoxicity against the M9-ENL1 cell line and against primary AML specimens as follows: M9-ENL1 cells were plated at a density of 106 cells/mL in alpha-MEM culture media (Invitrogen) supplemented with 5% human plasma, 20% FBS, and the cytokines SCF, IL-3, IL-7, and FLT3 (Peprotech). For primary AML specimens, cells were obtained from volunteer donors. Informed consent was obtained in accordance with the Declaration of Helsinki. Cells were isolated from the samples using Ficoll-Paque (GE Healthcare Bio-Sciences, Pittsburgh, PA) density gradient separation. In some cases, cells were cryopreserved in freezing medium of Iscove modified Dulbecco medium (IMDM), 40% fetal bovine serum (FBS), and 10% dimethylsulfoxide (DMSO) or in CryoStor CS-10 (VWR,West Chester, PA). Cells were cultured in serum-free medium (SFM), prepared with Iscove's MDM supplemented with 20% BIT 9500 serum substitute (StemCell Technologies), LDL, and beta-mercaptoethanol, for 1 hour before the addition of parthenolide or its derivatives. Drugs were diluted from a DMSO stock into PBS such that the final concentration of DMSO did not exceed 0.5%. Flow cytometric analysis was performed by co-staining with Annexin V and 7-AAD, according to manufacturer specifications, to identify the percentage of non-apoptotic cells which was defined as the population with negative staining for both labels. The percentage of non-apoptotic cells observed was normalized to that of the vehicle control and dose-response curves were analyzed using GraphPad Prism software to determine EC50 values. All analyses were conducted in triplicate.
Cytotoxicity screening with normal hematopoietic progenitor cells isolated from human umbilical cord blood
The cytotoxicity of compounds 7b-7f and 7h to normal hematopoietic progenitor cells was carried out utilizing human umbilical cord blood (CB) obtained from the National Disease Research Interchange (NDRI). Each compound was screened for cytotoxicity as follows: live mononuclear cells were isolated by subjecting samples to a Ficoll-Paque (GE Healthcare Life Sciences) gradient and plated at a density of 106 cells/mL in SFM. Drugs were diluted from a DMSO stock such that the final concentration of DMSO did not exceed 0.5%. Flow cytometric analysis was performed by co-staining with fluorescent antibodies recognizing the surface markers CD34 and CD45 as well as Annexin V, according to manufacturer specifications, to identify the percentage of non-apoptotic hematopoietic progenitor cells, defined as the population with negative staining for Annexin V but positive staining for CD34 and CD45. The percentage of non-apoptotic cells observed in the CD34+CD45dim compartment was normalized to that of the vehicle control and dose-response curves were analyzed using GraphPad Prism software to determine EC50 values where possible. All analyses were conducted in triplicate.
Comparison of NCI screening and colony formation assay
It should be noted that the NCI drug screening and the colony formation assay measure very different, cancer-relevant endpoints, respectively, metabolic and growth inhibition and clonogenic death. Because of this, both assays can facilitate selecting which compounds should be translated forward into testing in animal models for specific cancer types, e.g. glioblastoma, hepatocellular carcinoma, etc. The NCI cancer screening encompasses a larger number of compounds and identifies which have the greatest toxicity for each cancer type. Once investigators decide which cancer type to study in animal models the smaller number of compounds that are highly toxic to this cancer type, can be tested in vitro using the colony formation assay. Arguably, the compounds that are most effective in rendering the cells clonogenically dead will have the greatest likelihood of treating ectopic or orthotopic tumors successfully, and are the ones that should progress on to animal studies.
ACKNOWLEDGMENTS
We are grateful to the NIH/National Cancer Institute (grant # R01 CA158275), the UAMS Translational Research Institute (TRI), grant UL1TR000039 through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences, and the UAMS Department of Radiology for supporting this research, and to the Arkansas Research Alliance for an Arkansas Scholar award, and to the NCI drug-screening program.
ABBREVIATIONS
- PTL
parthenolide
- MMB
melampomagnolide B
- DMAPT
dimethylamino parthenolide
- TLC
thin layer chromatography
- EC50
concentration yielding 50% efficacy
- GI50
concentration yielding 50% growth inhibition
- LC50
concentration yielding 50% lethality
- SFM
serum-free media
- DMSO
dimethyl sulfoxide
- AML
acute myelogenous leukemia
- TEA
triethylamine
- MCL
micheliolide
- HRMS
high resolution mass spectrometry
Footnotes
ASSOCIATED CONTENT
Supporting Information
1H and 13C NMR spectra and HRMS (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Knight DW. Feverfew: chemistry and biological activity. Nat. Prod. Rep. 1995;12:271–276. doi: 10.1039/np9951200271. [DOI] [PubMed] [Google Scholar]
- 2.Skalska J, Brookes PS, Nadtochiy SM, Hilchey SP, Jordan CT, Guzman ML, Maggirwar SB, Briehl MM, Bernstein SH. Modulation of cell surface protein free thiols: a potential novel mechanism of action of the sesquiterpene lactone parthenolide. PLoS One. 2009;4:e8115. doi: 10.1371/journal.pone.0008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasim S, Crooks PA. Antileukemic activity of aminoparthenolide analogs. Bioorg. Med. Chem. Lett. 2008;18:3870–3873. doi: 10.1016/j.bmcl.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 4.Hewamana S, Alghazal S, Lin TT, Clement M, Jenkins C, Guzman ML, Jordan CT, Neelakantan S, Crooks PA, Burnett AK, Pratt G, Fegan C, Rowntree C, Brennan P, Pepper C. The NF-kappaB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood. 2008;111:4681–4689. doi: 10.1182/blood-2007-11-125278. [DOI] [PubMed] [Google Scholar]
- 5.Oka D, Nishimura K, Shiba M, Nakai Y, Arai Y, Nakayama M, Takayama H, Inoue H, Okuyama A, Nonomura N. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-kappaB. Int. J. Cancer. 2007;120:2576–2581. doi: 10.1002/ijc.22570. [DOI] [PubMed] [Google Scholar]
- 6.Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappa B. FEBS Lett. 1997;402:85–90. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]
- 7.Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J. Biol. Chem. 2002;277:38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- 8.Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J. Biol. Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin. Cancer. Res. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 10.Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol. Cancer. Ther. 2005;4:587–594. doi: 10.1158/1535-7163.MCT-04-0215. [DOI] [PubMed] [Google Scholar]
- 11.Nozaki S, Sledge Jr GW, Nakshatri H. Repression of GADD153/CHOP by NF-kappaB: a possible cellular defense against endoplasmic reticulum stress-induced cell death. Oncogene. 2001;20:2178–2185. doi: 10.1038/sj.onc.1204292. [DOI] [PubMed] [Google Scholar]
- 12.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman ML, Jordan CT. Feverfew: weeding out the root of leukaemia. Expert. Opin. Biol. Ther. 2005;5:1147–1152. doi: 10.1517/14712598.5.9.1147. [DOI] [PubMed] [Google Scholar]
- 14.Dai Y, Guzman ML, Chen S, Wang L, Yeung SK, Pei XY, Dent P, Jordan CT, Grant S. The NF (Nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br. J. Haematol. 2010;151:70–83. doi: 10.1111/j.1365-2141.2010.08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YR, Eom JI, Kim SJ, Jeung HK, Cheong JW, Kim JS, Min YH. Myeloperoxidase expression as a potential determinant of parthenolide-induced apoptosis in leukemia bulk and leukemia stem cells. J. Pharmacol. Exp. Ther. 2010;335:389–400. doi: 10.1124/jpet.110.169367. [DOI] [PubMed] [Google Scholar]
- 16.Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–2107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 17.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 18.Lowenberg B, Suciu S, Archimbaud E, Haak H, Stryckmans P, de Cataldo R, Dekker AW, Berneman ZN, Thyss A, van der Lelie J, Sonneveld P, Visani G, Fillet G, Hayat M, Hagemeijer A, Solbu G, Zittoun R. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy--the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J. Clin. Oncol. 1998;16:872–881. doi: 10.1200/JCO.1998.16.3.872. [DOI] [PubMed] [Google Scholar]
- 19.Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, Martinelli G, Conte R, Cocco L, McCubrey JA, Martelli AM. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21:427–438. doi: 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 21.Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathema VB, Koh YS, Thakuri BC, Sillanpaa M. Parthenolide, a sesquiterpene lactone, expresses multiple anticancer and anti-inflammatory activities. Inflammation. 2012;35:560–565. doi: 10.1007/s10753-011-9346-0. [DOI] [PubMed] [Google Scholar]
- 23.Steele AJ, Jones DT, Ganeshaguru K, Duke VM, Yogashangary BC, North JM, Lowdell MW, Kottaridis PD, Mehta AB, Prentice AG, Hoffbrand AV, Wickremasinghe RG. The sesquiterpene lactone parthenolide induces selective apoptosis of B-chronic lymphocytic leukemia cells in vitro. Leukemia. 2006;20:1073–1079. doi: 10.1038/sj.leu.2404230. [DOI] [PubMed] [Google Scholar]
- 24.Pei S, Minhajuddin M, Callahan KP, Balys M, Ashton JM, Neering SJ, Lagadinou ED, Corbett C, Ye H, Liesveld JL, O'Dwyer KM, Li Z, Shi L, Greninger P, Settleman J, Benes C, Hagen FK, Munger J, Crooks PA, Becker MW, Jordan CT. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J. Biol. Chem. 2013;288:33542–33558. doi: 10.1074/jbc.M113.511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004;23:7330–7344. doi: 10.1038/sj.onc.1207995. [DOI] [PubMed] [Google Scholar]
- 26.Janganati V, Penthala NR, Cragle CE, MacNicol AM, Crooks PA. Heterocyclic aminoparthenolide derivatives modulate G(2)-M cell cycle progression during Xenopus oocyte maturation. Bioorg. Med. Chem. Lett. 2014;24:1963–1967. doi: 10.1016/j.bmcl.2014.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woods JR, Mo H, Bieberich AA, Alavanja T, Colby DA. Fluorinated amino-derivatives of the sesquiterpene lactone, parthenolide, as (19)f NMR probes in deuterium-free environments. J. Med. Chem. 2011;54:7934–41. doi: 10.1021/jm201114t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, Vaughan A, Sweeney CJ, Matthews W, Carroll M, Liesveld JL, Crooks PA, Jordan CT. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.S. El-Feraly F. Melampolides from Magnolia grandiflora. Phytochemistry. 1984;23:2372–2374. [Google Scholar]
- 30.Nasim S, Pei S, Hagen FK, Jordan CT, Crooks PA. Melampomagnolide B: a new antileukemic sesquiterpene. Bioorg. Med. Chem. 2011;19:1515–1519. doi: 10.1016/j.bmc.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 31.Macías FA. Potential allelopathic activity of several sesquiterpene lactone models. Phytochemistry. 1992;31:1969–1977. [Google Scholar]
- 32.Janganati V, Penthala NR, Madadi NR, Chen Z, Crooks PA. Anticancer activity of carbamate derivatives of melampomagnolide B. Bioorg. Med. Chem. Lett. 2014;24:3499–502. doi: 10.1016/j.bmcl.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura M, Cordell GA, Farnsworth NR. Anticancer sesquiterpene lactones of Michelia compressa (magnoliaceae) Phytochemistry. 1978;17:957–961. [Google Scholar]
- 34.Jacobsson U, Kumar V, Saminathan S. Sesquiterpene lactones from Michelia champaca. Phytochemistry. 1995;39:839–843. [Google Scholar]
- 35.Sethi VK, Thappa RK, Dhar KL, Atal CK. Constituents of Michelia champaca and Lewis acid catalysed transformations of parthenolide into guaianolides. Planta. Med. 1984;50:364. doi: 10.1055/s-2007-969739. [DOI] [PubMed] [Google Scholar]
- 36.Ma WW, Shi QQ, Ding YH, Long J, Zhang Q, Chen Y. Synthesis of micheliolide derivatives and their activities against AML progenitor cells. Molecules. 2013;18:5980–5992. doi: 10.3390/molecules18055980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsunokawa Y, Iwasaki S, Okuda S. A new oxygenating method using 1-alkoxycarbonyl-1, 2, 4-triazoles and hydrogen peroxide relative reactivity of o-alkylperoxycarbonic acids. Chem. Pharm. Bull. 1983;31:4578–4581. [Google Scholar]
- 38.Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J. Natl. Cancer. Inst. 1990;82:1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 39. http://dtp.nci.nih.gov/branches/btb/ivclsp.html.
- 40.Henderson SD, Kimler BF, Morantz RA. Radiation therapy of 9L rat brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 1981;7:497–502. doi: 10.1016/0360-3016(81)90136-x. [DOI] [PubMed] [Google Scholar]
- 41.Borrelli MJ, Thompson LL, Dewey WC. Evidence that the feeder effect in mammalian cells is mediated by a diffusible substance. Int. J. Hyperthermia. 1989;5:99–103. doi: 10.3109/02656738909140436. [DOI] [PubMed] [Google Scholar]