Abstract
Buchnera aphidicola is an obligate endosymbiont that provides aphids with several essential nutrients. Though much is known about aphid-Buchnera interactions, the effect of the host plant on Buchnera population size remains unclear. Here we used quantitative PCR (qPCR) techniques to explore the effects of the host plant on Buchnera densities in the cotton-melon aphid, Aphis gossypii. Buchnera titers were significantly higher in populations that had been reared on cucumber for over 10 years than in populations maintained on cotton for a similar length of time. Aphids collected in the wild from hibiscus and zucchini harbored more Buchnera symbionts than those collected from cucumber and cotton. The effect of aphid genotype on the population size of Buchnera depended on the host plant upon which they fed. When aphids from populations maintained on cucumber or cotton were transferred to novel host plants, host survival and Buchnera population size fluctuated markedly for the first two generations before becoming relatively stable in the third and later generations. Host plant extracts from cucumber, pumpkin, zucchini, and cowpea added to artificial diets led to a significant increase in Buchnera titers in the aphids from the population reared on cotton, while plant extracts from cotton and zucchini led to a decrease in Buchnera titers in the aphids reared on cucumber. Gossypol, a secondary metabolite from cotton, suppressed Buchnera populations in populations from both cotton and cucumber, while cucurbitacin from cucurbit plants led to higher densities. Together, the results suggest that host plants influence Buchnera population processes and that this may provide phenotypic plasticity in host plant use for clonal aphids.
INTRODUCTION
Aphids harbor two types of bacterial endosymbionts: the obligate symbiont Buchnera aphidicola, which occurs in almost all aphids, and facultative bacteria belonging to a variety of taxa that are not essential for aphid survival and typically are found in some but not all individuals of a population (1–3). The association between Buchnera and aphids was established 80 to 150 million years ago (4), and because transmission is strictly maternal, bacteria and host phylogenies are perfectly congruent. Buchnera is essential for the aphid, and if the symbiont is experimentally removed, the host grows very slowly and cannot reproduce (5–7). Buchnera synthesizes essential amino acids and other substances that are absent in the aphid's phloem diet (1, 8–13) and is active in purine metabolism (14, 15). The symbiont's activities can be disrupted by heat shock, and it has been shown that variation in the promoter of a Buchnera heat shock gene impacts aphid thermal tolerance (16, 17). The genome of Buchnera is much smaller than those of its free-living relatives (18, 19) and in many ways it now resembles an organelle, highly integrated within the physiology of its host.
Densities of Buchnera (as measured by quantitative PCR [qPCR] relative to the host) are affected by host age and genotype and by environmental factors (20, 21). The numbers increase over the juvenile stages and then drop after the aphid becomes an adult (7, 22). Heat stress reduces Buchnera titers (23, 24), as does parasitism by hymenopteran wasps, though the extent of this depends on the intrinsic resistance of the aphid clone to the parasitoid (25, 26). The presence of facultative symbionts such as Hamiltonella defensa, Serratia symbiotica, and Rickettsia spp. is associated with lower Buchnera densities, perhaps because of competition for favored sites in the host (7, 27). However, in one specific pea aphid lineage, the presence of H. defensa led to an increase in Buchnera densities (26).
Variation in Buchnera densities within an individual aphid affects host fitness. In the cowpea aphid, Aphis craccivora, the density of Buchnera is reduced at relatively high and low temperatures, which seems to have a negative effect on aphid reproduction (23). Vogel and Moran (20) found evidence that aphid fitness was greatest for intermediate titers of Buchnera in natural populations of the pea aphid, Acyrthosiphon pisum. Reductions in Buchnera density in adult pea aphids are also associated with the development of flight muscles in winged (alate) morphs (28). There is some evidence from the pea aphid that host plant species and the availability of dietary nitrogen impact Buchnera densities and functioning (29, 30), but the nature of this relationship and its mechanistic basis are both unclear.
The cotton-melon aphid, Aphis gossypii Glover, is a cosmopolitan pest damaging many agricultural crops and ornamental plants, especially those in the families Cucurbitaceae, Malvaceae, Solanaceae, and Rutaceae (31). This aphid harbors the obligate endosymbiont Buchnera, while the facultative bacteria found commonly in the pea aphid and related species, such as Regiella insecticola, H. defensa, and S. symbiotica, are absent or rare (32–34). The taxon Aphis gossypii (like the pea aphid) is composed of a series of host plant races or biotypes that are to various degrees specialized on different host plant groups. Those associated with cotton and cucurbits are the most widespread and agriculturally important (35–37).
Here we used A. gossypii to study the effect of the host plant on the endosymbiont Buchnera. We compared Buchnera titers in clones of A. gossypii that have been collected on cotton and cucumber and reared on this host plant for 10 years and in the same clones but now reared for several generations on novel host plants. We also measured Buchnera densities in natural populations of A. gossypii collected from hibiscus and cotton (Malvaceae) and zucchini (courgette) and cucumber (both Curcurbitaceae) in Nanjing, China, and titers in different genotypes of A. gossypii reared on the same host plant. Finally, we explored the effect of host plant extracts and secondary metabolites (gossypol and cucurbitacin) on the population size of Buchnera in the clones collected on cotton and cucumber and reared on artificial diets. We found that both the host clone and food plant affect Buchnera densities, and we discuss how symbionts may mediate the interaction between herbivorous insects and their host plants.
MATERIALS AND METHODS
Experimental plants.
Six species of host plant were used in this study: Gossypium hirsutum L. cultivar Suzamian 3 (cotton), Cucumis sativus L. cultivar Lufeng (cucumber), Cucurbita pepo L. cultivar Kangzaoqing 1 (zucchini), Vigna unguiculata L. cultivar Chonglong (cowpea), Cucurbita moschata L. cultivar Miben (pumpkin), and hibiscus Hibiscus syriacus L. Plants of the first five species were grown from seeds planted in plastic cups (top diameter, 75 mm; bottom diameter, 55 mm; height, 100 mm) containing perlite and supplied with a growth medium made up of a solution of 2.55 mM KNO3, 1.89 mM KH2PO3, 2.14 mM Mg2SO4·7H2O, 6.27 mM Ca(NO3)2·4H2O, 19.2 μM NaFeEDTA, and1.73 mM KCl. Plants were used for experiments at the 4- to 6-leaf stage. Branches of hibiscus were cut from shrubs on the campus of Nanjing Agricultural University on 4 April 2013 and put into pots with the growth medium described above. One- to 2-centimeter-long leaves, sprouting from the branches, were used for rearing aphids and in the experiments.
Experimental aphids.
Cultures of clones of A. gossypii collected on cotton and cucumber were reared for 12 years on these host plants in growth chambers at 24 ± 1°C with a 14 h/10 h light/dark cycle and relative humidity (RH) of 70 to 80% (37). The cultures were founded from aphids collected in agricultural fields in July 2001 in Nanjing and subsequently maintained as asexual lineages.
Natural populations of A. gossypii were sampled from five species of host plant: aphids from hibiscus were collected on 23 April 2013 on the campus of Nanjing Agricultural University (n [number of aphids collected] = 22), and then aphids from cotton (n = 24), cucumber (n = 24), pumpkin (n = 24), and zucchini (n = 24) were all collected on 8 September 2013 at the Nanjing Agricultural University Experimental Station near Nanjing. Only one individual per tree or seedling was collected in order to increase the probability of sampling the full genetic diversity. Aphids were reared on fresh-cut leaves of their original host plant in petri dishes (diameter, 90 mm; height, 20 mm) at 24°C. The leaf petioles were wrapped in cotton wool dipped in the plant growth medium to keep the leaf fresh. Dishes were covered with Parafilm, which was punctured with dissecting needles to allow ventilation. Leaves were replaced before they withered every 3 to 4 days. After they had produced sufficient offspring to initiate laboratory colonies, the aphids collected from the different crops were preserved in 75% ethanol at −20°C and later assayed for facultative bacterial endosymbionts. A sample of 5-day-old aphids from the first generation on each host plant leaf was taken to assess Buchnera density. All samples were genotyped and offspring from an aphid of each genotype used to found a lineage that was reared on zucchini.
Characterization of bacteriocytes.
Samples consisting of 32 5-day-old aphids were fixed in Bouin's solution for 24 h and then washed in 70% ethanol and ultrapure water before being stored in 80% ethanol at 4°C for subsequent dissection and bacteriocyte characterization. We observed and counted the suspended bacteriocytes following the method described by Douglas and Dixon (38). Fixed aphids were dissected and macerated in a drop of ultrapure water using an insect needle to release the bacteriocytes, which could be clearly observed under a stereomicroscope. After the numbers of bacteriocytes were counted, the suspended cells on the slide were air dried. A fine insect needle was used to pick up individual bacteriocytes attached to the slide. One thousand bacteriocytes were collected and placed in a 1.5-ml Eppendorf tube to extract bacterial DNA, from which the Buchnera dnaK gene was PCR amplified using the ef1α gene from the cotton-melon aphid as a negative control (Table 1). The PCR diagnosis confirmed that these bacteriocytes contained Buchnera. The density of bacteriocytes was used to assess Buchnera population size.
TABLE 1.
Specific primers for diagnosing symbiont species in A. gossypii
| Genus or species | Primer | Primer sequence (5′→3′) | Annealing temp (°C) | Reference |
|---|---|---|---|---|
| Buchnera | Buch16S1F | GGATGAGCCCAGAAGAGATTAGC | 60 | This study |
| Buch16S1R | CTTTCTTCGGGTAACGTCAGGA | |||
| DnaK-F | CCGCAGTTCAAGGAGGAG | 58 | This study | |
| DnaK-R | GAAGTCATTACACCACCCATAGT | |||
| Arsenophonus | 16SA1 | AGAGTTTGATCMTGGCTCAG | 55 | 62 |
| Ars16sR | TTAGCTCCGGAGGCCACAGT | 63 | ||
| Hamiltonella | Ham-F | TGAGTAAAGTCTGGAATCTGG | 55 | 64 |
| Ham-R | AGTTCAAGACCGCAACCTC | |||
| Regiella | U1279F | CGAACGTAAGCGAACCTCAT | 55 | 65 |
| 35R | CCTTCATCGCCTCTGACTGC | 66 | ||
| Serratia | R1279F | CGAGAGCAAGCGGACCTCAC | 56 | 65 |
| 35R | CCTTCATCGCCTCTGACTGC | 66 | ||
| Wolbachia | WspF | GGGTCCAATAAGTGATGAAGAAAC | 55 | 67 |
| WspR | TTAAAACGCTACTCCAGCTTCTGC | |||
| Rickettsia | 16SA1 | AGAGTTTGATCMTGGCTCAG | 55 | 62 |
| Ric16SR1 | TTTGAAAGCAATTCCGAGGT | 68 | ||
| Rickettsiella | RCL16S-211F | GGGCCTTGCGCTCTAGGT | 55 | 69 |
| RCL16S-470R | TGGGTACCGTCACAGTAATCGA | |||
| Spiroplasma | 16SA1 | AGAGTTTGATCMTGGCTCAG | 55 | 62 |
| Spi16SR | ATCATCAACCCTGCCTTTGG | 68 | ||
| X type | 10F | AGTTTGATCATGGCTCAGATTG | 55 | 70 |
| X420R | GCAACACTCTTTGCATTGCT | 71 | ||
| Aphis gossypii | ef1α-1F | GTGAGCA CGCTCTATTGGCTTTC | 58 | This study |
| ef1α-1R | CACGACCTACTGGGACTGTTC |
Extraction of DNA from aphids.
Total aphid genomic DNA was extracted according to the method of An et al. (39). Aphids stored in a 1.5-ml Eppendorf tube with a 20-μl mixture of STE buffer (25 mM NaCl, 50 mM Tris-HCl, 25 mM Na2EDTA, 1% SDS, pH 8.0) were kept at −20°C for 4 min and then macerated. The homogenate was incubated at 65°C for 45 min, transferred to 120 μl 3 M CH3COONa, and then placed under ice for 1 h. The mixture was centrifuged at 12,000 rpm at 4°C for 10 min and the supernatant liquid transferred to a new 1.5-ml Eppendorf tube. The liquid was extracted twice using equal volumes of phenol-chloroform-isoamyl alcohol (25:24:1) (pH 8.0) and centrifuged at 12,000 rpm at 4°C for 10 min. The aqueous phase was transferred into a fresh tube, mixed with twice the volume of 50% ethanol, and kept at −20°C for at least an hour. The mixture was then centrifuged again at 15,000 rpm at 4°C for 15 min. The precipitate was collected, washed with 1 ml 75% ethanol, and recentrifuged at 15,000 rpm at 4°C for 15 min. The resultant precipitate was collected and dissolved in sterile ultrapure water, and the DNA concentration and quality were measured using a NanoDrop 2000/2001 spectrophotometer (Thermo Scientific). High-quality DNA samples were stored at −20°C for the experiments described below. All DNA samples were treated with RNase I at 37°C for 30 min before use.
Microsatellite genotyping and establishment of aphid lineages.
Adult female aphids collected from the five host plants were genotyped based on the seven microsatellite loci Ago24, Ago53, Ago59, Ago66, Ago69, Ago84, and Ago89 (40). The 106 aphids collected in the wild belonged to just four genotypes (genotypes I to IV) (Table 2), and the genetic similarity of these four genotypes was 28 to 44% based on these seven loci. We maintained aphid colonies founded from a single mother of each genotype on zucchini in order to study host genetic effects on the Buchnera titer. Genotype I was originally collected from hibiscus and genotypes II, III, and IV from zucchini. Ten aphids each from the laboratory populations maintained on cotton and cucumber were also genotyped, using the method described above, and were found to belong to genotypes I and IV, respectively.
TABLE 2.
Genotype and endosymbionts in A. gossypii collected from different host plants
| Host plant | No. of samples | Symbiont(s) | Genotype | Allelic combination at microsatellite locus: |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ago24 | Ago53 | Ag59 | Ago66 | Ago69 | Ago84 | Ago89 | ||||
| Hibiscus | 1 | Buchnera + Arsenophonus | I | 114-114 | 118-145 | 165-185 | 152-166 | 107-122 | 116-116 | 154-160 |
| 2 | Buchnera | I | 114-114 | 118-145 | 165-185 | 152-166 | 107-122 | 116-116 | 154-160 | |
| 12 | Buchnera + Arsenophonus | II | 114-155 | 118-145 | 165-185 | 152-166 | 117-117 | 120-124 | 154-160 | |
| 7 | Buchnera | II | 114-155 | 118-145 | 165-185 | 152-166 | 117-117 | 120-124 | 154-160 | |
| Zucchini | 5 | Buchnera | II | 114-155 | 118-145 | 165-185 | 152-166 | 117-117 | 120-124 | 154-160 |
| 12 | Buchnera | III | 153-153 | 118-145 | 181-206 | 152-166 | 117-117 | 116-120 | 154-160 | |
| 3 | Buchnera | IV | 155-210 | 118-145 | 181-206 | 152-166 | 117-117 | 116-120 | 154-160 | |
| Cucumber | 7 | Buchnera | II | 114-155 | 118-145 | 165-185 | 152-166 | 117-117 | 120-124 | 154-160 |
| 15 | Buchnera | IV | 155-210 | 118-145 | 181-206 | 152-166 | 117-117 | 116-120 | 154-160 | |
| 2 | Buchnera + Arsenophonus | IV | 155-210 | 118-145 | 181-206 | 152-166 | 117-117 | 116-120 | 154-160 | |
| Cotton | 8 | Buchnera | II | 114-155 | 118-145 | 165-185 | 152-166 | 117-117 | 120-124 | 154-160 |
| 6 | Buchnera + Arsenophonus | II | 114-155 | 118-145 | 165-185 | 152-166 | 117-117 | 120-124 | 154-160 | |
| 4 | Buchnera + Arsenophonus | III | 153-153 | 118-145 | 181-206 | 152-166 | 117-117 | 116-120 | 154-160 | |
| 2 | Buchnera | III | 153-153 | 118-145 | 181-206 | 152-166 | 117-117 | 116-120 | 154-160 | |
| Pumpkin | 4 | Buchnera | II | 114-155 | 118-145 | 165-185 | 152-166 | 117-117 | 120-124 | 154-160 |
| 16 | Buchnera | IV | 155-210 | 118-145 | 181-206 | 152-166 | 117-117 | 116-120 | 154-160 | |
Detection of endosymbionts in aphids.
The presence of endosymbionts in the aphids collected from different host plants and from the laboratory populations maintained on cotton and cucumber was determined by diagnostic PCR with specific primers based on 16S rRNA gene sequences from Buchnera, R. insecticola, H. defensa, S. symbiotica, Arsenophonus, Rickettsia, Rickettsiella, Spiroplasma, and X type and the wsp gene from Wolbachia (Table 1). PCRs for screening symbionts were conducted in a 25-μl liquid volume containing 2 μl DNA templates, 12.5 μl Taq premix (TaKaRa), 1 μl forward and reverse primers (10 μM), and 8.5 μl double-distilled H2O. PCR cycling parameters were 94°C for 2 min, followed by 35 cycles of 94°C for 1 min, the annealing temperature (Table 1) for 1 min, and 72°C for 1 min, with 72°C for 10 min at the end. Ten microliters of PCR products was subject to electrophoreses on a 1.0% agarose gel, and the presence of a specific target band was treated as diagnostic for symbiont infection. The species of facultative symbionts found in these samples were further confirmed by fully sequencing the 16S rRNA gene. Genotypes of Buchnera were studied by sequencing the ATP synthase β-subunit gene (atp) and the gluconate-6-phosphate dehydrogenase gene (gnd). The primers used were gnd-F (5′-CGCGGATCCGGWCCWWSWATWATGCCWGGWGG-3′), gnd-R (5′-CGCGGGCCCGTATGWGCWCCAAAATAATCWCKTTGWGCTTG-3′), atp-F (5′-CGGGGATCCTGCAGTTTGGWGGWGCWGGWGTWGGWAAAAC-3′), and atp-R (5′-CGGGGATCCGTCGACGCATCWARATGWGCAAAWGTWGTWGCWGG-3′). The gnd genes of Buchnera in aphids from the populations maintained on cotton and cucumber were identical, while there was only one base pair difference (out of 542) in the atp gene. Based on this result, we did not further consider Buchnera genotype in this study.
qPCR detection of the population size of Buchnera.
DNA was extracted from groups of five aphids, each 5 days old. The population size of Buchnera was quantified by the ratio of the copy number of the Buchnera 16S rRNA gene to that of the ef1α gene of the aphid (24, 41). Quantitative PCR (qPCR) was performed using SYBR Ex Taq premix (TaKaRa, Japan) in an ABI 7500 real-time PCR detection system (Applied Biosystems/Life Technologies, USA). The forward primer, 16SrRNA-F (5′-GGACCTTAAAAGGCCTCATGC-3′), and the reverse primer, 16SrRNA-R (5′-GCTGGTTATCCTCTCAGACCAG-3′), amplified a 111-bp fragment of the 16S rRNA gene with 100% efficiency. The forward primer, ef1α-2F (5′-CTTTCGTTCCCATCTCTGGATG-3′), and the reverse primer, ef1α-2R (5′-CCGTCAGCCTTTCCTTCTTTACG-3′), amplified a 109 bp fragment of ef1α with 98.96% efficiency. The real-time qPCR total reaction volume was 20 μl and contained 10 μl SYBR green PCR Mastermix (TaKaRa), 2 μl (14.5 ng μl−1) DNA template, 0.4 μl (10 μM) forward primer, 0.4 μl reverse primer, 0.4 μl ROX reference dye II, and 6.8 μl sterile distilled water. The PCR cycling conditions were an initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. These two pairs of primers had high amplification specificity, and their melting curves had unique peaks. Standard curves (log concentration of DNA on the x axis and PCR cycle number on the y axis) for the 16S rRNA and ef1α genes were constructed using serial dilutions (100, 10−1, 10−2, 10−3, and 10−4 ng μl−1) of plasmid DNA cloned with target amplicons. The DNA in different samples was quantified using the standard curve for each gene, while the gene copy number was calculated using the method described by Whelan et al. (42).
Buchnera population sizes in aphids feeding on different host plants.
The population sizes of Buchnera in the aphids maintained in the laboratory on cotton and cucumber were measured. The total DNA of five 5-day-old aphids was extracted to detect Buchnera densities by qPCR as described above. Four samples of DNA from four clones of each population were analyzed, and DNA amounts were estimated four times for each sample.
Because genotypes II, III, and IV could not to be maintained on cotton, we were unable to examine the effect of this host plant on Buchnera abundance. Aphids of genotype I reared for 46 generations on zucchini in the laboratory and aphids of genotypes II, III, and IV reared for 23 generations in the same way were transferred to fresh leaves of zucchini, cowpea, and cucumber in a plastic dish at 25°C. Four replicates of each combination were established. Leaves were replaced every 3 to 4 days to prevent a decline in food plant quality. After five generations on these host plants, five aphids that had just produced their first offspring were collected to extract DNA to examine the Buchnera population size by qPCR as described above.
Buchnera population sizes in aphids transferred to novel host plants.
Twenty to 25 adult aphids from the populations maintained on cotton (genotype I) and cucumber (genotype IV) were transferred onto new cotton and cucumber leaves in plastic dishes and maintained at 25°C. The offspring they produced within 10 h were counted and kept, and the mother aphids were removed. Five 5-day-old aphids (called G0) were collected and kept in 75% ethanol at −20°C to assess the Buchnera population size using qPCR, with the remaining aphids allowed to become adults. Fifteen to 20 of these adults were then transferred to fresh zucchini, pumpkin, cowpea, cotton, or cucumber leaf and allowed to produce first-generation nymphs (G1) over a period of 10 h; the adults were then removed. When G1 aphids were 5 days old, five of them were collected and retained to measure the Buchnera population size, and the others were left to produce the second-generation nymphs (G2). Third (G3)-, fourth (G4)-, and fifth (G5)-generation samples were obtained in the same way. Four replicates of all combinations were conducted and aphid survival to 5 days recorded.
Effects of host plant extract on Buchnera population size.
Plant extracts from cotton, cucumber, zucchini, cowpea, and pumpkin leaves were prepared to assess their effects on Buchnera densities when added to artificial diets. Fresh leaves of each plant species were collected, washed with ultrapure water, and then placed in an oven at 37°C for 48 h to dry. Dried leaves of cotton (100 g), cowpea (100 g), pumpkin (100 g), zucchini (50 g), and cucumber (50 g) were ground using a Swing Medicinal material grinder (Kefeng Equipment Co., Ltd., Zhengzhou, China). The leaf powder was soaked in absolute ethanol for 72 h in the dark at ambient temperatures (27.5 to 33.3°C; average, 28.6°C) and then filtered. The ethanol extract was concentrated to 1 g ml−1 using a vacuum rotatory evaporator (Qingpu Huxi Instrument Factory, Shanghai, China) at 40°C. Each leaf extract was then diluted with ethanol to obtain four concentrations: 0%, 25%, 50%, and 100%. Ten microliters of each was added to 990 μl artificial diets (43). To feed aphids, the artificial diets (150 μl) were placed between two layers of thin Parafilm (Polisciences, Inc., Warrington, PA, USA) which was fixed to one end of a glass tube (30 mm in diameter and 120 mm high). Thirty first-instar aphids from the populations maintained on cotton (genotype I) were starved for 5 h and then placed on the Parafilm in the tube, with the other end of the tube being covered by insect-proof nylon mesh net. The aphids pierce the thin Parafilm with their stylets and ingest the artificial diet, which was replaced every 3 days. Survival of the aphids in the tube was recorded after 1, 3, and 5 days, and five random aphids that had been reared for 5 days on the artificial diet were collected to assess the Buchnera population size. The experiments for each concentration of each leaf extract were repeated four times. Because young aphids from the population maintained on cucumber (genotype IV) showed poor survival on this kind of artificial diet, only 5-day-old aphids which had been starved for 5 h were exposed to the artificial diet treatments. After 3 days, five random aphids were collected to assess Buchnera densities. None of these aphids survived on the artificial diet containing pumpkin or cowpea extract.
Effects of plant secondary metabolites on Buchnera population size.
Artificial diets containing gossypol acetic acid (Sigma) and cucurbitacin B (Sigma) were also prepared. Gossypol or cucurbitacin B was dissolved in absolute ethanol, and the solution was added to the artificial diets to feed aphids. The concentrations of gossypol and cucurbitacin in the artificial diets were 0, 10, 25, and 50 ppm. Newborn aphids from the population maintained on cotton (genotype I) were starved for 5 h and then allowed to feed on the artificial diets with gossypol or cucubitacin. Five 1-, 3-, and 5-day-old aphids were collected to assess the Buchnera population size. Five-day-old aphids from the population maintained on cucumber (genotype IV) were starved for 5 h and then left for 0.5, 1, and 3 days on the artificial diets with gossypol or cucubitacin; five randomly collected aphids were then collected to assess Buchnera densities. Four replicates were preformed in all cases.
Statistical analysis.
Standard statistical tests (Student t test and one-, two-, and three-way analysis of variance [ANOVA]) were used to explore the effects of different factors on Buchnera population size in aphids, with Tukey's test employed to explore post facto multiple comparisons of means. All statistical analyses were performed with SAS software (44).
RESULTS
Aphid genotype and endosymbionts.
Four genotypes (I, II, III, and IV) were found in the collections of 106 A. gossypii individuals from hibiscus, cotton, cucumber, pumpkin, and zucchini in Nanjing, China (Table 2). Aphids on hibiscus and cotton mainly belonged to genotype II, whereas aphids on cucumber and pumpkin chiefly belonged to genotype IV. Aphids on zucchini were the most diverse, with genotypes II, III, and IV all being present and III being the most common (Table 2).
As expected, all aphids harbored Buchnera, but none were infected with the facultative endosymbionts R. insecticola, H. defensa, S. symbiotica, Rickettsia, Rickettsiella, Spiroplasma, X type, and Wolbachia. Totals of 40.0, 59.1, and 8.3% of aphids from cotton, hibiscus, and cucumber were infected with Arsenophonus, respectively (Table 2). Only aphids that contained Buchnera but not Arsenophonus were used to examine the factors affecting Buchnera population size to eliminate the effect of any interaction between primary symbiont and secondary symbiont.
Effect of host plant of aphid on Buchnera.
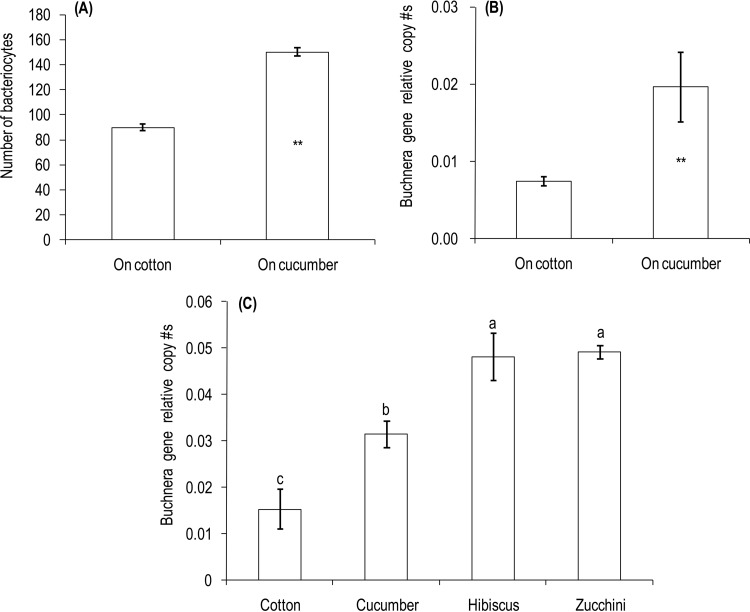
Average numbers of bacteriocytes in the aphids maintained on cucumber were significantly higher than numbers in those maintained on cotton (t = 14.09, df = 62, P < 0.0001) (Fig. 1A). The Buchnera population size, as measured by the ratio of symbiont and host gene density, was 2.7 times higher in the cucumber than in the cotton populations (t = 5.36, df = 6, P = 0.0017) (Fig. 1B). The correspondence between the bacteriocyte and qPCR results indicates that the latter can be used to assess the population size of Buchnera in aphids.
FIG 1.
Bacteriocyte number (A) and relative copy number of the Buchnera aphidicola 16S rRNA gene compared to the aphid ef1a gene (mean ± standard error [SE]; n = 4) from A. gossypii populations maintained on cotton or cucumber (B) and from natural populations collected from cotton, hibiscus, cucumber and zucchini (C). **, significant difference between the cotton and cucumber population aphids at P = 0.01. The different letters indicate significant differences among aphids from the four host plants (P = 0.05, Tukey test).
The population sizes of Buchnera also strongly differed in natural populations of A. gossypii collected from cotton, cucumber, hibiscus, and zucchini (F3,12 = 18.40, P < 0.0001), with aphids from hibiscus and zucchini showing higher population numbers than those from cotton and cucumber (Fig. 1C).
Effect of aphid genotype on Buchnera.
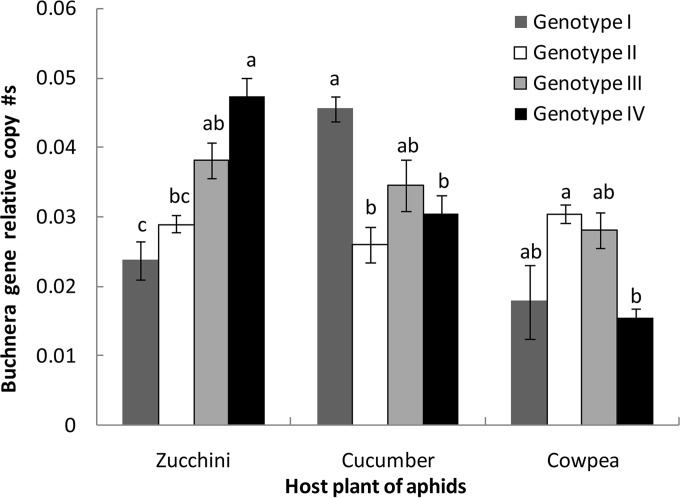
There were significant differences in the population size of Buchnera in aphids belonging to different genotypes when reared on the same host plant (Fig. 2) (zucchini, F3,12 = 18.76, P = 0.0001; cucumber, F3,12 = 9.35, P = 0.0018; cowpea, F3,12 = 5.81, P = 0.0072). In a two-way ANOVA, the population size of Buchnera was significantly affected by host plant but not by genotype, though there was a significant interaction (Table 3).
FIG 2.
Relative copy number of the Buchnera aphidicola 16S rRNA gene compared to the aphid ef1a gene in four genotypes of A. gossypii reared on zucchini, cucumber, and cowpea for six generations. The different lowercase letters above the bars indicate significant differences among aphid genotypes on the same host plant (P = 0.05, Tukey test).
TABLE 3.
Effect of host plant and aphid genotype on Buchnera titersa
| Source of variation | df | Mean square | F | P |
|---|---|---|---|---|
| Host plant | 2 | 691.37 | 22.81 | <0.0001 |
| Aphid genotype | 3 | 64.73 | 2.14 | 0.1127 |
| Host plant × aphid genotype | 6 | 433.66 | 14.31 | <0.0001 |
Determined by two-way ANOVA.
Effect of switching host plant on Buchnera densities and aphid survival.
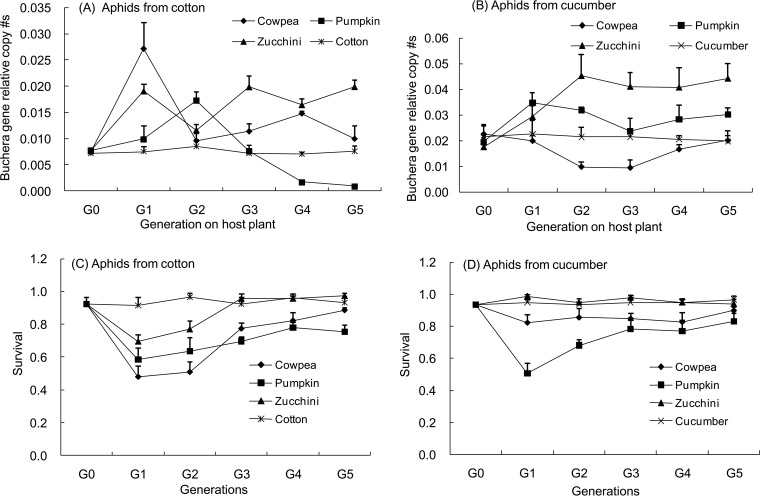
In the experiments where populations maintained on cotton and cucumber were moved onto new host plants, the population origin, host plant, and time (number of generations on the new plant) all significantly affected the population size of Buchnera. There was also a significant interaction between the three factors (Table 4). Buchnera densities in aphid populations originating from cotton showed significant variation across the six generations (G0 to G5) when reared on cowpea (F5,18 = 8.93, P = 0.0002), pumpkin (F5,18 = 18.86, P < 0.0001), and zucchini (F5,18 = 14.33, P < 0.0001). The same was found for the populations from cucumber reared on cowpea (F5,18 = 4.96, P = 0.005) and zucchini (F5,18 = 3.22, P = 0.0299). Buchnera densities in both populations varied greatly in the first two generations after transfer onto the novel host plant (Fig. 3A and B), as did aphid survival (Fig. 3C and D). However, the size of Buchnera populations from cotton became relatively stable after two or three generations, and there were no significant differences between the third, fourth, and fifth generations on cowpea (F2, 9 = 2.23, P = 0.1632) and zucchini (F2,9 = 1.56, P = 0.2625) or between the fourth and fifth generations on pumpkin (F1,6 = 5.49, P = 0.06). The population size of Buchnera in the aphids from the cucumber population also stabilized, and there were no differences in titers in the third, fourth, and fifth generations on zucchini (F2,9 = 0.09, P = 0.9169) or in the fourth and fifth generations on cowpea (F1,6 = 1.64, P = 0.2482) (Fig. 3A and B). When aphids from cotton were moved onto cotton leaves or those from cucumber moved onto the curcurbit, cucumber, or pumpkin leaves, no fluctuations in Buchnera densities were observed, and bacterial densities remained relatively constant over the six generations (cotton to cotton, F5,18 = 0.40, P = 0.8419; cucumber to cucumber, F5,18 = 0.07, P = 0.9960; cucumber to pumpkin, F5,18 = 2.32, P = 0.086). The survival of aphid populations maintained on cotton significantly decreased in the first generation after they were moved onto cowpea but then increased before plateauing after the third generation (F4,15 = 14.94, P < 0.0001). A similar pattern was found when aphids maintained on cotton were transferred to zucchini (F4,15 = 14.76, P < 0.0001) and when those maintained on cucumber were moved onto pumpkin (F4,15 = 5.32, P = 0.0072). No significant differences in survival were found when aphids from the cucumber population were moved onto cowpea (F4,15 = 0.46, P = 0.7647), zucchini (F4,15 = 1.24, P = 0.3348), or cucumber (F4,15 = 0.26, P = 0.9008) (Fig. 3C and D). Note that overall Buchnera densities were lower in aphids from the populations maintained on cotton than in those from cucumber regardless of what plant they fed on.
TABLE 4.
Effect of population origin (maintained on cotton or cucumber), host plant, and generation since transfer on Buchnera titers and host survival after transfer of aphids to a new host planta
| Effect |
Buchnera titer |
Aphid survival |
||||||
|---|---|---|---|---|---|---|---|---|
| df | Mean square | F | P | df | Mean square | F | P | |
| Population origin (A) | 1 | 10,081.56 | 251.72 | <0.0001 | 1 | 0.1954 | 29.11 | <0.0001 |
| Host plant (B) | 3 | 1,395.93 | 34.85 | <0.0001 | 3 | 0.5354 | 79.74 | <0.0001 |
| Rearing generation (C) | 5 | 196.22 | 4.90 | <0.0004 | 4 | 0.1397 | 20.81 | <0.0001 |
| A × B | 3 | 832.68 | 20.79 | <0.0001 | 3 | 0.0464 | 6.91 | 0.0002 |
| A × C | 5 | 78.24 | 1.95 | 0.0891 | 4 | 0.0375 | 5.59 | 0.0004 |
| B × C | 15 | 186.68 | 4.66 | <0.0001 | 8 | 0.0191 | 2.84 | 0.0018 |
| A × B × C | 15 | 113.14 | 2.82 | 0.0007 | 8 | 0.0250 | 3.72 | <0.0001 |
Determined by three-way ANOVA.
FIG 3.
Relative copy number of the Buchnera aphidicola 16S rRNA gene compared to the aphid ef1a gene (A and B) and survival rate (C and D) of the aphids from the populations maintained on cotton (A and C) and on cucumber (B and D) reared for different numbers of generations on four host plants.
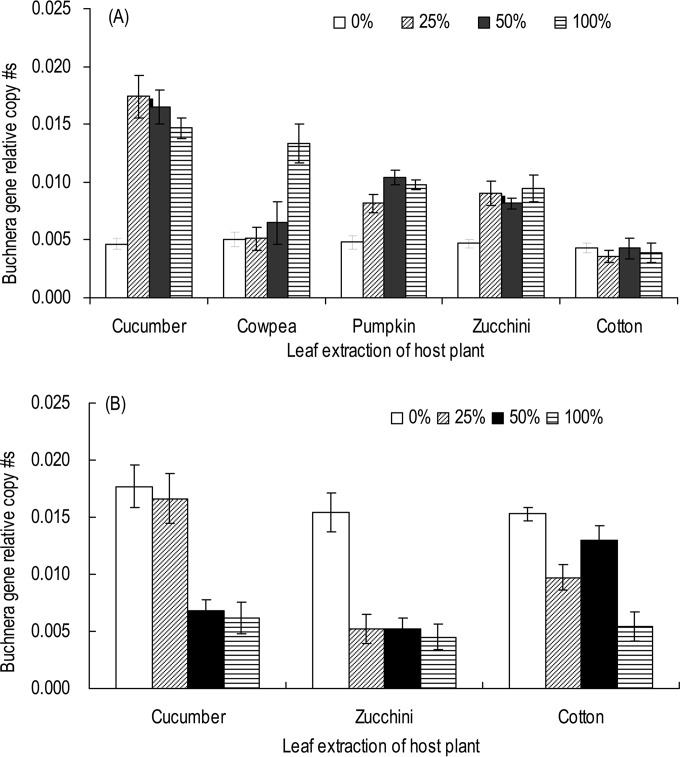
Effects of plant extracts on Buchnera.
Buchnera titers in aphids from both the populations maintained on cotton and cucumber were significantly affected by the addition of extracts from different host plant species when they were added to artificial diets (Table 5). Buchnera densities in the cotton population aphids significantly increased following the addition of cucumber, pumpkin, zucchini, or cowpea extracts, but there was no significant effect of cotton extract (Fig. 4A). Buchnera densities in the cucumber population aphids were lower when aphids were fed artificial diets with cotton or zucchini extracts or high-concentration cucumber leaf extract. Lower concentrations of cucumber extract did not impact Buchnera (Fig. 4B).
TABLE 5.
Effect of plant extract and concentration on Buchnera titers in aphids from populations maintained on cotton or cucumber and fed artificial dietsa
| Effect | Aphids maintained on cotton |
Aphids maintained on cucumber |
||||||
|---|---|---|---|---|---|---|---|---|
| df | Mean square | F | P | df | Mean square | F | P | |
| Plant extraction | 4 | 0.0001714 | 42.92 | <0.0001 | 2 | 0.0000706 | 10.20 | 0.0004 |
| Concn | 3 | 0.0001129 | 28.27 | <0.0001 | 3 | 0.0002370 | 34.21 | <0.0001 |
| Plant extraction × concn | 12 | 0.0000310 | 7.75 | <0.0001 | 6 | 0.0000364 | 5.10 | 0.0010 |
Determined by two-way ANOVA.
FIG 4.
Relative copy number of the Buchnera aphidicola 16S rRNA gene compared to that of the aphid ef1a gene in the aphids from the populations maintained on cotton (A) and cucumber (B) when fed an artificial diet containing different concentrations of leaf extract from cucumber, cowpea, pumpkin, zucchini, and cotton.
Effects of plant secondary metabolites on Buchnera.
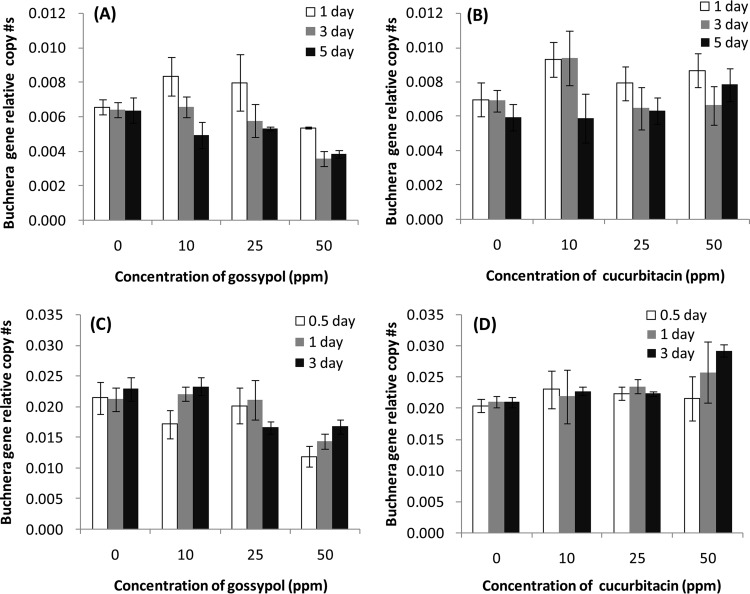
The addition of the plant secondary metabolites gossypol and cucurbitacin to artificial diets significantly affected the population size of Buchnera in both the cotton and cucumber aphid populations, though there was not a significant effect of chemical concentration (Table 6). Gossypol at a high concentration (50 ppm) suppressed Buchnera titers in aphids from both populations (Fig. 5A and C), while cucurbitacin led to higher densities (Fig. 5B and D). The length of time that aphids were allowed to feed on the supplemented artificial diets affected Buchnera densities in aphids from the cotton population but not those from the cucumber population (Table 6): population sizes of Buchnera in the cotton population aphids were significantly lower after feeding on gossypol for 5 days compared to 1 day (Fig. 5A). In the statistical analysis of Buchnera density, a significant interaction was found between plant secondary metabolite type and concentration but not between any of the other two-way interactions or the three-way interaction (Table 6).
TABLE 6.
Effect of secondary metabolite type (gossypol and cucurbitacin B), concentration, and length of exposure on Buchnera titers in aphids from populations maintained on cotton or cucumber and fed artificial dietsa
| Effect | Aphids maintained on cotton |
Aphids maintained on cucumber |
||||||
|---|---|---|---|---|---|---|---|---|
| df | Mean square | F | P | df | Mean square | F | P | |
| Plant secondary metabolite (A) | 1 | 0.00003993 | 11.11 | 0.0015 | 1 | 0.00031426 | 16.55 | 0.0001 |
| Concn (B) | 3 | 0.00000685 | 1.91 | 0.1382 | 3 | 0.00001200 | 0.63 | 0.5970 |
| Length of exposure (C) | 2 | 0.00002256 | 6.27 | 0.0034 | 2 | 0.00003630 | 1.91 | 0.1561 |
| A × B | 3 | 0.00001153 | 3.21 | 0.0293 | 3 | 0.00013989 | 7.37 | 0.0003 |
| A × C | 2 | 0.00000071 | 0.20 | 0.8221 | 2 | 0.00000133 | 0.07 | 0.9326 |
| B × C | 6 | 0.00000501 | 1.39 | 0.2317 | 6 | 0.00002258 | 1.19 | 0.3234 |
| A × B × C | 6 | 0.00000105 | 0.29 | 0.9388 | 6 | 0.00001214 | 0.64 | 0.6982 |
Determined by three-way ANOVA.
FIG 5.
Relative copy number of the Buchnera aphidicola 16S rRNA gene compared to that of the aphid ef1a gene in the aphids from the populations maintained on cotton (A and B) and cucumber (C and D) when fed on artificial diet containing concentrations of 0 to 50 ppm of the plant secondary metabolites gossypol (A and C) and cucurbitacin (B and D) for 0.5, 1, 3, and 5 days. A diet without plant secondary metabolites (0 ppm) was the control.
DISCUSSION
Host plant affects endosymbiont population density in aphids.
We demonstrate here that the host plant has a substantial influence on Buchnera population densities in A. gossypii. Previous studies with Aphis fabae have shown that the densities of Buchnera and an unidentified secondary symbiont were higher in aphids reared on Lamium purpureum (Lamiaceae) than in those reared on Chenopodium album (Chenopodiaceae) and Papaver dubium (Papaveraceae) (29). Aphis fabae also harbored more Buchnera and the secondary symbionts Hamiltonella and Regiella on L. purpureum than on Vicia faba (Fabaceae) (45). In the whitefly Bemisia tabaci, the relative densities of the primary symbiont Portiera and secondary symbionts Hamiltonella, Rickettsia, and Cardinium were strongly affected by the host plant. Whitefly populations living on cabbage (Brassica, Brassicaceae) harbored more Portiera than those populations on cucumber and cotton (46). In the present study, we found that the population size of a primary symbiont, Buchnera, was significantly higher in a laboratory population that had been reared for over 10 years on cucumber than in a population that had been reared for the same amount of time on cotton. Buchnera densities in natural clones of A. gossypii living on hibiscus and zucchini were significantly higher than those in clones collected on cucumber and cotton. When aphids were transferred to new host plants, Buchnera densities became unstable and showed pronounced fluctuations. These results clearly show that the host plant can have a significant effect on the population size of primary endosymbionts such as Buchnera.
There are at least two hypotheses that might explain how the host plant could affect endosymbionts in herbivorous insects. The first is that plant phytotoxins or secondary metabolites suppress or promote the population growth of endosymbionts. A number of plant secondary metabolites have been shown to have bacteriostatic and bactericidal properties (47), and if this activity varied across host plants, it might explain the observed differences in bacterial symbiont densities. In this study, we found that plant extracts added to artificial diets had a significant impact on Buchnera population size. Extracts from cucumber, zucchini, pumpkin, and cowpea leaves led to an increase in Buchnera densities in the aphids from the cotton populations, while those from cotton leaves had no effect. In contrast, plant extracts (from cucumber, zucchini, and cotton) tended to reduce Buchnera densities in the aphids from cucumber. Moreover, a high concentration (50 ppm) of gossypol, a secondary metabolite in cotton, suppressed the Buchnera population density in aphids from both the population maintained on cotton and that maintained on cucumber, while cucurbitacin, a secondary metabolite found in the Cucurbitaceae, had the opposite effect. The data indicate that plant secondary metabolites might influence endosymbiont dynamics in herbivorous insects. The host plant might influence Buchnera dynamics directly or indirectly through the aphid host, for example, by effects on the expression of aphid carboxypeptidase vitellogenic-like protein or by affecting bacteriocyte development and lysozyme production (48, 49).
The second hypothesis is that the host may manipulate its endosymbiont titer to compensate for specific deficiencies in the nutrient profile of its host plant (8, 50, 51). There is considerable variation in the amino acid compositions of different plant species that might affect optimum symbiont density (52, 53). Some association has been found between the essential amino acid requirements of different pea aphid genotypes and their Buchnera titer (20). Insects have the potential to regulate their symbiont density through control of nutrient transport to bacteriocytes (54). For example, Buchnera densities have been shown to increase with higher levels of nitrogen in their host's diet (30), and this might be manipulated by the aphid, though further study is needed to determine if this is a viable means of symbiont control.
In this study, the population sizes of Buchnera in cotton-melon aphids with identical genetic backgrounds differed significantly when they were reared on Malvaceae, Cucurbitaceae, or Fabaceae. These three families of plant have clear differences in their nutritional, phytotoxin, and secondary metabolite profiles (55–57). Moreover, the population size of Buchnera in aphids that had been maintained for over a decade on cucumber changed to different degrees when they were transferred to zucchini, pumpkin, and cucumber, all species that belong to the same family, Cucurbitaceae. Plant extracts from these different species administered through artificial diets also affected Buchnera titers. Together these observations suggest that both qualitative and quantitative aspects of host plant chemistry are likely to be important in determining growth and densities of Buchnera symbionts.
Insect genotype and population density of endosymbionts.
Previous studies have shown that the host genotype can affect endosymbiont growth and abundance. Examples of this have been found in Wolbachia (58, 59) and Spiroplasma infecting Drosophila (60). In pea aphids, significant among-genotype variation in Buchnera densities was found across eight clones (20, 26). However, our results indicate that the effect of aphid genotype on Buchnera density in A. gossypii was also dependent on the host plant and that there was a strong interaction between host plant and aphid genotype. For example, high Buchnera population densities were found in genotypes I and IV, but only when they fed on cucumber and zucchini, respectively. Both in pea aphid and in the whitefly Bemisia tabaci, the presence of different secondary endosymbionts has been shown to be influenced by both host genotype and host plant (61). There thus seems to be a complex interaction between host and host plant that influences the structure of the endosymbiont community in these insects.
Relationship of Buchnera density and host use of aphids.
After transfer to a new host plant, the densities of Buchnera in A. gossypii typically show marked fluctuations before settling to a new equilibrium that may differ across host plants. These fluctuations did not occur if aphids were transferred to leaves from the same species on which they had been maintained in the laboratory. For example, Buchnera densities in aphids that had been reared for over 10 years on cotton fluctuated wildly when they were transferred onto zucchini and cowpea but varied little when they were left on cotton. Concomitant with the stabilization of Buchnera numbers after two or three generations on the novel host plant, aphid survival improved and also became less variable. Though not tested in this study, previous work has shown that A. gossypii populations maintained on cotton or cucumber become adapted to these hosts and are not able to use the other species (35). As the populations are clonal, it is likely that the adaptation is occurring at the level of the primary symbiont rather than the aphids and may involve nongenetic changes, for example, in symbiont density or location. The fluctuations observed as the aphids are transferred to new host plants might reflect disruption of this phenotypic adaptation to the host plant.
Here, we found that the densities of Buchnera in aphids from populations maintained on cucumber were significantly higher than those in aphids maintained on cotton. However, when aphids from the cotton populations were reared on zucchini for five generations and those from the cucumber populations were reared on cowpea for one generation, they were able to use the new plant host, which suggests an expansion in diet width (37). When cotton population aphids were reared on zucchini, Buchnera densities significantly increased and approached the level in aphids from the cucumber populations. In contrast, Buchnera densities in aphids from the cucumber populations declined to the level in cotton aphids when they were reared on cowpea. These results imply that the capacity of A. gossypii to use different host plants might be related to the population density of its primary endosymbiont, Buchnera. Changes in Buchnera population processes thus provide a level of phenotypic plasticity that may be particularly valuable in a largely clonal organism such as an aphid.
ACKNOWLEDGMENTS
We thank Zhi-Juan Sun for collecting aphid samples. We are grateful to Jacob Russell for his valuable comments on an early version of the manuscript.
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20141368), the National Natural Science Foundation of China (31070377), and the Scientific Research and Innovation Project for Graduate Students in Jiangsu Province (grant KYLX-0578 to Yuan-Chen Zhang).
REFERENCES
- 1.Douglas AE. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol 43:17–37. doi: 10.1146/annurev.ento.43.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Moran NA, Dunbar HE, Wilcox JL. 2005. Regulation of transcription in a reduced bacterial genome: nutrient-provisioning genes of the obligate symbiont Buchnera aphidicola. J Bacteriol 187:4229–4237. doi: 10.1128/JB.187.12.4229-4237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady CM, Asplen MK, Desneux N. 2014. Worldwide populations of the aphid Aphis craccivora are infected with diverse facultative bacterial symbionts. Microb Ecol 67:195–204. doi: 10.1007/s00248-013-0314-0. [DOI] [PubMed] [Google Scholar]
- 4.von Dohlen CD, Moran NA. 2000. Molecular data support a rapid radiation of aphids in the Cretaceous and multiple origins of host alternation. Biol J Linn Soc 71:689–717. doi: 10.1006/bijl.2000.0470. [DOI] [Google Scholar]
- 5.Houk EJ, Griffiths GW. 1980. Intracellular symbiotes of Homoptera. Annu Rev Entomol 25:161–187. doi: 10.1146/annurev.en.25.010180.001113. [DOI] [Google Scholar]
- 6.Douglas AE. 1996. Reproductive failure and the free amino acid pools in pea aphids (Acyrthosiphon pisum) lacking symbiotic bacteria. J Insect Physiol 42:247–255. doi: 10.1016/0022-1910(95)00105-0. [DOI] [Google Scholar]
- 7.Koga R, Tsuchida T, Fukatsu T. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc Biol Sci 270:2543–2550. doi: 10.1098/rspb.2003.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas AE, Minto LB, Wilkinson TL. 2001. Quantifying nutrient production by the microbial symbionts in an aphid. J Exp Biol 204:349–358. [DOI] [PubMed] [Google Scholar]
- 9.Baumann P. 2005. Biology of bacteriocyte-associated endosymionts of plant sap-sucking insects. Annu Rev Microbiol 59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- 10.Gündüz EA, Douglas AE. 2009. Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proc Biol Sci 276:987–991. doi: 10.1098/rspb.2008.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark MA, Baumann P. 1997. The (F1F0) ATP synthase of Buchnera aphidicola (endosymbiont of aphids): genetic analysis of the putative ATP operon. Curr Microbiol 35:84–89. doi: 10.1007/s002849900217. [DOI] [PubMed] [Google Scholar]
- 12.Nakabachi A, Ishikawa H. 1999. Provision of riboflavin to the host aphid, Acyrthosiphon pisum, by endosymbiotic bacteria, Buchnera. J Insect Physiol 45:1–6. doi: 10.1016/S0022-1910(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 13.Moran NA, Degnan H. 2006. Fuctional genomics of Buchnera and the ecology of aphid hosts. Mol Ecol 15:1251–1261. [DOI] [PubMed] [Google Scholar]
- 14.Thomas GH, Zucker J, Macdonald SJ, Sorokin A, Goryanin I, Douglas AE. 2009. A fragile metabolic network adapted for cooperation in the symbiotic bacterium Buchnera aphidicola. BMC Syst Biol 3:24. doi: 10.1186/1752-0509-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsey JS, Macdonald SJ, Jander G, Nakabachi A, Thomas GH, Douglas AE. 2010. Genomic evidence for complementary purine metabolism in the pea aphid, Acyrthosiphon pisum, and its symbiotic bacterium Buchnera aphidicola. Insect Mol Biol 19:241–248. doi: 10.1111/j.1365-2583.2009.00945.x. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar HE, Wilson ACC, Ferguson NR, Moran NA. 2007. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol 5:e96. doi: 10.1371/journal.pbio.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke GR, McLaughlin HJ, Simon JC, Moran NA. 2010. Dynamics of a recurrent Buchnera mutation that affects thermal tolerance of pea aphid hosts. Genetics 186:367–372. doi: 10.1534/genetics.110.117440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charles H, Ishikawa H. 1999. Physical and genetic map of the genome of Buchnera, the primary endosymbiont of the pea aphid Acyrthosiphon pisum. J Mol Evol 48:142–150. doi: 10.1007/PL00006452. [DOI] [PubMed] [Google Scholar]
- 19.van Ham RC, Kamerbeek J, Palacios C, Rausell C, Abascal F, Bastolla U, Tamames J. 2003. Reductive genome evolution in Buchnera aphidicola. Proc Natl Acad Sci U S A 100:581–586. doi: 10.1073/pnas.0235981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel KJ, Moran NA. 2011. Effect of host genotype on symbiont titer in the aphid-Buchnera symbiosis. Insects 2:423–434. doi: 10.3390/insects2030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas A, Wilkinson T. 1998. Host cell allometry and regulation of the symbiosis between pea aphids, Acyrthosiphon pisum, and bacteria, Buchnera. J Insect Physiol 44:629–635. doi: 10.1016/S0022-1910(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 22.Komaki K, Ishikawa H. 2000. Genomic copy number of intracellular bacterial symbionts of aphids varies in response to developmental stage and morph of their host. Insect Biochem Mol Biol 30:253–258. doi: 10.1016/S0965-1748(99)00125-3. [DOI] [PubMed] [Google Scholar]
- 23.Chen CY, Lai CY, Kuo MH. 2009. Temperature effect on the growth of Buchnera endosymbiont in Aphis craccivora (Hemiptera: Aphididae). Symbiosis 49:53–59. doi: 10.1007/s13199-009-0011-4. [DOI] [Google Scholar]
- 24.Lu WN, Chiu MC, Kuo MH. 2014. Host life stage-and temperature-dependent density of the symbiont, Buchnera aphidicola, in a subtropical pea aphid (Acyrthosiphon pisum) population. J Asia Pac Entomol 17:537–541. doi: 10.1016/j.aspen.2014.03.012. [DOI] [Google Scholar]
- 25.Cloutier C, Douglas AE. 2003. Impact of a parasitoid on the bacterial symbiosis of its aphid host. Entomol Exp Appl 109:13–19. doi: 10.1046/j.1570-7458.2003.00087.x. [DOI] [Google Scholar]
- 26.Martinez AJ, Weldon SR, Oliver KM. 2014. Effects of parasitism on aphid nutritional and protective symbioses. Mol Ecol 23:1594–1607. doi: 10.1111/mec.12550. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T. 2005. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl Environ Microbiol 71:4069–4075. doi: 10.1128/AEM.71.7.4069-4075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hongoh Y, Ishikawa H. 1994. Changes of mycetocyte symbiosis in response to flying behavior of alatiform aphid (Acyrthosiphon pisum). Zool Sci 11:731–735. [Google Scholar]
- 29.Wilkinson TL, Adams D, Minto LB, Douglas AE. 2001. The impact of host plant on the abundance and function of symbiotic bacteria in an aphid. J Exp Biol 204:3027–3038. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson TL, Koga R, Fukatsu T. 2007. Role of host nutrition in symbiont regulation: impact of dietary nitrogen on proliferation of obligate and facultative bacterial endosymbionts of the pea aphid Acyrthosiphon pisum. Appl Environ Microbiol 73:1362–1366. doi: 10.1128/AEM.01211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebert TA, Cartwright B. 1997. Biology and ecology of Aphis gossypii Glover (Homotera: Aphididae). Southwest Entomol 22:116–153. [Google Scholar]
- 32.Carletto J, Gueguen G, Fleury F, Vanlerberghe-Masutti F. 2008. Screening the bacterial endosymbiotic community of sap feeding insects by terminal restriction fragment length polymorphism analysis. Entomol Exp Appl 129:228–234. doi: 10.1111/j.1570-7458.2008.00760.x. [DOI] [Google Scholar]
- 33.Jones RT, Bressan A, Greenwell AM, Fierer N. 2011. Bacterial communities of two parthenogenetic aphid species cocolonizing two host plants across the Hawaiian Islands. Appl Environ Microbiol 77:8345–8349. doi: 10.1128/AEM.05974-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Najar-Rodríguez AJ, McGraw EA, Mensah RK, Pittman GW, Walter GH. 2009. The microbial flora of Aphis gossypii: patterns across host plants and geographical space. J Invertebr Pathol 100:123–126. doi: 10.1016/j.jip.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Liu XD, Zhai BP, Zhang XX. 2008. Specialized host-plant performance of the cotton aphid is altered by experience. Ecol Res 23:919–925. doi: 10.1007/s11284-007-0458-9. [DOI] [Google Scholar]
- 36.Vanlerberghe-Masutti F, Chavigny P. 1998. Host-based genetic differentiation in the aphid Aphis gossypii Glover, evidenced from RAPD fingerprints. Mol Ecol 7:905–914. doi: 10.1046/j.1365-294x.1998.00421.x. [DOI] [Google Scholar]
- 37.Wu W, Liang XL, Zhao HY, Xu TT, Liu XD. 2013. Special plant species determines diet breadth of phytophagous insects: a study on host plant expansion of the host-specialized Aphis gossypii Glover. PLoS One 8:e60832. doi: 10.1371/journal.pone.0060832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douglas AE, Dixon AFG. 1987. The mycetocyte symbiosis of aphids: variation with age and morph in virginoparae of Megoura viciae and Acyrthosiphon pisum. J Insect Physiol 33:109–113. doi: 10.1016/0022-1910(87)90082-5. [DOI] [Google Scholar]
- 39.An RS, Tan SJ, Chen XF. 2002. Improvement in grinding tissue during extracting DNA from small insects. Entomol Knowledge 39:311–312. [Google Scholar]
- 40.Vanlerberghe-Masutti F, Chavigny P, Fuller SJ. 1999. Characterization of microsatellite loci in the aphid species Aphis gossypii Glover. Mol Ecol 8:693–695. doi: 10.1046/j.1365-294x.1999.00876.x. [DOI] [PubMed] [Google Scholar]
- 41.Oliver KM, Moran NA, Hunter MS. 2006. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc Biol Sci 273:1273–1280. doi: 10.1098/rspb.2005.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whelan JA, Russel NB, Whelan MA. 2003. A method for the absolute quantification of cDNA using real time PCR. J Immunol Methods 278:261–269. doi: 10.1016/S0022-1759(03)00223-0. [DOI] [PubMed] [Google Scholar]
- 43.Mittler TE, Dadd RH. 1964. An improved method for feeding aphids on artificial diets. Ann Entomol Soc Am 57:139. [Google Scholar]
- 44.SAS Institute. 2001. SAS software, release 8.2. SAS Institute, Cary, NC, USA. [Google Scholar]
- 45.Chandler SM, Wilkinson TL, Douglas AE. 2008. Impact of plant nutrients on the relationship between a herbivorous insect and its symbiotic bacteria. Proc Biol Sci 275:565–570. doi: 10.1098/rspb.2007.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan H, Su Q, Jiao X, Zhou L, Liu B, Xie W, Wang S, Wu Q, Xu B, Zhang Y. 2013. Relative amount of symbionts in Bemisia tabaci (Gennadius) Q changes with host plant and establishing the method of analyzing free amino acid in B. tabaci. Commun Integr Biol 6:e23397. doi: 10.4161/cib.23397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harborne JB. 1993. Introduction to ecological biochemistry, 4th ed Academic Press, London, United Kingdom. [Google Scholar]
- 48.Nishikori K, Kubo T, Morioka M. 2009. Morph-dependent expression and subcellular localization of host serine carboxypeptidase in bacteriocytes of the pea aphid associated with degradation of the endosymbiotic bacterium Buchnera. Zool Sci 26:415–420. doi: 10.2108/zsj.26.415. [DOI] [PubMed] [Google Scholar]
- 49.Nishikori K, Morioka K, Kubo T, Morioka M. 2009. Age- and morph-dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. J Insect Physiol 55:351–357. doi: 10.1016/j.jinsphys.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Mittler TE. 1971. Dietary amino acid requirement of the aphid Myzus persicae affected by antibiotic uptake. J Nutr 101:1023–1028. [DOI] [PubMed] [Google Scholar]
- 51.Wilson AC, Ashton PD, Calevro F, Charles H, Colella S, Febvay G, Douglas AE. 2010. Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol Biol 19:249–258. doi: 10.1111/j.1365-2583.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 52.Sandstrom J, Pettersson J. 1994. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid (Acyrthosiphon pisum) performance. J Insect Physiol 40:947–955. doi: 10.1016/0022-1910(94)90133-3. [DOI] [Google Scholar]
- 53.Douglas AE. 2003. The nutritional physiology of aphids. Adv Insect Physiol 31:73–140. doi: 10.1016/S0065-2806(03)31002-1. [DOI] [Google Scholar]
- 54.Hansen AK, Moran NA. 2014. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol Ecol 23:1473–1496. doi: 10.1111/mec.12421. [DOI] [PubMed] [Google Scholar]
- 55.Mattson WJ. 1980. Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161. doi: 10.1146/annurev.es.11.110180.001003. [DOI] [Google Scholar]
- 56.Obst JR. 1998. Special (secondary) metabolites from wood, p 151–165. In Bruce A, Palferyman JW (ed), Forest products biotechnology. Taylor & Francis, Philadelphia, PA. [Google Scholar]
- 57.Paré PW, Tumlinson JH. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–332. doi: 10.1104/pp.121.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondo N, Shimada M, Fukatsu T. 2005. Infection density of Wolbachia endosymbiont affected by coinfection and host genotype. Biol Lett 1:488–491. doi: 10.1098/rsbl.2005.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mouton L, Henri H, Charif D. 2007. Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol Lett 3:210–213. doi: 10.1098/rsbl.2006.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anbutsu H, Goto S, Fukatsu T. 2008. High and low temperatures differently affect infection density and vertical transmission of male-killing Spiroplasma symbionts in Drosophila hosts. Appl Environ Microbiol 74:6053–6059. doi: 10.1128/AEM.01503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, Inbar M, Ghanim M. 2007. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Br Entomol Res 97:407–413. doi: 10.1017/S0007485307005159. [DOI] [PubMed] [Google Scholar]
- 62.Fukatsu T, Nikoh N. 1998. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl Environ Microbiol 64:3599–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. 2002. Diversity and geographic distrbution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol Ecol 11:2123–2135. doi: 10.1046/j.1365-294X.2002.01606.x. [DOI] [PubMed] [Google Scholar]
- 64.Zchori-Fein E, Brown JK. 2002. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann Entomol Soc Am 95:711–718. doi: 10.1603/0013-8746(2002)095[0711:DOPAWB]2.0.CO;2. [DOI] [Google Scholar]
- 65.Russell JA, Latorre A, Sabater-Munoz B, Moya A, Moran NA. 2003. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol Ecol 12:1061–1075. doi: 10.1046/j.1365-294X.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 66.Russell JA, Moran NA. 2005. Horizontal transfer of bacterial symbionts: heritability and fitness effects in a novel aphid host. Appl Environ Microbiol 71:7987–7994. doi: 10.1128/AEM.71.12.7987-7994.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kondo N, Ijichi N, Shimada M, Fukatsu T. 2002. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Mol Ecol 11:167–180. doi: 10.1046/j.0962-1083.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- 68.McLean AHC, van Asch M, Ferrari J, Godfray HCJ. 2011. Effects of bacterial secondary symbionts on host plant use in pea aphids. Proc Biol Sci 278:760–766. doi: 10.1098/rspb.2010.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon JC, Fukatsu T. 2010. Symbiotic bacterium modifies aphid body color. Science 330:1102–1104. doi: 10.1126/science.1195463. [DOI] [PubMed] [Google Scholar]
- 70.Sandström JP, Russell JA, White JP, Moran NA. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol Ecol 10:217–228. doi: 10.1046/j.1365-294X.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 71.Ferrari J, West JA, Via S, Godfray HCJ. 2012. Population genetic structure and secondary symbionts in host-associated populations of the pea aphid complex. Evolution 66:375–390. doi: 10.1111/j.1558-5646.2011.01436.x. [DOI] [PubMed] [Google Scholar]