Abstract
BACKGROUND
Bone marrow (BM) dysfunction is common in severely injured trauma patients, resulting from elevated catecholamines and plasma granulocyte colony-stimulating factor (G-CSF) as well as prolonged mobilization of hematopoietic progenitor cells (HPCs). We have previously shown that propranolol (β-blocker [BB]) reduces HPC mobilization in a rodent model of injury and hemorrhagic shock. We hypothesize that BB would prevent BM dysfunction in humans following severe injury.
METHODS
Forty-five severely injured trauma patients were studied in a prospective, randomized pilot trial. Twenty-five patients received BB, and 20 served as untreated controls. The dose of propranolol was adjusted to decrease the heart rate by 10% to 20% from baseline. Blood was analyzed for the presence of HPC (blast-forming unit erythroid cells [BFU-E] and colony-forming unit erythroid cells) and G-CSF. Demographic data, Injury Severity Score (ISS), hemoglobin, reticulocyte number, and outcome data were obtained.
RESULTS
The mean age of the study population was 33 years; 87% were male, with a mean ISS of 29. There is a significant increase in BFU-E in peripheral blood immediately following traumatic injury, and this mobilization persists for 30 days. The use of BB significantly decreases BFU-E and colony-forming unit erythroid cells at all time points. G-CSF is significantly elevated in both groups on admission; the use of BB decreases G-CSF levels by 51% as compared with 37% for controls. The average hemoglobin is nearly 1 g higher on the day of discharge with propranolol treatment (BB, 9.9 ± 0.4 g/dL vs. no BB, 9.1 ± 0.6 g/dL).
CONCLUSION
Following severe trauma, early treatment with propranolol following resuscitation is safe. The use of propranolol blunts early tachycardia, reduces HPC mobilization, and results in a faster return to baseline of the G-CSF peak seen after injury. There is also a trend toward faster recovery and resolution of anemia. Propranolol may be the first therapeutic agent to show improved BM function after severe injury.
LEVEL OF EVIDENCE
Therapeutic study, level III.
Keywords: Bone marrow, β blockade, anemia, hemoglobin, G-CSF
Patients experiencing major trauma develop anemia, and subsequent transfusions are independent risk factors for infection, organ failure, and death.1 Previously, we have shown that bone marrow (BM) dysfunction is common in severely injured trauma patients, and this BM dysfunction is accompanied by persistent elevation of norepinephrine (NE), plasma granulocyte colony-stimulating factor (G-CSF), and the prolonged mobilization of hematopoietic progenitor cells (HPCs) into the peripheral blood.2,3 HPCs include granulocyte-erythrocyte-monocyte-macrophage progenitor cells, blast-forming unit erythroid cells (BFU-E), and colony-forming unit erythroid cells (CFU-E), which are the precursors to red blood cells. Under normal homeostatic conditions, HPCs are found within the BM and participate in erythropoiesis, and there is a reduced number of HPCs in the peripheral blood.4 G-CSF plays a direct role in the release of HPCs from the BM to the peripheral blood, and this G-CSF release is mediated by NE.4 Therefore, the combined exaggerated loss of HPCs from the BM and the suppression of HPC growth in BM after trauma contribute to prolonged BM dysfunction, which manifests as a persistent anemia that can persist for several months.5–7
The sympathetic response to severe traumatic injury is associated with a persistent systemic elevation of epinephrine and NE.2,8–11 This trauma-induced catecholamine surge has been shown to independently predict mortality, especially when there is concomitant head injury.11 In animal models, NE causes a dose-dependent reduction of BM HPC growth both in vitro and in vivo.12,13 In addition, plasma G-CSF levels are significantly elevated after trauma and shock, and this elevation is likely linked to the persistent elevation of NE.3,14 In a rodent model of injury and hemorrhagic shock, the use of propranolol reduced G-CSF and prevented excess HPC mobilization.5,14,15 Propranolol inhibits the action of endogenous catecholamines by competitively binding to all β-adrenergic receptors. Recent studies have demonstrated an association between A-blockade use and improved outcomes after injury.16,17 To date, however, propranolol has not been tested in trauma patients to examine its potential benefit on hematologic outcomes.
Therefore, we hypothesize that β-blockade would prevent prolonged HPC mobilization, persistent elevation of G-CSF, and anemia following severe injury. This study examines the effects of propranolol given after injury on BM function.
PATIENTS AND METHODS
Subjects
This study was approved the institutional review board of Rutgers-New Jersey Medical School and University Hospital, and written informed consent was obtained from the patient or an appointed surrogate. This study is a prospective, randomized, controlled trial of 45 patients treated at a Level I trauma center from September 2011 through March 2013. All patients admitted to the university hospital following traumatic injury were evaluated for enrollment within 24 hours of admission. Inclusion and exclusion criteria are shown in Figure 1.
Figure 1.

Inclusion and exclusion criteria.
Study Design
Randomization was performed with the use of a computer-generated random number scheme. Once resuscitated and the lactate level is 4 mg/dL or less, the patients randomized to the propranolol group received their first dose of propranolol within 24 hours of admission. The initial dosing of propranolol was titrated to cause a 10% to 20% reduction in the average initial heart rate (HR) (Tables 1A and B, Supplemental Digital Content, at http://links.lww.com/TA/A420). If participants did not achieve their goal HR 30 minutes following the administration of propranolol, the patient received additional 0.5 mg intravenously, and dosing every 6 hours was increased by 0.5 mg. Dose escalation was continued until the goal HR was achieved. Throughout their hospital stay, propranolol dosing was titrated to maintain the initial goal HR. The protocol dictated that if the participant’s HR was less than 60 beats per minute or mean arterial pressure (MAP) was less than 50 mm Hg, the next dose would be held, and the subsequent dose would be lowered by one algorithm level. Propranolol was converted from intravenous to oral administration once the patient tolerated a diet. The study was complete after 30 days in the hospital or upon discharge, which ever occurred first. Blood was collected in a heparinized tube (Becton Dickinson, Franklin Lakes, NJ) on admission and on Days 1, 5, 10, 14, and 30 after injury.
TABLE 1.
Demographic and Outcome Data by Group
| Characteristic | Control | Propranolol | p |
|---|---|---|---|
| n | 20 | 25 | |
| Male/female | 20/0 | 19/6 | <0.05* |
| Age, y | 30 ± 7 | 35 ± 7 | 0.18 |
| Ethnicity | |||
| Black, n (%) | 14 (70) | 17 (68) | 0.89 |
| White, n (%) | 6 (30) | 8 (47) | |
| Mechanism of injury | |||
| Blunt, n (%) | 4 (20) | 15 (60) | <0.05* |
| Penetrating, n (%) | 17 (80) | 10 (40) | |
| ISS | 31 ± 3 | 27 ± 3 | 0.31 |
| Admission base deficit | −7.4 ± 1.5 | −7.4 ± 1.4 | 0.99 |
| Lactate, mg/dL | 6 ± 1 | 5 ± 1 | 0.26 |
| Initial HR, beats/min | 111 ± 6 | 104 ± 4 | 0.47 |
| Initial SBP, mm Hg | 108 ± 7 | 113 ± 6 | 0.38 |
| Admission GCS score | 14 ± 1 | 13 ± 1 | 0.33 |
| 30-d mortality, n | 0 | 0 | |
| pRBC transfusion, n (95% CI) | 2.4 ± 0.6 (1.3–3.6) | 2 ± 0.6 (0.88–3.1) | 0.5 |
| ICU LOS (95% CI), d | 14 ± 4 (6–21) | 9 ± 2 (6–12) | 0.79 |
| Ventilator days (95% CI) | 11 ± 7 (4–17) | 7 ± 2 (3.9–9.9) | 0.97 |
| Infections, n (%) | |||
| Pneumonia | 13 (38) | 11 (48) | 0.64 |
| Urinary tract infection | 1 (3) | 5 (22) | 0.51 |
| Wound infection | 2 (6) | 1 (4) | 0.73 |
| Bacteremia | 4 (12) | 1 (4) | 0.35 |
| Abdominal abscess | 11 (32) | 3 (13) | <0.05* |
p < 0.05.
GCS, Glasgow Coma Scale; LOS, length of stay; pRBC, packed red blood cells.
Demographic Information
Patient charts and the trauma registry were reviewed for demographic data on age, sex, mechanism of injury, ISS, the presence of shock on admission (defined as a systolic blood pressure [SBP] < 90 mm Hg), and initial blood transfusion requirements. Initial laboratory values including hemoglobin (Hgb), lactate, and arterial base deficit were collected. Additional data collected throughout the hospital stay included Hgb, reticulocyte count, number of packed red blood cells required after initial resuscitation, number of surgical interventions, number of ventilator days, and intensive care unit (ICU) length of stay. Outcome data collected included mortality and incidence of infection.
Measurement of Vital Signs
HR, systolic blood pressure, and diastolic blood pressure were measured hourly with the use of electrocardiographic monitor and arterial catheter if available or noninvasive blood pressure monitoring. The average HR was determined in the initial 24 hours before the start of propranolol administration. After initiation of propranolol, HR data and blood pressure data were recorded hourly each day and then averaged daily throughout the first 14 days of the study or until the day of discharge.
Peripheral Blood HPC Cultures
Whole blood from each data point was plated for cultures of HPCs following Stemcell Technologies MethoCult protocol.18 Briefly, mononuclear cells in the peripheral blood were separated by Ficoll-Hypaque density gradient (Sigma-Aldrich, St. Louis, MO) then resuspended in IMDM + glutamine (Gibco Life Technologies, Grand Island, NY) containing 10% fetal calf serum (FCS, Hyclone Laboratories, Greeley, CO). The number of mononuclear cells was enumerated using an inverted microscope and then plated (1 × 106 cells/mL) in MethoCult H4034 Optimum (Stemcell Technologies, Tukila, WA). Cultures were incubated at 37°C in 5% CO2. Colonies (clusters of > 10 cells) were enumerated at the end of incubation (Day 14 for BFU-E and Day 7 for CFU-E) by an observer blinded to the origin of the samples.
Measurement of Plasma G-CSF
Peripheral blood samples were centrifuged at 10,000 rpm for 10 minutes at 10-C to obtain plasma, which was stored at −80°C. Plasma samples were analyzed for G-CSF using commercially colorimetric sandwich enzyme-linked immunosorbent assay kits (Human G-CSF Quantikine ELISA kit, R&D systems, Minneapolis, MN). Assays were performed according to the provided manufacturer’s instructions. All standards and samples were assayed in duplicate.
Statistical Analysis
Data are presented as mean T SEM. Final analysis was based on intention to treat. Statistical analyses were performed using Mann-Whitney U-test and Fisher’s exact test. *p < 0.05 was considered statistically significant.
RESULTS
Patient Population
During the study period, 88 eligible trauma patients were screened. Forty-three patients were excluded from the study (2 failed to stabilize hemodynamically, 38 declined consent, 3 had no appointed surrogates). Forty-five patients were enrolled in the study, 25 were randomized to receive propranolol, and 20 served as untreated controls.
The mean age of the study population was 33 years; 87% were male, with a mean ISS of 29. Approximately 42% presented with blunt injury, and 36% presented to the emergency department in shock (SBP < 90 mm Hg). Patient characteristics are listed in Table 1. There was no significant difference in age, ISS, admission base deficit, or lactate between the two groups (Table 1).
Vital Signs
Propranolol decreased the HR by 10% to 20% as compared with the patient’s baseline HR. There was one patient who had one dose held because of a MAP less than 50 mm Hg, and another patient developed bradycardia, which resolved after the dose was decreased. One third of the participants failed to reach their HR goal with dose escalation, and the most common reason was missed doses of propranolol. The average number of missed doses was five per patient, and the most common reason was a trip to the operating room.
There was a statistically significant difference in HR on Day 1 in the propranolol group as compared with the controls (Fig. 2). At the end of 2 weeks, the HR trend remains lower with propranolol treatment but is not statistically significant. There was no difference in MAP at baseline between the two groups (BB, 68 ± 5 mm Hg vs. no BB, 65 ± 5 mm Hg). After approximately 2 weeks of treatment, there was no significant change in MAP between the groups (BB, 68 ± 2 mm Hg vs. no BB, 73 ± 3 mm Hg).
Figure 2.
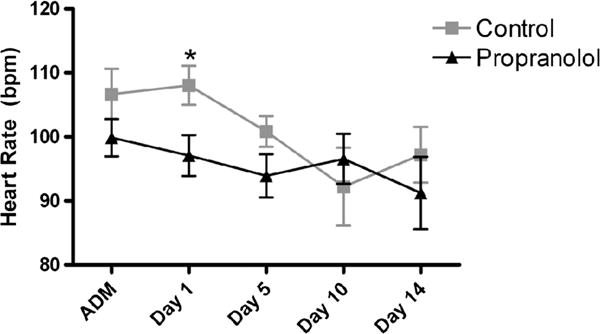
Mean HR by group on admission (ADM), Day 1, Day 5, and Day 10. *p < 0.05.
HPC Mobilization to the Peripheral Blood
HPC (BFU-E and CFU-E) growth in the peripheral blood is a marker of mobilization from BM. On admission, there is significant HPC mobilization to the peripheral blood in both groups, and BFU-E growth is similar in both groups (Fig. 3A). HPC presence in peripheral blood is reduced at all four time points after propranolol administration. On Day 1, there is a significant decrease in HPC mobilization (BFU-E) with propranolol treatment that is maintained through Day 14 (Fig. 3A). On Day 1, BFU-E growth is decreased by 50% with propranolol treatment, and propranolol maintains a 90% decrease in BFU-E growth on Days 10 and 14 as compared with the untreated controls (Fig. 3A).
Figure 3.
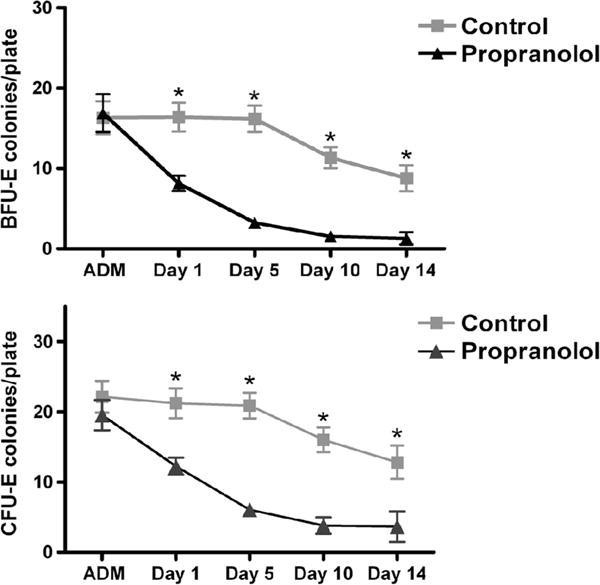
A, The effect of propranolol therapy on HPC mobilization. BFU-E growth in peripheral blood in each group on admission (ADM) and at each time point (Days 1, 5, 10, and 14). B, CFU-E growth in peripheral blood in each group on admission (ADM) and at each time point (Days 1, 5, 10, and 14). *p < 0.05.
Similarly, on admission, there is significant HPC mobilization in the peripheral blood but no difference in CFU-E growth in either group (Fig. 3B). On Day 1, CFU-E growth is significantly decreased with propranolol treatment (12 ± 1* vs. 21 ± 2). Throughout Days 5, 10, and 14, there is a 70% to 80% decrease in CFU-E growth as compared with the untreated controls (Fig. 3B).
Plasma G-CSF
After severe injury, there is a marked increase in plasma levels of G-CSF in both groups on admission. On Day 1, the use of BB decreases plasma levels of G-CSF levels 51% as compared with 37% for the control group (Fig. 4). By day 10, the use of BB decreases G-CSF by 90%. Untreated controls had G-CSF levels that remained elevated from Day 1 to Day 14. On the day of discharge, untreated controls had G-CSF levels that were persistently elevated (BB, 0.8 ± 0.8 pg/mL vs. no BB, 168 ± 85 pg/mL).
Figure 4.
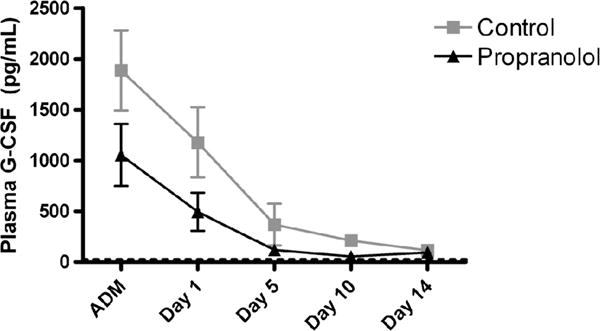
Effect of propranolol therapy on G-CSF levels on admission (ADM) and at each time point (Days 1, 5, 10, and 14).
Hematologic Parameters
There was no difference in admission Hgb levels between the two groups (BB, 12.2 ± 0.5 g/dL vs. no BB, 12.2 ± 0.4 g/dL). On Day 1 following resuscitation, there is a 2-g drop in Hgb in both groups (Table 2). Hgb trends are similar in both groups over time. However, while not statistically significant, the average Hgb is nearly 1 g higher on the date of discharge in those patients receiving propranolol (BB, 9.9 ± 0.4 g/dL vs. no BB, 9.1 ± 0.6 g/dL). There is no difference in the average number of late packed red blood cell transfusions in both groups (BB, 2 ± 0.6 vs. no BB, 2.4 ± 0.6).
TABLE 2.
Effect of Propranolol Therapy on Hematologic Parameters on Admission and at Each Time Point (Day 1, 5, 10, and 14) Until Discharge
| Control
|
Propranolol
|
|||
|---|---|---|---|---|
| Hgb g/dL (95% CI) | Reticulocyte Count % RBCs (95% CI) | Hgb g/dL (95% CI) | Reticulocyte Count % RBCs (95% CI) | |
| Admission | 12.2 ± 0.4 (11.3–13) | 1.2 ± 0.2 (0.9–1.5) | 12.2 ± 0.5 (11.2–13.0) | 1.2 ± 0.1 (0.9–1.4) |
| Day 1 | 9.8 ± 0.3 (9.1–10.5) | 1.7 ± 0.1 (1.4–1.9) | 10.2 ± 0.5 (9.2–11.2) | 1.5 ± 0.2 (1.2–1.8) |
| Day 5 | 8.6 ± 0.3 (8.1–9.3) | 2.7 ± 0.3 (2.1–3.2) | 9.2 ± 0.6 (8.4–9.9) | 2.7 ± 0.2 (2.2–3.1) |
| Day 10 | 8.8 ± 0.4 (8–9.6) | 3.1 ± 0.4 (2.5–4.1) | 9 ± 0.4 (8.1–9.8) | 3.5 ± 0.3 (2.9–4.1) |
| Day 14 | 9.1 ± 0.4 (8.4–9.9) | 3.3 ± 0.4 (2.6–4) | 8.9 ± 0.5 (7.9–9.7) | 4.0 ± 0.6 (2.8–5.3) |
| Discharge | 9.1 ± 0.6 (8.4–10.3) | 3.3 ± 0.3 (2.9–3.9) | 9.9 ± 0.4 (8.6–10.4) | 4.6 ± 0.4 (3.7–5.0) |
RBCs, red blood cells.
The reticulocyte count increases over time in both groups (Table 2). By Day 14, those patients receiving propranolol have a fourfold increase in their reticulocyte count from baseline as compared with the control group, which has only a threefold increase in their reticulocyte count from baseline (Table 2). On the day of discharge, reticulocyte counts continue to increase in the propranolol group, but in the untreated control group, the reticulocyte count has plateaued (BB, 4.6% ± 0.4% vs. no BB, 3.4% ± 0.3%).
Outcomes
There were no mortalities for the 30 days of the study (Table 1). There was one death in the untreated control group that occurred after 30 days when the family withdrew care. The patients in the propranolol group had a shorter ICU stay and less ventilator days, but neither was statistically significant (Table 1). There was no statistical difference in the number of pnuemonias, wound infections, or bacteremias. There was significantly less intra-abdominal abscess in the propranolol treatment group (Table 1), but there were also significantly fewer abdominal surgeries in that group.
DISCUSSION
Following severe trauma, there is an injury-associated persistent anemia that is linked to BM dysfunction. This is the first study in severely injured trauma patients that demonstrates that propranolol administration improves BM dysfunction by measuring BM surrogates, HPC growth in peripheral blood, and Hgb. Early use of propranolol after injury is safe and blunts early tachycardia. In 25 severely injured trauma patients given propranolol, there is prevention of persistent HPC mobilization, a reduction in the persistent elevation of G-CSF, and a trend toward faster resolution of anemia.
Major trauma and thermal injuries induce a significant and sustained release of catecholamines.2,8–11 Catecholamines induce hypermetabolism, tachycardia, increased oxygen demand, and immunologic changes.19–21 In addition to these effects, NE is a mediator of BM dysfunction.2,3,6,12–15 In addition, NE is a key regulator of G-CSF-induced HPC mobilization.4 Plasma G-CSF is markedly elevated after injury, and this associated with prolonged mobilization of HPC.3 The use of propranolol, a nonselective BB, has been shown to have beneficial cardioprotective and metabolic effects as well as improved outcomes in burn patients.22,23 In rodents, we have demonstrated improved BM function with the use of propranolol after injury and hemorrhagic shock.5,14,15,24 This improved BM function is manifested as restored growth of HPCs in BM, prevention of HPC mobilization, reduced G-CSF levels, and improved Hgb levels.5,14,15,24 Furthermore, this improvement of BM function seems to be mediated by β-2 and β-3 receptors.24
In this prospective, randomized trial, early propranolol use led to a reduction in HR. The early use of propranolol in this study was safe but does require continuous hemodynamic monitoring. We had a specific therapeutic goal of decreasing the HR by 10% to 20% from their admission baseline HR. This goal was chosen based on the success of propranolol use in burned children and in rodent studies.20,22,25 In rodents, propranolol exhibited its protective effects in both a time- and dose-dependent fashion.25 The protective effects of propranolol on BM have been guided by the ability of propranolol to decrease HR by 20% in rodents.25 In this trial, propranolol treatment did decrease HR, but only one third of patients maintained their HR goal for the entire duration of the study. This is likely because an average of five doses per patient were missed.
To our knowledge, there has been no study looking at hematologic outcomes with propranolol use in trauma patients. By measuring the amount of HPC in peripheral blood, this study demonstrates that the use of propranolol prevents prolonged HPC mobilization. HPCs have been shown to mobilize following acute injury, and prolonged HPC mobilization is associated with anemia.3 The untreated control group has significant mobilization of HPCs to the peripheral blood similar to previous studies.3 Propranolol use significantly reduced both BFU-E and CFU-E growth in peripheral blood within 24 hours. Continued propranolol treatment prevented late HPC mobilization. The reduction in the sustained release of HPCs into the peripheral blood would likely improve BM function. In this pilot study, direct BM analysis was not performed to confirm this assumption.
To correlate with HPC mobilization, G-CSF levels were measured in both groups of patients. We confirmed that G-CSF peaks early and is markedly elevated after severe trauma.3 Untreated controls had a persistent increase in G-CSF from admission to the day of discharge. These results correlate with similar data in burn patients, demonstrating a peak in G-CSF on Day 2 after burn, and levels that remain elevated through 3 weeks of injury.26 Propranolol treatment reduced G-CSF, and this reduction in G-CSF with propranolol treatment mirrors the decrease seen in HPC mobilization. This data support the idea that severe trauma results in a prolonged stress response that increases NE, which mediates the release of G-CSF and leads to sustained release of HPCs to the peripheral blood; all of which is abrogated by the use of propranolol.
Critically ill trauma patients remain anemic in the absence of any blood loss for several weeks following their initial injury.27–29 This is the first study that demonstrates a potential faster resolution of anemia following trauma with the use of propranolol. In this pilot study, there is a clinically significant increase in Hgb level on the day of discharge with propranolol treatment (9.9 ± 0.4 g/dL vs. 9.1 ± 0.6 g/dL). There was no difference in the number of late packed red blood cell transfusions in either group. Therefore, the observation of the higher discharge Hgb levels is not caused by treatment bias. The lack of statistical significance in Hgb differences may be secondary to the length of follow-up for this study. In this study, patients had an Hgb measurement on the day of discharge. Consequently, not all patients had an Hgb measurement on postinjury Day 30. Four- and six-week time points after injury would provide the most accurate assessment of improvement in Hgb levels.
Increased production of red blood cells should be reflected by an increase in reticulocyte number. Hgb levels and reticulocyte counts have been shown to be related following trauma, and the measurement of reticulocytes is helpful in predicting the rise in Hgb after acute blood loss.30 This study demonstrates that reticulocyte counts increase with time in both groups. However, the rate of increase of reticulocytes is greater in those patients receiving propranolol. These data correlate with the trend toward improved Hgb with propranolol treatment.
When examining the overall outcomes, propranolol use did not lead to mortality. There was no statistically significant difference in ICU length of stay, number of ventilator days, or infections. There is a trend toward a reduced number of ventilator days (7 ± 2 days vs. 11 ± 7 days) and a reduced ICU length of stay (9 ± 2 days vs. 14 ± 4 days) with propranolol treatment. Friese et al.16 and Arbabi et al.17 found that preinjury BB exposure resulted in improved outcomes. These studies did not control for the use of a particular BB, and most commonly metoprolol was the agent prescribed.
The findings of our study must be interpreted within the context of its limitations. First, the treatment team was not blinded to the use of propranolol. However, the researchers were blinded to the origin of the samples during analysis. In addition, only one third of patients met their HR goal for the duration of the study. More aggressive propranolol dosing schedules and appropriate dosing during surgery or procedures might have achieved a statistically significant decrease in HR throughout the study. Although our analysis showed improvement in G-CSFand Hgb levels as well as ventilator days and ICU length of stay with the use of propranolol, this improvement was not statistically significant. This may be explained by both a small sample size and a high variability. While there was no difference in age or ISS between the groups, the higher percentage of penetrating trauma in the control group may also be a confounding variable. We do not believe that the sex ratio played a role in our findings since on repeated analysis, with the females removed from the study, there was no change in the results.
In summary, using surrogate measures of BM function, HPC growth and G-CSF levels in the peripheral blood, as well as Hgb and reticulocyte counts, this study demonstrates that early use of propranolol after severe injury improves BM function. When propranolol is given in doses that decrease the HR by 10% to 20% from baseline values and with hemodynamic monitoring, propranolol is safe, easily administered, and effective. Further clinical investigation of this therapeutic hematologic benefit is warranted.
Supplementary Material
Acknowledgments
DISCLOSURE
This research was supported by the National Institutes of Health grant T32 GM069330.
Footnotes
This study was presented at the 27th Annual Meeting of the Eastern Association for the Surgery of Trauma, January 14–18, 2014, in Naples, Florida.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
AUTHORSHIP
L.E.B., L.V.P., A.V.G., and A.M.M. performed the literature search. Z.C.S., D.H.L., and A.M.M. provided the study design. L.E.B., L.V.P., W.D.A., K.J.S., and A.V.G. performed the data collection. L.E.B., L.V.P., W.D.A., K.J.S., and A.V.G. performed the data analysis. L.E.B., L.V.P., Z.C.S., D.H.L., and A.M.M. performed the data interpretation. L.E.B., L.V.P., Z.C.S., and A.M.M. wrote the article. L.E.B., L.V.P., Z.C.S., D.H.L., and A.M.M. provided critical revision.
References
- 1.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898–905. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect. 2004;5:385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 3.Cook KM, Sifri ZC, Baranski GM, Mohr AM, Livingston DH. The role of plasma granulocyte colony stimulating factor and bone marrow dysfunction after severe trauma. J Am Coll Surg. 2013;216:57–64. doi: 10.1016/j.jamcollsurg.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katayama Y, Battista M, Kao W, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohr AM, Elhassan IO, Hannoush EJ, Sifri ZC, Offin MD, Alzate WD, Rameshwar P, Livingston DH. Does beta blockade post injury prevent bone marrow suppression? J Trauma. 2011;70:1043–1050. doi: 10.1097/TA.0b013e3182169326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bateman AP, McArdle F, Walsh TS. Time course of anemia duringsix months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med. 2009;37:1906–1912. doi: 10.1097/CCM.0b013e3181a000cf. [DOI] [PubMed] [Google Scholar]
- 8.Woolf PD, McDonald JV, Feliciano DV, Kelly MM, Nichols D, Cox C. The catecholamine response to multisystem trauma. Arch Surg. 1992;127:899–903. doi: 10.1001/archsurg.1992.01420080033005. [DOI] [PubMed] [Google Scholar]
- 9.Goodall MC, Stone C, Haynes BW., Jr Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg. 1957;145:479–487. doi: 10.1097/00000658-195704000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–1187. doi: 10.1016/s0039-6109(16)41685-3. [DOI] [PubMed] [Google Scholar]
- 11.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. High circulating adrenaline levels at admission predict increased mortality after trauma. J Trauma Acute Care Surg. 2012;72:428–436. doi: 10.1097/ta.0b013e31821e0f93. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–889. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 13.Penn A, Mohr AM, Shah SG, Sifri ZC, Kaiser VL, Rameshwar P, Livingston DH. Dose-response relationship between norepinephrine and erythropoiesis: evidence for a critical threshold. J Surg Res. 2010;163:e85–e90. doi: 10.1016/j.jss.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baranski GM, Offin MD, Sifri ZC, Elhassan IO, Hannoush EJ, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. A-blockade protection of bone marrow following trauma: the role of G-CSF. J Surg Res. 2011;170:325–331. doi: 10.1016/j.jss.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elhassan IO, Hannoush EJ, Sifri ZC, Jones E, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Beta-blockade prevents hematopoietic progenitor cell suppression after hemorrhagic shock. Surg Infect. 2011;12:273–278. doi: 10.1089/sur.2010.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friese RS, Barber R, McBride D, Bender J, Gentilello LM. Could beta blockade improve outcomes after injury by modulating inflammatory profiles? J Trauma. 2008;64:1061–1068. doi: 10.1097/TA.0b013e3181684cf0. [DOI] [PubMed] [Google Scholar]
- 17.Arbabi S, Campion EM, Hemmila MR, Barker M, Dimo M, Ahrns KS, Niederbichler AD, Ipaktchi K, Wahl WL. Beta-blocker use is associated with improved outcomes in adult trauma patients. J Trauma. 2007;62:56–62. doi: 10.1097/TA.0b013e31802d972b. [DOI] [PubMed] [Google Scholar]
- 18.Song H, Vita M, Sallam H, Tehranchi R, Nilsson C, Sidén A, Hassan Z. Effect of Cdk-inhibitor roscovitine on mouse hematopoietic progenitors in vivo and in vitro. Cancer Chemother Pharmacol. 2007;60:841–849. doi: 10.1007/s00280-007-0431-x. [DOI] [PubMed] [Google Scholar]
- 19.Bryan RM., Jr Cerebral blood flow and energy metabolism during stress. Am J Physiol. 1990;259:J269–J280. doi: 10.1152/ajpheart.1990.259.2.H269. [DOI] [PubMed] [Google Scholar]
- 20.Herndon DN, Barrow RE, Rutan TC, Minifee P, Jahoor F, Wolfe RR. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–492. doi: 10.1097/00000658-198810000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufamn TM, Horton JW. Characterization of cardiac beta-adrenergic receptors in the guinea pig heart: application to study of beta-adrenergic receptors in shock models. J Surg Res. 1993;55:516–523. doi: 10.1006/jsre.1993.1177. [DOI] [PubMed] [Google Scholar]
- 22.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 23.Arbabi S, Ahrns KS, Wahl WL, Hemmila MR, Wang SC, Brandt MM, Taheri PA. Beta-blocker use is associated with improved survival in adult burn patients. J Trauma. 2004;56:265–269. doi: 10.1097/01.TA.0000109859.91202.C8. [DOI] [PubMed] [Google Scholar]
- 24.Beiermeister KA, Keck BM, Sifri ZC, ElHassan IO, Hannoush EJ, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 receptors after injury. J Trauma. 2010;69:338–343. doi: 10.1097/TA.0b013e3181e5d35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranski GM, Pasupuleti LV, Sifri ZC, Cook KM, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Beta blockade protection of bone marrow following injury: a critical link between heart rate and immunomodulation. J Bone Marrow Res. 2013;1:124. doi: 10.4172/2329-8820.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG. Serum cytokine differences is severely burned children with and without sepsis. Shock. 2007;27:4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 27.Shander A, Gandhi NR, Goodnough LT. Anemia, erythropoietin, and the trauma patient. ITACCS. 2008;18:29–34. [Google Scholar]
- 28.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 29.Marque S, Cariou A, Chiche JD, Mallet VO, Pene F, Mira JP, Dhainaut JF, Claessens YE. Risk factors for post-ICU red blood cell transfusion: a prospective study. Crit Care. 2006;10:R129. doi: 10.1186/cc5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otterman ML, Nijboer JM, van der Horst IC, van Meurs M, ten Duis HJ, Nijsten MW. Reticulocyte counts and their relation to hemoglobin levels in trauma patients. J Trauma. 2009;67:121–124. doi: 10.1097/TA.0b013e318187a848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


