ABSTRACT
A chemolithoautotrophic strain of the family Beggiatoaceae, Beggiatoa sp. strain 35Flor, was found to oxidize molecular hydrogen when grown in a medium with diffusional gradients of oxygen, sulfide, and hydrogen. Microsensor profiles and rate measurements suggested that the strain oxidized hydrogen aerobically when oxygen was available, while hydrogen consumption under anoxic conditions was presumably driven by sulfur respiration. Beggiatoa sp. 35Flor reached significantly higher biomass in hydrogen-supplemented oxygen-sulfide gradient media, but hydrogen did not support growth of the strain in the absence of reduced sulfur compounds. Nevertheless, hydrogen oxidation can provide Beggiatoa sp. 35Flor with energy for maintenance and assimilatory purposes and may support the disposal of internally stored sulfur to prevent physical damage resulting from excessive sulfur accumulation. Our knowledge about the exposure of natural populations of Beggiatoaceae to hydrogen is very limited, but significant amounts of hydrogen could be provided by nitrogen fixation, fermentation, and geochemical processes in several of their typical habitats such as photosynthetic microbial mats and submarine sites of hydrothermal fluid flow.
IMPORTANCE Reduced sulfur compounds are certainly the main electron donors for chemolithoautotrophic Beggiatoaceae, but the traditional focus on this topic has left other possible inorganic electron donors largely unexplored. In this paper, we provide evidence that hydrogen oxidation has the potential to strengthen the ecophysiological plasticity of Beggiatoaceae in several ways. Moreover, we show that hydrogen oxidation by members of this family can significantly influence biogeochemical gradients and therefore should be considered in environmental studies.
INTRODUCTION
Members of the family Beggiatoaceae are colorless sulfur bacteria known to oxidize reduced sulfur compounds and organic substances for chemolithoautotrophic, chemoorganoheterotrophic, and mixotrophic growth (1). The use of various organic substances, such as mono- and dicarboxylic acids, sugars, amino acids, and alcohols, has been studied repeatedly in different strains of the family (2–6), but inorganic electron donors other than reduced sulfur compounds were never reported to support growth. The only indication of the oxidation of a nonsulfuric, inorganic electron donor was the stimulation of sulfur reduction by molecular hydrogen in a microaerophilic Beggiatoa strain under short-term anoxic conditions (7). Hydrogen oxidation or hydrogen-supported growth has been reported for many other well-known sulfur oxidizers such as members of the families Chromatiaceae (8), Acidithiobacillaceae (9, 10), Aquificaceae (11–13), and Sulfolobaceae (14), the genus Sulfurimonas (15, 16), the SUP05 clade (17), and endosymbionts of mussels (18). This suggests that hydrogen oxidation may be a widespread metabolic trait among sulfur oxidizers and as such may also be realized in the family Beggiatoaceae.
Substantial amounts of molecular hydrogen are produced and consumed in many microbial habitats, so H2 is considered to be an important electron transfer agent in oxic and anoxic environments (19). Nevertheless, there is little information about the environmental exposure of Beggiatoaceae populations to hydrogen and the potential importance of hydrogen oxidation for members of the family in situ. Despite high conversion rates, in situ studies on hydrogen cycling and availability are difficult due to the generally very low ambient concentrations (20). Steep biogeochemical gradients, which are typical for habitats of Beggiatoaceae, pose an additional problem because these necessitate a sampling resolution on the micrometer scale for meaningful conclusions. Microsensors are typically used for this purpose, and a microsensor for hydrogen has been available for more than two decades (21). However, the hydrogen microsensor has the critical disadvantage of being sensitive to hydrogen sulfide (22). This cross-reactivity disqualifies the sensor from many in situ applications, in particular, from measurements in habitats of sulfur bacteria, where the concentrations of sulfide are usually considerably higher than those of hydrogen.
In the present study, we investigated the consumption of molecular hydrogen in cultures of a chemolithoautotrophic Beggiatoa strain using microsensors. Culture-based experiments allowed us to adjust the concentrations of hydrogen and sulfide to levels at which reliable measurements with the hydrogen microsensor are possible. We discuss here how hydrogen oxidation can contribute to the ecophysiological plasticity of the strain and point out environmental settings in which members of the family Beggiatoaceae may be able to use hydrogen as an electron donor and energy source.
MATERIALS AND METHODS
Organisms and cultivation.
All experiments were conducted with the marine chemolithoautotrophic bacterium Beggiatoa sp. strain 35Flor, which was maintained in a defined coculture with Pseudovibrio sp. strain FO-BEG1, a heterotrophic and metabolically versatile bacterium (23). The coculture was grown in a medium with opposed gradients of oxygen and sulfide as described previously (24, 25). The concentration of NiCl2 in top agar and bottom agar was increased to 7 μM to provide a sufficient amount of nickel for the synthesis of the [NiFe]-hydrogenase cofactor. Bottom agar sulfide concentrations were adjusted to 6 mM (low sulfide flux) or 16 mM (high sulfide flux), depending on the experiment. Ammonium chloride in a concentration of 200 μM was added to the top agar only when the influence of a fixed nitrogen source on hydrogen oxidation was to be tested; nitrate was never added to the medium.
The setup for cultivation in the presence of a diffusional hydrogen gradient was as follows (Fig. 1). A glass tube with a conical ground cone (nominal size [NS] 29/32; 26 by 130 mm; inner diameter 22 mm; all glassware was obtained from Lenz Laborglas GmbH & Co. KG, Wertheim, Germany) was closed toward the cone with a 20-mm-high plug of silicone (RTV-2 silicone, 13 ShA; Silikonfabrik.de, Ahrensburg, Germany) and loosely capped on top with a lid of thick aluminum foil. The sterilized tube was placed on the central socket of a 100-ml three-neck flask. The screw thread adapters on the side necks (NS 14/23) of the flask were closed with butyl stoppers and apertured caps. All joints were greased with medium-viscosity Baysilone paste (GE Bayer Silicones GmbH & Co. KG, Leverkusen, Germany) and fixed in place with steel clips. Bottom agar (4-ml) and top agar (17-ml) layers were poured consecutively onto the silicone plug. The gas reservoir was flushed with either nitrogen or hydrogen gas for 30 min immediately after the pouring of the top agar. In cultures with a high sulfide flux, a lower hydrogen partial pressure was achieved by replacing 12 ml of the nitrogen-filled gas reservoir with hydrogen. Gradients were allowed to establish for 1 day prior to inoculation with 300 μl Beggiatoa filament suspension prepared from mats of 9-to-16-day-old precultures (25). Hydrogen-supplemented and -unsupplemented cultures were prepared in parallel and inoculated with the very same homogeneous filament suspension. The cultures were incubated at room temperature, and the gas reservoirs were refreshed every 3 to 4 days.
FIG 1.
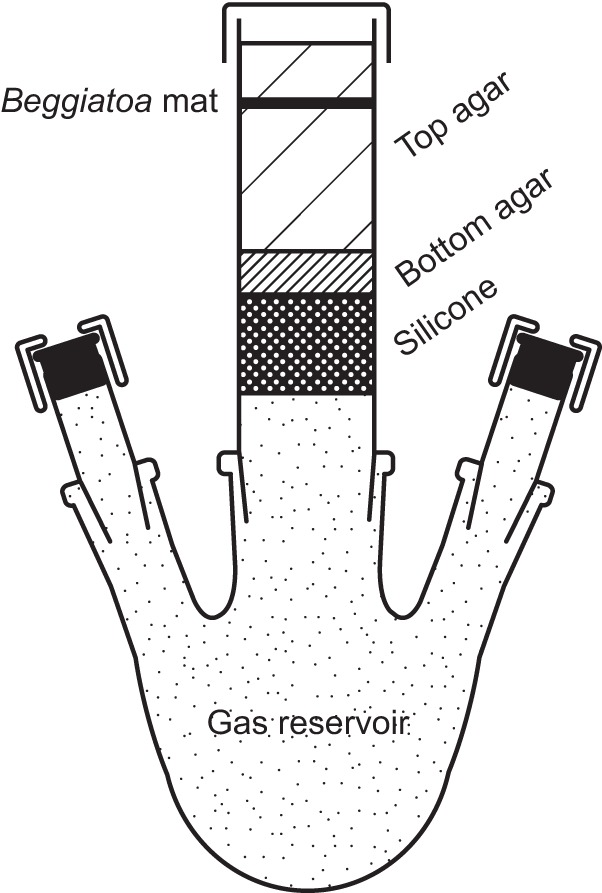
Setup for the incubation of Beggiatoa sp. 35Flor in the presence of diffusional gas gradients. The Beggiatoa mat grows between opposed diffusional gradients of oxygen, sulfide, and (if the latter is provided) hydrogen. Oxygen is supplied to the mat by diffusion from the headspace above, and sulfide is provided by diffusion from the bottom agar below. Hydrogen can be added to the gas reservoir and can reach the mat by diffusion through the silicone plug.
Microsensor measurements.
Microsensors for pH (pH-10), H2 (H2-10), O2 (OX-10), and H2S (H2S-10) with tip diameters of 8 to 12 μm and response times of <10 s were purchased from Unisense A/S (Aarhus, Denmark) and calibrated directly before and after the measurements as described by Schwedt et al. (25). The hydrogen sensor was calibrated in artificial seawater (25) by stepwise addition of a hydrogen-saturated stock solution, the concentration of which was calculated as described by Gordon et al. (26). Profiles of total sulfide (Stot = H2S + HS− + S2−) were calculated from the corresponding H2S and pH profiles as described previously (25, 27). Measured hydrogen profiles were corrected for the H2S-derived background recorded by the cross-reactive hydrogen sensor. This background was estimated from profiles measured in hydrogen-unsupplemented parallel cultures. In the case of cultures with a low sulfide flux, the average background for a given H2S concentration was calculated from the H2S and apparent H2 concentrations measured in the very same hydrogen-unsupplemented cultures at the very same depths. The H2S-derived background in hydrogen-supplemented cultures was then calculated based on the measured H2S profiles and was subtracted from the measured H2 profiles. The estimated H2S-derived background accounted for ≤12% of the recorded hydrogen signal in all hydrogen-supplemented cultures and was ≤5% in most cases. In cultures with a high sulfide flux, the average H2S-derived background profile measured in hydrogen-unsupplemented cultures was directly subtracted from the H2 profiles measured in hydrogen-supplemented cultures. This was possible because the oxygen-sulfide interfaces were located at similar depths and H2S profiles were essentially congruent in hydrogen-supplemented and -unsupplemented cultures with a high sulfide flux. It has to be noted that both corrections could overestimate the contribution of the H2S-induced background, because genuine H2 signals present in hydrogen-unsupplemented cultures would wrongly be ascribed to H2S and subtracted. It is indeed possible that hydrogen-unsupplemented cultures contained H2, because Beggiatoa sp. 35Flor fixes nitrogen under standard cultivation conditions (A.-T. Henze, unpublished data) and this process is associated with the evolution of H2 (reviewed in reference 28). However, we assume that the hydrogen concentrations were not significant in hydrogen-unsupplemented cultures due to the slow growth and high hydrogen oxidation rates of Beggiatoa sp. 35Flor. Correspondingly, there was no notable difference in the H2 profiles measured in hydrogen-supplemented nitrogen-fixing and non-nitrogen-fixing Beggiatoa sp. 35Flor cultures (see Fig. S1 in the supplemental material).
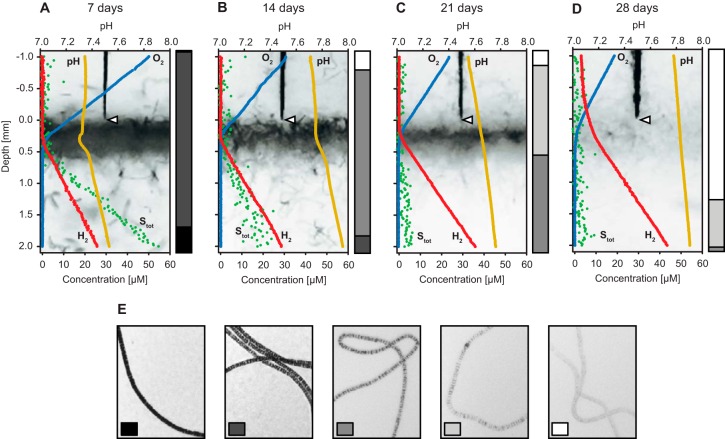
Oxygen, total sulfide, and hydrogen fluxes were calculated according to Fick's first law of diffusion (J = –D ∂c/∂x). The diffusion coefficient (D) was 1.52 × 10–9 m2 s–1 for sulfide (29), 2.06 × 10–9 m2 s–1 for oxygen (29), and 3.67 × 10–9 m2 s–1 for hydrogen (30). The flux of a compound into the mat was equal to its consumption rate when the compound was depleted within the mat. When a compound diffused through the mat, its consumption rate was calculated as the absolute difference in the fluxes above and below the mat. Volumetric rates for hydrogen oxidation were calculated by assuming a constant mat thickness of 0.5 to 0.6 mm during the first 3 weeks. This thickness was estimated from images of 7-day-old cultures, which were the cultures in which the filaments can be seen best due to the high sulfur globule content (see Fig. 3A).
FIG 3.
Development of chemical gradients and sulfur inclusion density in hydrogen-supplemented Beggiatoa sp. 35Flor cultures over 4 weeks. The cultures were grown in the presence of a low sulfide flux. (A to D) Microsensor profiles were recorded 7, 14, 21, and 28 days after inoculation. Profiles of oxygen (blue), hydrogen (red), pH (orange), and total sulfide (Stot; green) were determined at a vertical resolution of 20 μm. Photographs of the profiled mat sections are shown in the background. For each set of profiles, the tip of the microsensor (triangle) indicates the zero position at the mat surface to which all sensors were aligned. The bar graph next to each set of profiles shows a semiquantitative estimation of the relative sulfur inclusion density in Beggiatoa filaments at that point of time. This estimation considered five degrees of sulfur inclusion density, examples of which are shown in panel E. The standard deviation of sulfur inclusion density estimates in triplicate cultures was consistently below 10%. The density of sulfur inclusions decreased over time, resulting in an increasingly transparent appearance of the Beggiatoa mat.
Protein determination.
Total cell protein was measured as a substitute for Beggiatoa biomass as described previously (24, 31–33). The semiliquid top agar of a culture was sampled by pouring the entire volume into a 50-ml polypropylene tube. Residual agar that adhered to the walls of the culture tube was transferred by rinsing with 10 ml sterile artificial seawater. Centrifugation in a swing-out rotor at 5,000 × g (20 min) yielded a dense agar pellet of about 8 ml, in which the entire biomass was concentrated. The density of accompanying Pseudovibrio sp. FO-BEG1 cells in the thoroughly vortex-mixed pellet was determined in triplicate 10-μl subsamples using a Neubauer counting chamber. The remaining agar was hydrolyzed, and the protein was precipitated through incubation in 10% (wt/vol) trichloroacetic acid for 20 min at 90°C (24) followed by cooling at 4°C overnight. Four 2-ml subsamples were taken from each sample and centrifuged at 20,817 × g (10 min, 4°C). The supernatant was removed, and each pellet was dissolved and incubated in 0.7 ml 0.1 M NaOH (20 min, 55°C) to measure the protein content. The colorimetric protein assay (34) contained 0.5 ml sample or standard in 0.1 M NaOH, 0.5 ml 0.15 M HCl, and 0.35 ml dye reagent concentrate (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Bovine serum albumin (2 to 10 μg ml−1) served as a standard. All measured protein concentrations were corrected for blanks (extractions from sterile top agar) and the contribution of Pseudovibrio sp. FO-BEG1 protein, considering the respective Pseudovibrio cell densities. The average protein content of Pseudovibrio sp. FO-BEG1 cells (290 ± 70 fg protein per cell) was determined separately. For this purpose, known amounts of axenically cultivated and washed Pseudovibrio sp. FO-BEG1 cells were added to a sterile mix of top agar and artificial seawater. The protein was extracted and quantified as described above.
Estimation of sulfur inclusion density in filaments.
The density of sulfur inclusions was estimated semiquantitatively in Beggiatoa sp. 35Flor filaments that were grown in hydrogen-supplemented cultures with a low sulfide flux. Bacterial sulfur inclusions are highly light refractive, so the sulfur inclusion density correlates with the opaqueness of the filaments. At each time point, 333 to 385 filaments from three parallel cultures (105 to 134 filaments per culture) were inspected microscopically and assigned to one of five predefined categories of sulfur inclusion density (see Fig. 3E).
Photography.
Photographs of culture tubes were taken with a Sony XCD-X710 digital camera (Sony, Tokyo, Japan), controlled by the image acquisition software IC Capture (The Imaging Source Europe GmbH, Bremen, Germany). Due to better visibility in print, negatives are shown. The brightness of all negatives was adjusted using the same modifications for all images contained in a figure. Different adjustments were used for different figures in order to achieve a good contrast when profiles were plotted on top of the photographs. Single filaments were photographed with a camera attached to a microscope (Sterni 2000-C, Zeiss, Germany), which was operated in bright-field mode.
RESULTS
Influence of molecular hydrogen on the migration behavior of Beggiatoa sp. 35Flor in gradient cultures with a low sulfide flux.
Beggiatoa sp. 35Flor filaments grew in dense, opaque mats at the transition from oxic to sulfidic conditions when cultivated in agar-stabilized gradient media (Fig. 2). Irrespective of the presence or absence of hydrogen, these mats migrated downward in course of a 4-week incubation period in response to the progressive depletion of the bottom sulfide reservoir. However, the downward migration was considerably less pronounced in the presence of a diffusional hydrogen gradient (Fig. 2). While mats in hydrogen-supplemented cultures had not left the upper third of the top agar even after 4 weeks of growth, mats in hydrogen-unsupplemented cultures had already reached the bottom agar layer (Fig. 2D). No mat formation or growth of Beggiatoa sp. 35Flor could be observed in fresh sulfide-free gradient media supplemented with only oxygen and hydrogen.
FIG 2.
Position and appearance of Beggiatoa sp. 35Flor mats in hydrogen-supplemented and -unsupplemented oxygen-sulfide gradient media. The cultures were grown under low-sulfide-flux conditions over 4 weeks. Culture tubes were photographed after 7, 14, 21, and 28 days of growth in the presence (left panels; +) and absence (right panels; –) of a diffusional hydrogen gradient. The scale bar on the left indicates the depth below the air-agar interface.
Hydrogen oxidation at the oxygen-sulfide interface in cultures with a low sulfide flux.
Oxygen, sulfide, and hydrogen that diffused into Beggiatoa sp. 35Flor mats were consumed completely during the first 3 weeks of incubation (Fig. 3A to C). After 4 weeks, hydrogen was still oxidized, but the consumption was not complete and some hydrogen diffused through the mat (Fig. 3D). The zones of hydrogen and oxygen consumption overlapped at all times, and microsensor profiles showed no evidence of hydrogen oxidation in the anoxic section of the mat. The density of sulfur inclusions in Beggiatoa sp. 35Flor filaments decreased over the course of the incubation (Fig. 3A to D). After 4 weeks, about 75% of the filaments were devoid of visible sulfur inclusions and the remainder contained only a low level (Fig. 3D). The presence of ammonium at a concentration previously shown to inhibit nitrogen fixation in Beggiatoa sp. 35Flor (200 μM in the top agar; Henze, unpublished) did not affect hydrogen consumption in mats at the oxygen-sulfide interface (see Fig. S1 in the supplemental material). Hydrogen was not oxidized in axenic gradient cultures of Pseudovibrio sp. FO-BEG1, whereas it was consumed efficiently in parallel cocultures of Beggiatoa sp. 35Flor and Pseudovibrio sp. FO-BEG1 (see Fig. S2B), in which the average Pseudovibrio cell density was only 13% higher (P = 0.12; see Fig. S2A).
Consumption rates of oxygen, sulfide, and hydrogen at the oxygen-sulfide interface.
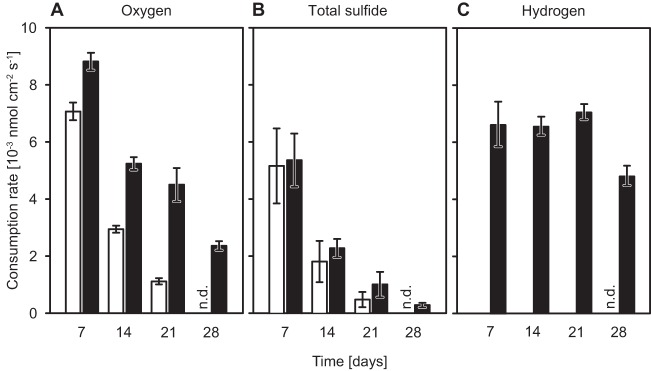
Average consumption rates of oxygen, total sulfide (Stot = H2S + HS− + S2−), and hydrogen were determined in hydrogen-supplemented and -unsupplemented Beggiatoa sp. 35Flor cultures over 4 weeks of incubation (Fig. 4). In both types of cultures, the consumption rates of total sulfide and oxygen decreased with incubation time due to the progressive depletion of the sulfide reservoir and the resulting downward migration of the mat. The average consumption rates of total sulfide were similar in the two types of cultures at all times (Fig. 4B). In contrast, the average oxygen consumption rate was always significantly higher (P ≤ 1.5 × 10−5) and decreased less over time in hydrogen-supplemented cultures (Fig. 4A). Measurements conducted within the first 3 weeks suggested a leveling off at a consumption rate of about 3 × 10−3 to 4 × 10−3 nmol O2 cm−2 s−1 in hydrogen-supplemented cultures, but a pronounced drop to circa 2.5 × 10−3 nmol O2 cm−2 s−1 occurred between weeks 3 and 4. The average hydrogen consumption rate in hydrogen-supplemented cultures remained fairly constant within the first 3 weeks but dropped markedly by week 4 (Fig. 4C), corresponding to the diffusion of hydrogen through the mat (Fig. 3D). A diffusion of hydrogen through the mat was always observed after about 4 weeks, but the fractions of hydrogen that passed through the mat at 28 days differed between independent cultivations.
FIG 4.
Average consumption rates of oxygen, total sulfide, and hydrogen in Beggiatoa sp. 35Flor mats over 4 weeks. Average consumption rates were determined weekly for hydrogen-supplemented (black bars) and -unsupplemented (white bars) cultures that were grown in the presence of a low sulfide flux. Rates of consumption of oxygen (A), total sulfide (B), and hydrogen (C) were calculated from profiles that were measured with a vertical resolution of 100 to 250 μm and that covered a distance of ca. 11 mm around the mat. The values for days 7, 14, and 21 are averages of consumption rates (± standard deviation) measured in six replicate cultures of two independent cultivations; values for day 28 are averages of consumption rates (± standard deviation) measured in triplicate cultures. Mats were absent from 28-day-old hydrogen-unsupplemented cultures, so consumption rates could not be determined (n.d.).
Influence of hydrogen oxidation on growth at the oxygen-sulfide interface.
Hydrogen-supplemented cultures grew faster and contained at least double the amount of Beggiatoa protein present in hydrogen-unsupplemented cultures at all time points (Fig. 5). In addition, hydrogen-supplemented cultures maintained growth for about 3 weeks, while hydrogen-unsupplemented cultures had already stopped growing after 2 weeks.
FIG 5.
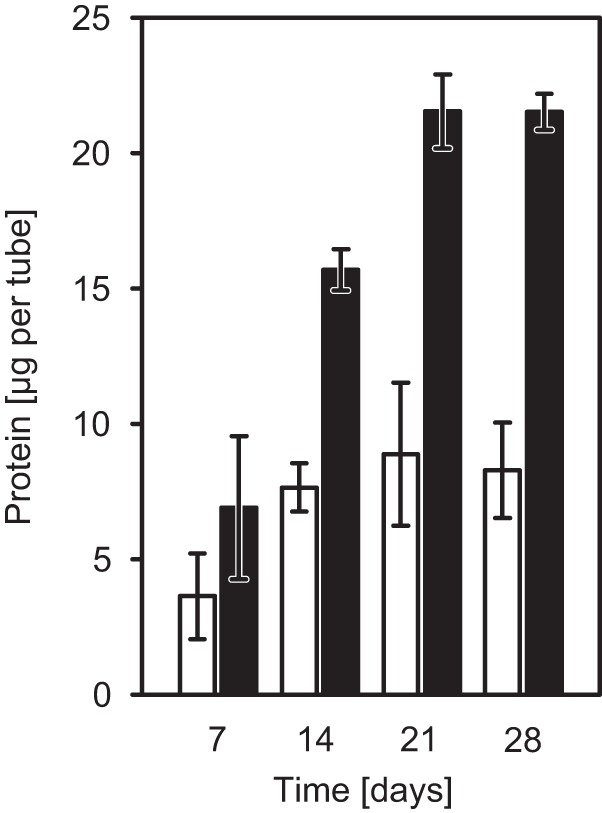
Influence of hydrogen oxidation on the growth of Beggiatoa sp. 35Flor. Beggiatoa sp. 35Flor protein levels were measured as a substitute for filament biomass in hydrogen-supplemented (black bars) and -unsupplemented (white bars) oxygen-sulfide gradient cultures. The cultures were grown in the presence of a low sulfide flux. The top agar from triplicate cultures was sampled weekly to determine the total protein content and the density of the accompanying Pseudovibrio sp. FO-BEG1 cells. Measured total protein amounts were subsequently corrected for the contribution of Pseudovibrio sp. FO-BEG1 protein, which accounted for 20% to 50% of the measured values (see Fig. S3 in the supplemental material).
Hydrogen oxidation under anoxic conditions in cultures with a high sulfide flux.
When Beggiatoa sp. 35Flor was grown in gradient cultures with a high sulfide flux, a subpopulation of filaments migrated from the oxygen-sulfide interface down into the anoxic section of the medium after about 1 week of incubation. Irrespective of the presence or absence of hydrogen, these filaments aggregated loosely in an anoxic horizon about 4 to 8 mm below the mat at the oxygen-sulfide interface. Microsensor profiles showed that added hydrogen was consumed within this horizon (Fig. 6). The profiles of H2S, pH, and total sulfide from hydrogen-supplemented cultures did not differ significantly from those of hydrogen-unsupplemented cultures, even though average H2S and total sulfide concentrations were minimally higher in the region of the anoxic subpopulation in hydrogen-supplemented cultures (see Fig. S4 in the supplemental material).
FIG 6.
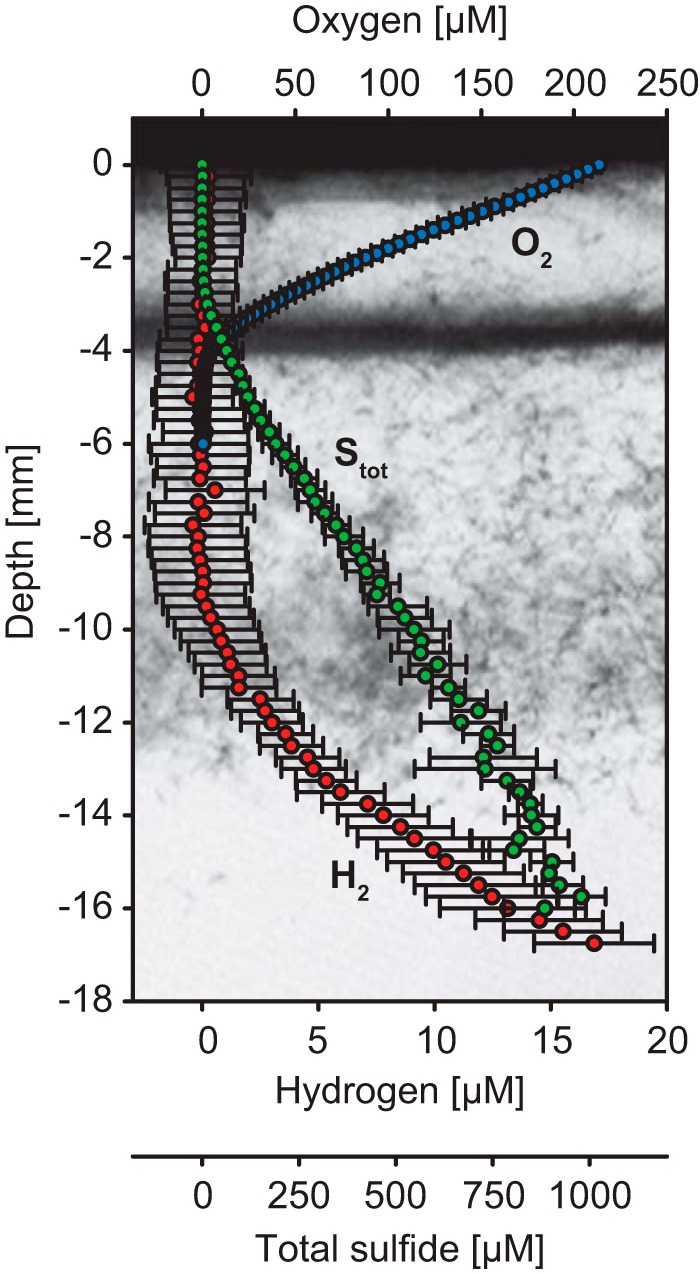
Hydrogen consumption under anoxic conditions in Beggiatoa sp. 35Flor cultures with a high sulfide flux. Oxygen (blue), hydrogen (red), and total sulfide (Stot; green) profiles were measured in hydrogen-supplemented cultures with vertical resolutions of 100 μm (O2) and 250 μm (H2, Stot). The plotted values are averages (± standard deviation) of measurements performed in three parallel cultures. The photograph in the background shows the filament distribution in a representative culture after 9 days of incubation when the profiles were measured. Depth values represent depth below the air-ager interface.
DISCUSSION
We showed that a chemolithoautotrophic strain of the family Beggiatoaceae, Beggiatoa sp. 35Flor, consumed molecular hydrogen at the oxygen-sulfide interface. Microsensor profiles and rate measurements suggested that the strain oxidized hydrogen aerobically. With 5 to 17 nmol H2 per μg protein and hour or 417 to 523 nmol H2 per cm3 mat volume and hour (7 to 21 days; see Fig. S5 in the supplemental material), the average hydrogen oxidation rates were substantial and in fact exceeded the sulfide oxidation rates at all times (Fig. 4).
Hydrogen is a valuable electron donor for Beggiatoa sp. 35Flor, as illustrated by the significantly higher protein content in hydrogen-supplemented cultures (Fig. 5). Similarly to other members of the family Beggiatoaceae (24, 35), Beggiatoa sp. 35Flor is capable of nitrogen fixation (Henze, unpublished). Because this process releases hydrogen as a byproduct (28), many diazotrophs couple the expression of nitrogenase to the expression of uptake hydrogenases on a transcriptional level (36–39). Hydrogen oxidation occurring under conditions of repression of nitrogen fixation (see Fig. S1 in the supplemental material) showed, however, that Beggiatoa sp. 35Flor does not merely recycle internally produced hydrogen but is able to use externally supplied hydrogen as a genuine electron donor.
Beggiatoa sp. 35Flor grew in a defined coculture with Pseudovibrio sp. FO-BEG1, but several lines of evidence suggest that the Pseudovibrio strain did not contribute to the consumption of hydrogen. We did not observe hydrogen oxidation in gradient cultures that contained only Pseudovibrio sp. FO-BEG1 (see Fig. S2 in the supplemental material), and hydrogen oxidation was never observed in liquid cultures of the strain, irrespective of the incubation conditions tested (V. Bondarev, unpublished data). In addition, hydrogenase genes could not be identified in the essentially closed genome of Pseudovibrio sp. FO-BEG1 (Bondarev, unpublished).
Hydrogen oxidation clearly influenced the mat position, oxygen consumption, and growth of Beggiatoa sp. 35Flor. This is of particular importance for environmental studies, because it illustrates that the measurement of oxygen and sulfide gradients alone does not necessarily suffice to gain a comprehensive picture of Beggiatoaceae metabolism. In contrast, the use of alternative electron donors such as hydrogen or electron acceptors such as nitrate (40–44) can significantly influence biogeochemical gradients as well as the position of Beggiatoaceae populations with respect to these.
Hydrogen versus sulfur as an electron donor at the oxygen-sulfide interface.
In order to assess the influence of hydrogen oxidation on the electron turnover in Beggiatoa sp. 35Flor, electron budgets were calculated on the basis of the measured consumption rates of oxygen, sulfide, and hydrogen as well as the estimated CO2 fixation rates (Fig. 7A). In hydrogen-unsupplemented cultures, the average contribution of sulfide oxidation to the total electron supply decreased from 36% to 20% within the first 3 weeks of incubation. The absolute rates of sulfide oxidation were similar in hydrogen-supplemented cultures, but the relative contribution to the total electron supply was lower because hydrogen-supplemented cultures showed an overall higher electron demand. Within the first 3 weeks, the average contribution of sulfide oxidation decreased from 30% to 11% of the total electron supply in hydrogen-supplemented cultures and dropped to only 6% after 4 weeks. Concurrently, the average contribution of hydrogen oxidation to the total electron supply increased from 36% after 1 week to 102% after 4 weeks. Hydrogen oxidation was already the main electron-supplying reaction after 2 weeks, fulfilling on average 59% of the total electron demand. Other electron donors and CO2 fixation as an electron sink were insignificant after 4 weeks such that hydrogen oxidation explained the total oxygen demand according to the Knallgas reaction (2H2 + O2 → 2H2O).
FIG 7.
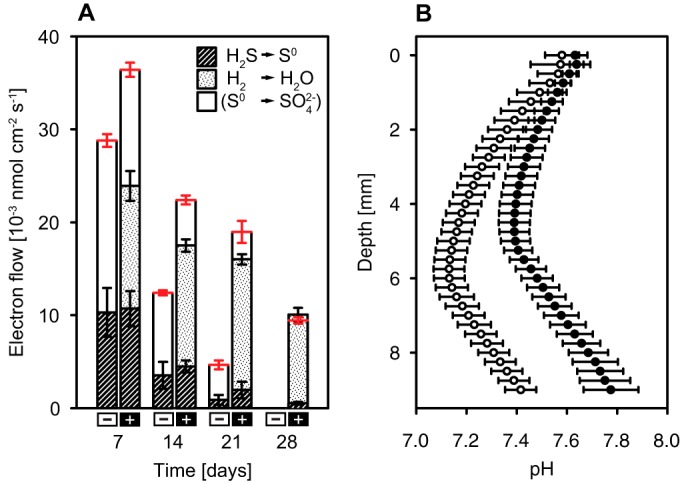
Influence of hydrogen oxidation on electron turnover and pH in Beggiatoa sp. 35Flor cultures. (A) Electron budgets in hydrogen-supplemented (+) and -unsupplemented (–) cultures over 4 weeks. The total electron demand (shown in red) was calculated based on the measured oxygen consumption rate and the estimated rate of CO2 fixation into biomass (<CH2O>). Weekly averages of CO2 fixation rates were estimated on the basis of the increase in Beggiatoa protein levels and a cell carbon-to-protein ratio of 1.13 (wt/wt; determined for closely related strain Beggiatoa sp. MS-81-6 under similar growth conditions; [31]). According to this estimation, CO2 fixation accounted for ≤6.4% of the total electron demand at all times. Hatched areas indicate the contribution of sulfide oxidation (2 electrons per H2S → S0) to the electron supply; dotted areas represent the contribution of hydrogen oxidation (2 electrons per H2). The electron demand, which cannot be fulfilled by the reactions described above, is most likely met by the oxidation of sulfur to sulfate (6 electrons per S0). (B) Average pH profiles (± standard deviation; n = 6) measured in hydrogen-supplemented (black) and -unsupplemented (white) cultures after 7 days of incubation. Mats in hydrogen-supplemented cultures were situated 5.2 to 6.2 mm below the air-agar interface; mats in hydrogen-unsupplemented cultures were located at a depth of 6.6 to 7.5 mm.
The total electron demand in hydrogen-supplemented and -unsupplemented cultures during the first 3 weeks was higher than what could be supplied by the oxidation of sulfide and hydrogen alone (Fig. 7A). This excess demand was most likely fulfilled by the oxidation of elemental sulfur to sulfuric acid as sulfur inclusions disappeared over time (Fig. 3), and pH profiles showed a pronounced acidification in the region of the mat (Fig. 7B). Sulfur oxidation in oxygen-sulfide gradient cultures of Beggiatoa sp. 35Flor was recently also demonstrated by the production of large amounts of sulfate (45). Notably, the excess electron demand was lower in hydrogen-supplemented cultures throughout the incubation (Fig. 7A). Together with a less pronounced acidification of the medium (Fig. 7B), this suggests that less sulfur was oxidized to sulfuric acid in the presence of hydrogen. In addition to the production of sulfuric acid, higher CO2 fixation rates (Fig. 5) contribute to higher pH values in hydrogen-supplemented cultures. However, it is unlikely that the observed pH difference resulted mainly from differences in CO2 fixation rates, because the estimated contribution of CO2 fixation to the total electron demand was low in general (≤6.4%; Fig. 7).
Overall, the influence of hydrogen oxidation on the sulfur metabolism of Beggiatoa sp. 35Flor points to a very efficient and purposeful use of the different electron donors in an environment, in which sulfide toxicity, competition for resources, and fluctuating supplies with oxidants and reductants are the major challenges. Sulfide and hydrogen, which cannot be stored, are oxidized immediately when available, while sulfur may be kept in reserve when the current energy requirements can be met by using other electron donors.
Aerobic hydrogen oxidation occurs in the presence of reduced sulfur compounds.
The presented results clearly show that Beggiatoa sp. 35Flor used energy from aerobic hydrogen oxidation for growth when reduced sulfur compounds were available. In contrast, growth on hydrogen in the absence of reduced sulfur compounds could not be shown. The apparent inability of hydrogen to support growth as an exclusive electron donor was unexpected, given that electrons from hydrogen (46, 47) enter the electron transport chain either on the same level as or upstream of electrons from reduced sulfur compounds (48–50) and thus should be able to support at least the same metabolic processes. So far, the reason for absent or discontinued growth of Beggiatoa sp. 35Flor on hydrogen and oxygen alone is unclear. Possible explanations are the potential inability to assimilate sulfate, the accumulation of waste products in older cultures, or the missing abiotic oxygen removal by sulfide and the resulting lack of a microoxic niche in fresh sulfide-free gradient media.
Beggiatoa sp. 35Flor oxidizes hydrogen also under anoxic conditions, presumably through sulfur respiration.
Beggiatoa sp. 35Flor filaments did not oxidize hydrogen only aerobically in mats at the oxygen-sulfide interface (Fig. 3). In cultures with a high sulfide flux, hydrogen was also oxidized in the fully anoxic section of the medium by a subpopulation of filaments that had migrated downward from the oxygen-sulfide interface (Fig. 6). Several members of the family Beggiatoaceae are known to store nitrate in large amounts and use it as an alternative electron acceptor under anoxic conditions (e.g., 40, 43, 44, 51). However, nitrate can be excluded as an electron acceptor in the present study. Gradient media for precultures and experiments were prepared without fixed nitrogen compounds, and Beggiatoa sp. 35Flor filaments from such precultures were previously shown to be free of NOX compounds (25). Hence, neither external nor internal nitrate was available for hydrogen oxidation under anoxic conditions.
In addition to the use of nitrate, several members of the family Beggiatoaceae are known to use stored sulfur as an electron acceptor under short-term anoxic conditions. Previous studies showed that sulfur respiration in Beggiatoaceae can be supported by organic electron donors such as acetate (52) and internally stored polyhydroxyalkanoates (7, 25) but also by molecular hydrogen (7). Sulfur respiration was recently shown in the strain Beggiatoa sp. 35Flor by Schwedt et al. (25) under incubation conditions very similar to the ones used here. Schwedt and colleagues showed that Beggiatoa sp. 35Flor filaments that had migrated into the anoxic section of a gradient medium under high sulfide fluxes reduced stored sulfur with stored polyhydroxyalkanoates. We assume that the same population of filaments as was studied by Schwedt et al. (25) used sulfur also as an electron acceptor for hydrogen oxidation under anoxic conditions in our experiments. Total sulfide profiles recorded in hydrogen-supplemented and -unsupplemented cultures did, however, not show significantly higher sulfide concentrations in the region of the anoxic subpopulation when hydrogen was present (see Fig. S3 in the supplemental material). This may have been due to the fact that the increase in sulfide production through hydrogen oxidation was too low compared to the background sulfide flux and the variability among replicate cultures. The average expected sulfide production rate, which is equal to the average measured hydrogen consumption rate (H2 + S0 → H2S; 1.16 × 10−3 nmol cm−2 s−1), was only 11% of the background sulfide flux (10.50 × 10−3 nmol cm−2 s−1) in hydrogen-supplemented cultures. The standard deviation of total sulfide concentrations was 7% to 18% (n = 3; hydrogen-supplemented cultures) and 9% to 23% (n = 3; hydrogen-unsupplemented cultures) of the average values in the region of the anoxic subpopulation located at a depth of 8 to 12 mm. For this reason, a significant increase in sulfide production through hydrogen oxidation may not have been detectable.
Sulfur respiration in Beggiatoaceae has been suggested to serve two purposes: the generation of metabolic energy under short-term anoxic conditions (7, 52) and the disposal of excess internal sulfur to avoid cell rupture (25). The use of hydrogen as an electron donor would enable an uncoupling of sulfur respiration from the oxidation of organic carbon compounds, thus leading to a higher flexibility in energy generation and sulfur disposal under anoxic conditions.
Thus, hydrogen oxidation has the potential of increasing the ecophysiological plasticity of Beggiatoa sp. 35Flor and possibly of other members of the family Beggiatoaceae in two ways, both of which are tightly coupled to the sulfur metabolism. In the presence of a low sulfide flux and electron acceptors with a more positive redox potential such as oxygen, hydrogen can partially replace sulfur as an electron donor and thereby increase the amount of sulfur available for storage. In contrast, hydrogen may support sulfur respiration and disposal under conditions of high sulfide flux and anoxia in order to provide metabolic energy and prevent physical damage from excessive sulfur accumulation.
Environmental relevance of hydrogen oxidation for members of the family Beggiatoaceae.
A variety of biotic and abiotic environmental processes are associated with the production of molecular hydrogen (19). Nevertheless, significant amounts of hydrogen are probably available to Beggiatoaceae in only certain environments. Members of this family are very often found in organic-rich sediments, in which hydrogen is produced by fermentation. However, the preferred habitat of Beggiatoaceae, the oxygen-sulfide interface, is usually well and permanently separated from the zone of hydrogen production in these sediments. Even though large quantities of hydrogen are produced by fermentative processes in deeper, anoxic layers, H2 is rapidly and efficiently reoxidized by the local community of hydrogenotrophic prokaryotes (20). Beggiatoaceae, which populate the oxygen-sulfide interface, are therefore unlikely to experience high concentrations or fluxes of hydrogen in such systems. In contrast, nitrate- or sulfur-respiring members of the family, which are residing in or traveling through fermenting sediment layers, could exploit hydrogen as an electron donor.
The hypersaline cyanobacterial mats of the Guerrero Negro evaporation lagoons (Baja California Sur, Mexico) are a prominent example of an environment in which large amounts of hydrogen are frequently available to members of the Beggiatoaceae. The biogeochemical conditions in these mats follow a strong diel cycle (53–56), which involves the presence of exceptionally high hydrogen concentrations at the mat surface during nighttime (57). Reacting to the changing biogeochemical conditions, filamentous Beggiatoaceae migrate to the anoxic and sulfidic surface of the Guerrero Negro mats at night (58, 59) and thus are regularly exposed to high hydrogen concentrations. Extensive cyanobacterial mats resembling those of the Guerrero Negro lagoons were present on earth for most of life's history, once dominating the biosphere (53, 60, 61). Substantial genetic exchange between cyanobacteria and Beggiatoaceae (62, 63) strikingly evidences a historically frequent co-occurrence of these taxa. This suggests that hydrogen transfer from nitrogen-fixing and fermenting cyanobacteria to members of the family Beggiatoaceae could indeed be an ancient and once-widespread process.
In addition, chemosynthetic ecosystems in the deep sea are sites at which hydrogen, specifically, H2 of geothermal origin, could potentially serve as a source of metabolic energy for Beggiatoaceae. Members of the family are regularly encountered in the deep sea at sites of hydrothermal fluid flow (e.g., 64–69), and hydrogen is extruded at several of such places (18, 70, 71). In fact, H2 of geothermal origin was suggested to be a key energy source in deep-seawater masses (17) and has been shown to fuel CO2 fixation in sulfide-oxidizing endosymbionts of deep sea mussels (18). Yet seep-dwelling populations of Beggiatoaceae have apparently never been tested for exposure to or even consumption of H2. Similarly to submarine sites of hydrothermal fluid flow, members of the Beggiatoaceae thrive in terrestrial sulfidic springs (1, 5, 72, 73), sites at which hydrogen is frequently emitted (74). However, further studies are necessary to evaluate the importance of molecular hydrogen for members of the family Beggiatoaceae on a broader scale. These studies will need to investigate the availability of H2 to environmental populations as well as the ability of different strains to oxidize this electron donor.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hans Røy and Rita Dunker for sharing the design of the gas incubation tubes as well as Martina Meyer for technical assistance. We are grateful to Mark Mußmann and Manuel Kleiner for comments on the manuscript. We thank Dirk de Beer for consultation on microsensor measurements.
We declare that the research was conducted in the absence of any commercial or financial relationships that could be a potential conflict of interest.
Funding Statement
The Max Planck Society provided funding to Anne-Christin Kreutzmann and Heide N. Schulz-Vogt. The German National Academic Foundation provided funding to Anne-Christin Kreutzmann. The funding organizations were not involved in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03818-15.
REFERENCES
- 1.Teske A, Nelson DC. 2006. The genera Beggiatoa and Thioploca, p 784–810. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes, vol 6 Springer, New York, NY. [Google Scholar]
- 2.Scoten KL, Stokes JL. 1962. Isolation and properties of Beggiatoa. Arch Mikrobiol 42:353–368. doi: 10.1007/BF00409071. [DOI] [PubMed] [Google Scholar]
- 3.Pringsheim EG. 1964. Heterotrophism and species concepts in Beggiatoa. Am J Bot 51:898–913. doi: 10.2307/2439898. [DOI] [Google Scholar]
- 4.Burton SD, Morita RY. 1964. Effect of catalase and cultural conditions on growth of Beggiatoa. J Bacteriol 88:1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson DC, Castenholz RW. 1981. Organic nutrition in Beggiatoa sp. J Bacteriol 147:236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jewell T, Huston SL, Nelson DC. 2008. Methylotrophy in freshwater Beggiatoa alba strains. Appl Environ Microbiol 74:5575–5578. doi: 10.1128/AEM.00379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt TM, Arieli B, Cohen Y, Padan E, Strohl WR. 1987. Sulfur metabolism in Beggiatoa alba. J Bacteriol 169:5466–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imhoff JF. 2006. The chromatiaceae, p 846–873. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes, vol 6 Springer, New York, NY. [Google Scholar]
- 9.Drobner E, Huber H, Stetter KO. 1990. Thiobacillus ferrooxidans, a facultative hydrogen oxidizer. Appl Environ Microbiol 56:2922–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallberg KB, Lindström EB. 1994. Characterization of Thiobacillus caldus sp. nov., a moderately thermophilic acidophile. Microbiology 140:3451–3456. doi: 10.1099/13500872-140-12-3451. [DOI] [PubMed] [Google Scholar]
- 11.Bonjour F, Aragno M. 1986. Growth of thermophilic, obligatory chemolithoautotrophic hydrogen-oxidizing bacteria related to Hydrogenobacter with thiosulfate and elemental sulfur as electron and energy source. FEMS Microbiol Lett 35:11–15. doi: 10.1111/j.1574-6968.1986.tb01490.x. [DOI] [Google Scholar]
- 12.Kawasumi TY, Igarashi TK, Minoda Y. 1984. Hydrogenobacter thermophilus gen. nov. sp. nov., an extremely thermophilic, aerobic, hydrogen-oxidizing bacterium. Int J Syst Evol Microbiol 34:5–10. doi: 10.1099/00207713-34-1-5. [DOI] [Google Scholar]
- 13.Huber R, Wilharm T, Huber D, Trincone A, Burggraf S, König H, Reinhard R, Rockinger I, Fricke H, Stetter KO. 1992. Aquifex pyrophilus gen. nov. sp. nov., represents a novel group of marine hyperthermophilic hydrogen-oxidizing bacteria. Syst Appl Microbiol 15:340–351. doi: 10.1016/S0723-2020(11)80206-7. [DOI] [Google Scholar]
- 14.Huber G, Drobner E, Huber H, Stetter KO. 1992. Growth by aerobic oxidation of molecular hydrogen in Archaea—a metabolic property so far unknown for this domain. Syst Appl Microbiol 15:502–504. doi: 10.1016/S0723-2020(11)80108-6. [DOI] [Google Scholar]
- 15.Takai K, Suzuki M, Nakagawa S, Miyazaki M, Suzuki Y, Inagaki F, Horikoshi K. 2006. Sulfurimonas paralvinellae sp. nov., a novel mesophilic, hydrogen- and sulfur-oxidizing chemolithoautotroph within the Epsilonproteobacteria isolated from a deep-sea hydrothermal vent polychaete nest, reclassification of Thiomicrospira denitrificans as Sulfurimonas denitrificans comb. nov. and emended description of the genus Sulfurimonas. Int J Syst Evol Microbiol 56:1725–1733. doi: 10.1099/ijs.0.64255-0. [DOI] [PubMed] [Google Scholar]
- 16.Grote J, Schott T, Bruckner CG, Glöckner FO, Jost G, Teeling H, Labrenz M, Jürgens K. 2012. Genome and physiology of a model Epsilonproteobacterium responsible for sulfide detoxification in marine oxygen depletion zones. Proc Natl Acad Sci U S A 109:506–510. doi: 10.1073/pnas.1111262109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anantharaman K, Breier JA, Sheik CS, Dick GJ. 2013. Evidence for hydrogen oxidation and metabolic plasticity in widespread deep-sea sulfur-oxidizing bacteria. Proc Natl Acad Sci U S A 110:330–335. doi: 10.1073/pnas.1215340110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen JM, Zielinski FU, Pape T, Seifert R, Moraru C, Amann R, Hourdez S, Girguis PR, Wankel S, Barbe V, Pelletier E, Fink D, Borowski C, Bach W, Dubilier N. 2011. Hydrogen is an energy source for hydrothermal vent symbioses. Nature 476:176–180. doi: 10.1038/nature10325. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz E, Friedrich B. 2006. The H2-metabolizing prokaryotes, p 496–563. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes, vol 2 Springer, New York, NY. [Google Scholar]
- 20.Hoehler TM, Alperin MJ, Albert DB, Martens CS. 1998. Thermodynamic control on hydrogen concentrations in anoxic sediments. Geochim Cosmochim Acta 62:1745–1756. doi: 10.1016/S0016-7037(98)00106-9. [DOI] [Google Scholar]
- 21.Witty JF. 1991. Microelectrode measurements of hydrogen concentrations and gradients in legume nodules. J Exp Bot 42:765–771. doi: 10.1093/jxb/42.6.765. [DOI] [Google Scholar]
- 22.Revsbech NP. 2005. Analysis of microbial communities with electrochemical microsensors and microscale biosensors. Methods Enzymol 379:147–166. [DOI] [PubMed] [Google Scholar]
- 23.Bondarev V, Richter M, Romano S, Piel J, Schwedt A, Schulz-Vogt HN. 2013. The genus Pseudovibrio contains metabolically versatile bacteria adapted for symbiosis. Environ Microbiol 15:2095–2113. doi: 10.1111/1462-2920.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson DC, Waterbury JB, Jannasch HW. 1982. Nitrogen fixation and nitrate utilization by marine and freshwater Beggiatoa. Arch Microbiol 133:172–177. doi: 10.1007/BF00414997. [DOI] [Google Scholar]
- 25.Schwedt A, Kreutzmann A-C, Polerecky L, Schulz-Vogt HN. 2011. Sulfur respiration in a marine chemolithoautotrophic Beggiatoa strain. Front Microbiol 2:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon LI, Cohen Y, Standley DR. 1977. The solubility of molecular hydrogen in seawater. Deep Sea Res 24:937–941. doi: 10.1016/0146-6291(77)90563-X. [DOI] [Google Scholar]
- 27.Kühl M, Steuckart C, Eickert G, Jeroschewski P. 1998. A H2S microsensor for profiling biofilms and sediments: application in an acidic lake sediment. Aquatic Microb Ecol 15:201–209. doi: 10.3354/ame015201. [DOI] [Google Scholar]
- 28.Burgess B, Lowe DJ. 1996. Mechanism of molybdenum nitrogenase. Chem Rev 96:2983–3011. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen BB, Revsbech NP. 1983. Colorless sulfur bacteria, Beggiatoa spp. and Thiovulum spp., in O(2) and H(2)S microgradients. Appl Environ Microbiol 45:1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz HD. 2006. Quantification of early diagenesis: dissolved constituents in pore water and signals in the solid phase, p 73–124. In Schulz HD, Zabel M (ed), Marine geochemistry, 2nd ed Springer, Berlin, Germany. [Google Scholar]
- 31.Nelson DC, Jannasch HW. 1983. Chemoautotrophic growth of a marine Beggiatoa in sulfide-gradient cultures. Arch Microbiol 136:262–269. doi: 10.1007/BF00425214. [DOI] [Google Scholar]
- 32.Nelson DC, Jørgensen BB, Revsbech NP. 1986. Growth pattern and yield of a chemoautotrophic Beggiatoa sp. in oxygen-sulfide microgradients. Appl Environ Microbiol 52:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagen KD, Nelson DC. 1997. Use of reduced sulfur compounds by Beggiatoa spp.: enzymology and physiology of marine and freshwater strains in homogeneous and gradient cultures. Appl Environ Microbiol 63:3957–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradford M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Polman JK, Larkin JM. 1988. Properties of in vitro nitrogenase activity in Beggiatoa alba. Arch Microbiol 150:126–130. doi: 10.1007/BF00425151. [DOI] [Google Scholar]
- 36.Brito B, Martínez M, Fernandéz D, Rey L, Cabrera E, Palacios JM, Imperial J, Ruiz-Argüezo T. 1997. Hydrogenase genes from Rhizobium leguminasorum bv. viciae are controlled by the nitrogen fixation regulatory protein NifA. Proc Natl Acad Sci U S A 94:6019–6024. doi: 10.1073/pnas.94.12.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Axelsson R, Oxelfelt F, Lindblad P. 1999. Transcriptional regulation of the Nostoc uptake hydrogenase. FEMS Microbiol Lett 170:77–81. doi: 10.1111/j.1574-6968.1999.tb13357.x. [DOI] [PubMed] [Google Scholar]
- 38.Elsen S, Dischert W, Colbeau A, Bauer CE. 2000. Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J Bacteriol 182:2831–2837. doi: 10.1128/JB.182.10.2831-2837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Happe T, Schütz K, Böhme H. 2000. Transcriptional and mutational analysis of the uptake hydrogenase of the filamentous cyanobacterium Anabena variabilis ATCC 29431. J Bacteriol 182:1624–1631. doi: 10.1128/JB.182.6.1624-1631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweerts J-PRA, de Beer D, Nielsen LP, Verdouw H, van den Heuvel JC, Cohen Y, Cappenberg TE. 1990. Denitrification by sulphur oxidizing Beggiatoa spp. mats on freshwater sediments. Nature 344:762–763. [Google Scholar]
- 41.Mussmann M, Schulz HN, Strotmann B, Kjær T, Nielsen LP, Rosselló-Mora RA, Amann RI, Jørgensen BB. 2003. Phylogeny and distribution of nitrate-storing Beggiatoa spp. in coastal marine sediments. Environ Microbiol 5:523–533. doi: 10.1046/j.1462-2920.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 42.Sayama M, Risgaard-Petersen N, Nielsen LP, Fossing H, Christensen PB. 2005. Impact of bacterial NO3- transport on sediment biogeochemistry. Appl Environ Microbiol 71:7575–7577. doi: 10.1128/AEM.71.11.7575-7577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamp A, Stief P, Schulz-Vogt HN. 2006. Anaerobic sulfide oxidation with nitrate by a freshwater Beggiatoa enrichment culture. Appl Environ Microbiol 72:4755–4760. doi: 10.1128/AEM.00163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinck S, Neu TR, Lavik G, Mussmann M, de Beer D, Jonkers HM. 2007. Physiological adaptation of a nitrate-storing Beggiatoa sp. to diel cycling in a phototrophic hypersaline mat. Appl Environ Microbiol 73:7013–7022. doi: 10.1128/AEM.00548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berg JS, Schwedt A, Kreutzmann A-C, Kuypers MMM, Milucka J. 2014. Polysulfides as intermediates in the oxidation of sulfide to sulfate by Beggiatoa spp. Appl Environ Microbiol 80:629–636. doi: 10.1128/AEM.02852-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandelia M-E, Lubitz W, Nitschke W. 2012. Evolution and diversification of group 1 [NiFe] hydrogenases. Is there a phylogenetic marker for O2-tolerance? Biochim Biophys Acta 1817:1565–1575. doi: 10.1016/j.bbabio.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Vignais PM, Billoud B, Meyer J. 2001. Classification and phylogeny of hydrogenases. FEMS Microbiol Rev 25:455–501. doi: 10.1111/j.1574-6976.2001.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 48.Dahl C, Friedrich CG, Kletzin A. 2008. Sulfur oxidation in prokaryotes. Encyclopedia of Life Sciences. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 49.Kappler U. 2011. Bacterial sulfite-oxidizing enzymes. Biochim Biophys Acta 1807:1–10. doi: 10.1016/j.bbabio.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Dahl C, Franz B, Hensen D, Kesselheim A, Zigann R. 2013. Sulfite oxidation in the purple sulfur bacterium Allochromatium vinosum: identification of SoeABC as a major player and relevance of SoxYZ in the process. Microbiology 159:2626–2638. doi: 10.1099/mic.0.071019-0. [DOI] [PubMed] [Google Scholar]
- 51.Schulz HN, Brinkhoff T, Ferdelman TG, Hernández Mariné M, Teske A, Jørgensen BB. 1999. Dense populations of a giant sulfur bacterium in Namibian shelf sediments. Science 284:493–495. doi: 10.1126/science.284.5413.493. [DOI] [PubMed] [Google Scholar]
- 52.Nelson DC, Castenholz RW. 1981. Use of reduced sulfur compounds by Beggiatoa sp. J Bacteriol 147:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Des Marais DJ. 1995. The biogeochemistry of hypersaline microbial mats, p 251–274. In Jones JG. (ed), Advances in microbial ecology, vol 14 Plenum, New York, NY. [DOI] [PubMed] [Google Scholar]
- 54.Dillon JG, Killer S, Bebout B, Hullar M, Pinel N, Stahl D. 2009. Spatial and temporal variability in a stratified hypersaline microbial mat community. FEMS Microbiol Ecol 68:46–58. doi: 10.1111/j.1574-6941.2009.00647.x. [DOI] [PubMed] [Google Scholar]
- 55.Canfield DE, Des Marais DJ. 1993. Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochim Cosmochim Acta 57:3971–3984. doi: 10.1016/0016-7037(93)90347-Y. [DOI] [PubMed] [Google Scholar]
- 56.Omoregie EO, Crumbliss LL, Bebout B, Zehr JP. 2004. Determination of nitrogen-fixing phylotypes in Lyngbya sp. and Microcoleus chthonoplastes cyanobacterial mats from Guerrero Negro, Baja California, Mexico. Appl Environ Microbiol 70:2119–2128. doi: 10.1128/AEM.70.4.2119-2128.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoehler TM, Bebout BM, Des Marais DJ. 2001. The role of microbial mats in the production of reduced gases on the early earth. Nature 412:324–327. doi: 10.1038/35085554. [DOI] [PubMed] [Google Scholar]
- 58.Jørgensen BB, Des Marais DJ. 1986. Competition for sulfide among colorless and purple sulfur bacteria in cyanobacterial mats. FEMS Microbiol Lett 38:179–186. doi: 10.1111/j.1574-6968.1986.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Pichel F, Mechling M, Castenholz RW. 1994. Diel migrations of microorganisms within a benthic, hypersaline mat community. Appl Environ Microbiol 60:1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Des Marais DJ. 2003. Biogeochemistry of hypersaline microbial mats illustrates the dynamics of modern microbial ecosystems and the early evolution of the biosphere. Biol Bull 204:160–167. doi: 10.2307/1543552. [DOI] [PubMed] [Google Scholar]
- 61.Jørgensen BB. 2001. Space for hydrogen. Nature 412:286–289. doi: 10.1038/35085676. [DOI] [PubMed] [Google Scholar]
- 62.Mussmann M, Hu FZ, Richter M, de Beer D, Preisler A, Jørgensen BB, Huntemann M, Glöckner FO, Amann R, Koopmann W, Lasken RS, Janto B, Hogg J, Stoodley P, Boissy R, Ehrlich GD. 2007. Insights into the genome of large sulfur bacteria revealed by analysis of single filaments. PLoS Biol 5:e230. doi: 10.1371/journal.pbio.0050230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacGregor BJ, Biddle JF, Teske A. 2013. Mobile elements in a single-filament orange Guaymas Basin Beggiatoa (“Candidatus Maribeggiatoa”) sp. draft genome: evidence for genetic exchange with cyanobacteria. Appl Environ Microbiol 79:3974–3985. doi: 10.1128/AEM.03821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jannasch HW, Nelson DC, Wirsen CO. 1989. Massive natural occurrence of unusually large bacteria (Beggiatoa sp.) at a hydrothermal deep-sea vent site. Nature 342:834–836. doi: 10.1038/342834a0. [DOI] [Google Scholar]
- 65.Kalanetra KM, Joye SB, Sunseri NR, Nelson DC. 2005. Novel vacuolate sulfur bacteria from the Gulf of Mexico reproduce by reductive division in three dimensions. Environ Microbiol 7:1451–1460. doi: 10.1111/j.1462-2920.2005.00832.x. [DOI] [PubMed] [Google Scholar]
- 66.de Beer D, Sauter E, Niemann H, Kaul N, Foucher J-P, Witte U, Schlüter M, Boetius A. 2006. In situ fluxes and zonation of microbial activity in surface sediments of the Håkon Mosby Mud Volcano. Limnol Oceanogr 51:1315–1331. doi: 10.4319/lo.2006.51.3.1315. [DOI] [Google Scholar]
- 67.Girnth A-C, Grünke S, Lichtschlag A, Felden J, Knittel K, Wenzhöfer F, de Beer D, Boetius A. 2011. A novel mat-forming Thiomargarita population associated with a sulfidic fluid flow from a deep-sea mud volcano. Environ Microbiol 13:495–505. doi: 10.1111/j.1462-2920.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 68.Grünke S, Lichtschlag A, de Beer D, Felden J, Salman V, Ramette A, Schulz-Vogt HN, Boetius A. 2012. Mats of psychrophilic thiotrophic bacteria associated with cold seeps of the Barents Sea. Biogeosciences 9:2947–2960. doi: 10.5194/bg-9-2947-2012. [DOI] [Google Scholar]
- 69.Grünke S, Felden J, Lichtschlag A, Girnth A-C, de Beer D, Wenzhöfer F, Boetius A. 2011. Niche differentiation among mat-forming, sulfide-oxidizing bacteria at cold seeps of the Nile Deep Sea Fan (Eastern Mediterranean Sea). Geobiology 9:330–348. doi: 10.1111/j.1472-4669.2011.00281.x. [DOI] [PubMed] [Google Scholar]
- 70.Welhan JA, Craig H. 1979. Methane and hydrogen in East Pacific Rise hydrothermal fluids. Geophys Res Lett 6:829–831. doi: 10.1029/GL006i011p00829. [DOI] [Google Scholar]
- 71.Lilley MD, de Angelis M, Gordon LI. 1982. CH4, H2, CO, and N2O in hydrothermal vent waters. Nature 300:48–50. doi: 10.1038/300048a0. [DOI] [Google Scholar]
- 72.Cohn F. 1875. Untersuchungen über Bacterien II. Beiträge zur Biologie der Pflanzen 1:141–207. [Google Scholar]
- 73.Caldwell DE, Caldwell SJ, Tiedje JM. 1975. An ecological study of the sulfur-oxidizing bacteria from the littoral zone of a Michigan lake and a sulfur spring in Florida. Plant Soil 43:101–114. doi: 10.1007/BF01928479. [DOI] [Google Scholar]
- 74.Aragno M. 1992. Thermophilic, aerobic, hydrogen-oxidizing (knallgas) bacteria, p 3917–3933. In Balows A, Tr̈uper HG, Dworkin M, Harder W, Schleifer K-H (ed), The prokaryotes, 2nd ed, vol 4 Springer, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.