Abstract
Pectobacteria are devastating plant pathogens that infect a large variety of crops, including members of the family Brassicaceae. To infect cabbage crops, these plant pathogens need to overcome the plant's antibacterial defense mechanisms, where isothiocyanates are liberated by hydrolysis of glucosinolates. Here, we found that a Pectobacterium isolate from the gut of cabbage root fly larvae was particularly resistant to isothiocyanate and even seemed to benefit from the abundant Brassica root metabolite 2-phenylethyl isothiocyanate as a nitrogen source in an ecosystem where nitrogen is scarce. The Pectobacterium isolate harbored a naturally occurring mobile plasmid that contained a sax operon. We hypothesized that SaxA was the enzyme responsible for the breakdown of 2-phenylethyl isothiocyanate. Subsequently, we heterologously produced and purified the SaxA protein and characterized the recombinant enzyme. It hydrolyzed 2-phenylethyl isothiocyanate to yield the products carbonyl sulfide and phenylethylamine. It was also active toward another aromatic isothiocyanate but hardly toward aliphatic isothiocyanates. It belongs to the class B metal-dependent beta-lactamase fold protein family but was not, however, able to hydrolyze beta-lactam antibiotics. We discovered that several copies of the saxA gene are widespread in full and draft Pectobacterium genomes and therefore hypothesize that SaxA might be a new pathogenicity factor of the genus Pectobacterium, possibly compromising food preservation strategies using isothiocyanates.
INTRODUCTION
Pectobacteria are phytopathogens that cause tuber soft rot and blackleg (stem rot) in many horticulturally and economically important crops during cultivation, transport, or storage. Most Pectobacterium spp., e.g., Pectobacterium carotovorum or P. wasabiae, can be detrimental to many different plants, such as potato, sugar beet, cabbage, wasabi, chicory, and Ornithogalum plants, whereas other Pectobacterium spp., e.g., P. atrosepticum and P. betavasulorum, have a more narrow host range of potato and sugar beet plants, respectively (1).
Several pathogenicity factors of soft-rot-causing Pectobacterium spp. have been found during the last decades (2–4). Of these, plant cell wall-degrading enzymes produced by Pectobacterium spp. have a large impact on the progress of the disease, as their production leads to the degradation of invaded plant tissue (2, 3). The production of these enzymes is dependent on cell density and regulated by quorum sensing through N-acetylhomoserine lactone (5) and intracellular regulators (6, 7). Motility and nutrient uptake are also factors influencing phytopathogenicity (4, 8–10). Besides these general pathogenicity factors, phytopathogens have to overcome the toxicity of plant allelochemicals. Important allelochemicals of members of the family Brassicaceae (cabbages and mustards) are isothiocyanates that are liberated by the glucosinolate-myrosinase defense system (11–13). The antimicrobial effect of isothiocyanates has been mainly attributed to their reactivity with thiol groups in proteins observed in vitro, but there is a variety of metabolic functions that have been found to be negatively influenced by isothiocyanates in vivo (recently reviewed in reference 14). As a consequence, isothiocyanates can be used as food preservatives to prevent microbial growth and spoilage. In microorganisms, general allelochemical defense systems are efflux pumps that decrease the intracellular concentration of toxic substances. Several studies identified TolC as a pathogenicity factor related to the extrusion of phytochemicals (4, 15, 16). TolC is an outer membrane protein that interacts with efflux pumps of the cytoplasmic membrane (17, 18). Although several phytochemicals have been tested as substrates of Pectobacterium TolC (4, 15, 16), none of the substrates tested included isothiocyanates. In addition to this, a transposon mutagenesis study of Pseudomonas syringae pv. tomato revealed several multidrug efflux pumps (Sax proteins) that could be associated with TolC and be crucial for the survival of P. syringae pv. tomato on isothiocyanate-containing Arabidopsis extract (19). It seems that TolC, together with (multidrug) efflux pumps, may also play a role in the defense of pectobacteria against isothiocyanates (14, 19). Another defense system against isothiocyanates may be their breakdown or chemical modification. Although the antimicrobial effects of isothiocyanates have been known for a long time, microbial isothiocyanate degradation pathways have not been described so far. In light of the use of isothiocyanates for food preservation and as antibiotic additives, a microbial enzymatic breakdown system may form the basis of microbial resistance or detoxification. There are indications that proteins from the Sax system identified in P. syringae pv. tomato degrade isothiocyanates (19) and that a distinct class of glutathione S-transferases may play a role in cyanobacteria (20, 21) and possibly in other microbes (14, 22).
In this study, we investigated a SaxA protein encoded by a plasmid found in a Pectobacterium isolate from the cabbage root fly larval gut microbiome (23). It belongs to the metallo-beta-lactamase family but is not active toward beta-lactam antibiotics. Instead, it efficiently catalyzed the hydrolysis of aromatic isothiocyanates and pectobacteria could take advantage of the liberated nitrogen compounds for metabolism and growth. We found that the saxA gene is widespread in many Pectobacterium genomes, sometimes even in up to three distinct copies per genome. In the light of phytopathogenicity, the Pectobacterium saxA gene may be an additional pathogenicity factor when Pectobacterium infects Brassica plants that are the natural sources of isothiocyanates.
MATERIALS AND METHODS
Bacterial strains and vectors.
Pectobacterium strain CW-5 was isolated from the cabbage root fly larval gut (23). The same source was used to obtain the saxA gene encoding the protein used in this study.
Growth characterization.
Pectobacterium strain CW-5 was grown in 100 ml of minimal medium (45 mM Na2HPO4, 22 mM KH2PO4, 8.5 mM NaCl, 1 mM MgSO4, 1 μM FeSO4, 100 μM CaCl2, 200 μl/liter vitamin solution [DSM medium 141, http://www.dsmz.de/microorganisms/medium/pdf/DSMZ_Medium141.pdf], 1 ml/liter trace element solution [24], 0.2% glucose, pH 7.0) with 1 mM NH4Cl or 1 mM 2-phenylethyl isothiocyanate as a nitrogen source. Optical density at 600 nm was determined in plastic cuvettes with a 1-cm light path.
Production and characterization of recombinant SaxA.
SaxA was produced in Escherichia coli BL21 Star (Life Technologies, Bleiswijk, The Netherlands) with the pASK-saxA vector (23), which was derived from the pASK-IBA3(+) vector (IBA, Goettingen, Germany). The recombinant protein was C terminally fused to Strep-tag and produced in 200 ml of maximal induction medium (25) (32 g liter−1 tryptone, 20 g liter−1 yeast extract) containing 1× M9 salts, 100 μM CaCl2, 1 mM MgSO4, 1 μM FeNH4 citrate, and 100 μM ZnSO4. The cells were grown to an optical density at 600 nm of 0.5, after which protein production was induced with 200 ng ml−1 anhydrotetracycline. After cell harvesting (6,000 × g, 15 min), cells were disrupted by suspension in CelLytic B (Sigma-Aldrich) according to the manufacturer's instructions. After centrifugation (15,000 × g, 15 min, 4°C), cell extract was applied to a 1-ml Strep-Tactin affinity chromatography column (IBA, Goettingen, Germany). Affinity chromatography was done in accordance with the manufacturer's instructions by using Tris-HCl buffer (100 mM Tris, 150 mM NaCl, pH 8). Protein content was determined with the Bradford assay (26), and protein-containing elution fractions were pooled.
Polyacrylamide gel electrophoresis (PAGE) was performed with a Criterion TGX 4 to 15% polyacrylamide gel (Bio-Rad, Veenendaal, The Netherlands) with SDS-Tris-glycine buffer according to the manufacturer's instructions. Protein samples of 5 μg of were applied to the gel, which was subsequently visualized by silver staining (27). Western blot analysis was performed with antibodies directed against Strep-tag (IBA, Göttingen, Germany). The recombinant SaxA protein was visualized by the reaction of horseradish peroxidase conjugated directly to the antibody with 4-chloro-1-naphthol and H2O2 as described by the manufacturer (IBA, Göttingen, Germany).
Enzyme assays.
Enzyme activity was determined in a discontinuous assay in a 5-ml reaction mixture that contained KP buffer (40 mM KH2PO4-K2HPO4, pH 7.0), 100 μM substrate (diluted in dimethyl sulfoxide [DMSO]), and 7.5 μg of SaxA at 20°C. At various time points within 30 min, 500-μl samples were taken from the reaction mixture and isothiocyanates were directly extracted with 100 μl of dichloromethane. One microliter of the dichloromethane sample was injected into an Interscience TRACE gas chromatograph (GC) 2000 (Interscience, Breda, The Netherlands) that contained an HP-5ms capillary column (30-m length, 0.25-mm inside diameter [ID], 0.25 μm; Agilent Technologies, Middelburg, The Netherlands) and an AL3000 autoinjector. This GC was connected to a Thermo Finnigan (Polaris Q) ion trap mass spectrometer (Interscience, Breda, The Netherlands). The gas chromatography conditions used for isothiocyanate analyses were 50°C for 1 min, followed by a temperature gradient of 50°C/min to 250°C for 1 min. The split ratio was 1:20. Peaks were integrated, and DMSO was used as an internal standard to quantify isothiocyanates. Control reactions without enzyme were performed for every enzyme assay to correct for the non-enzyme-mediated volatility or reactivity of the substrate.
Degradation of the beta-lactam antibiotics ampicillin and cefotaxime was assayed by high-performance liquid chromatography (HPLC; Agilent 1100; Agilent, Amstelveen, The Netherlands) with a Merck Lichrocart 250-4 RP-18 (5 μm) column. One milliliter of KP buffer containing 1 mM ampicillin or cefotaxime and 10 μg of SaxA was incubated at 15°C in an HPLC reaction vial, and every 7.2 min, a 10-μl sample was injected into the HPLC column. An isocratic mixture of 0.05 M KH2PO4 (pH 4.0) and acetonitrile (50:50) was used for elution. Peaks were detected with a photodiode array detector with 360 nm as the integration wavelength. A control sample without SaxA was measured to ensure the stability of the antibiotics under the conditions used.
Breakdown product analysis.
For the analysis of carbonyl sulfide (COS) production, the enzyme assay was performed with a rubber-stoppered 15-ml serum bottle as described for enzyme assays. The solution was stirred with a Micro Stir Bar at 400 rpm at room temperature. Samples were taken for 2 min every 11.5 min with a solid-phase microextraction (SPME) fiber (100-μm polydimethylsiloxane, fused silica 24-gauge needle; Sigma-Aldrich, Bellefonte, PA, USA). Immediately after sampling, the fiber was desorbed in the GC injection port (250°C) and analyzed by gas chromatography coupled to a high-resolution mass spectrometer (JEOL AccuTOF-GCv JMS-100GCv equipped with an Agilent 7890A GC that contains an HP-5ms column [30 m by 0.25 mm, 0.25 μm]). The gas chromatography program was set to 50°C for 1.5 min, followed by a temperature gradient of 50°C/min to 300°C for 1 min; a split ratio of 1:10 was used. Peaks were detected by using the total ion current (TIC) and a selected ion trace with a mass range of m/z 59.85 to 60.15 for the determination of COS. Quantification was performed with comparison to a calibration curve based on a COS standard (≥96%; Aldrich). As other volatiles (e.g., isothiocyanate) in the reaction mixture may interfere with adsorption of COS to the SPME fiber, which would not be the case for measurement of the calibration curve, this method should be regarded as semiquantitative and likely overestimates the amount of COS in the reaction mixture (28). The other expected reaction product was phenylethylamine. Below a pH of 9.83 (pKa of phenylethylamine), the compound is protonated and cannot be extracted with dichloromethane from water. For this reason, the pH of 1 ml of the sample mixture (assay performed according to the enzyme assay procedure) was adjusted with 10 μl of 10 M sodium hydroxide to adjust the pH to >10; this was followed by extraction with 100 μl of dichloromethane. A 1-μl volume of the dichloromethane phase was analyzed by high-resolution gas chromatography-mass spectrometry (50°C for 1.5 min, followed by a temperature gradient of 50°C/min to 300°C for 1 min; split ratio of 1:10; detector voltage of 2,000 V). Peaks were detected by TIC and a selected ion trace with a mass range of m/z 56.00 to 56.10 for the determination of phenylethylamine. As a standard, a phenylethylamine sample (Fluka) was analyzed by high-resolution gas chromatography-mass spectrometry under the same conditions as the dichloromethane phase from the enzyme sample.
Genome analyses.
For the investigation of the presence or absence of the saxA gene in fully sequenced genomes of the Pectobacterium clade, we used the genome sequences that were available in the Kyoto Encyclopedia of Genes and Genomes (http://www.genome.jp/kegg/) to look for orthologues. At the time of the analysis (June 2015), this comprised eight genomes (eca, patr, pato, pct, pcc, pcv, pwa, and pec).
To determine the distribution of the saxA gene in draft genome sequences of members of the Pectobacterium clade, the Drgb-derived SaxA protein (Drgb-SaxA, Delia radicum gut bacteria) sequence (GenBank accession number ALG88671) was used as a query in a BLASTp search of NCBI (http://www.ncbi.nlm.nih.gov/, June 2015) that targeted only the genus Pectobacterium (taxid:122277). Those hits with high bit scores (>250) and E values of <1.0 × e−50 were considered further in the analysis. The protein sequences were extracted and compared by multiple-sequence alignment (http://www.ebi.ac.uk/Tools/msa/clustalo/). Using this information, a phylogenetic tree was calculated with MEGA6 (29).
RESULTS AND DISCUSSION
In this study, we investigated a new mechanism for the phytopathogen Pectobacterium to overcome the toxicity of isothiocyanates. We showed previously that a Pectobacterium isolate from the gut of cabbage root fly larvae (Delia radicum L.) feeding on kohlrabi and rutabaga, two Brassica crops, contained a plasmid encoding a SaxA protein (23). The present study aimed at characterizing the Pectobacterium SaxA protein with respect to the breakdown of the toxic isothiocyanate and highlights an important additional function, the provision of ammonium in nitrogen-deprived ecosystems.
Pectobacterium strain CW-5 uses isothiocyanate as a nitrogen source.
Cabbage roots have a more woody structure than other annual plants, with a high lignin content and a very low N/C ratio (30) that makes nitrogen scarce for cabbage root-feeding insects and phytopathogenic root bacteria. As the cabbage root fly larvae from which the Pectobacterium strain CW-5 isolate investigated here was obtained were fed on cabbage roots, Pectobacterium strain CW-5 likely also experienced nitrogen limitation. Therefore, we investigated whether additional nitrogen could be assimilated from nitrogen-containing isothiocyanates. We performed growth experiments with minimal medium containing 1 mM NH4+, 1 mM 2-phenylethyl isothiocyanate, and both compounds as a combined nitrogen source (Fig. 1). It appeared that both NH4+ and 2-phenylethyl isothiocyanate served equally well as nitrogen sources, leading to the same amount of biomass, as measured by determining the final optical density. The growth rate was higher in the NH4+ cultures. When both nitrogen sources were combined, the final optical density was twice as high. This illustrates that 2-phenylethyl isothiocyanate could indeed be used as a nitrogen source, either independently or together with other nitrogen sources like ammonium. 2-Phenylethyl isothiocyanate was not used as carbon or energy source.
FIG 1.
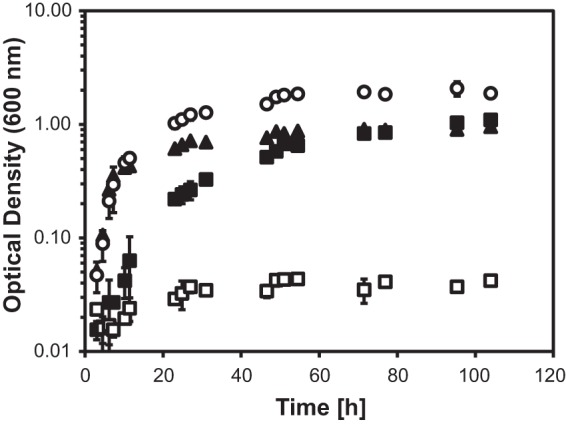
Growth curves determined by measuring the optical density at 600 nm of Pectobacterium strain CW-5 on minimal medium with different nitrogen sources. Symbols: ▲, 1 mM ammonium as a sole nitrogen source; ■, 1 mM 2-phenylethyl isothiocyanate as a sole nitrogen source; ○, both ammonium (1 mM) and 2-phenylethyl isothiocyanate (1 mM) as nitrogen sources; □, no nitrogen source. Average values ± standard deviations were calculated from two independent experiments.
To elucidate how nitrogen was released from 2-phenylethyl isothiocyanate, we envisioned different intermediates of 2-phenylethyl isothiocyanate breakdown that may yield NH4+ as a by-product (Fig. 2). When we compared the growth of Pectobacterium strain CW-5 with the two potential intermediates of 2-phenylethyl isothiocyanate breakdown—phenylethylamine and thiocyanate—as nitrogen sources, it became apparent that 2-phenylethyl isothiocyanate and phenylethylamine could be used equally well as nitrogen sources, whereas thiocyanate did not support growth. There are two pathways described for the breakdown of phenylethylamine, one that is oxygen dependent using an oxidase and one that uses an oxygen-independent dehydrogenase. We found that growth with phenylethylamine as a nitrogen source was dependent on the presence of oxygen, whereas anaerobic growth on the same medium with ammonium as a nitrogen source was also possible. We concluded that the release of nitrogen, probably in the form of ammonium, was dependent on the action of phenylethylamine oxidase (31).
FIG 2.
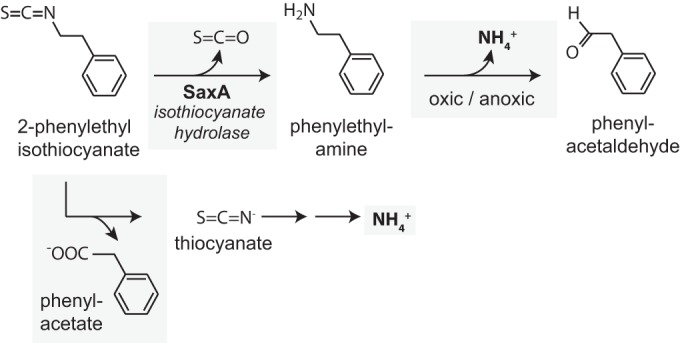
Possible pathway of 2-phenylethyl isothiocyanate degradation for provision of ammonium in Pectobacterium strain CW-5. In vivo experiments support the upper pathway for the provision of nitrogen for the growth of Pectobacterium strain CW-5. In vitro experiments with purified SaxA from the same organism demonstrated that it catalyzes the hydrolysis of 2-phenylethyl isothiocyanate to phenylethylamine and COS.
Drgb-SaxA catalyzes the hydrolysis of aromatic isothiocyanates.
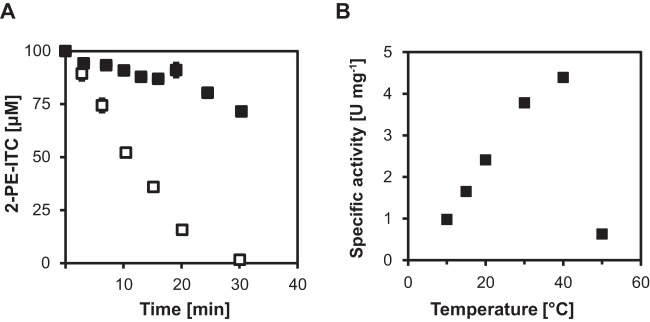
The SaxA protein that was investigated in this study was obtained from a Pectobacterium strain isolated from the D. radicum gut (23). Here, we purified and characterized this protein. The Strep-tag-fused protein appeared as a single band with an approximate molecular mass of 30 kDa on an SDS-polyacrylamide gel after silver staining. The identity of the protein was confirmed by Western blotting with an antibody directed against Strep-tag. The protein could be purified from the E. coli culture in high yields (2 to 5 mg/100-ml culture volume). The activity of the enzyme was recorded in a discontinuous assay after extraction of the isothiocyanates with dichloromethane and measurement of the isothiocyanate content by gas chromatography coupled to mass spectrometry (Fig. 3A). Enzyme assays were performed with 2-phenylethyl isothiocyanate, which is one of the main root volatiles of Brassica plants (32). The temperature optimum was found at 40°C with 4.4 U mg−1 (Fig. 3B), whereas at 50°C, enzyme activity was essentially inactivated. Pectobacteria infect crops at a variety of temperatures (stored versus field crops), so it is noteworthy that the temperature spectrum at which Drgb-SaxA is active is relatively broad and reasonable activity can still be detected at only 10°C with about 1.0 U mg−1. When we tested other plant volatiles as substrates (Table 1), it became apparent that the aromatic benzyl isothiocyanate was also a substrate for the Drgb-SaxA protein. In contrast, Drgb-SaxA was hardly active toward the aliphatic allyl and ethyl isothiocyanates. Cinnamic acid is a widespread plant secondary metabolite not limited to Brassica plants that has an aromatic ring structure and an activated carbon atom in an aliphatic side chain, similar to 2-phenylethyl isothiocyanate. We found that it was not hydrolyzed by the Drgb-SaxA protein. As SaxA belongs to the metallo-beta-lactamase fold enzyme class, we also determined whether it can hydrolyze beta-lactam antibiotics. Neither ampicillin (of the penicillin class) nor cefotaxime (of the cephalosporin class) was hydrolyzed (Table 1).
FIG 3.
Biochemical characterization of the recombinant Drgb-SaxA enzyme. (A) Discontinuous enzyme activity measurement (□) and control without enzyme (■). The assay mixture contained 5 ml of buffer (40 mM K2HPO4-KH2PO4, pH 7.0), 7.5 μg of Drgb-SaxA, and 100 μM 2-phenylethyl isothiocyanate (2-PE-ITC), and the assay was performed at 20°C. (B) Temperature dependence of Drgb-SaxA enzyme activity. The highest activity was found at a temperature of 40°C.
TABLE 1.
Substrates tested with the recombinant Drgb-SaxA enzyme
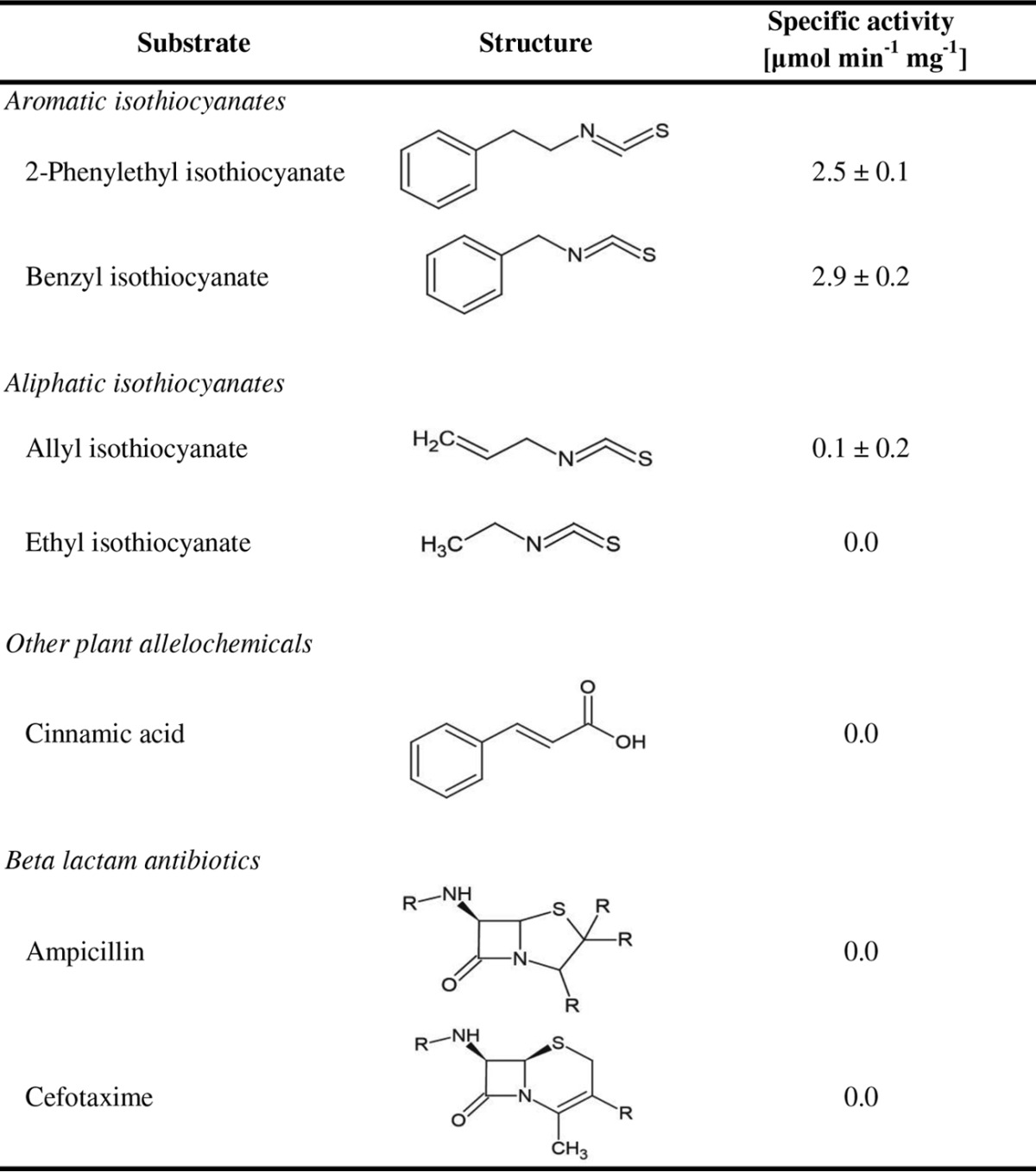
Drgb-SaxA hydrolyzes 2-phenylethyl isothiocyanate to COS and phenylethylamine.
The in vivo assays with Pectobacterium strain CW-5 indicated that SaxA hydrolyzes the N=C double bond of the R—N=C=S moiety of 2-phenylethyl isothiocyanate (Fig. 2). Therefore, in vitro assays with Drgb-SaxA and 2-phenylethyl isothiocyanate as the substrate should yield the breakdown products COS and phenylethylamine. We were able to identify COS in nearly stoichiometric amounts. Phenylethylamine was identified unambiguously on a qualitative scale. Enzymatic assays were run until the endpoint of 30 min until all of the 2-phenylethyl isothiocyanate was converted and breakdown products were analyzed. For the measurement of COS, an SPME fiber was exposed to the closed headspace of the enzyme assay to adsorb gaseous compounds. The fiber was subsequently injected into the GC coupled to a high-resolution mass spectrometer, where adsorbed substances were liberated. A clear peak was observed at 1.2 min with an accurate mass of 59.9673 mass units that matched well the monoisotopic expected mass of COS (59.9669 mass units). The difference in measured mass (0.0003 mass unit) is within the range of the specified inaccuracy of the instrument (0.0020 mass unit). Furthermore, a molecule isotope peak of 34S was visible at the expected abundance in the spectrum that confirmed the presence of sulfur. COS was the only molecule we found that explains the above-mentioned measurements. After a COS calibration curve was recorded by the same semiquantitative method, we found that 135% of the substrate 2-phenylethyl isothiocyanate was converted to COS, indicating that all of the substrate was converted to COS in a 1:1 stoichiometry. Without enzyme, no COS production was observed. The second breakdown product, phenylethylamine, was detected by extraction with dichloromethane, followed by high-resolution gas chromatography coupled to mass spectrometry. During the analysis of the sample, a peak was detected with the same retention time (6.3 min) as the phenylethylamine standard and an identical mass spectrum. This peak had a measured accurate mass of 121.0903 mass units that differed by only 0.0012 mass unit from the calculated monoisotopic mass (121.0891 mass units). During an enzyme assay with SaxA, the respective phenylethylamine peak increased while the 2-phenylethyl isothiocyanate peak decreased, which strongly suggests that phenylethylamine is the reaction product of 2-phenylethyl isothiocyanate hydrolysis. We also investigated whether thiocyanate would be formed in any of our fractions, but that compound could not be detected. Therefore, we established that 2-phenylethyl isothiocyanate is hydrolyzed to phenylethylamine and COS.
Drgb-SaxA is the first member of the new class of isothiocyanate hydrolases for which an enzymatic activity has been experimentally demonstrated. Therefore, comparison to other isothiocyanate hydrolases is not yet possible. As Drgb-SaxA contained a metallo-beta-lactamase fold, we compared its catalytic activity to that of related enzymes in the same superfamily. The most closely related enzymes were the beta-lactamases of Shigella flexneri and Serratia marcescens, which catalyzed the hydrolysis of the beta-lactam antibiotic imipenem at 14.1 and 61.0 U mg protein−1, respectively (33, 34). These specific activity values are about 10-fold higher than the specific activities that we have found for Drgb-SaxA. More distant beta-lactamases, however, exhibit considerably higher specific activity (e.g., 272 U mg protein−1 reported in reference 35), highlighting that catalytic parameters within the enzyme superfamily are not directly comparable.
SaxA may compromise isothiocyanate-utilizing food preservation strategies.
Allyl isothiocyanate has been tested as a preservative for a wide variety of foods, including water (36), spinach leaves (37), cherry tomatoes (38), fresh cut onions (39), cantaloupe (40), kimchi (41), bread (42), chicken meat (43, 44), catfish (45), and ham (46). The application of allyl isothiocyanate is usually via microencapsulation into different matrices to trap the strong odor of allyl isothiocyanate. Other isothiocyanates, including benzyl isothiocyanate and 2-phenylethyl isothiocyanate, have been proposed for use as food preservatives because of their strong growth inhibition of various pathogenic bacteria (47–50) but have not yet been tested as single compounds in food preservation. Because of their antimicrobial effect, isothiocyanates could also be used as synergists with antibiotics, lowering the doses of conventionally used antibiotics (51–53). Here we show that Drgb-SaxA hydrolyzes isothiocyanates in vitro and in vivo and may therefore be regarded as a potential resistance protein compromising the use of isothiocyanates in food preservation and as synergists for antibiotics.
Comparative genome analysis of pectobacteria for saxA genes.
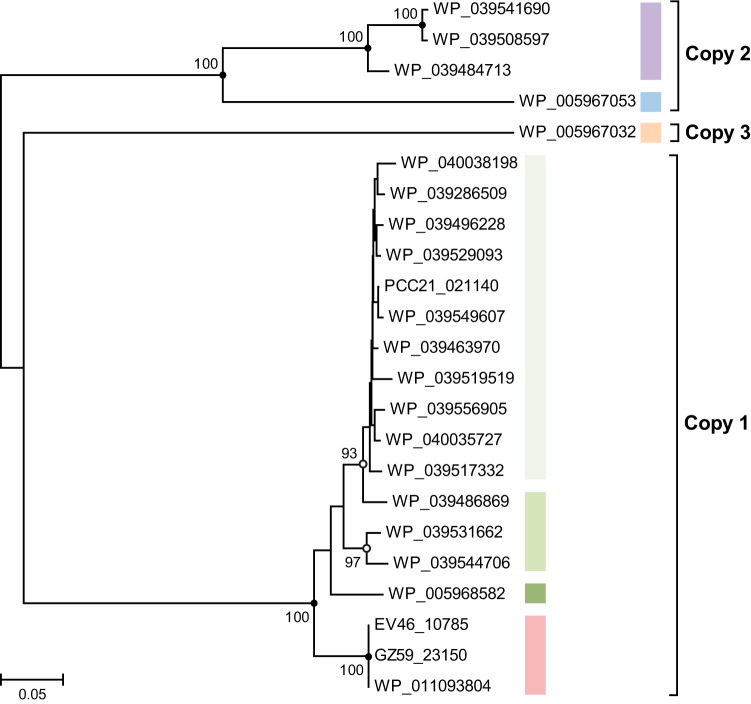
Pectobacteria are important phytopathogens with many representatives whose genomes have been sequenced. In a first step to analyze the occurrence of the saxA gene, we compared Pectobacterium spp. whose genomes have been fully sequenced with an available isolation source for the presence of saxA genes. We observed that all of the isolates that were cultivated from Brassica and wasabi plants contained saxA genes, whereas only part of the strains isolated from potato (Solanum tuberosum) plants contained a saxA gene in their genomes. In a second step, we investigated the distribution of saxA genes in draft genome sequences of pectobacteria. Draft genome sequences lacking a saxA gene were not included in the analysis; rather, we focused on those genomes that contained one or more copies of the saxA gene. It became apparent that P. wasabiae strains isolated from wasabi (Eutrema wasabi) plants contained three copies of the saxA gene in their genomes (Table 2) whose corresponding protein sequences were remarkably distinct from each other (Fig. 4). At the same time, three distinct saxA copies was also the maximum number that we found in a single Pectobacterium genome. The first and second saxA copies occurred individually or in combination in other draft genomes, whereas the third copy seems to be unique to the P. wasabiae clade, as it was not present in any of the other genomes investigated. Regarding the distribution of the first two copies, we found that P. carotovorum subsp. carotovorum strains contained either one or both of the two copies in their genomes and were isolated predominantly from Brassica rapa. All of the draft genome sequences of P. carotovorum subsp. brasiliense and P. atrosepticum investigated contained only the first copy of the saxA gene. When the three different copies were compared on the amino acid level, it became apparent that there was a remarkable difference between the second copy and the other two. All of the proteins that were encoded by the second copy contained a signal peptide for periplasmic translocation of the SaxA protein, whereas the other two copies encoded cytoplasmic enzymes, which is unusual for metallo-beta-lactamase class proteins. All of the copies contain conserved amino acids for the binding of two zinc ions (His67, His69, Asp71, His72, His128). The overall sequence identity of all of the amino acid sequences investigated was as low as 31%. The closely related class B beta-lactamases also show weak sequence similarity and therefore great structural variety. At the same time, they often exhibit similar catalytic behavior (54), which is why we think that their low overall amino acid conservation does not contradict a similar substrate range for the SaxA enzymes. The sequence identity within the different copy groups was high: the proteins derived from copy one were 83% identical to each other, and those from copy two were 66% identical to each other, excluding the signal peptide, which was functionally but not structurally conserved (16% identity). Only one sequence of copy three was available. These results demonstrate that the saxA gene is widespread in phytopathogenic pectobacteria, specifically, in those strains that were isolated from Brassica or wasabi vegetables. This analysis cannot reveal the possible roles or substrates of the different enzyme groups, which await further biochemical studies.
TABLE 2.
Analysis of full and draft Pectobacterium genome sequences for the presence of SaxA-encoding genes, including isolation sites and geographic locations
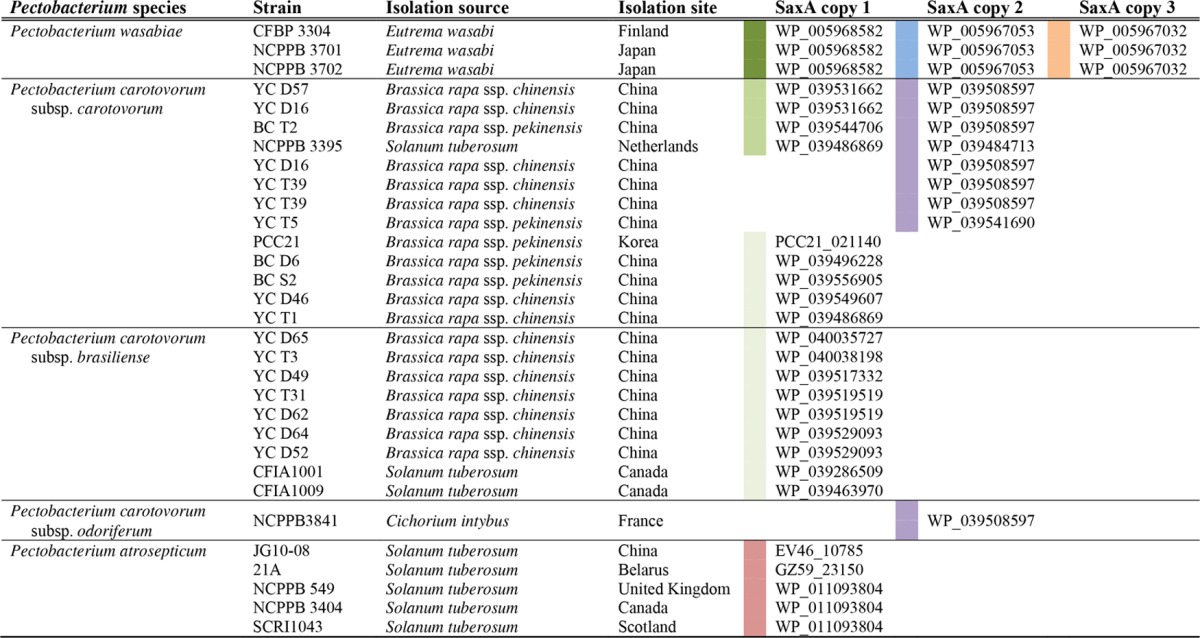
FIG 4.
Neighbor-joining tree of SaxA homologues found in full and draft Pectobacterium genome sequences. Amino acid sequences were aligned with Clustal Omega, and phylogenetic trees were calculated in MEGA6 (29) by using 500 bootstrap replicates. Analysis of the data set by the maximum-likelihood method resulted in the same tree topology. The values on the branches are the percentages of replicate trees in which the associated taxa clustered together in the bootstrap test for values of >75. Branches are drawn to scale, and branch lengths are in the same units as the evolutionary distances used to infer the phylogenetic tree. The color coding corresponds to that in Table 2.
ACKNOWLEDGMENTS
We thank Peter van Galen and Liesbeth Pierson for technical support.
This work was supported by a DAAD postdoctoral fellowship to C.U.W., an ERASMUS fellowship to J.F.R., and SIAM gravitation grant 24002002. The funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Ma B, Hibbing ME, Kim HS, Reedy RM, Yedidia I, Breuer J, Breuer J, Glasner JD, Perna NT, Kelman A, Charkowski AO. 2007. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology 97:1150–1163. doi: 10.1094/PHYTO-97-9-1150. [DOI] [PubMed] [Google Scholar]
- 2.Pérombelon MCM. 2002. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol 51:1–12. doi: 10.1046/j.0032-0862.2001.Shorttitle.doc.x. [DOI] [Google Scholar]
- 3.Toth IK, Bell KS, Holeva MC, Birch PRJ. 2003. Soft rot erwiniae: from genes to genomes. Mol Plant Pathol 4:17–30. doi: 10.1046/j.1364-3703.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Lim JA, Lee J, Roh E, Jung K, Choi M, Oh C, Ryu S, Yun J, Heu S. 2013. Characterization of genes required for the pathogenicity of Pectobacterium carotovorum subsp. carotovorum PCC21 in Chinese cabbage. Microbiology 159:1487–1496. doi: 10.1099/mic.0.067280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Põllumaa L, Alamae T, Mae A. 2012. Quorum sensing and expression of virulence in pectobacteria. Sensors 12:3327–3349. doi: 10.3390/s120303327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Y, Chatterjee A, Hasegawa H, Chatterjee AK. 2006. Erwinia carotovora subspecies produce duplicate variants of ExpR, LuxR homologs that activate rsmA transcription but differ in their interactions with N-acylhomoserine lactone signals. J Bacteriol 188:4715–4726. doi: 10.1128/JB.00351-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laasik E, Ojarand M, Pajunen M, Savilahti H, Mäe A. 2005. Novel mutants of Erwinia carotovora subsp carotovora defective in the production of plant cell wall degrading enzymes generated by Mu transpososome-mediated insertion mutagenesis. FEMS Microbiol Lett 243:93–99. doi: 10.1016/j.femsle.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 8.Pirhonen M, Saarilahti H, Karlsson M-B, Palva ET. 1991. Identification of pathogenicity determinants of Erwinia carotovora subsp. carotovora by transposon mutagenesis. Mol Plant Microbe Interact 4:276–283. doi: 10.1094/MPMI-4-276. [DOI] [Google Scholar]
- 9.Urbany C, Neuhaus HE. 2008. Citrate uptake into Pectobacterium atrosepticum is critical for bacterial virulence. Mol Plant Microbe Interact 21:547–554. doi: 10.1094/MPMI-21-5-0547. [DOI] [PubMed] [Google Scholar]
- 10.Mole B, Habibi S, Dangl JL, Grant SR. 2010. Gluconate metabolism is required for virulence of the soft-rot pathogen Pectobacterium carotovorum. Mol Plant Microbe Interact 23:1335–1344. doi: 10.1094/MPMI-03-10-0067. [DOI] [PubMed] [Google Scholar]
- 11.Winde I, Wittstock U. 2011. Insect herbivore counteradaptations to the plant glucosinolate-myrosinase system. Phytochemistry 72:1566–1575. doi: 10.1016/j.phytochem.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Finch S, Ackley CM. 1977. Cultivated and wild host plants supporting populations of the cabbage root fly. Ann Appl Biol 85:13–22. doi: 10.1111/j.1744-7348.1977.tb00626.x. [DOI] [Google Scholar]
- 13.Halkier BA, Gershenzon J. 2006. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 14.Dufour V, Stahl M, Baysse C. 2015. The antibacterial properties of isothiocyanates. Microbiology 161:229–243. doi: 10.1099/mic.0.082362-0. [DOI] [PubMed] [Google Scholar]
- 15.Barabote RD, Johnson OL, Zetina E, San Francisco SK, Fralick JA, San Francisco MJD. 2003. Erwinia chrysanthemi tolC is involved in resistance to antimicrobial plant chemicals and is essential for phytopathogenesis. J Bacteriol 185:5772–5778. doi: 10.1128/JB.185.19.5772-5778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Karablieh N, Weingart H, Ullrich MS. 2009. The outer membrane protein TolC is required for phytoalexin resistance and virulence of the fire blight pathogen Erwinia amylovora. Microb Biotechnol 2:465–475. doi: 10.1111/j.1751-7915.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federici L, Walas F, Luisi B. 2004. The structure and mechanism of the TolC outer membrane transport protein. Curr Sci 87:190–196. http://www.iisc.ernet.in/currsci/jul252004/190.pdf. [Google Scholar]
- 18.Piddock LJ. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat Rev Microbiol 4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Crooks C, Creissen G, Hill L, Fairhurst S, Doerner P, Lamb C. 2011. Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science 331:1185–1188. doi: 10.1126/science.1199707. [DOI] [PubMed] [Google Scholar]
- 20.Wiktelius E, Stenberg G. 2007. Novel class of glutathione transferases from cyanobacteria exhibit high catalytic activities towards naturally occurring isothiocyanates. Biochem J 406:115–123. doi: 10.1042/BJ20070328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey T, Singh SK, Chhetri G, Tripathi T, Singh AK. 2015. Characterization of a highly pH stable chi-class glutathione S-transferase from Synechocystis PCC 6803. PLoS One 10:e0126811. doi: 10.1371/journal.pone.0126811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakobsen TH, Bragason SK, Phipps RK, Christensen LD, van Gennip M, Alhede M, Skindersoe M, Larsen TO, Hoiby N, Bjarnsholt T, Givskov M. 2012. Food as a source for quorum sensing inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl Environ Microbiol 78:2410–2421. doi: 10.1128/AEM.05992-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welte CU, de Graaf RM, van den Bosch TJ, Op den Camp HJ, van Dam NM, Jetten MS. 31 July 2015. Plasmids from the gut microbiome of cabbage root fly larvae encode SaxA that catalyses the conversion of the plant toxin 2-phenylethyl isothiocyanate. Environ Microbiol doi: 10.1111/1462-2920.12997. [DOI] [PubMed] [Google Scholar]
- 24.van de Graaf AA, de Bruijn P, Robertson LA, Jetten MSM, Kuenen JG. 1996. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142:2187–2196. doi: 10.1099/13500872-142-8-2187. [DOI] [Google Scholar]
- 25.Mott JE, Grant RA, Ho YS, Platt T. 1985. Maximizing gene expression from plasmid vectors containing the lambda PL promoter: strategies for overproducing transcription termination factor rho. Proc Natl Acad Sci U S A 82:88–92. doi: 10.1073/pnas.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Blum H, Beier H, Gross HJ, Blum H. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99. doi: 10.1002/elps.1150080203. [DOI] [Google Scholar]
- 28.Murray RA. 2001. Limitations to the use of solid-phase microextraction for quantitation of mixtures of volatile organic sulfur compounds. Anal Chem 73:1646–1649. doi: 10.1021/ac001176m. [DOI] [PubMed] [Google Scholar]
- 29.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaves B, De Neve S, Hofman G, Boeckx P, Van Cleemput O. 2004. Nitrogen mineralization of vegetable root residues and green manures as related to their (bio)chemical composition. Eur J Agron 21:161–170. doi: 10.1016/j.eja.2003.07.001. [DOI] [Google Scholar]
- 31.Hanlon SP, Hill TK, Flavell MA, Stringfellow JM, Cooper RA. 1997. 2-Phenylethylamine catabolism by Escherichia coli K-12: gene organization and expression. Microbiology 143:513–518. doi: 10.1099/00221287-143-2-513. [DOI] [PubMed] [Google Scholar]
- 32.Crespo E, Hordijk CA, de Graaf RM, Samudrala D, Cristescu SM, Harren FJ, van Dam NM. 2012. On-line detection of root-induced volatiles in Brassica nigra plants infested with Delia radicum L. root fly larvae. Phytochemistry 84:68–77. doi: 10.1016/j.phytochem.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 33.O'Hara K, Haruta S, Sawai T, Tsunoda M, Iyobe S. 1998. Novel metallo beta-lactamase mediated by a Shigella flexneri plasmid. FEMS Microbiol Lett 162:201–206. [DOI] [PubMed] [Google Scholar]
- 34.Marumo K, Takeda A, Nakamura Y, Nakaya K. 1995. Purification and characterization of metallo-beta-lactamase from Serratia marcescens. Microbiol Immunol 39:27–33. doi: 10.1111/j.1348-0421.1995.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 35.Crawford PA, Sharma N, Chandrasekar S, Sigdel T, Walsh TR, Spencer J, Crowder MW. 2004. Over-expression, purification, and characterization of metallo-beta-lactamase ImiS from Aeromonas veronii bv. sobria. Protein Expr Purif 36:272–279. doi: 10.1016/j.pep.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Mushantaf F, Blyth J, Templeton MR. 2012. The bactericidal effects of allyl isothiocyanate in water. Environ Technol 33:2461–2465. doi: 10.1080/09593330.2012.671855. [DOI] [PubMed] [Google Scholar]
- 37.Seo HS, Bang J, Kim H, Beuchat LR, Cho SY, Ryu JH. 2012. Development of an antimicrobial sachet containing encapsulated allyl isothiocyanate to inactivate Escherichia coli O157:H7 on spinach leaves. Int J Food Microbiol 159:136–143. doi: 10.1016/j.ijfoodmicro.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Yun J, Fan X, Li X. 2013. Inactivation of Salmonella enterica serovar typhimurium and quality maintenance of cherry tomatoes treated with gaseous essential oils. J Food Sci 78:M458–464. doi: 10.1111/1750-3841.12052. [DOI] [PubMed] [Google Scholar]
- 39.Piercey MJ, Mazzanti G, Budge SM, Delaquis PJ, Paulson AT, Truelstrup Hansen L. 2012. Antimicrobial activity of cyclodextrin entrapped allyl isothiocyanate in a model system and packaged fresh-cut onions. Food Microbiol 30:213–218. doi: 10.1016/j.fm.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Jin TZ, Gurtler JB, Geveke DJ, Fan X. 2012. Inactivation of Salmonella on whole cantaloupe by application of an antimicrobial coating containing chitosan and allyl isothiocyanate. Int J Food Microbiol 155:165–170. doi: 10.1016/j.ijfoodmicro.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Ko JA, Kim WY, Park HJ. 2012. Effects of microencapsulated allyl isothiocyanate (AITC) on the extension of the shelf-life of kimchi. Int J Food Microbiol 153:92–98. doi: 10.1016/j.ijfoodmicro.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Jideani VA, Vogt K. 2015. Antimicrobial packaging for extending the shelf life of bread—a review. Crit Rev Food Sci Nutr doi: 10.1080/10408398.2013.768198. [DOI] [PubMed] [Google Scholar]
- 43.Olaimat AN, Fang Y, Holley RA. 2014. Inhibition of Campylobacter jejuni on fresh chicken breasts by kappa-carrageenan/chitosan-based coatings containing allyl isothiocyanate or deodorized oriental mustard extract. Int J Food Microbiol 187:77–82. doi: 10.1016/j.ijfoodmicro.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Dias MV, Soares Nde F, Borges SV, de Sousa MM, Nunes CA, de Oliveira IR, Medeiros EA. 2013. Use of allyl isothiocyanate and carbon nanotubes in an antimicrobial film to package shredded, cooked chicken meat. Food Chem 141:3160–3166. doi: 10.1016/j.foodchem.2013.05.148. [DOI] [PubMed] [Google Scholar]
- 45.Pang YH, Sheen S, Zhou S, Liu L, Yam KL. 2013. Antimicrobial effects of allyl isothiocyanate and modified atmosphere on Pseudomonas aeruginosa in fresh catfish fillet under abuse temperatures. J Food Sci 78:M555–559. doi: 10.1111/1750-3841.12065. [DOI] [PubMed] [Google Scholar]
- 46.Dussault D, Vu KD, Lacroix M. 2014. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci 96:514–520. doi: 10.1016/j.meatsci.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Jang M, Hong E, Kim GH. 2010. Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J Food Sci 75:M412–M416. doi: 10.1111/j.1750-3841.2010.01725.x. [DOI] [PubMed] [Google Scholar]
- 48.Aires A, Mota VR, Saavedra MJ, Monteiro AA, Simoes M, Rosa EA, Bennett RN. 2009. Initial in vitro evaluations of the antibacterial activities of glucosinolate enzymatic hydrolysis products against plant pathogenic bacteria. J Appl Microbiol 106:2096–2105. doi: 10.1111/j.1365-2672.2009.04181.x. [DOI] [PubMed] [Google Scholar]
- 49.Sofrata A, Santangelo EM, Azeem M, Borg-Karlson AK, Gustafsson A, Putsep K. 2011. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PLoS One 6:e23045. doi: 10.1371/journal.pone.0023045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MG, Lee HS. 2009. Growth-inhibiting activities of phenethyl isothiocyanate and its derivatives against intestinal bacteria. J Food Sci 74:M467–471. doi: 10.1111/j.1750-3841.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- 51.Freitas E, Aires A, Rosa EAD, Saavedra MJ. 2013. Antibacterial activity and synergistic effect between watercress extracts, 2-phenylethyl isothiocyanate and antibiotics against 11 isolates of Escherichia coli from clinical and animal source. Lett Appl Microbiol 57:266–273. doi: 10.1111/lam.12105. [DOI] [PubMed] [Google Scholar]
- 52.Palaniappan K, Holley RA. 2010. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int J Food Microbiol 140:164–168. doi: 10.1016/j.ijfoodmicro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Saavedra MJ, Borges A, Dias C, Aires A, Bennett RN, Rosa ES, Simoes M. 2010. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med Chem 6:174–183. doi: 10.2174/1573406411006030174. [DOI] [PubMed] [Google Scholar]
- 54.Meini MR, Llarrull LI, Vila AJ. 2015. Overcoming differences: the catalytic mechanism of metallo-beta-lactamases. FEBS Lett 589:3419–3432. doi: 10.1016/j.febslet.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]