Abstract
Objectives:
To examine anti-KIR4.1 antibodies by 2 different assays in Japanese patients with multiple sclerosis (MS) or neuromyelitis optica (NMO).
Methods:
One hundred sixty serum samples from 57 patients with MS, 40 patients with NMO/NMO spectrum disorder (NMOSD), and 50 healthy controls (all were Japanese) were tested with ELISA using a synthetic peptide of the first extracellular portion of human KIR4.1. In addition, we attempted to detect anti-KIR4.1 immunoglobulin G in the serum by the luciferase immunoprecipitation systems (LIPS) with the full length of human KIR4.1 produced in a human cell line, which is highly sensitive to single or multiple epitopes.
Results:
We failed to detect antibodies to the peptide fragment KIR4.183–120 in any case of MS and NMO/NMOSD using ELISA. Antibodies to the recombinant full length of KIR4.1 protein were detected in only 2 patients with MS and none in the patients with NMO/NMOSD by the LIPS assay.
Conclusions:
We developed 2 different methods (ELISA and LIPS) to measure autoantibodies to KIR4.1 in serum. We detected anti-KIR4.1 immunoglobulin G at a very low frequency in Japanese patients with MS or NMO/NMOSD. Serologic testing for human KIR4.1-specific antibodies is unlikely to improve the diagnosis of MS or NMO/NMOSD in Japanese patients.
The causes and disease pathways of multiple sclerosis (MS) remain poorly understood; especially unclear is the role of B cells in the pathogenesis of MS. Oligoclonal bands are recognized as a key immunopathologic feature of MS and other neuroinflammatory diseases.
Neuromyelitis optica (NMO) has been regarded as a variant of MS, but with demonstrated distinctive pathologic features. It is another devastating CNS demyelinating disease characterized by severe optic neuritis and transverse, longitudinally extensive myelitis. The discovery of a pathogenic antibody against the astrocyte water channel protein aquaporin-4 (AQP4), with a high diagnostic sensitivity and specificity for NMO, indicates that this condition is distinct from MS.
Srivastava et al.1 reported that antibodies against the inward rectifying potassium channel 4.1 (KIR4.1) were detected in the serum of patients with MS. The authors found anti-KIR4.1 antibodies in serum samples from 47% of patients with MS, 1% with other neurologic diseases, and in none of the healthy controls. Accordingly, KIR4.1 is a candidate pathogenic autoantigen in MS, but subsequent studies have not confirmed the association.2,3 In addition, the coexpression of KIR4.1 and AQP4 channels on astrocyte endfeet may suggest a relationship between anti-KIR4.1 antibodies and NMO/NMO spectrum disorder (NMOSD).
In this study, we used 2 different assays to measure autoantibodies to KIR4.1 in serum from Japanese patients with MS and NMO, and investigated the clinical features of seropositive patients in Japan. In addition, we have recently developed the luciferase immunoprecipitation systems (LIPS) assay to measure antibodies to KIR4.1, which can detect protein–protein interactions with high sensitivity.4–6
METHODS
Standard protocol approvals, registrations, and patient consents.
The ethics committee of Nagasaki Kawatana Medical Center (Nagasaki, Japan) approved this study. Informed consent was obtained from each participant, in person, before participation in the study. Participants provided written informed consent, and this process was documented on an approved consent form.
Patients and controls.
Fifty-seven patients with MS were recruited from the Hokkaido Medical Center and Sapporo Neurology Clinic. Recruitment was limited to patients who were not receiving disease-modifying therapy. The diagnosis of MS was confirmed using 2005 and 2010 revisions to the McDonald criteria.7,8 Patient demographic data were as follows: median age, 40.6 ± 11.5 years; male/female ratio, 11/46; onset age, 27.8 ± 8.4 years; and Expanded Disability Status Scale score, 2.7 ± 2.3. Patients were classified as having relapsing-remitting MS (n = 45), secondary progressive MS (n = 11), or an unclassified (n = 1) disease course by 2 trained neurologists. Clinical sampling phases were as follows: onset = 2; relapse = 16; remission = 30; clinically isolated syndrome = 1; unclassified = 11.
Forty patients with NMO/NMOSD were recruited from Tohoku University Graduate School of Medicine. Recruitment was limited to patients who were seropositive for anti-AQP4 antibodies.9 The diagnosis of NMO/NMOSD was confirmed using the revised NMO criteria.10 Demographic data were as follows: median age, 50.8 ± 14.3 years; male/female ratio, 1/39; onset age, 43.0 ± 13.7 years; and NMO/NMOSD, 21/19. Clinical sampling phases were as follows: acute phase = 13 (onset = 1; relapse = 12); chronic phase = 27. Thirty-six of 40 patients with NMO/NMOSD were administered oral prednisolone.
An additional 50 serum samples from healthy controls were tested (mean age, 35.5 ± 9.2 years, 11 men and 39 women). They were recruited from the Nagasaki Kawatana Medical Center. All 160 serum samples were obtained separately from 2002 to 2013 at 3 locations and cryopreserved at −80°C until use in this study.
ELISA system using the 38-mer synthetic peptide of human KIR4.1.
According to the method described,1 a 38-mer peptide (NH2-G-V-V-W-Y-L-V-A-V-A-H-G-D-L-L-E-L-D-P-P-A-N-H-T-P-C-V-V-Q-V-H-T-L-T-G-A-F-L-COOH) representing the extracellular loop and adjacent intramembrane regions of human KIR4.1 was synthesized and its amino terminus was tagged with biotin (Peptide Institute, Inc., Osaka, Japan). The peptide (10 μg/mL) was dissolved in phosphate-buffered saline (PBS) supplemented with 0.05% Tween 20 (PBST), and 100 μL of the peptide solution was layered onto streptavidin-coated microtiter plates (Nunc, Roskilde, Denmark) at room temperature for 3 hours. After blocking the wells with PBST supplemented with 3% bovine serum albumin, 100 μL of diluted human sera at 100-fold with PBST was added to each well and allowed to react with the peptide at 4°C overnight. After thoroughly washing the wells with PBST, 100 μL of 1:2,500 goat anti-human immunoglobulin G (IgG) (H+L) antibody horseradish peroxidase conjugate (Promega, Madison, WI) was added and incubated for 1 hour at room temperature. After extensive washing, 100 μL of the substrate solution in the TMB Peroxidase EIA Substrate Kit (Bio-Rad Laboratories, Hercules, CA) was added, and the reaction was stopped by adding 100 μL of 1 N H2SO4. A microplate reader was used to measure the optical density at 450 nm. Empty wells were used as a negative control in this assay. The control values were subtracted from the KIR4.1-derived peptide-specific values. ELISA assays were independently performed 3 times. As a positive control for the system, antiserum was prepared from a rabbit immunized with a synthetic peptide of human KIR4.1 (amino acids [aa] 92–105) (Peptide Institute, Inc., Osaka, Japan). Preimmune serum was also collected from the same animal before immunization with the peptide (Peptide Institute, Inc.).
LIPS assay for full-length human KIR4.1.
For LIPS, Gaussia luciferase (GL) was used as a luciferase reporter. Briefly, the LIPS procedure for human KIR4.1 was as follows. A GL reporter for a human KIR4.1 (KIR4.1-GL) expression plasmid was constructed with both human cDNAs encoding the full-length KIR4.1 (GenBank accession no. AB384828) and GL (from pGLuc-Basic; New England BioLabs, Ipswich, MA) in pcDNA3.1-Myc/His (A) (Invitrogen, Carlsbad, CA). The KIR4.1-GL expression plasmid was transfected to human 293F cells (Invitrogen) with FuGENE6 (Promega). Two days later, transfected cells cultured on a 100-mm culture dish were solubilized with 1 mL of lysis buffer (20 mM Tris-HCl, pH 8.0; 100 mM NaCl; and 1% Triton X-100), and a soluble fraction was prepared as a GL reporter sample. Next, 100 μL of the reporter fraction and 15 μL of human serum were mixed for 1 hour at 4°C. To the mixture, 600 μL of PBS supplemented with 0.05% Tween 20, 3% bovine serum albumin, and 15 μL (volume of resin) of Protein G Sepharose (GE Healthcare, Little Chalfont, UK) was added; the mixture was then incubated for 1 hour at 4°C with rotation. The Protein G Sepharose was precipitated at a relative centrifugal force of 9,391 for a few seconds and extensively washed with PBST up to 2 times. A 50-μL substrate solution was added to the precipitate, and bioluminescence was measured by a Lumat LB 9507 Luminometer (Berthold Technologies, Zug, Switzerland). The luminometer output was expressed in relative luminescence units (RLU). Rabbit antiserum against a synthetic peptide of human KIR4.1 (residues 92–105) was used as a positive control to confirm the results of the LIPS assay. A cutoff value was calculated as the mean +4 SDs based on the measurement values of healthy controls (n = 50). LIPS assays were independently performed 3 times.
Statistical analysis.
Commercially available statistics software was used for data analysis (SigmaPlot; HULINKS Inc., Tokyo, Japan). One-way analysis of variance was used for comparisons of antibody titers in the LIPS test and optical density values in the ELISA between the healthy controls and patient groups.
RESULTS
Detection of autoantibodies against KIR4.1 in patients with MS and NMO/NMOSD using ELISA.
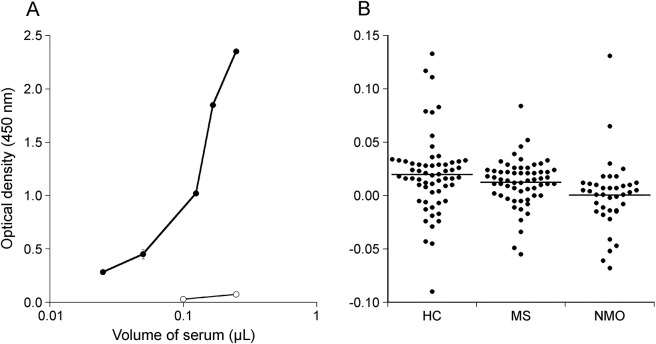
To verify the high clinical sensitivity for anti-KIR4.1 antibodies reported in MS,1 we used the ELISA system with a synthetic 38-mer peptide (aa 83–120 of human KIR4.1). This peptide is highly insoluble, perhaps because of its clusters of hydrophobic amino acids at both ends. Accordingly, one of our concerns was whether the wells in a microtiter plate were properly coated with the peptide. To confirm that 38-mer synthetic peptides bind in the wells of a microtiter plate, we examined the binding of a rabbit antiserum raised against an internal portion (aa 92–105) of human KIR4.1 to the 38-mer synthetic peptide-coated microtiter plate. As shown in figure 1A, anti-KIR4.192–105 rabbit antiserum, but not its preimmune serum, bound to the plate in a dose-dependent manner (figure 1A). Based on the cutoff value established from data for healthy controls, anti-KIR4.1 antibodies were not detected in any cases of MS or NMO/NMOSD (figure 1B).
Figure 1. Detection of KIR4.1 autoantibodies using ELISA with the synthetic peptide.
(A) To ensure that each well was coated with the synthetic peptide, the ELISA assay was performed with anti-KIR4.192–105 rabbit antiserum. The antibody bound in a dose-dependent manner. The x-axis indicates the amount of rabbit antiserum for the KIR4.192–105 peptide used. The lines with open and closed circles are the results using preimmune serum and antiserum for the KIR4.192–105 peptide, respectively. The y-axis indicates the optical density at 450 nm. (B) No patient with MS was positive for the antibody (0%). No patient was positive for the antibody among 40 patients with anti-aquaporin-4 antibody–positive NMO spectrum disorder (0%). There were no positive samples in the HC group. HC = healthy controls; KIR4.1 = inward rectifying potassium channel 4.1; MS = multiple sclerosis; NMO = neuromyelitis optica.
Detection of autoantibodies to KIR4.1 using LIPS for patients with MS and NMO/NMOSD.
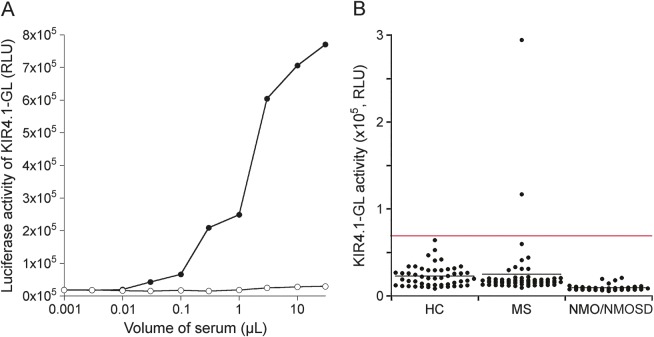
To search for epitopes in the full-length human KIR4.1 polypeptide, we used the LIPS for the anti-KIR4.1 antibody. It has been demonstrated that LIPS is effective for detecting antibodies against several membrane proteins.2–4 To confirm the validity of the LIPS assay for anti-human KIR4.1 antibodies, anti-KIR4.192–105 rabbit antiserum for KIR4.1 was used as a positive control (figure 2A). The anti-KIR4.192–105 rabbit antiserum bound KIR4.1-GL in a dose-response manner, but not in preimmune serum, suggesting that anti-KIR4.1 antibodies in rabbit antiserum recognize the first extracellular portion in the full-length human KIR4.1 fused to GL. As shown in figure 2B, based on a cutoff of 0.708 × 105 RLU (mean + 4 SDs of 50 serum samples from healthy controls), only 2 patients with MS were positive for the anti-KIR4.1 antibody based on the LIPS assay (3.3%), while none of the patients with NMO/NMOSD or the healthy controls were autoantibody-positive. There were no statistically significant differences among the 3 groups (p = 0.278). To exclude the possibility that an antibody binding to GL is detected in this assay, we further performed LIPS using both KIR4.1-GL and GL. Two KIR4.1 antibody–positive sera of patients with MS bound to KIR4.1-GL in a dose-dependent manner. Conversely, the binding activities of these sera to GL were apparently lower than those to KIR4.1-GL, indicating that immunoglobulin G binding KIR4.1 was contained in the sera of 2 patients with MS (figure e-1 at Neurology.org/nn).
Figure 2. Detection of KIR4.1 autoantibodies using LIPS.
(A) LIPS assay for KIR4.1 by rabbit antiserum for the KIR4.192–105 peptide. Anti-KIR4.1 antibodies bound KIR4.1-GL in a dose-dependent manner. The x-axis indicates the amount of antiserum for the KIR4.192–105 peptide. The y-axis indicates KIR4.1-GL activity. The lines with open and closed circles are the results using preimmune serum and antiserum for the KIR4.192–105 peptide, respectively. (B) LIPS assay for KIR4.1. Two patients with multiple sclerosis were positive for the anti-KIR4.1 antibody based on the LIPS assay (3.3%); none of the patients with NMO or NMOSD, or HCs, were autoantibody-positive. There were no statistical differences among the 3 groups (p = 0.469). The y-axis indicates the luciferase activity of KIR4.1-GL (in RLU). Red and black lines indicate the cutoff value and the mean values, respectively. GL = Gaussia luciferase; HC = healthy controls. KIR4.1 = inward rectifying potassium channel 4.1; LIPS = luciferase immunoprecipitation systems; NMO = neuromyelitis optica; NMOSD = NMO spectrum disorder; RLU = relative luminescence units.
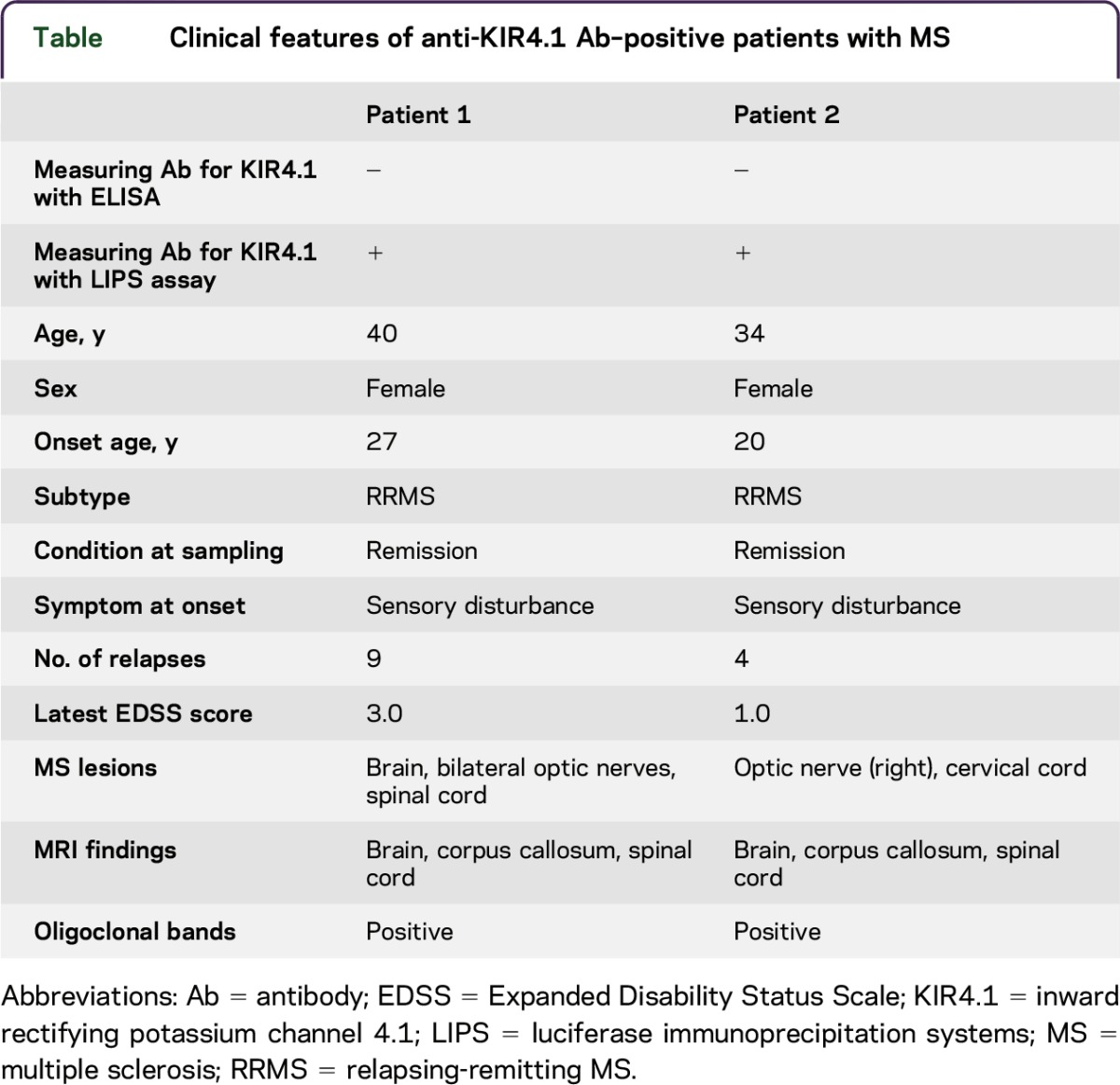
Clinical profile of anti-KIR4.1 antibody–positive patients with MS or NMO/NMOSD.
The table summarizes the clinical profiles of 2 anti-KIR4.1 antibody–positive patients with MS. The patient characteristics were rather typical of MS (patients 1 and 2). We found no specific clinical features in these patients other than the basic disease.
Table.
Clinical features of anti-KIR4.1 Ab–positive patients with MS
DISCUSSION
It had been reported that there was a strong correlation between the serum antibody reactivities measured by ELISA with the recombinant full length of KIR4.1 and measured by ELISA with the peptide fragment KIR4.183–120.1 In accordance with their findings, we also used the 2 types of antigens to measure serum antibody to KIR4.1. One was the peptide fragment KIR4.183–120 designed by a previous study for ELISA.1 Another was the recombinant full length of KIR4.1 synthesized in a human cell line for LIPS. In the ELISA method, we failed to detect anti-KIR4.1 IgG in Japanese patients with MS and NMO/NMOSD. Meanwhile, anti-KIR4.1 antibody positivity was detected in 2 patients with MS using the LIPS method. In the LIPS, recombinant proteins are produced in human cells and are directly tagged with the highly sensitive GL reporter enzyme. One of the key advantages of our LIPS test is the large dynamic range of detection of antibodies because of the solution-phase assay format and the high signal-to-noise ratio of the GL reporter. These results differ markedly from those of the previous report1 in which KIR4.1 was identified as a candidate autoantigen in MS. However, the results of this study were consistent with the low prevalence of anti-KIR4.1 antibodies observed in patients with MS by several groups.2,3 By contrast, the other group recently detected anti-KIR4.1 antibodies in the serum of patients with MS and NMO, at a significantly higher frequency and at higher levels compared to healthy controls.11 This discrepancy is difficult to explain since analogous ELISA techniques used in each study were not identical and the samples were ethnically different. To clarify the differences, it is necessary to perform various assays to detect KIR4.1 IgG using the same coded samples in an international multicenter study.12
KIR4.1 is an inwardly rectifying potassium channel for the osmotic homeostasis of extracellular potassium, which is expressed on oligodendrocytes and the perivascular and perisynaptic endfeet of astrocytes.13 It is localized with AQP4 at the interface of astrocytes and blood vessels, where KIR4.1 cooperates with AQP4 for the balance of potassium and water transport. This suggests a functional link between the 2 channels. Several studies suggested NMO as an autoimmune astrocytopathy in which damage to astrocytes exceeds both myelin and neuronal damage.14,15 We also studied 50 samples from patients with NMO/NMOSD positive for anti-AQP4 antibodies to confirm the prevalence of anti-KIR4.1 antibodies in the astrocytopathy. However, we found that none of the patients with NMO/NMOSD had anti-KIR4.1 antibodies. There was no serologic evidence that autoantibodies target glial KIR4.1 in patients with NMO/NMOSD.
The other research group in Japan16 already screened for anti-KIR4.1 antibodies using ELISA in a cohort of Japanese patients with idiopathic CNS demyelinating disease.16 They also showed that anti-KIR4.1 antibodies are not specific to MS; the antibody positivity rate in MS was only 3.9% for 180 patients with MS, which is much lower than the 46.9% previously described.1 They mentioned a need for replication studies using Japanese patients residing in other regions, including the northern part of Japan.16 Therefore, we collected sera from patients with MS residing at 2 hospitals located at high latitudes, where the incidence of MS is the highest in Japan. Taken together, serologic testing for anti-KIR4.1 antibodies is unlikely to aid in the diagnosis of MS or NMO/NMOSD in a Japanese population.
ACKNOWLEDGMENT
This work was supported in part by grants from the Neuroimmunological Disease Research Committee; the Ministry of Health, Labour and Welfare, Japan; and the Ministry of Education, Culture, Sports, Science and Technology of Japan in the form of a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI grant 24590720, 25461305).
GLOSSARY
- AQP4
aquaporin-4
- GL
Gaussia luciferase
- IgG
immunoglobulin G
- KIR4.1
inward rectifying potassium channel 4.1
- LIPS
luciferase immunoprecipitation systems
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorder
- PBS
phosphate-buffered saline
- PBST
phosphate-buffered saline with Tween 20
- RLU
relative luminescence units
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: O.H., S.N., M.N., K.F., H.M. Performed the experiments: O.H., W.S., Y.M. Analyzed the data: O.H., S.N., M.N., T.T., T.F., S.K., K.F., H.M. Wrote the manuscript: O.H., S.N., M.N., K.F., H.M.
STUDY FUNDING
This work was supported in part by grants from the Neuroimmunologic Disease Research Committee; the Ministry of Health, Labour and Welfare, Japan; and the Ministry of Education, Culture, Sports, Science and Technology of Japan in the form of a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI grant 24590720, 25461305).
DISCLOSURE
O. Higuchi received research support from the Japan Society for the Promotion of Science. S. Nakane, W. Sakai, and Y. Maeda report no disclosures. M. Niino received travel funding and/or speaker honoraria from Biogen, Bayer Schering Pharma, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company, served on the editorial board for Immunology and Immunogenetics Insights, Multiple Sclerosis International, received research support from the Ministry of Health, Labour and Welfare of Japan. T. Takahashi received speaker honoraria from Biogen Idec, Cosmic Corporation. T. Fukazawa served on the scientific advisory board for Biogen Idec, Novartis Pharma, received travel funding and/or speaker honoraria from Bayer Pharma, Biogen Idec, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company, Novartis Pharma. S. Kikuchi received travel funding and/or speaker honoraria from Otsuka Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Abbott Japan, Sumitomo Dainippon Pharma, Nihon Medi-Physics, Fujifilm RI Pharma, GlaxoSmithKline K.K., Eisai, Japan Blood Products Organization, Nihon Pharmaceutical, Kyowa Hakko Kirin, Takeda Pharmaceutical Ltd. K. Fujihara serves on the scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, Alexion Pharmaceuticals, MedImmune and Medical Review, received travel funding and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Eisai Inc., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Astellas Pharma Inc., Takeda Pharmaceutical Company Ltd., Asahi Kasei Medical Co., Daiichi Sankyo, Nihon Pharmaceutical, is on the editorial board for Clinical and Experimental Neuroimmunology, is an advisory board member for Sri Lanka Journal of Neurology, received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Medical, The Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, Genzyme Japan, Ministry of Education, Science and Technology of Japan, Ministry of Health, Welfare and Labour of Japan. H. Matsuo is on the editorial board for Transfusion and Apheresis Science. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Srivastava R, Aslam M, Kalluri SR, et al. . Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med 2012;367:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nerrant E, Salsac C, Charif M, et al. . Lack of confirmation of anti-inward rectifying potassium channel 4.1 antibodies as reliable markers of multiple sclerosis. Mult Scler 2014;20:1699–1703. [DOI] [PubMed] [Google Scholar]
- 3.Brickshawana A, Hinson SR, Romero MF, et al. . Investigation of the KIR4.1 potassium channel as a putative antigen in patients with multiple sclerosis: a comparative study. Lancet Neurol 2014;13:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. Antibody profiling by luciferase immunoprecipitation systems (LIPS). J Vis Exp 2009;32:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakane S, Higuchi O, Koga M, et al. . Clinical features of autoimmune autonomic ganglionopathy and the detection of subunit-specific autoantibodies to the ganglionic acetylcholine receptor in Japanese patients. PLoS One 2015;10:e0118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 2011;69:418–422. [DOI] [PubMed] [Google Scholar]
- 7.Polman CH, Reingold SC, Edan G, et al. . Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 8.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Fujihara K, Nakashima I, et al. . Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J Exp Med 2006;210:307–313. [DOI] [PubMed] [Google Scholar]
- 10.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 11.Brill L, Goldberg L, Karni A, et al. . Increased anti-KIR4.1 antibodies in multiple sclerosis: could it be a marker of disease relapse?. Mult Scler 2015;21:572–579. [DOI] [PubMed] [Google Scholar]
- 12.Waters PJ, McKeon A, Leite MI, et al. . Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology 2012;78:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J Neurosci 2015;35:13827–13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucchinetti CF, Guo Y, Popescu BF, Fujihara K, Itoyama Y, Misu T. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol 2014;24:83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeka B, Hastermann M, Hochmeister S, et al. . Highly encephalitogenic aquaporin 4-specific T cells and NMO-IgG jointly orchestrate lesion location and tissue damage in the CNS. Acta Neuropathol 2015;130:783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe M, Yamasaki R, Kawano Y, Imamura Y, Kira J. Anti-KIR4.1 antibodies in Japanese patients with idiopathic central nervous system demyelinating diseases. Clin Exp Neuroimmunol 2013;4:241–242. [Google Scholar]