Abstract
BACKGROUND
Severe injury results in increased mobilization of hematopoietic progenitor cells (HPC) from the bone marrow (BM) to sites of injury, which may contribute to persistent BM dysfunction after trauma. Norepinephrine is a known inducer of HPC mobilization, and nonselective β-blockade with propranolol has been shown to decrease mobilization after trauma and hemorrhagic shock (HS). This study will determine the role of selective β-adrenergic receptor blockade in HPC mobilization in a combined model of lung contusion (LC) and HS.
METHODS
Male Sprague-Dawley rats were subjected to LC, followed by 45 minutes of HS. Animals were then randomized to receive atenolol (LCHS + β1B), butoxamine (LCHS + β2B), or SR59230A (LCHS + β3B) immediately after resuscitation and daily for 6 days. Control groups were composed of naive animals. BM cellularity, %HPCs in peripheral blood, and plasma granulocyte-colony stimulating factor levels were assessed at 3 hours and 7 days. Systemic plasma-mediated effects were evaluated in vitro by assessment of BM HPC growth. Injured lung tissue was graded histologically by a blinded reader.
RESULTS
The use of β2B or β3B following LCHS restored BM cellularity and significantly decreased HPC mobilization. In contrast, β1B had no effect on HPC mobilization. Only β3B significantly reduced plasma G-CSF levels. When evaluating the plasma systemic effects, both β2B and β3B significantly improved BM HPC growth as compared with LCHS alone. The use of β2 and β3 blockade did not affect lung injury scores.
CONCLUSION
Both β2 and β3 blockade can prevent excess HPC mobilization and BM dysfunction when given after trauma and HS, and the effects seem to be mediated systemically, without adverse effects on subsequent healing. Only treatment with β3 blockade reduced plasma G-CSF levels, suggesting different mechanisms for adrenergic-induced G-CSF release and mobilization of HPCs. This study adds to the evidence that therapeutic strategies that reduce the exaggerated sympathetic stimulation after severe injury are beneficial and reduce BM dysfunction.
Keywords: Norepinephrine, atenolol, butoxamine, SR59230A, rats
Under normal homeostatic conditions, small numbers of hematopoietic progenitor cells (HPCs) are detectable in the bloodstream. Following trauma and hemorrhagic shock (HS), there is an increased mobilization of HPCs from the bone marrow (BM) to sites of injury.2,3 The exaggerated loss of HPCs from the BM and suppression of BM HPC growth after trauma may contribute to BM dysfunction, which manifests as a persistent anemia seen for more than 2 weeks after injury.4,5
The mobilization of HPCs from BM has been shown to be mediated by an increase in plasma G-CSF, which then activates proteases in the BM such as stromal-derived factor 1 or CXCL12 to release the HPCs into the peripheral blood.6 The sympathetic nervous system, particularly norepinephrine (NE), has been proposed as a crucial regulator of HPC mobilization.6–9 Katayama et al.8 found that HPC mobilization following G-CSF injection was significantly reduced following chemical sympathectomy with 6-hydroxydopamine. 6-Hydroxydopamine causes degeneration of dopaminergic and noradrenergic neurons, thus reducing NE levels. Their findings suggest that G-CSF alone is not adequate to induce HPC mobilization and that NE is an essential cofactor.
NE has been shown to be persistently elevated up to 2 to 10 times normal more than 7 days after trauma.10 Previously, we have shown that NE causes a dose-dependent reduction of BM HPC growth both in vitro and in vivo animal models.11,12 In addition, after trauma and HS, plasma G-CSF levels are significantly elevated in both humans and rodent models.3,13 The elevation in G-CSF after trauma and HS is also likely linked to the persistent elevation of NE, which correlates with increased HPC mobilization. We have also shown that the stress response after trauma and HS leads to increased HPC mobilization from the BM, which is abrogated by the use of propranolol, a nonselective β-blocker.3,5,14 Propranolol inhibits the action of endogenous catecholamines by competitively binding to all β-adrenergic receptors (ref). β-adrenergic receptors are subdivided into three types as follows: β1-, β2-, and β3-adrenergic receptors. While β1-adrenergic receptors predominate in the heart, β2-adrenergic receptors in the respiratory system, and β3-adrenergic receptors in adipose tissues, β2- and β3-adrenergic receptors have also been shown to be present on leukocytes and in BM.6,15 In addition, acute critical illness is characterized by a hypermetabolic and catabolic response where profound endocrine, metabolic, and immunologic changes are initiated and sustained through the activation of β-adrenergic receptors.15 The reduction of HPC mobilization when propranolol was given after trauma and HS is potentially linked to decreased NE binding to available β-adrenergic receptors.
Despite the evidence supporting the role of the sympathetic nervous system in HPC growth and trafficking, the exact mechanism involved and its role following trauma and HS have not been well elucidated. Therefore, the aim of this study was to define the role of the specific β-adrenergic receptors in HPC mobilization following trauma and HS. We will examine the effects of selective β-blockade (BB) given after injury on HPC mobilization, in vitro BM HPC growth, and postinjury lung healing.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 300 g to 400 g were housed at 25°C under barrier-sustained conditions with 12-hour light-dark cycles. Animals were provided ad libitum access to water and chow (Teklad22/5 Rodent Diet W-8640, Harlan Teklad, Madison, WI). The animal facility environment and animals were maintained in accordance with the regulations detailed in the Guide for the Care and Use of Laboratory Animals. The New Jersey Medical School Animal Care and Use Committee approved all animal protocols.
Reagents
Bovine serum albumin and 2-mercaptoethanol were purchased from Sigma-Aldrich (St. Louis, MO). Methylcellulose was purchased from Stemcell Technologies (Vancouver, Canada). Fetal bovine serum, Iscove’s Modified Dulbecco’s Medium, glutamine, penicillin/streptomycin, and trypan blue were obtained from Invitrogen (Carlsbad, CA). All cytokines rhEpo, rhIL-3, rhGM-CSF were purchased from R&D Systems (Minneapolis, MN). Sodium pentobarbital was purchased from Lundbeck Inc. (Deerfield, IL), and heparin was obtained from Hospira Inc. (Lakefront, IL). Atenolol (β1B), butoxamine (β2B), and SR59230A (β3B) were purchased from Sigma-Aldrich.
Experimental Groups
Animals were randomly allocated to either control or the following experimental groups (n = 6 per group): lung contusion followed by HS (LCHS), lung contusion followed by HS and treatment with either atenolol (LCHS/β1B), butoxamine (LCHS/β2B) or SR59230A (LCHS/β3B). The respective doses were 10-mg/kg atenolol,5-mg/kg butoxamine, and 5-mg/kg SR59230A. Control groups were composed of naive animals. The immediate effects of LCHS and selective βB treatment were assessed after a single dose of medication after resuscitation, and animals were sacrificed at 3 hours. The sustained effects of LCHS and selective BB therapy were assessed by once daily dosing and then sacrificing animals on Day 7.
Combined Lung Contusion and HS Model
Experimental animals were anesthetized with intraperitoneal injections of sodium pentobarbital (50 mg/kg). Unilateral lung contusion (LC) was inflicted using a blast wave percussive nail gun (Craftsman 968514 Stapler, Sears Brands, Chicago, IL) applied to a 12-mm metal plate adherent to the right axilla of the rat. This LC model has been shown to produce a clinically significant lung injury as demonstrated by histology and radiography.16 With the use of aseptic surgical technique, the right internal jugular vein and femoral artery were then cannulated with polyethylene (PE-50, Becton Dickinson and Co., Sparks, MD) and Silastic (Dow Corning Corp., Midland, MI) tubing, respectively. To prevent clotting, all tubing was flushed with 10-U/mL heparinized saline. Continuous blood pressure monitoring (BP-2 Digital Blood Pressure Monitor, Columbus Instruments, Columbus, OH) was performed. Animals were bled to a mean arterial blood pressure of 30 mm Hg to 35 mm Hg for 45 minutes. Temperature was maintained at approximately 37°C with the use of a heating pad. Shed blood was reinfused at a rate of 1 mL/min following the shock period. Selective BB was administered after resuscitation (when mean arterial pressure > 80 mm Hg) via intraperitoneal injection.
BM Cellularity
BM cells were obtained by removing the femoral epiphysis post mortem and aspirating the BM with an 18-gauge needle on a 5-mL syringe filled with of 1-mL Iscove’s Modified Dulbecco’s Medium supplemented with 10% fetal bovine serum. A suspension was prepared by passing cells through a 40-μm sterile nylon strainer to remove particulate matter. Total viable cell counts were then determined by 0.4% Trypan blue staining using a hemocytometer.
Flow Cytometry
The frequency of CD71+ and CD117+ cells was quantified in unfractionated peripheral blood samples using an established, single-platform enumeration method. Briefly, 100 μL of peripheral blood (one million cells) was labeled with 10 μL of BD PharmingenTM mouse antirat CD71 antibody conjugated with fluorescein isothiocyanate and 10 μL of BD PharmingenTM rat antimouse CD117 (c-Kit) antibody conjugated to phycoerythrin (BD Biosciences, Franklin Lakes, NJ) for 30 minutes. Following erythrocyte lysis, cells were then centrifuged at 300 G for 5 minutes, and the supernatant was discarded. Cells were washed three times and fixed with BD CytofixTM solution (BD). Cells were analyzed using BD FACSCalibur flow cytometer (BD) equipped with CellQuest software (BD). Samples from each group were stained and run in duplicate, and an event count of 30,000 was obtained for each run. Following acquisition of data, further analysis was performed using Flow Jo version 7.2.4 (Tree Star, Ashland, OR).
BM HPC Cultures
To study the systemic effect of circulating plasma from experimental groups on normal BM progenitor cell growth, BM HPC growth was evaluated using an in vitro culture method. Normal BM was harvested from all rodent control femurs by removing the epiphysis and flushing each femur with 5 mL of cold MEM-alpha medium. The BM suspension was then centrifuged at 1,500 rpm (400 G) for 15 minutes, the supernatant was discarded, and the remaining pellet was resuspended in 1 mL of Dulbecco’s Modified Eagle’s Medium containing 10% fetal calf serum. Two percent plasma (vol/vol) from all experimental groups was added to the BM suspension. BM mononuclear cells were then plated in duplicate (2 × 106) in Iscove’s media containing 30% fetal calf serum, 2% bovine serum albumin, 1% methylcellulose, rat growth factor, penicillin/streptomycin, 2 × 10−4 mol/L 2-mercaptoethanol, and glutamine. To select for particular erythropoietic progenitor cell lineages, cultures were supplemented with the cytokines 1.3-U/mL rhEpo and 6-U/mL rhIL-3 for erythroid blast-forming unit (BFU-E) and erythroid colony-forming unit (CFU-E) and 3-U/mL rhGM-CSF for granulocyte erythrocyte monocyte megakaryocyte progenitor cells (GEMM). Cultures were incubated at 37°C in 5% CO2, and colonies were counted at 7, 14, and 18 days for CFU-E, BFU-E, and GEMM, respectively, by an observer blinded to the origin of the samples.
Measurement of Plasma G-CSF
Following sacrifice, peripheral blood samples at 3 hours and 7 days were centrifuged at 10,000 rpm for 10 minutes at 10°C to obtain plasma, which was collected and stored at −80°C. Plasma samples were analyzed for G-CSF using commercial colorimetric sandwich enzyme-linked immunosorbent assay kits (R&D Systems Inc., Minneapolis, MN). Assays were performed according to the provided manufacturer’s instructions. All standards and samples were assayed in duplicate.
Lung Histology
The contused right lungs were harvested at 3 hours and 7 days and fixed in 10% buffered formalin solution to obtain lung injury scores for all experimental groups. Briefly, after fixation, the samples were dehydrated and embedded in paraffin blocks. Sections with 4-μm thickness were cut and stained by hematoxylin and eosin. Lung tissue was assessed using a modified quantitative lung injury score that accounted for inflammatory cells, interstitial edema, pulmonary edema, and alveolar integrity.17 The lung injury score ranges from 0 to 11. Slides were evaluated under the standard light microscope, and 30 random fields in each sample were graded in a blinded fashion.
Statistical Analysis
All data are expressed as mean ± SEM. Statistical analyses were performed using one-way analysis of variance followed by Tukey-Kramer’s multiple comparison posttest with GraphPad Prism (version 4.0, San Diego, CA). Results were considered significant if*p < 0.05 versus control or **p < 0.05 versus LCHS.
RESULTS
BM Cellularity
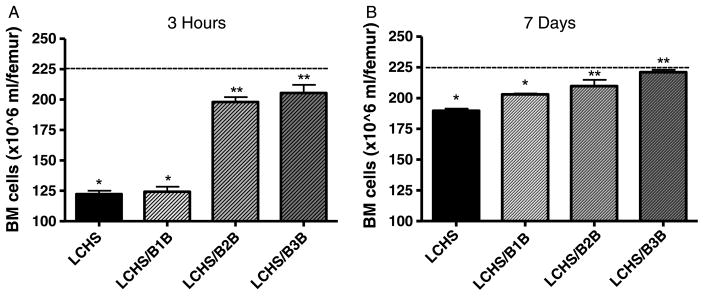
Within 3 hours, LCHS resulted in a statistically significant decrease in overall BM cellularity as compared with controls (122 ± 6* vs. 220 ± 7). Administration of either β2B or β3B after LCHS prevented the decrease in BM cellularity seen with LCHS alone and restored BM cellularity to within 10% of control values (Fig. 1A). Administration of β1B after LCHS had no effect on BM cellularity compared with LCHS alone (124 ± 10 vs. 122 ± 6). The effect of both β2B and β3B at 7 days was a continued protection of BM and maintenance of normal BM cellularity (Fig. 1B). After 7 days of administration, LCHS animals given β1B still had significantly less BM cellularity similar to LCHS-alone animals.
Figure 1.
A and B, The effect of selective BB on BM cellularity after trauma and shock. Dashed line represents control levels. n = 6 animals per group. LCHS/β1B, LCHS followed by β1 blockade; LCHS/β2B, LCHS followed by β2 blockade; LCHS/β3B, LCHS followed by β3 blockade. *p < 0.05 versus control. **p < 0.05 versus LCHS.
HPC Mobilization by Flow Cytometry
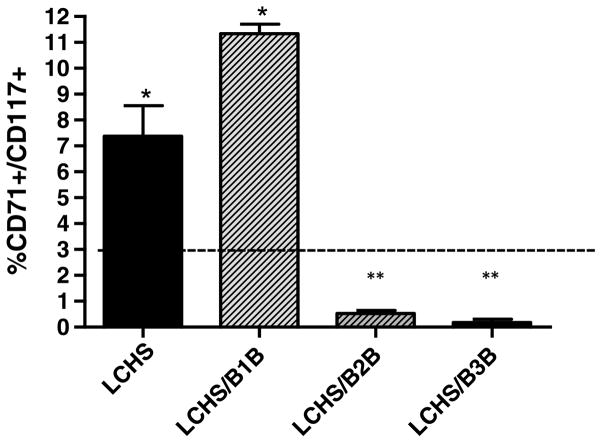
Three hours after LCHS, there was a statistically significant increase in HPCs in peripheral blood compared with controls (7.4 ± 2.3* vs. 0.5 ± 0.2). However, administration of either β2B or β3B after LCHS prevented this egress of HPCs at 3 hours (Fig. 2). The use of β1B after LCHS had no effect on HPC egress from BM similar to LCHS alone (11.3 ± 0.8 vs. 7.4 ± 2.3). Seven days following LCHS, the percentage of HPCs in peripheral blood of all experimental groups returned to baseline control levels of less than 1%.
Figure 2.
Selective β2B and β3B prevent HPC egress from BM after injury and shock. LCHS prevented by β2B and β3B. Dashed line represents control levels. n = 6 animals per group. LCHS/β1B, LCHS followed by β1 blockade; LCHS/β2B, LCHS followed by β2 blockade; LCHS/β3B, LCHS followed by β3 blockade. *p < 0.05 versus control. **p < 0.05 versus LCHS.
Plasma G-CSF
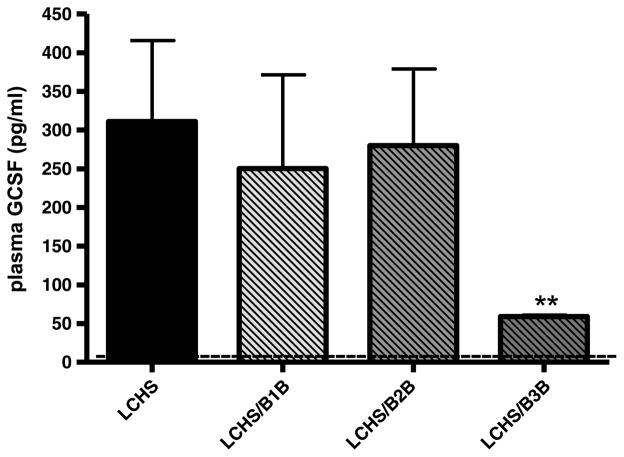
Three hours after LCHS, there was a significant increase in plasma G-CSF levels compared with controls, but only administration of β3B after LCHS caused a significant reduction in plasma G-CSF (59 ± 4** vs. 311 ± 234) (Fig. 3). Treatment with either β1B or β2B had no effect on plasma G-CSF levels 3 hours after LCHS. Seven days following LCHS, plasma G-CSF levels of all experimental groups returned to baseline control levels of less than 50 pg/mL, which correlates with the decrease in HPC mobilization seen at 7 days.
Figure 3.
Effect of selective BB on plasma G-CSF levels following trauma and shock. Dashed line represents control levels. n = 6 animals per group. LCHS/β1B, LCHS followed by β1 blockade; LCHS/β2B, LCHS followed by β2 blockade; LCHS/β3B, LCHS followed by β3 blockade. **p < 0.05 versus LCHS.
Systemic Effects of Selective BB on BM HPC Growth
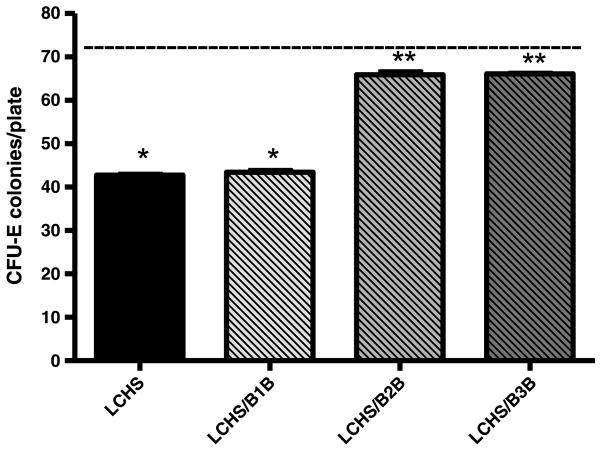
Plasma from animals 3 hours after LCHS incubated with normal BM produces a significant suppression in BM CFU-E colony growth versus control plasma (44 ± 1* vs. 73 ± 1) (Fig. 4). Plasma from animals given either β2B or β3B immediately after LCHS did not suppress BM CFU-E colony growth as compared with LCHS alone (66 ± 2** and 66 ± 1* vs. 44 ± 1). Plasma from animals given β1B after LCHS suppressed BM CFU-E growth similar to plasma from LCHS alone.
Figure 4.
Systemic effects of selective BB on BM CFU-E growth. Dashed line represents control levels. n = 6 animals per group. LCHS/β1B, LCHS followed by β1 blockade; LCHS/β2B, LCHS followed by β2 blockade; LCHS/β3B, LCHS followed by β3 blockade. *p < 0.05 versus control. **p < 0.05 versus LCHS.
More primitive BM progenitors, BFU-E and CFU-GEMM, show similar protection with the use of β2 or β3 blockade 3 hours after LCHS. Plasma from animals given either β2B or β3B immediately after LCHS prevented BM BFU-E and CFU-GEMM colony growth suppression compared with LCHS alone (59 ± 1** and 60 ± 1* vs. 40 ± 1 and 31 ± 1** and 31 ± 1* vs. 16 ± 0, respectively). Plasma from animals given β1B after LCHS had no protection of BM and suppressed BM BFU-E and CFU-GEMM growth similar to LCHS alone.
Effects on Lung Injury Scores
Treatment with selective β-blockers had no adverse effects on lung injury score as measured by lung histology either at 3 hours or 7 days after LCHS. Three hours after LCHS, lung injury scores are modestly elevated for all groups (Table 1). There is a slight decrease in lung injury scores with the use of β2 or β3 blockade; however, this did not reach statistical significance. Despite decreased HPC mobilization with β2 or B3 blockade, there is a similar expected degree of lung healing on Day 7 after LCHS (Table 2).
TABLE 1.
Three-Hour Lung Injury Scores
| Group | Pulmonary Edema | Interstitial Edema | Alveolar Integrity | Inflammation | Total |
|---|---|---|---|---|---|
| (0–2) | (0–3) | (0–2) | (0–4) | ||
| LCHS | 1.5 ± 0.6 | 1.5 ± 0.6 | 1.3 ± 0.5 | 3.3 ± 0.5 | 7.6 ± 1.3 |
| LCHS/β1B | 1.7 ± 0.5 | 1.2 ± 0.4 | 1.3 ± 0.8 | 3.0 ± 0.9 | 7.2 ± 1.6 |
| LCHS/β2B | 1.8 ± 0.4 | 1.4 ± 0.5 | 0.2 ± 0.4 | 2.0 ± 0.7 | 5.4 ± 1.7 |
| LCHS/β3B | 1.0 ± 0.7 | 1.2 ± 0.4 | 0.8 ± 0.8 | 2.8 ± 0.8 | 5.8 ± 2.3 |
p < 0.05 versus LCHS.
Grading of lung injury scores 3 hours and 7 days after injury and shock. n = 6 animals per group.
LCHS/β1B, LCHS followed by β1 blockade; LCHS/β2B, LCHS followed by β2 blockade; LCHS/β3B, LCHS followed by β3 blockade.
TABLE 2.
Seven-Day Lung Injury Scores
| Group | Pulmonary Edema | Interstitial Edema | Alveolar Integrity | Inflammation | Total |
|---|---|---|---|---|---|
| (0–2) | (0–3) | (0–2) | (0–4) | ||
| LCHS | 1.0 ± 0.8 | 2.5 ± 0.6 | 1.0 ± 0 | 1.1 ± 1.7 | 5.6 ± 1.5 |
| LCHS/β1B | 1.0 ± 1.2 | 2.0 ± 0.8 | 1.0 ± 0.8 | 1.1 ± 1.3 | 5.1 ± 3.0 |
| LCHS/β2B | 0.5 ± 0.6 | 1.4 ± 0.6 | 1.3 ± 1.0 | 1.5 ± 0.6 | 4.7 ± 1.5 |
| LCHS/β3B | 0.8 ± 0.5 | 1.2 ± 0.5 | 1.0 ± 0 | 1.0 ± 0 | 4.0 ± 0.6 |
n = 6 animals per group.
LCHS/β1B, LCHS followed by β1 blockade; LCHS/β2B, LCHS followed by β2 blockade; LCHS/β3B, LCHS followed by β3 blockade.
DISCUSSION
The use of nonselective BB after injury with propranolol has previously been shown to reduce HPC mobilization to the peripheral blood and protect the BM following tissue injury and trauma.5 This is the first study to examine the effects of selective BB on HPC mobilization and plasma G-CSF levels when given after trauma and HS. This study demonstrates that both β2B and β3B can prevent excess HPC mobilization and BM dysfunction when given after trauma and HS. The effects of β2B and β3B seem to be mediated systemically, without adverse effects on subsequent lung injury healing.
Lung injury combined with HS has been shown to significantly decrease BM cellularity and inhibit BM HPC growth and significantly increase HPC mobilization to peripheral blood.3,18 While HPC mobilization and homing play a role in tissue repair at the sites of injury,2 an excessive release of HPCs with a corresponding decrease in BM cellularity may contribute to persistent BM dysfunction. In this study, when β2B and β3B were administered after LCHS, there was a significant increase in BM cellularity after 3 hours, and this protection of BM cellularity was maintained at 7 days after injury. Associated with this increase in BM cellularity, the use of β2B and β3B after LCHS prevented the excess egress of HPC cells to peripheral blood seen after combined tissue injury and HS. These findings were similar to what was demonstrated in an LC-alone model.19 In the absence of HS, daily retreatment of β2B or β3B before LC led to a decrease in HPC mobilization to peripheral blood and homing to injured lung tissue.19 Despite a more significant decrease in BM cellularity and a more profound egress of HPCs to peripheral blood with the addition of HS to LC as compared with LC alone model, both β2B and β3B were able to restore BM protection at the same dose used previously.19
The administration of β1B after LCHS had no effect on BM cellularity or HPC mobilization. These findings are not unexpected because β1 receptors are located primarily in the heart and the kidneys. Our previous work demonstrated that the use of atenolol at 10 mg/kg for 7 days following LCHS produced a cardiovascular response and effectively reduced heart rate by 10% but this was not associated with BM protection.20 Atenolol use while associated with a reduction in heart rate was not associated with a change in mean arterial pressure, so the shock state was not propagated by the use of β1B.20
Propranolol administration after LCHS has been shown to prevent HPC mobilization, which was also associated with reduced plasma G-CSF levels.3 In this study, only β3B was shown to reduce plasma G-CSF levels after LCHS. While only β3B reduced G-CSF levels, both β2B and β3B improved BM cellularity and prevented HPC mobilization from BM after LCHS. This suggests different mechanisms by which β2 and β3 blockade affect HPC release. Recent work by Méndez-Ferrer et al.6 also shows that the β2- and β3-adrenergic receptors may be regulating the BM by distinct mechanisms. β3 receptors are found not only on adipocytes but also in the bone and the BM environment. β3 receptors are found on stromal cells and have been shown to regulate the release of HPCs to the bloodstream.7 The β3 receptor may down-regulate stromal-derived factor 1, allowing for HPC release from the BM, while stimulation of β2 receptors induces clock gene expression in stromal cells.6,21 Circadian effects on the sympathetic nervous system are mediated by clock gene expression in many peripheral organs throughout the body.21,22 A double deficiency of β2- and β3-adrenergic receptors significantly decreased mobilization of HPCs to the peripheral blood.6 The cooperation of both β2- and β3-adrenergic receptors for HPC mobilization is thought to be caused by their function in both hematopoietic and stromal compartments of the BM.6 Recent evidence has shown that the daily use of β3B following LCHS, not β2B, resulted in an improvement in hemoglobin at 7 days.20 These findings further support the varying roles of the β2- and β3-adrenergic receptors in BM function following trauma.
Livingston et al.4 has previously shown that plasma from trauma patients suppressed BM HPC colony growth by 40% to 60% up to 2 weeks after injury compared with cultures incubated with normal plasma. This inhibition of the BM seemed to be mediated by systemic effects on the BM stroma.4 This 2-week inhibition of human BM HPC colony growth parallels the profound elevation of catecholamines seen in severely injured trauma patients.10 Therefore, the protective effects of β2B and β3B and decreased HPC mobilization may be linked to a blockade of NE following severe injury.11,12 Since the hyperadrenergic state following severe traumatic injury is a systemic process, we hypothesized that the protection of BB is not limited to the BM. This study confirms that β2 and β3 blockade seem to work systemically. LCHS plasma reduced BM HPC colony growth. However, plasma from LCHS + β2B or LCHS + β3B animals improved BM HPC colony growth, suggesting that both β2B and β3B have protective systemic effects. Baranski et al.18 showed similar improvement in HPC colony growth with LCHS plasma of animals given with propranolol.
Since β2 and β3 blockade decrease HPC mobilization, there is a concern about potential adverse effects on injured tissue and subsequent tissue healing. Previous work in an LC-alone model showed that 7 days after pretreatment with propranolol, β2 blockade, or β3 blockade, all groups had similar lung injury scores.19 Likewise, our current results show that there are no adverse effects on lung injury scores after LCHS after treatment with selective BB. Thus, both β2 and β3 blockades offer systemic protection after LCHS without adversely affecting the healing of injured tissue.
These data continue to suggest that NE may be a key systemic mediator in potentiating BM dysfunction following severe trauma. Following severe traumatic injury, both epinephrine and NE are markedly elevated for more than a week.10 In addition, the adrenal gland and the nervous system are not the sole producers and releasers of catecholamines.22 There is mounting evidence that key mediators of the stress response following injury are the lymphocytes and phagocytes that synthesize and release neuropeptides, neurotransmitters, hormones, and cytokines.23,24 At these exaggerated levels, NE has been shown to not only have α- but also β-adrenergic effects.25 While the exact mechanisms involved are not well understood, it seems that a systemic reduction in a severe hyperadrenergic state and NE may help to alleviate posttraumatic BM dysfunction.
In summary, the use of β2 and β3 blockade attenuates HPC mobilization from BM following injury and shock without worsening lung healing. The protective effects of β2 and β3 blockade seem to be mediated systemically via the plasma but may prevent BM dysfunction via different mechanisms. Additional studies are needed to further delineate the mechanisms by which β2 and β3 blockades affect HPC mobilization.
Footnotes
This study was part of the oral presentation at the 26th annual meeting of the Eastern Association for the Surgery of Trauma, January 15–19, 2013, in Scottsdale, Arizona.
DISCLOSURE
This research was supported by the National Institutes of Health grant K08 NIH GM078304 and the Clowes American College of Surgeons/American Association for the Surgery of Trauma Award.
References
- 1.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19:702–714. [PubMed] [Google Scholar]
- 2.Shah S, Ulm J, Sifri ZC, Mohr AM, Livingston DH. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma. 2009;67:315–321. doi: 10.1097/TA.0b013e3181a5c9c7. [DOI] [PubMed] [Google Scholar]
- 3.Baranski GM, Offin MD, Sifri ZC, Elhassan IO, Hannoush EJ, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. β-blockade protection of bone marrow following trauma: the role of G-CSF. J Surg Res. 2011;170:325–331. doi: 10.1016/j.jss.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohr AM, Elhassan IO, Hannoush EJ, Sifri ZC, Offin MD, Alzate WD, Rameshwar P, Livingston DH. Does beta blockade post injury prevent bone marrow suppression? J Trauma. 2011;70:1043–1050. doi: 10.1097/TA.0b013e3182169326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Méndez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139–144. doi: 10.1111/j.1749-6632.2010.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Hematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–448. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 8.Katayama Y, Battista M, Kao W, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MJ, Shankar R, Stevenson J, Fernandez R, Gamelli RL, Jones SB. Bone marrow norepinephrine mediates development of functionally different macrophages after thermal injury and sepsis. Ann Surg. 2004;240:132–141. doi: 10.1097/01.sla.0000130724.84914.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect. 2004;5:385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–889. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 12.Penn A, Mohr AM, Shah SG, Sifri ZC, Kaiser VL, Rameshwar P, Livingston DH. Dose-response relationship between norepinephrine and erythropoiesis: evidence for a critical threshold. J Surg Res. 2010;163:e85–e90. doi: 10.1016/j.jss.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook KM, Sifri ZC, Baranski GM, Mohr AM, Livingston DH. The role of plasma granulocyte colony stimulating factor and bone marrow dysfunction after severe trauma. J Am Coll Surg. 2012;216:57–64. doi: 10.1016/j.jamcollsurg.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhassan IO, Hannoush EJ, Sifri ZC, Jones E, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Beta-blockade prevents hematopoietic progenitor cell suppression after hemorrhagic shock. Surg Infect. 2011;12:273–278. doi: 10.1089/sur.2010.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laukova M, Vargovic P, Csaderova L, Chovanova L, Vicek M, Imrich R, Krizanonva O, Kvetnansky R. Acute stress differently modulates β1, β2 and β3 adrenorecptors in T cells, but not in B cells, from the rat spleen. Neuroimmunomodulation. 2012;19:69–78. doi: 10.1159/000329002. [DOI] [PubMed] [Google Scholar]
- 16.Badami CD, Livingston DH, Sifri ZC, Caputo FJ, Bonilla L, Mohr AM, Deitch EA. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63:596–600. doi: 10.1097/TA.0b013e318142d231. [DOI] [PubMed] [Google Scholar]
- 17.Claridge JA, Enelow RI, Young JS. Hemorrhage and resuscitation induce delayed inflammation and pulmonary dysfunction in mice. J Surg Res. 2000;92:206–213. doi: 10.1006/jsre.2000.5899. [DOI] [PubMed] [Google Scholar]
- 18.Baranski GM, Sifri ZC, Cook KM, Alzate WD, Livingston DH, Mohr AM. Is the sympathetic system involved in shock-induced gut and lung injury? J Trauma Acute Care Surg. 2012;73:343–350. doi: 10.1097/TA.0b013e31825a785a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beiermeister KA, Keck BM, Sifri ZC, ElHassan IO, Hannoush EJ, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 receptors after injury. J Trauma. 2010;69:338–343. doi: 10.1097/TA.0b013e3181e5d35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasupuleti LV, Cook KM, Sifri ZC, Kotamarti S, Calderon GM, Alzate WD, Livingston DH, Mohr AM. Does selective beta-1 blockade provide bone marrow protection after trauma/hemorrhagic shock? Surgery. 2012;152:322–330. doi: 10.1016/j.surg.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, Pévet P, Buijs RM. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS One. 2009;4:e5650. doi: 10.1371/journal.pone.0005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Le Tulzo Y, Shenkar R, Kaneko D, Moine P, Fantuzzi G, Dinarello CA, Abraham E. Hemorrhage increases cytokine expression in lung mononuclear cells in mice: involvement of catecholamines in nuclear factor-kappaB regulation and cytokine expression. J Clin Invest. 1997;99:1516–1524. doi: 10.1172/JCI119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. Catecholamines-Crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening Pandora’s box? Mol Med. 2008;14:195–204. doi: 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodde OE, Daul A, Michel MC. Subtype-selective modulation of human beta 1-and beta 2-adrenoreceptor function by beta-adrenoreceptor agonists and antagonists. Clin Physiol Biochem. 1990;8:11–17. [PubMed] [Google Scholar]