Abstract
Microsporidia, a latent opportunistic infection associated with mild inflammation, is characterized by a strong CD8 T cell response, which has been shown to be CD4 T cell dependent. In this manuscript, we demonstrate for the first time that CD4 help is provided via IL-21 production, a common gamma chain cytokine closely related to IL-2. The peak of IL-21 expression, observed during the acute infection, is associated with an elevated IL-21+ CD4 T subset and these cells bear a phenotypic resemblance to T follicular helper cells. We observed that, during per-oral microsporidial infection, IL-21 was critical for the generation of an optimal effector CD8 T cell immunity. Sharply decreased effector KLRG1+ CD8 response was observed in IL-21R knockout mice and while these cells exhibited reduced functional properties they retained the ability to proliferate. The role of IL-21 in the generation of CD8 effectors was cell intrinsic, as stronger defects were observed in the IL-21 deficient compartment from the bone marrow chimeric mice (IL-21R KO/wild type). These findings are different from those reported for viral infections where IL-21 has been primarily associated with the generation and maintenance of CD8 memory response. To the best of our knowledge, this report demonstrates a critical role for IL-21 in the generation of a primary effector CD8 T cells response to an infectious disease model.
Introduction
IL-21 is a member of the common gamma chain family, which is composed of IL-2, IL-4, IL7, IL-9 and IL-15. This pleiotropic cytokine is produced by activated CD4 T cells, in particular follicular T helper (Tfh), Th17 and activated natural killer T (NKT) cells (1). In a murine model of Lymphocytic Choriomeningitis virus (LCMV) infection, IL-21 produced by virus specific CD4 T cells was essential for sustaining the CD8 T cell response after they lost their effector abilities (2-4). In patients with HIV-1 infection, reduced IL-21 production could be a contributing factor to the compromised cellular and humoral response (5). Collectively, these studies underline the importance of IL-21 in long-term CD8 T cell immunity needed for restricting the chronic infection, but the absence of IL-21 does not seem to affect the development of a potent effector immunity against viral infections.
Microsporidia causes a self-limiting disease in immunocompetent individuals but results in progressive infection in HIV infected and other immunocompromised individuals (6). Symptoms in these high-risk groups can be severe, ranging from chronic diarrhea to encephalitis and hepatitis (7). Evidences have recently emerged suggesting that microsporidiosis is a latent infection. In an animal model, corticosteroid induced immunosuppression led to the reactivation and dissemination of the parasite (8), confirming older data, which document the frequent relapse of infection in HIV patients who discontinued therapy after pathogen clearance (9, 10). In a mouse model of microsporidial infection using Encephalitozoon cuniculi, induction of a robust CD8 T cell immunity has been observed, and the CD8 T cells exhibit polyfunctional characteristics manifested by IFNγ production and cytolytic activity against infected targets (11). Studies from our laboratory have demonstrated that protective immunity against intraperitoneal (i.p.) infection is exclusively dependent on CD8 T cell immunity. However, during per-oral or natural route of infection, depletion of CD8 T cells alone does not result in the mortality of infected animals and both CD4 and CD8 T cells are needed for protection (12). Consequently, the generation of effector CD8 T cell immunity against microsporidia is an important component of protective immunity and factors needed for eliciting this response need to be studied. Interestingly, the data presented in this manuscript shows that amongst the γ chain cytokines only IL-21 was elevated during the acute infection and played an important intrinsic role in the development of polyfunctional CD8 effector response against the pathogen. In the absence of functional IL-21, CD8 T cell effectors were unable to acquire polyfunctional ability and were unable to expand optimally. The data demonstrates a critical role for IL-21 in the development of a robust effector CD8 T cells during microsporidial infection.
Material and Methods
Mice
C57BL/6J (wild type WT) and B6.SJL-Ptprca/BoyAiTac (CD45.1) 6-8 week old mice were purchased from Taconic. IL21R knockout (IL-21R KO) and CD4 knockout (CD4 KO) mice, purchased from The Jackson Laboratory (B6N.129-Il21rtm1Kopf/J) were bred in house. Mice were housed under specific pathogen free conditions at the Animal Research Facility at The George Washington University (Washington, DC). All animal experiments were approved by the George Washington School of Medicine and Health Sciences Institutional Animal Care and Use Committee.
For some experiments, WT/IL-21R KO mixed bone marrow chimera animals were generated. Bone marrow was extracted from IL-21R KO (CD45.2) and B6.SJL (CD45.1) mice as previously described (11) and 5×106 total cells (at a 1: 1 ratio for WT: IL-21R KO) were injected i.v. into lethally irradiated recipients (8Gy/20g of body weight). Recipient animals received sulfamethoxazole and trimethoprim (Hi-Tech Pharmacal) supplemented water for 5 weeks post-transfer. Experiments were performed 8 weeks post-reconstitution.
For BrdU experiments, mice were injected with bromodeoxyuridine (BrdU) (1mg/mouse) intraperitoneally starting at day 7 p.i. and every other day thereafter until the end of the experiment.
Parasites
A rabbit isolate of E. cuniculi (genotype III) was maintained as previously described (13). Animals were infected with 2×107 spores/mouse per orally.
Antigenic extract was prepared by mechanical disruption of freshly harvested E. cuniculi spores in presence of 0.5mm zirconia/silica beads (BioSpec Products Inc) using 6 pulses of 1 min each in a mini bead beater. Insoluble antigen and residual spores were removed by centrifugation and solution was sterile filtered before use.
Flow cytometry analysis
Splenocytes were prepared as previously described (11). Cell suspension was labeled for surface markers before fixation (IC fixation buffer, Invitrogen).
For in vitro function assay, overnight restimulation of splenocytes was performed in presence of 20μg/ml of E. cuniculi specific antigenic extract, followed by 4 h incubation in presence of protein transport inhibitor cocktail as well as fluorochrome conjugated anti-CD107a. Surface staining was followed by fixation with IC fixation buffer/IC permeabilization buffer (Invitrogen) according to manufacturer's instruction and intracellular staining for IFNγ and Ki67.
Intracellular staining for IL-21 was performed using recombinant IL-21R/Fc fusion protein (R&D Systems) followed by PE conjugated F(ab)2 goat anti-human Fcγ (Jackson ImmunoResearch Laboratories) according to previously published report (4).
For T-bet detection, splenocytes were stimulated overnight with antigenic extract as described above and labeled for surface antigens. T-bet staining was performed following fixation and permeabilization with Foxp3/transcription factor staining buffer set (affimetrix eBioscience).
Annexin V staining was performed according to manufacturer's instruction after 4 hours incubation at 37°C (Biolegend).
Antibodies used for xflow cytometry analysis: CD8β (eBioH35-17.2), CD4 (GK1.5), KLRG1 (2F1), CD44 (IM7), CD62L (MEL-14), CD11a (M17/4), CD127 (A7R34), IL-21R (eBio4A9), ICOS (7E.17G9), IFNγ (XMG1.2), Ki67 (SolA15), CD107a (eBio1D4B) and T-bet (eBio4B10) were purchased from affymetrix eBioscience. Antibodies for CXCR5 (L38D7) and PD-1 (RMP1-14) were obtained from Biolegend. Live/Dead Aqua staining (Invitrogen) was systematically performed prior to any flow cytometry analysis. Cell acquisition was performed with a FACS Calibur cytometer with a Cytek upgrade (Becton Dickinson, Cytek Development Inc). Data analysis was accomplished using FlowJo software (TreeStar). After data analysis, computation of different functions (IFNγ, Ki67 and CD107a) was performed using SPICE program (provided by M. Roederer, NIH, Bethesda, Maryland, USA). Fluorescence minus one controls (FMO) were performed for all experiments.
BrdU staining was performed according to manufacturer's instruction (BD Pharmingen).
Common γ chain cytokine mRNA detection
For real time PCR detection of IL-21, IL-7, IL-15 and Il-2, total RNA was extracted from spleen using Trizol (Invitrogen). After DNAse I treatment and RNA cleanup with RNeasy kit (Qiagen), cDNA was prepared using SuperScript II reverse Transcriptase (Invitrogen). The primers used were: IL-21, 5’-TCA TCA TTG ACC TCG TGG CCC-3’ and 5’-ATC GTA CTT CTT CAC TTG CAA TCC C-3’; IL-2, 5’-AGC AGC TGT TGA TGG ACC TA-3’ and 5’-CGC AGA GGT CCA AGT TCA T-3’; IL-15, 5’-ATC AAC ACG TCC TGA CT AND 5’-GTC TGA GAC GAG CTC TT-3’; IL-7, 5’-TCC TCC ACT GAT CCT TGT TC-3’ and 5’-CTT CAA CTT GCG AGC AGC AC-3’; b actin 5’-CTC TGG CTC CTA GCA CCA TGA AGA-3’ AND 5’-GTA AAA CGC AGC TCA GTA ACA GTC CG-3’. Real-Time PCR analysis was performed with a myIQ PCR (Biorad) using published primers (14). Amplification was performed for 40 cycles as follow: 950C for 45s, 61.4°C for 50 sec and 72°C for 45 s. Gene expression was normalized using β actin as endogenous control.
Statistical analysis
Results are presented as mean ± s.d. Comparison between two groups was performed by Student's t-test throughout the study.
Results
IL-21 expression during microsporidia infection
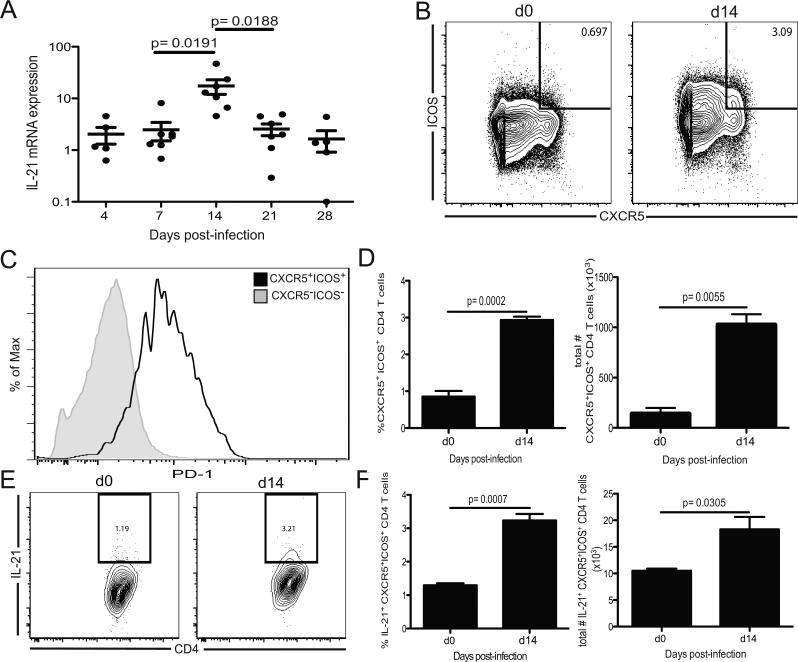
The central role of IL-21 in memory CD8 T cells maintenance in a number of chronic viral infections is well accepted (2-4, 15, 16). To assess if IL-21 was upregulated during microsporidia infection, the message levels of the cytokine was analyzed in the spleens of infected animals. As shown in figure 1 A, IL-21 mRNA transcript was transiently but significantly increased in the spleen of infected mice at day 14 p.i. (acute phase) and returned to background level as early as day 21 p.i. There was no significant increase in the message levels of other common γ chain cytokines like IL-2, IL-7 or IL-15 (supp figure 1), which have been reported to be important for the development and maintenance of CD8 T cell immunity (17). As Tfh are known to be major producers of IL-21 (18), their induction in response to infection was measured. Increase in both frequency and number of Tfh cells (based on CXCR5, ICOS and PD1 expression), was observed at day 14 p.i. (figure 1 B-D), which correlates with the peak of IL-21 message. Using intracellular staining, we observed that at day 14 p.i., CXCR5+ICOS+ CD4 T cells expressed significantly increased levels of IL-21 compared to the same subset from naïve animals (figure 1 E-F). On the other hand, IL-21 staining was not observed in CD8, γδ, NK or NKT cells after E. cuniculi infection (data not shown). These data suggest that per-oral E. cuniculi infection leads to up-regulated Tfh response, and these cells are an important source of IL-21 during the acute phase of the infection.
Figure 1. IL-21 production during E. cuniculi infection.
A) IL-21 mRNA expression was assessed by real time PCR in the spleen of C57B/6 mice at different time points post infection. Data represent two experiments with 3-4 mice per time point. B) Splenic CXCR5+ICOS+ CD4 T cells were analyzed at day 14 p.i. Plots are gated on CD4 T cells. C) Histogram illustrates PD-1 expression from CXCR5+ICOS+ and CXCR5−ICOS− CD4 T cells at day 14 p.i. D) Graph represents the total number of splenic CXCR5+ICOS+ CD4 T cells per mouse at the same time point. E) IL-21 expression by CXCR5+ICOS+ CD4 T cell subset was measured by intracellular staining. Plots are gated on CXCR5+ICOS+ CD4 T cells at day 0 and 14 p.i. F) Graph represents the frequency and total numbers of IL-21 producing CXCR5+ICOS+ CD4 T cells. Results (B-F) are representative of at least two independent experiments.
Defective IL-21 signaling affects the KLRG1+ CD8 T cell response
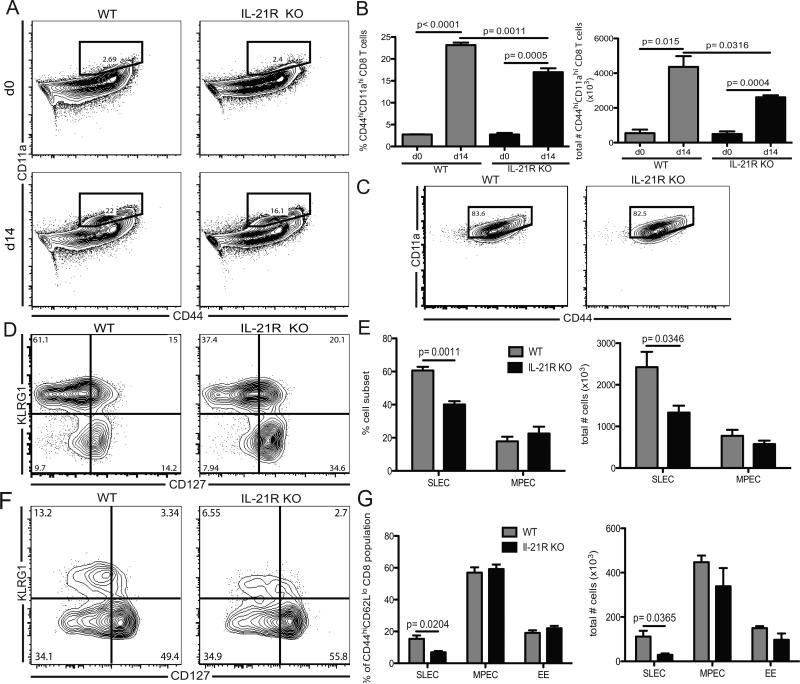
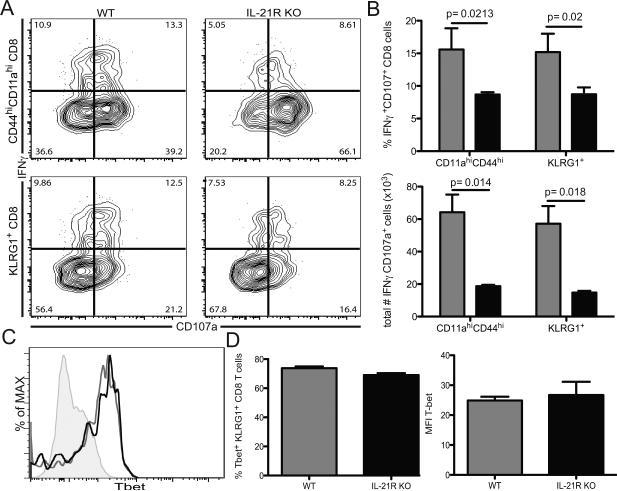
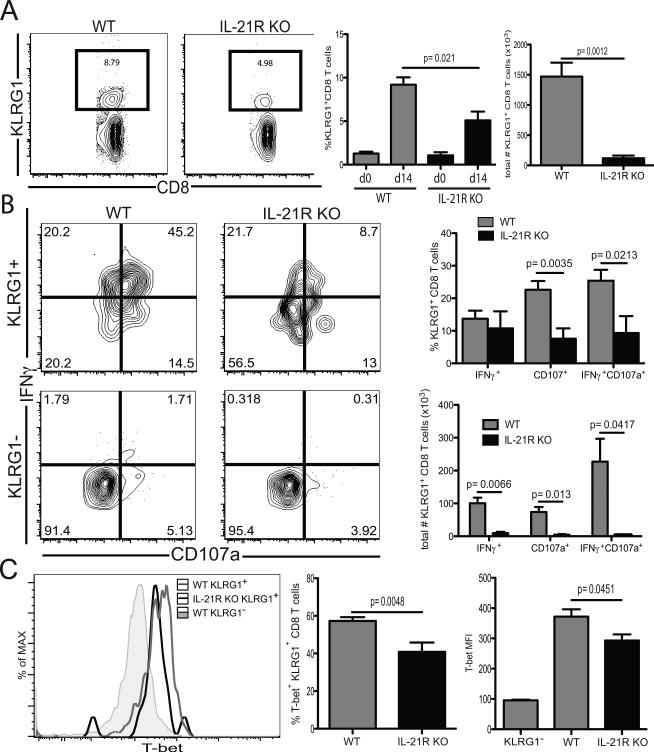
Next, we determined the role of IL-21 in the generation of antigen specific CD8 T cells, which represent a critical component of the protective immunity during microsporidial infection (19). Although the role of IL-21 in the survival of a primary CD8 T cell response has been suggested (20, 21), its contribution to the induction of a CD8 T cell response against a parasite has not been studied. For this purpose, IL-21R knockout (IL-21R KO) and wild type (WT) mice were infected and the CD8 T cell response evaluated at day 14 p.i. As tetramers for E. cuniculi CD8 T cells are not yet available, the antigen specific response was measured using a previously described surrogate markers strategy (22). First, to validate this approach, analysis of CD44 and CD11a expression by CD8 T cells from WT animals was assessed (supp figure 2). As expected, we observed an increase in CD44hiCD11ahi CD8 T cells at day 14 p.i. and this subset exhibited strong functionality characteristic (supp figure 2 A-B). In addition, IFNγ+CD107a+ CD8 T cells expressed elevated CD11a and CD44 markers (supp figure 2 C-D) confirming that CD44hiCD11ahi CD8 T cells represent the antigen specific CD8 T cell population. As shown in figure 2 A, a significant increase in the frequency of CD44hiCD11ahi CD8 T cells in response to the infection was detected in both WT and IL-21R KO animals at day 14 p.i. However, this response was significantly lower in the absence of IL-21 signaling (figure 2 A-B). Furthermore, the majority of the antigen-specific CD8 T cells expressed KLRG1 but not CD127, a hallmark of short-lived effector population (SLEC) (11), as expected during the acute phase of the infection (figure 2 B). However, yet again, lack of IL-21 signaling led to lower KLRG1 expression amongst antigen-specific CD8 population (figure 2 F). However, KLRG1+ CD8 T cells from both IL-21R KO and WT exhibit the same phenotypic attribute for antigen specificity (figure 2 C). Subsequently, to further dissect the defect in induction of CD8 T cell effectors in the absence of IL-21 signaling, we investigated early precursors (EE: CD44hi, CD62Llo, KLRG1lo, CD127lo and CD11ahi) at day 5 p.i. This cell type has been categorized as the first effector population and govern the differentiation of all other effector subsets (23). There was no significant difference in frequency or total numbers of EE between IL-21R KO and WT mice at day 5 p.i. (figure 2 G). Also, there was no change in frequency of memory precursor effector cells (MPEC) between WT and mutant animals. However, IL-21R KO SLEC were significantly reduced compare to WT as early as day 5 p.i. (figure 2 F). Therefore, our data suggest that the lack of IL-21 signaling exclusively compromises the development of antigen-specific KLRG1+ CD8 response against E. cuniculi and this defect cannot be attributed to reduced generation of early precursor population. Since previous studies from our laboratory reported that the majority of polyfunctional CD8 T cells belongs to the KLRG1+ CD8 T cell subset (11), this population was subsequently analyzed in both IL-21R KO mice and WT mice. At day 14 p.i., IFNγ+CD107a+ IL-21R KO effector KLRG1+ CD8 T cells was significantly reduced compared to WT controls (figure 3 A-B). These data strongly suggest that IL-21 play a role in the initiation of the effector CD8 immunity in response to microsporidia infection. A recent study revealed that IL-21 was involved in the induction of T-bet in CD8 T cells and this transcription factor has been reported to be critical for the development of the Th1 response against different pathogens (24). Interestingly, lack of IL-21 signaling did not hamper the Th1 differentiation of CTL response as KLRG1+ CD8 T cells from both WT and knock out animals exhibited similar expression of T-bet (figure 3 C-D). To establish the role of CD4 in the development of KLRG1+ CD8 T cell response, experiments were performed using CD4 KO mice. Similar to IL-21R KO, CD4 deficient mice exhibited reduced antigen specific CD8 response at day 14 p.i. (supplementary figure 3 A-B). Also, KLRG1 expression on the antigen-specific CD8 T cells from these mutant animals was significantly decreased (supplementary figure 3 C-E) and they displayed lower bi-functionality in response to the infection (supplementary figure 3 F-G). These results demonstrate an important role for CD4 T cells in the induction of the effector CD8 T cell response, which is most likely mediated by IL-21.
Figure 2. Impaired effector CD8 T cell response in absence of IL-21 signaling.
Splenic CD8 T cell response from WT and IL-21R KO mice was analyzed at day 14 post-E. cuniculi infection. A) Antigen specific CD8 T cell response from WT and IL-21R KO mice was analyzed at day 14 p.i. via surrogate markers staining (CD44hiCD11ahi). Plots are gated on total CD8 T cells. B) Graphs represent frequency and absolute number of CD44hiCD11ahi CD8 T cells for either WT or IL-21R KO animals. C) CD44 and CD11a expression by WT and IL-21R KO KLRG1+ CD8 T cells. Plots are gated on KLRG1+ CD8 T cells. D) KLRG1 and CD127 expression by antigen specific CD8 T cells was analyzed by flow cytometry at day 14 p.i. Plots are gated on CD44hiCD11ahi CD8 T cells. E) Graphs represent frequency and total number of SLEC and MPEC amongst CD44hiCD11ahi CD8 T cells. F) Splenic CD44hiCD62Llo CD8 T cells from WT and IL-21R KO mice were analyzed for KLRG1 and CD127 expression at day 5 p.i. Plots are gated on CD44hiCD62Llo CD8 T cells. G) Bar graphs depict frequency and total number of SLEC, MPEC and early precursor (EE: KLRG1-CD127−CD11ahi) amongst CD44hiCD62lo CD8 T cells from infected WT and IL-21R KO mice at day 14 p.i. Experiments were performed at least twice and data are representative of one experiment.
Figure 3. Lack of IL-21 signaling impairs KLRG1+ CD8 T cell response.
A) Functionality of KLRG1+ CD8 T cells from WT and IL-21R KO animals was measured by flow cytometry analysis of IFNγ and CD107a at day 14 pi. Plots are gated on KLRG1+ CD8 T cells. B) Graphs represent frequency and total number of IFNγ+CD107a+ cells amongst KLRG1+ CD8 T cells for each strain at day 14p.i. C) T-bet expression by KLRG1+ CD8 T cells was assessed by flow cytometry at day 14 p.i. D) Graphs represent frequency of T-bet+ cells and MFI for T-bet expression amongst KLRG1+ CD8 T cells. Experiments were performed at least twice and data are representative of one experiment.
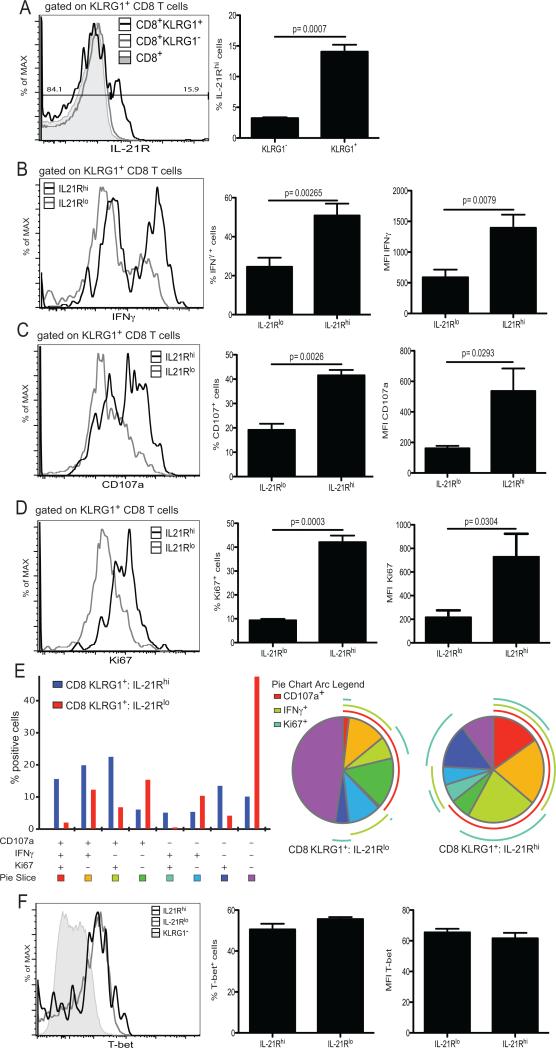
To establish that polyfunctional attributes of CD8 effectors are dependent on IL-21 signaling, we analyzed their IL-21R expression post-microsporidial infection. At day 14 p.i., a fraction of KLRG1+ CD8 T cells from WT animals exhibited significantly higher staining for IL-21R compared to KLRG1− or total CD8 T cell population (figure 4 A). Also, IL-21RhiKLRG1+ subset exhibited significantly higher frequency of IFNγ+ (figure 4 B), CD107a+ (figure 4 C) and Ki67+ (figure 4 D) population than IL-21RloKLRG1+ CD8 T cells. The increased functionality of IL-21RhiKLRG1+ CD8 population was also reflected by the significantly higher mean fluorescent intensity (MFI) for IFNγ, CD107a and Ki67. The up-regulation of IL-21R by KLRG1+ CD8 subset was correlated with their increased polyfunctionality capability (figure 4 E). Similar to the above findings (with KLRG1+ CD8 T cells from IL-21R KO mice), IL-21Rhi and IL-21Rlo KLRG1+ CD8 T cells displayed comparable frequencies of T-bet+ expressing cells and expression for T-bet as shown by MFI was also comparable (figure 4 F). Nevertheless, the data obtained from these studies demonstrate that IL-21 signaling is required for the optimal generation of polyfunctional KLRG1+ CD8 effector response, but the induction of Th1 effector response as demonstrated by T-bet expression is not affected.
Figure 4. IL-21R expression by KLRG1+ CD8 T cells is important for polyfunctionality.
A) IL-21R expression by splenic KLRG1+ CD8 T cells from WT animals was analyzed by flow cytometry at day 14 p.i. Histogram represents IL-21R expression by total CD8 T cells and KLRG1+ or KLRG1− CD8 T cell subsets from infected animals. Graph shows frequency of IL-21Rhi cells among KLRG1+ and KLRG1− CD8 T cells. B-D)- IFNγ (B), CD107a (C) and KI67 (D) expression by IL-21Rhi and IL-21RloKLRG1+ CD8 T cells was analyzed at day 14 p.i. Graphs show frequency or MFI of IFNγ+ (B), CD107a+ (C) and Ki67+ (D) cells amongst IL-21Rhi and IL-21Rlo KLRG1+ CD8 T cells. E SPICE analysis of CD107a, IFNγ and Ki67 markers for IL-21Rhi and IL-21RloKLRG1+ CD8 T cells from infected animals is represented as bar graph and pie chart. F) T-bet expression by IL-21Rhi and IL-21RloKLRG1+ CD8 T cells was analyzed at day 14 p.i. Graph shows T-bet+ cells amongst IL-21Rhi and IL-21RloKLRG1+ CD8 T cells. Experiments were performed at least twice and data are representative of one experiment.
IL-21 plays a cell intrinsic role in the induction of optimal effector CD8 T cells during microsporidial infection
To determine if IL-21 plays a cell intrinsic role in the induction of optimal KLRG1+ CD8 response, a well established mixed bone marrow chimeric approach was used (11). Bone marrow from WT (CD45.1) and IL-21R KO (CD45.2) donors were injected at 1:1 ratio into lethally irradiated WT animals (CD45.1). As shown in figure 5 A, in mixed bone marrow chimera, frequency of IL21R KO KLRG1+ CD8 T cells was significantly lower than WT population. Comparable to the data obtained from IL-21R KO mice, frequency of bifunctional KLRG1+ CD8 T cells in IL-21R KO compartment was significantly reduced in the chimeric animals (figure 5 B). Similar to previously described reports demonstrating the importance IL-21 in long-term CD8 T cell response (2-4), the reduced bifunctionality of CD8 T cells in the IL-21R KO compartment from the chimeric mice was observed at day 30 p.i. (supp figure 4). Interestingly, as compared to WT CD8 KLRG1+ cells, both frequency and MFI for T-bet was significantly decreased in the cells from the IL-21R KO compartment (figure 5 C). These findings are different from those observed in IL-21R KO mice (figure 3 C-D) and could be attributed to the existence of a compensatory mechanism in the knock out animals. IL-2 and IL-21 are closely related cytokines and the role of IL-2 in the generation of effector CD8 T cell response is well documented (25). To determine if normal T-bet levels in the CD8 T cells from the IL-21R KO mice can be attributed to increased IL-2 levels in these animals, the cytokine response was evaluated by real time PCR and intracellular staining. However, no upregulation of the cytokine message in the total splenocyte population or protein expression by CD4 or CD8 T cells was detected in IL-21R KO mice (data not shown). Overall, our data demonstrates a cell intrinsic role for IL-21 signaling in the generation of bifunctional KLRG1+ CD8 effector response during E. cuniculi infection.
Figure 5. IL-21 plays an intrinsic role in KLRG1+ CD8 T cell response.
WT/IL-21R KO mixed bone marrow chimera were generated and CD8 T cell response analyzed at day 14 p.i. A) KLRG1 expression by WT and IL-21R KO CD8 T cells from mixed bone marrow chimera is analyzed by flow cytometry. Plots are gated on total CD8 T cells from WT or IL-21R KO compartment from infected chimeric mice. Bar graphs represent frequency and total number of KLRG1+ cells amongst either WT or IL-21R KO total CD8 T cells. B) Response of KLRG1+ (top row) and KLRG1− (bottom row) CD8 T cells from WT or IL-21R KO compartment from mixed bone marrow chimera is measured by flow cytometry analysis of IFNγ and CD107a staining. Graphs show frequency and total number of IFNγ+, IFNγ+CD107a+ or CD107+ among KLRG1+ CD8 T cells for either WT or IL-21R KO compartments. C) Histogram displays T bet expression by WT and IL-21R KO KLRG1+ CD8 T cells from mixed bone marrow chimera. Graphs represent frequency and MFI for T-bet+ cells for KLRG1+ CD8 T cells from WT and IL-21R KO compartment. Experiments were performed at least twice and data are representative of one experiment.
IL-21 signaling is important for the expansion of KLRG1+ CD8 T cells
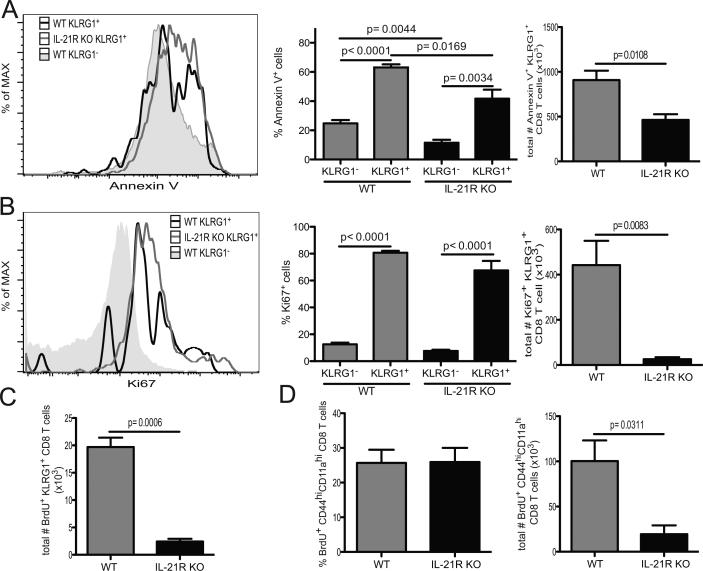
Previous studies have reported the role of IL-21 in promoting T cell survival in the absence of antigen (26). Furthermore, in vivo studies with an acute infection model demonstrated that IL-21 signaling was essential for the survival of activated CD8 T cells (21). Therefore, assays were performed to better assess the role of IL-21 signaling in the elicitation of an optimal KLRG1+ CD8 response during E. cuniculi infection using the mixed bone marrow approach mentioned above. As expected, terminally differentiated WT KLRG1+ CD8 T cells exhibited high levels of annexin V (figure 5A). However, surprisingly, a smaller frequency of early apoptotic (live cells expressing annexin V) KLRG1+ CD8 T cells was detected in IL-21R KO compartment (figure 6 A). Since IL-21 deficient signaling did not affect survival of KLRG1+ CD8 T cells, the proliferative capability of these cells was analyzed via expression of Ki67, a nuclear protein expressed in all active phases of cell division. Previous reports have shown that IL-21 can synergize with either IL-7 or IL-15 to induce proliferation of CD8 T cells in vitro (27, 28). As shown in figure 6 B, using mixed bone marrow chimera model, frequencies of Ki67+KLRG1+ CD8 T cell were similarly increased for both WT and IL21R KO at day 14 p.i. These results were confirmed with in vivo BrdU assay (figure 6 C) and no difference in frequency of BrdU+ antigen specific CD8 T cells between WT and IL-21R KO mice was observed. However, the total number of BrdU+ CD44hiCD11ahi CD8 T cells from mutant mice was significantly lower than WT (figure 6 D). Thus, our findings establish that in a microsporidial model, lack of IL-21 signaling led to increased survival of KLRG1+ CD8 T cells and the expansion of the population from the mutant animals was similar to WT animals as demonstrated by Ki67 expression and BrdU incorporation. The fact that lower numbers of KLRG1+ CD8 T cells in response to infection was noted as early as day 5 p.i. strongly suggests a role for IL-21 in the generation of KLRG1+ CD8 effector response during microsporidial infection.
Figure 6. Lack of IL-21 signaling affects expansion of KLRG1+ CD8 T cells.
A) Early apoptosis of KLRG1+ and KLRG1− CD8 T cells was assessed by flow cytometry analysis of annexin V after 4 hours incubation. Histogram is gated on live WT or IL-21R KO KLRG1+ CD8 T cells from mixed bone marrow chimera at day 14 p.i. Annexin V expression for WT CD8 T cells from uninfected mice is showed as control. Bar graphs depict frequency and total number of annexin V+ amongst live KLRG1+ CD8 T cells for both WT and IL-21R compartments of infected chimeric mice. B) Proliferative response of different CD8 T cell subsets was analyzed via Ki67 expression. Histogram is gated on KLRG1+ or KLRG1− CD8 T cells from WT and IL-21R KO compartment of mixed bone marrow chimera. Graphs represent frequency and total number of Ki67+ cells amongst KLRG1+ or KLRG1− from different subsets. C) Bar graph shows total number of WT and IL-21R KO BrdU+ KLRG1+ CD8 T cells. D) Bar graphs represent frequency and total number of WT and IL-21R KO BrdU+ CD44hiCD11ahi CD8 T cells. Experiments were performed at least twice and data are representative of one experiment.
Discussion
The role of IL-21 in the maintenance of memory CD8 T cells during chronic infectious disease models (especially in viral infections) is well established. In this regard, several studies conducted with LCMV infection have demonstrated that while mice with defective IL-21 signaling are able to develop an initial CD8 T cell immunity, the memory CD8 T cell response was subsequently impaired, leading to their inability to control the chronic infection (2-4). Using a microsporidial infection model, the evidences presented in this manuscript demonstrate an important role for IL-21 in the development of a robust antigen-specific CD8 T cell effector immunity against E. cuniculi. The induction of IL-21 was observed during the acute phase of infection and led to the generation of optimal levels of CD8 T cell effectors. To the best of our knowledge, this novel report establishes the critical function of IL-21 in the development of CD8 effectors. Using a mixed bone marrow chimeric approach, we established a cell intrinsic role for IL-21 in the development of KLRG1+ CD8 effector response during microsporidial infection. While a robust functional effector CD8 T cell immunity in the cells from the WT compartment of the mixed bone marrow chimera was noted, the response was severely impaired in the knock out effector CD8 T cells. Interestingly, in contrast to CD8 effectors from knockout mice, the IL-21R KO cells from the chimeric mice exhibited lower expression of T-bet (an important transcription factor for effector CD8 T cells) as compared to their WT counterparts. This could be explained by compensatory mechanisms in the knock out mice, which could, to a certain extent, rescue the CD8 effector response in the absence of functional IL-21. This is also emphasized by the fact that although, CD8 T cell effector response in IL-21R KO mice was subdued compared to WT animals, the differences in the chimeric animals (between WT and IL-21R KO compartment) were more pronounced. The interpretation that can be drawn from these observations is as follows: Although the compensatory mechanisms taking place in the knock out mice are able to support the CD8 effector immunity and the cells exhibit normal levels of T-bet, IL-21 signaling is still cirtical for the development of an optimal effector CD8 response.
Infections, like LCMV or Listeria monocytogenes, induce strong inflammatory cytokines and the primary CD8 T cells response is relatively independent of CD4 T cells (29). However, CD8 effector response against non-inflammatory antigens is reliant on CD4 T cell help and IL-2, a cytokine primarily produced by CD4 T cells (30), is often important for the generation of this help-dependent primary CD8 T cell response (31). Early CD25 upregulation, which was critically dependent on third cytokine signaling (32), combined with increased IL-2 expression is known to promote effector CD8 T cell differentiation (33, 34). Furthermore, in an infectious model using mixed bone marrow chimeric mice, it was demonstrated that IL-2Rα deficiency impaired the differentiation of effector CD8 T cells and these cells exhibited reduced T-bet expression and CTL response (34). Also, in a low inflammatory situation, induction of the transcription factor B lymphocyte-induced maturation protein 1 (Blimp-1) (required for proper acquisition of effector functions by SLEC (35)) was contingent on IL-2 expression (36). In the case of microsporidia infection, which does not induce a strong inflammation, CD4 T cells appear to play a very important role in the control of the pathogen. Depletion of both CD4 and CD8 T cells led to host mortality, while the mice treated with anti-CD4 or anti-CD8 alone survived the challenge (12). Interestingly, the role of IL-2 in priming the effector CD8 T cell response appears to be minimal, as this cytokine was not detected either by real time PCR or intracellular staining of T cells from infected animals (data not shown). On the other hand, in the case of microsporidial infection, we observed an increase in CXCR5+ICOS+ CD4 T in the WT mice at day 14 p.i. and this population produced IL-21 after infetion. Based on these findings, we can interpret that one of the important roles of CD4 T cells during microsporidial infection is their ability to secrete IL-21, which is needed for the generation of a robust effector CD8 T cell response. However, the ability of IL-21 to generate this response cannot be generalized given that this cytokine limits the terminal differentiation of effector CD8 T cells in a cancer model, favoring the development of immature effector cells that are more effective in suppressing tumor growth (37). Conversely, several studies have demonstrated the role of IL-21 in the survival, expansion and/or function of CD8 T cells in general, alone or in combination with IL-2. Recent observations have shown that IL-21 was able to rescue CD8 T cells cultured under IL-2 deprivation conditions. In these studies, blockade of both IL-2 and IL-21 signaling pathway led to reduced CD8 T cell survival (38). Similarly, in another study, IL-21 alone promoted expansion and cytolytic development of OT1 T cells (39). Comparable to these in vitro studies, our data revealed that mice with defective IL-21 signaling displayed lower frequency of antigen specific and KLRG1+ CD8 T cells. Studies conducted in vitro have demonstrated that IL-21 can cause an upregulation of the transcription factor T-bet in CD8 T cells, which promotes their functionality (24). In another study, addition of IL-21 in vitro rapidly up-regulated IFNγ, T-bet, IL-2Rα and IL-12Rβ2 mRNA synthesis, all genes associated with a Th1 response (40). The data presented in this manuscript demonstrates that although effector CD8 T cells from IL-21R KO mice exhibited normal levels of T-bet, significantly lower expression was observed in the IL-21R KO compartment from the chimeric animals. As mentioned above, the discrepancy in these observations can be attributed to the existence of compensatory mechanism in the knock out mice. As it has been recently reported that IL-21 can inhibit IL-2 production by conventional T cells (41) we suspected that in the absence of functional IL-21, upregulation of IL-2 may rescue the CD8 T cell effector response during microsporidial infection. However, this was not the case, as increase in message for IL-2 was not observed and CD4 or CD8 T cells from infected animals failed to secrete the protein as determined by flow cytometry and therefore, IL-2 was not involved in the rescue of KLRG1+ CD8 effectors generated in the absence of IL-21 signaling.
Although IL-21R KO KLRG1+ CD8 T cells proliferate as well as their WT counterpart, they exhibited higher survival rate. This is somewhat unexpected as KLRG1+ CD8 T cells are terminally differentiated and subsequently die after the peak of the response (42). As far as the role of IL-21 on T cell apoptosis is concerned, the situation is unclear and most likely dependent on the nature of stimulus. In one of the studies using a non-infectious model, it was reported that the cytokine can promote T lymphocyte survival by activating the phosphatidylinositol-3 kinase cascade and inducing Bcl-2 expression (26). On the other hand, in an SIV model of infection, IL-21 was reported to drive CD8 T cell to apoptosis by down-regulating Bcl-2 (43). Similar to the observations made with SIV, in our model, a lower frequency of annexin V+ KLRG1+ CD8 T cells was observed in the absence of IL-21 signaling, which could be attributed to pro-apoptotic properties of the cytokine in this model. Also, as fewer effector CD8 T cells are generated in the knockout compartment, the decreased apoptosis may be due to a delay in the contraction phase. The potential role of IL-21 in the contraction of CD8 T cell effector response is an interesting area that needs to be investigated.
Overall, our findings demonstrate an important and previously unidentified role for IL-21 in the development of a polyfunctional effector CD8 T cell response in an infectious disease model. These studies extend the role of IL-21 to the generation of CD8 effectors T cells in addition to its already established importance in the development and maintenance of the memory response (2-4). The data obtained with chimeric animals clearly underline the critical importance of IL-21 in the generation of CD8 T cell response and strongly suggest that, like type I IFN and IL-12, it could be considered a third signal important for the development of effector CD8 T cells against intracellular infection. The findings presented in this manuscript raise important questions related to the alternative mechanisms responsible for CD8 T cell effector development in the IL-21R KO mice. As our studies rule out IL-2 in the elicitation of CD8 effectors in these animals, it is possible that other CD4 subsets like Th1 or Th17 may be able to compensate in the absence of IL-21. Furthermore, how IL-21 plays a role in the generation of KLRG1+ population is an interesting question, which will be addressed in future studies conducted in our laboratory. Ongoing studies in our laboratory will provide answers to these important questions. Our observations have significant implications for HIV infected population where IL-21 producing Tfh population are major targets (44) and as a result depleted cytokine levels could severely compromise the CD8 T cell effectors against this important opportunistic infection.
Supplementary Material
Acknowledgments
This work was supported by NIH grant (AI-096978 and AI-102711) to IAK.
References
- 1.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13:379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 2.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 4.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iannello A, Tremblay C, Routy JP, Boulassel MR, Toma E, Ahmad A. Decreased levels of circulating IL-21 in HIV-infected AIDS patients: correlation with CD4+ T-cell counts. Viral Immunol. 2008;21:385–388. doi: 10.1089/vim.2008.0025. [DOI] [PubMed] [Google Scholar]
- 6.Didier ES. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 2005;94:61–76. doi: 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006;19:485–492. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotkova M, Sak B, Kvetonova D, Kvac M. Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole. PLoS One. 2013;8:e60941. doi: 10.1371/journal.pone.0060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr A, Marriott D, Field A, Vasak E, Cooper DA. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256–261. doi: 10.1016/S0140-6736(97)07529-6. [DOI] [PubMed] [Google Scholar]
- 10.Molina JM, Chastang C, Goguel J, Michiels JF, Sarfati C, Desportes-Livage I, Horton J, Derouin F, Modai J. Albendazole for treatment and prophylaxis of microsporidiosis due to Encephalitozoon intestinalis in patients with AIDS: a randomized double-blind controlled trial. J Infect Dis. 1998;177:1373–1377. doi: 10.1086/515268. [DOI] [PubMed] [Google Scholar]
- 11.Bhadra R, Moretto MM, Castillo JC, Petrovas C, Ferrando-Martinez S, Shokal U, Leal M, Koup RA, Eleftherianos I, Khan IA. Intrinsic TGF-beta signaling promotes age-dependent CD8+ T cell polyfunctionality attrition. J Clin Invest. 2014;124:2441–2455. doi: 10.1172/JCI70522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretto M, Weiss LM, Khan IA. Induction of a rapid and strong antigen-specific intraepithelial lymphocyte response during oral Encephalitozoon cuniculi infection. J Immunol. 2004;172:4402–4409. doi: 10.4049/jimmunol.172.7.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawlor EM, Moretto MM, Khan IA. Optimal CD8 T-cell response against Encephalitozoon cuniculi is mediated by Toll-like receptor 4 upregulation by dendritic cells. Infect Immun. 2010;78:3097–3102. doi: 10.1128/IAI.00181-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan AH, Lam KP. Pharmacologic inhibition of MEK-ERK signaling enhances Th17 differentiation. J Immunol. 2010;184:1849–1857. doi: 10.4049/jimmunol.0901509. [DOI] [PubMed] [Google Scholar]
- 15.Publicover J, Goodsell A, Nishimura S, Vilarinho S, Wang ZE, Avanesyan L, Spolski R, Leonard WJ, Cooper S, Baron JL. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest. 2011;121:1154–1162. doi: 10.1172/JCI44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol. 2011;85:2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhadra R, Guan H, Khan IA. Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One. 2010;5:e10842. doi: 10.1371/journal.pone.0010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 19.Khan IA, Schwartzman JD, Kasper LH, Moretto M. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J Immunol. 1999;162:6086–6091. [PubMed] [Google Scholar]
- 20.Barker BR, Gladstone MN, Gillard GO, Panas MW, Letvin NL. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur J Immunol. 2010;40:3085–3096. doi: 10.1002/eji.200939939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci U S A. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland AP, Joller N, Michaud M, Liu SM, Kuchroo VK, Grusby MJ. IL-21 promotes CD8+ CTL activity via the transcription factor T-bet. J Immunol. 2013;190:3977–3984. doi: 10.4049/jimmunol.1201730. [DOI] [PubMed] [Google Scholar]
- 25.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 26.Ostiguy V, Allard EL, Marquis M, Leignadier J, Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. Journal of leukocyte biology. 2007;82:645–656. doi: 10.1189/jlb.0806494. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Lizee G, Lou Y, Liu C, Overwijk WW, Wang G, Hwu P. IL-21 synergizes with IL-7 to augment expansion and anti-tumor function of cytotoxic T cells. International immunology. 2007;19:1213–1221. doi: 10.1093/intimm/dxm093. [DOI] [PubMed] [Google Scholar]
- 28.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. The Journal of experimental medicine. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 30.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 31.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 32.Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. The Journal of experimental medicine. 2014;211:105–120. doi: 10.1084/jem.20130901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulet S, Daudelin JF, Labrecque N. IL-2 induction of Blimp-1 is a key in vivo signal for CD8+ short-lived effector T cell differentiation. J Immunol. 2014;193:1847–1854. doi: 10.4049/jimmunol.1302365. [DOI] [PubMed] [Google Scholar]
- 37.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khattar M, Miyahara Y, Schroder PM, Xie A, Chen W, Stepkowski SM. Interleukin-21 is a critical regulator of CD4 and CD8 T cell survival during priming under Interleukin-2 deprivation conditions. PLoS One. 2014;9:e85882. doi: 10.1371/journal.pone.0085882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casey KA, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 40.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 41.Attridge K, Wang CJ, Wardzinski L, Kenefeck R, Chamberlain JL, Manzotti C, Kopf M, Walker LS. IL-21 inhibits T cell IL-2 production and impairs Treg homeostasis. Blood. 2012;119:4656–4664. doi: 10.1182/blood-2011-10-388546. [DOI] [PubMed] [Google Scholar]
- 42.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 43.Barker BR, Parvani JG, Meyer D, Hey AS, Skak K, Letvin NL. IL-21 induces apoptosis of antigen-specific CD8+ T lymphocytes. J Immunol. 2007;179:3596–3603. doi: 10.4049/jimmunol.179.6.3596. [DOI] [PubMed] [Google Scholar]
- 44.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. The Journal of experimental medicine. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.