Abstract
Thyroid cancer is on the rise. Novel approaches are needed to improve the outcome of patients with recurrent and advanced metastatic thyroid cancers. FDA approval of suberoylanilide hydroxamic acid (SAHA; vorinostat), an inhibitor of histone deacetylase, for the treatment of hematologic malignancies led to the clinical trials of vorinostat for advanced thyroid cancer. However, patients were resistant to vorinostat treatment. To understand the molecular basis of the resistance, we tested the efficacy of SAHA in two mouse models of metastatic follicular thyroid cancer: ThrbPV/PV and ThrbPV/PVPten+/− mice. In both, thyroid cancer is driven by over-activation of PI3K-AKT signaling. But the latter exhibit more aggressive cancer progression due to haplodeficiency of the tumor suppressor, the Pten gene. SAHA had no effects on thyroid cancer progression in ThrbPV/PV mice, indicative of resistance to SAHA. Unexpectedly, thyroid cancer progressed in SAHA-treated ThrbPV/PVPten+/− mice with accelerated occurrence of vascular invasion, anaplastic foci, and lung metastasis. Molecular analyses showed further activated PI3K-AKT in thyroid tumors of SAHA-treated ThrbPV/PVPten+/− mice, resulting in the activated effectors, p-Rb, CDK6, p21Cip1, p-cSrc, ezrin and matrix metalloproteinases to increase proliferation and invasion of tumor cells. Single molecule DNA analysis indicated that the wild-type allele of the Pten gene was progressively lost while carcinogenesis progressed in SAHA-treated ThrbPV/PVPten+/− mice. Thus, the present studies have uncovered a novel mechanism by which SAHA-induced loss of the tumor suppressor Pten to promote thyroid cancer progression. Effectors downstream of the Pten loss-induced signaling may be potential targets to overcome resistance of thyroid cancer to SAHA.
Keywords: Thyroid hormone receptors, tumorigenesis, genetically engineered mouse model, thyroid cancer, vorinostat, SAHA, tumor suppressor PTEN
Introduction
Thyroid cancer is the most common malignancy of the endocrine organs. It consists of an array of several different histologic and biologic types, but the majority of clinically important human thyroid cancers are the differentiated papillary and follicular types. Papillary thyroid cancer commonly metastasizes to lymph nodes, whereas follicular thyroid cancer shows blood-borne metastases. While overall survival of patients with these types of tumor is generally better than many other cancers, approximately 30% of patients do not survive beyond 20 years, even with successful primary surgical therapy. The treatment of recurrent and metastatic thyroid carcinoma is still a major challenge. Radioactive iodine therapy is currently the treatment of choice for patients whose thyroid cancer has spread to distant sites. However, an ominous histologic sign in these tumors is the appearance of anaplastic foci (areas of spindle cell or embryonic dedifferentiation), and once these anaplastic foci have developed, the prognosis is considerably worse. One consequence of dedifferentiation of tumor cells is the loss of the ability to uptake radioiodine, thus severely limiting treatment options (Reiners, et al. 2011). Intensive efforts have been made to search for effective ways to re-differentiate the dedifferentiated tumor cells to regain iodine uptake ability to improve patients’ outcome.
One approach that has offered great promise is via modulation of epigenetic events by inhibition of histone deacetylation. Histone acetylation by histone acetyl transferases results in open chromatin structures to facilitate transcription activation. The reverse process of histone deacetylation by histone deacetylases (HDACs) leads to compact chromatin structures, resulting in transcription repression (Grunstein 1997; Khan and La Thangue 2012; Marks, et al. 2001). Because aberrant HDAC activity is implicated in a variety of cancers, HDAC inhibitors have been developed as potential anticancer therapies. Suberoylanilide hydroxamic acid (SAHA; vorinostat) is one of the HDAC inhibitors approved for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma (Mann, et al. 2007; Olsen, et al. 2007). Several preclinical studies have demonstrated the efficacy of SAHA treatment to induce cell cycle arrest and apoptosis in thyroid cancer cell lines (Borbone, et al. 2010; Luong, et al. 2006; Mitsiades, et al. 2005). SAHA also restores thyroid-specific gene expression such as sodium/iodide symporter as well as radioiodine uptake (Hou, et al. 2010). These promising preclinical findings led to clinical trials of SAHA and another inhibitor in patients with radioactive iodine-refractory thyroid carcinoma (Sherman, et al. 2013; Woyach, et al. 2009), but the patients showed no major responses (Sherman et al. 2013) and the cancer of a few even progressed during SAHA treatment (Woyach et al. 2009). The outcome of these clinical trials, while very limited, suggested that in contrast to the positive findings from cell-based studies, patients are resistant to such treatment. At present, how patients’ thyroid cancer is resistant and even progresses further during SAHA treatment is not clear.
The availability of mouse models of follicular thyroid cancer (FTC) allowed us to dissect the molecular basis in the resistance of thyroid cancer to SAHA treatment. The ThrbPV/PV mouse, harboring a dominant negative thyroid hormone receptor β (TRβPV), spontaneously develops FTC similar to human thyroid cancer with a pathologic progression from hyperplasia to capsular invasion, vascular invasion, and eventually metastasis (Suzuki, et al. 2002). Extensive molecular analyses of altered signaling pathways during thyroid carcinogenesis further confirmed that the ThrbPV/PV mouse is a preclinical mouse model of FTC. As found in human FTC, ThrbPV/PV mice exhibit aberrant signaling pathways that include constitutive activation of phosphatidylinositol 3-kinase (PI3K)-AKT (Furuya, et al. 2006; Furuya, et al. 2007) and integrin–cSrc–MAPK signaling (Beroukhim, et al. 2010) and aberrant accumulation of the oncogenic pituitary tumor transforming gene protein (Ying, et al. 2006) and β-catenin (Guigon, et al. 2008). Another mutant mouse that spontaneously develops FTC is the ThrbPV/PVPten+/− mouse (Guigon, et al. 2009). PTEN (phosphatase and tensin homologue deleted from chromosome 10) functions as a tumor suppressor by opposing the PI3K-AKT signaling pathway (Li, et al. 1997). PTEN haplodeficiency further exacerbates the overactivated PI3K-AKT signaling, leading to a more aggressive cancer phenotype with decreased survival and increased distant metastasis (Guigon et al. 2009). The use of ThrbPV/PV and ThrbPV/PVPten+/− mice allowed us to understand the effect of SAHA on thyroid cancer progression with different genetic changes. Using these two mouse models, we found that thyroid cancer progression in ThrbPV/PV mice was resistant to SAHA treatment. Unexpectedly, SAHA treatment significantly increased thyroid tumor growth of ThrbPV/PVPten+/− mice. In addition, SAHA promoted carcinogenesis by increasing the occurrence of vascular invasion, anaplastic foci, and distant lung metastasis. Molecular analysis showed that PI3K-AKT signaling was further exacerbated in SAHA-treated ThrbPV/PVPten+/− mice. Moreover, the extent of thyroid tumor growth was correlated to the progressive loss of the wild-type Pten allele in the ThrbPV/PVPten+/− mice. Thus, the present study uncovered that the loss of the Pten gene is one mechanism by which SAHA induced more aggressive thyroid cancer in ThrbPV/PVPten+/− mice.
Materials and Methods
Animals and treatment of SAHA
The National Cancer Institute Animal Care and Use Committee approved the protocols for animal care and handling in the present study. Mice harboring the ThrbPV gene (ThrbPV/PV mice) were previously described (Kaneshige, et al. 2000). Pten+/− mice were kindly provided by Dr. Ramon Parsons (Columbia University, New York, NY). ThrbPV/PVPten+/− mice were obtained by crossing Pten+/− mice with ThrbPV/+ mice, followed by crossing ThrbPV/+Pten+/− with ThrbPV/+Pten+/+ mice. Vorinostat (SAHA) (Selleckchem, Cat #: S1047) was dissolved in water to make a 10 mg/ml stock and administered by oral gavage daily at a dose of 50 mg/kg body weight/day starting at the age of 6 weeks for 8 weeks. The thyroids and lungs were dissected after mice were euthanized for weighing, histologic analysis, and biochemical studies.
Western blot analysis
The Western blot analysis was carried out as described by Zhu et al (Zhu, et al. 2014). Primary antibodies for p-AKT (#9271), total-AKT (#9272), PTEN (#9552), CDK4 (#2906), CDK6 (#3136), p-RB (#9307), MMP7 (#3801), and GAPDH (#2118) were purchased from Cell Signaling Technology (Danvers, MA). The p21 primary antibody (sc-6246), Rb (sc-50), and MMP2 (sc-10736) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Primary antibody against ERBB2 (RB-103-P0) was purchased from Neomarkers (Fremont, CA). Antibodies were used at a concentration recommended by the manufacturers. For control of protein loading, the blot was probed with the antibody against GAPDH.
Histological analysis and immunohistochemistry
Thyroid glands, heart, and lung were dissected and embedded in paraffin. Five-micrometer-thick sections were prepared and stained with hematoxylin and eosin (H&E). For each mouse, single random sections through the thyroid, lung, and heart were examined. Immunohistochemistry was performed with paraffin sections by standard methods. Primary antibodies for p-AKT (S473) (#4060, 1:120 dilution) and PTEN (#9552, 1:120 dilution) were purchased from Cell Signaling Technology.
Hormone assays
The serum level of total T4 (TT4) and T3 (TT3) were determined by using a Gamma Coat T4 and T3 assay RIA kit. TSH levels in serum were measured as described (Zhao, et al. 2012).
RNA extraction and real-time RT-PCR
Total RNA from thyroids was isolated using TRIzol (Invitrogen, CA), as indicated by the protocol of the manufacturer. Real-time RT-PCR was performed using a QuantiTect SYBR green RT-PCR kit from Qiagen, following the instructions of the manufacturer (Qiagen, Valencia, CA). Primers were as follows: for mouse Pten, forward, 5′-TGGGGAAGTAAGGACCAGAG-3′; reverse, 5′- TCACCTTTAGCTGGCAGACC-3′; for the endogenous control gene mouse glyceraldehyde-3-phosphate dehydrogenase (Gapdh), forward, 5′-cgtcccgtagacaaaatggt-3′; reverse, 5′-gaatttgccgtgagtggagt-3′.
Single molecule DNA analysis by droplet digital PCR reaction
In single molecule DNA analysis, by using the genomic DNA instead of mRNA as templates, the droplet digital PCR (ddPCR) enables us to quantify the loss of the Pten gene in mouse thyroid tumors. Genomic DNA from fresh-frozen thyroid tissue samples was extracted using the Qiagen blood and tissue kit according to the manufacturer's protocol (Qiagen, Hilden, Germany). DNA concentration was measured with the NanoDrop 2000c spectrophotometer (Thermo Scientific, Wilmington, DE). For EvaGreen-based ddPCR, reaction mixtures contained 1× ddPCR EvaGreen Supermix (Bio-Rad, Hercules, CA), 100 nM primers (Integrated DNA Technologies, Coralville, IA) and mouse thyroid genomic DNA template in a final volume of 22 µL. Droplet digital PCR were performed in the CCR Genomics Core using QX200 autoDG droplet digital PCR system. Primers were as follows: for mouse Pten, forward, 5′-TGGGGCAGGAAAAGATTATG-3′; reverse, 5′- CTGCACACAGGTCCACTGAT-3′; for the control mouse telomerase reverse transcriptase (Tert) gene, forward, 5′-CTGGCTGATGGACACATACG-3′; reverse, 5′-TGCTCCACACACTCTTACGG-3′. The numbers of positive droplets for the wild-type Pten gene and the control telomerase reverse transcriptase (Tert) gene were read with the QX200 droplet-reader. The number of copies of the wild-type Pten gene per genome was calculated by dividing the number of the wild-type Pten copies by the number of the Tert gene copies. The number of copies of the wild-type Pten gene per diploid genome was calculated by multiplying the number of copies per genome by 2.
Statistical analysis
All data are expressed as means ± standard errors. Statistical analysis was performed and p < 0.05 was considered significant. All statistical tests were two-sided. GraphPad Prism version 5.0 for Mac OS X was used to perform analyses of variances.
Results
SAHA increases thyroid tumor growth in ThrbPV/PVPten+/− mice
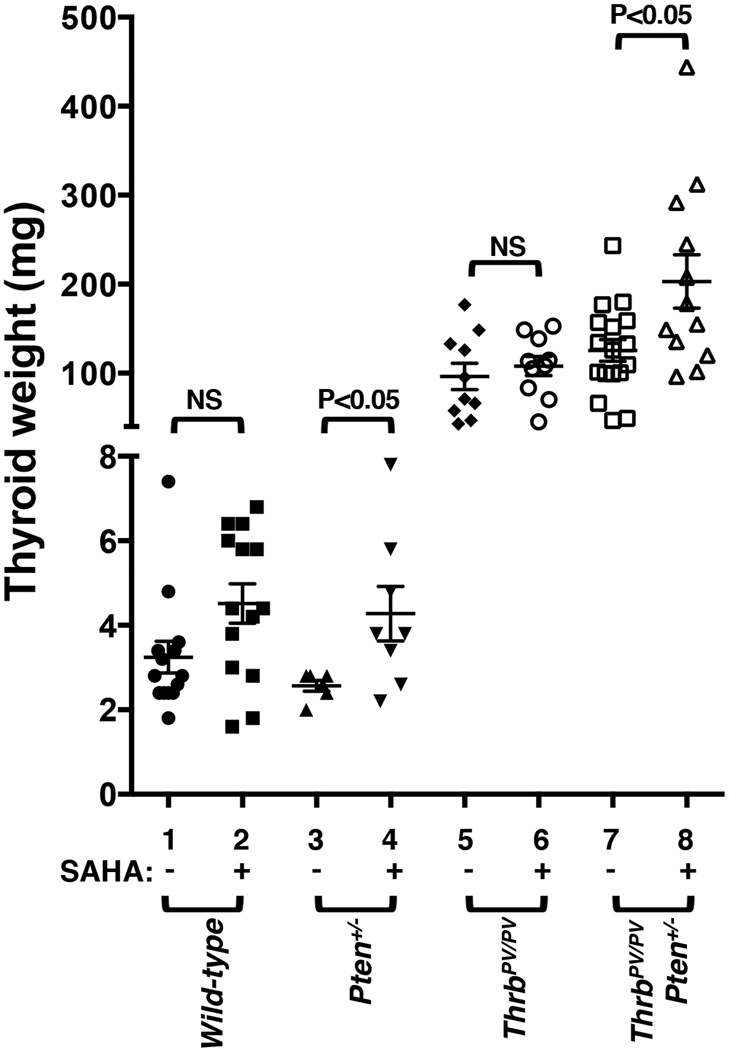
We treated wild-type mice, Pten+/− mice, ThrbPV/PV, and ThrbPV/PVPten+/− mice with SAHA for 2 months and evaluated its effect on thyroid carcinogenesis. SAHA had no effect on the thyroid growth of wild-type mice (3.2 ± 0.4 mg, n=14, versus 4.5 ± 0.5 mg, n=14, without or with SAHA, respectively, Figure 1). SAHA treatment increased the weight of thyroid from 2.6 ± 0.1 mg (n=6) to 4.3 ± 0.4 mg (n=8) in Pten+/− mice (data set 3 versus 4). No significant differences in tumor weight were detected in SAHA-treated (108.4 ± 10.8 mg, n=10) or untreated ThrbPV/PV mice (96.4 ± 14.8 mg, n=10). However, ThrbPV/PVPten+/− mice treated with SAHA had a 1.6-fold increase in thyroid tumor weight as compared with no SAHA treatment (203.0 ± 30.0 mg, n=12, versus 125.5±12.2 mg, n=17, data set 8 versus 7, Figure 1). These results indicate that while SAHA had no effects on thyroid tumor growth of ThrbPV/PV mice, it increased thyroid tumor growth in ThrbPV/PVPten+/− mice.
Figure 1. SAHA increases thyroid tumor growth in ThrbPV/PVPten+/− mice.
Thyroid glands of wild-type, Pten+/−, ThrbPV/PV, and ThrbPV/PVPten+/− mice without or with SAHA treatment were dissected and compared in mice of the same age. The differences in thyroid weights of the ThrbPV/PVPten+/− mice without or with SAHA treatment was significant (p<0.05), as determined by analysis of variance (ANOVA).
Increased thyroid growth in ThrbPV/PVPten+/− mice is not mediated by altered TSH levels
TSH is the primary growth stimulator of thyroid epithelial cells (Rivas and Santisteban 2003). To evaluate whether TSH could contribute to the increased thyroid growth in ThrbPV/PVPten+/− mice treated with SAHA, we compared serum TSH of the mice without or with SAHA treatment (Figure 2A). There were no significant differences in serum TSH levels of wild-type, Pten+/−, and ThrbPV/PV mice between the mice without and those with SAHA treatment (wild-type: 43.8 ± 3.3 ng/ml, n=15, versus 43.5 ± 6.5 ng/ml, n=14; Pten+/−: 38.9 ± 4.9 ng/ml, n=8, versus 45.4 ± 4.6 ng/ml, n=6; ThrbPV/PV: 19529 ± 2700 ng/ml, n=11, versus 16662 ± 2349 ng/ml, n=11). In ThrbPV/PVPten+/− mice, the serum level of TSH in mice with SAHA treatment (12899 ± 1446 ng/ml, n=12) was significantly lower than that in those without SAHA treatment (26276 ± 4382 ng/ml, n=11) (Figure 2A). There were no significant differences in serum total T4 (Figure 2B) and total T3 (Figure 2C) between the mice treated with SAHA or without SAHA. The findings that SAHA treatment significantly decreased TSH levels in ThrbPV/PVPten+/− mice indicated that increased thyroid tumor growth in ThrbPV/PVPten+/− mice was not an effect of TSH (Figure 2A).
Figure 2. Serum TSH, total T4, and total T3 levels in wild-type and mutant mice treated with or without SAHA.
Serum total TSH (A), TT4 (B), and TT3 (C) of wild-type, Pten+/−, ThrbPV/PV, and ThrbPV/PVPten+/− mice with or without SAHA treatment were determined as described in Materials and Methods. The p values are marked (n= 5–14); NS, not significant.
SAHA promotes thyroid tumor progression in ThrbPV/PVPten+/− mice
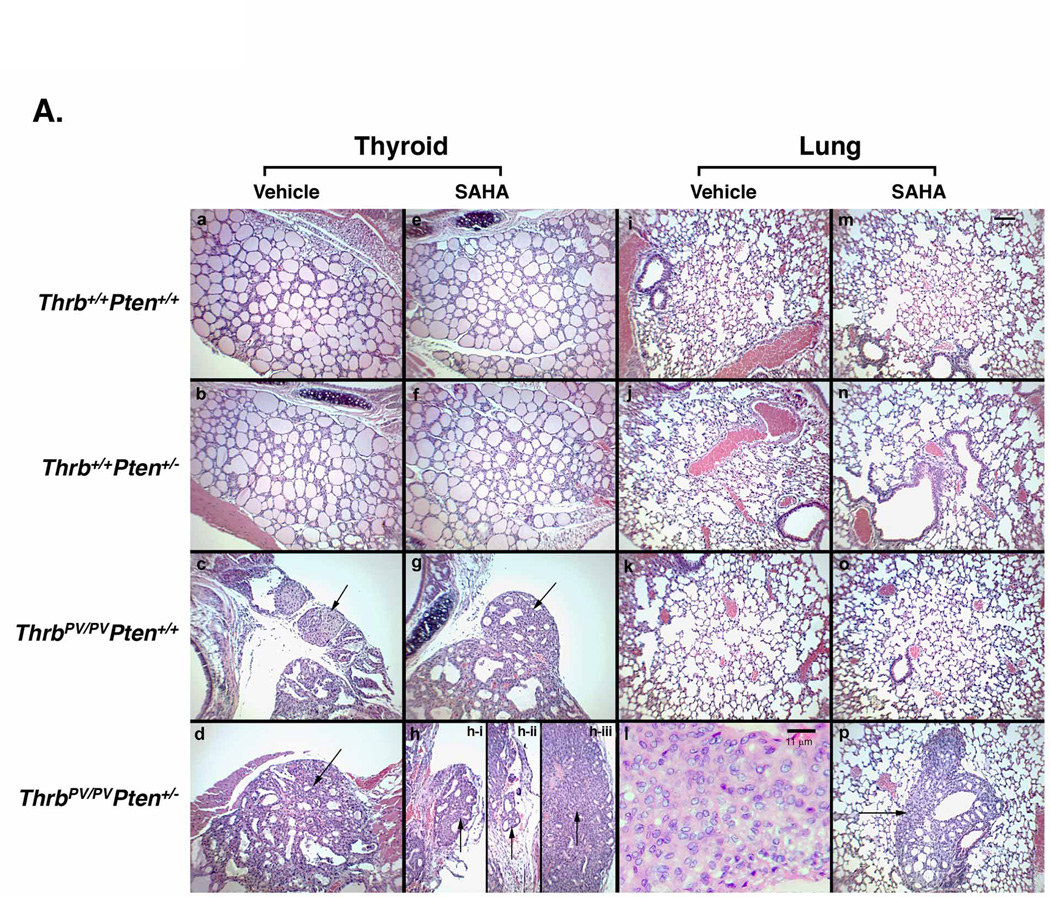
We treated wild-type mice, Pten+/−, ThrbPV/PV, and ThrbPV/PVPten+/− mice for 8 weeks after they were weaned at 6 weeks. Pathohistological analysis was performed to determine the effects of SAHA on thyroid carcinogenesis in mice at the same age (Figure 3). Thyroids and lungs of the wild-type mice treated with SAHA exhibited no apparent abnormalities (Figure 3A-a, e, i, and m). Although the thyroid weight of the Pten+/− mice treated with SAHA was increased, morphology of the thyroid and lung after SAHA treatment was similar to that of mice without SAHA treatment (Figure 3A-b, f, j, and n). Thyroids of ThrbPV/PVPten+/+ mice displayed extensive hyperplasia. Only a few of the ThrbPV/PV mice displayed capsular invasion (Figure 3A-c), but was not affected by SAHA treatment (Figure 3A-g). No lung metastasis was detected in ThrbPV/PV mice (panels k and o). In thyroids of ThrbPV/PVPten+/− mice with no SAHA treatment, capsular invasion was frequently observed (Figure 3A-d). None of ThrbPV/PVPten+/− mice was found to have lung metastasis (Figure 3A-l). After SAHA treatment, the thyroids of ThrbPV/PVPten+/− mice showed capsular invasion (Figure 3A-h-i), vascular invasion (Figure 3A-h-ii), and anaplastic foci (Figure 3A-h-iii). Moreover, lung metastases were frequently detected in the SAHA-treated ThrbPV/PVPten+/− mice (Figure 3A-p).
Figure 3. Histopathological features of thyroids of wild-type mice and mutant mice treated with or without SAHA.
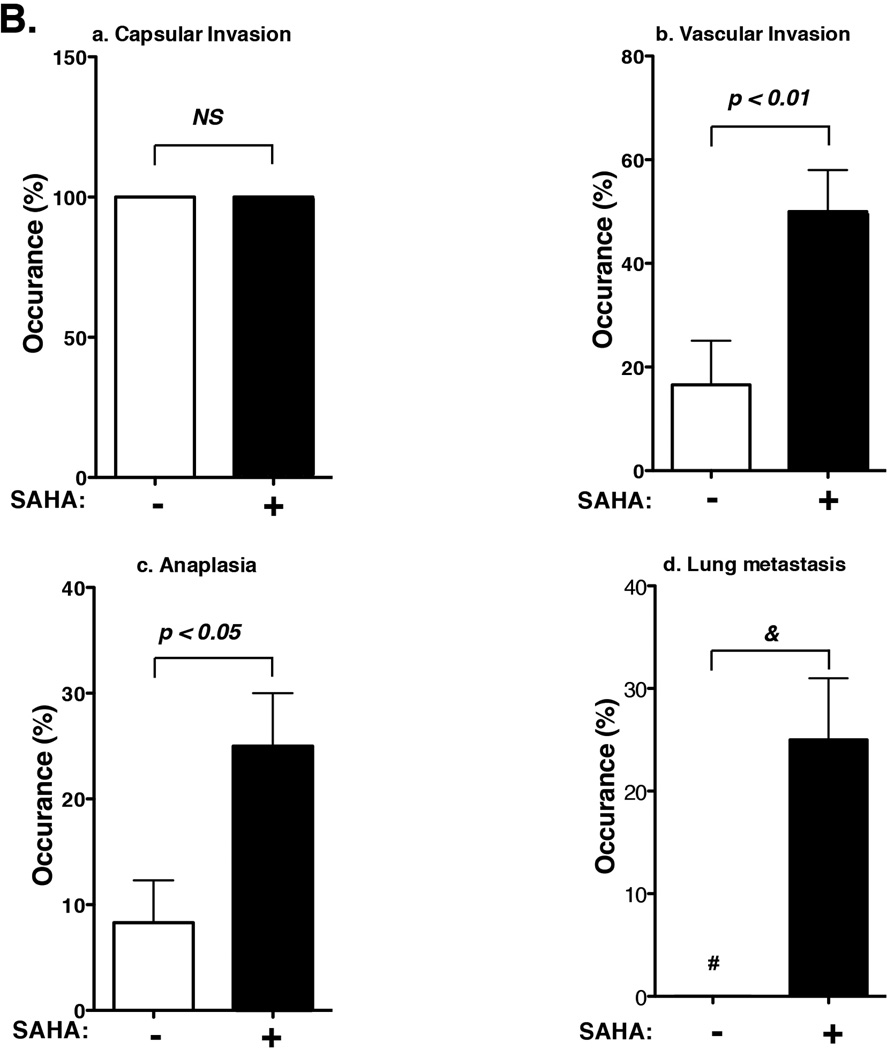
(A) After mice were treated with SAHA for 8 weeks as described in Methods and Materials, thyroids (panels a–h) and lung (panels i–p) were dissected and stained with H&E: wild-type (a, e, i, m), Pten+/− (b, f, j, n), ThrbPV/PV(c, g, k, o) and ThrbPV/PVPten+/− (d, h, l, p) mice. Panels show hyperplasia (c, d, g, h), capsular invasion (c, d, g, h-i, arrow), vascular invasion (h-ii, arrow), anaplastic foci, (h-iii, arrow), microscopic lung metastases (p, arrow). The anaplastic focus is shown at a higher magnification in panel I. (B) Quantitative analysis of occurrence frequency (%) of (a) capsular invasion, (b) vascular invasion, (c) anaplastic foci, and (d) lung metastasis of ThrbPV/PVPten+/− mice with or without SAHA treatment. Sections of thyroids and lungs were stained with H&E and analyzed for pathological progression. The data are expressed as the percentage of occurrence frequency of the mice examined. The # indicates zero occurrence frequency (%). The p values are indicated. “&” in panel d shows that statistics analysis could not be analyzed as there was no occurrence (zero) of lung metastasis in the absence of SAHA.
The effect of SAHA treatment on the occurrence frequency of pathohistological features shown above (Figure 3A) in ThrbPV/PVPten+/− mice is summarized in figure 3B. All ThrbPV/PVPten+/− mice, who were the same age, had developed capsular invasion for which the frequency of occurrence was not affected by SAHA treatment (Figure 3B-a). Seventeen percent of thyroid tumors in untreated ThrbPV/PVPten+/− mice had developed vascular invasion. SAHA treatment accelerated the occurrence of vascular invasion from 17% to 50% (Figure 3B-b). While only 8% of the untreated ThrbPV/PVPten+/− mice had developed anaplasia, the frequency of occurrence of anaplasia was increased to 25% by SAHA treatment (Figure 3B-c). In the absence of SAHA, no lung metastasis was developed in ThrbPV/PVPten+/− mice. However, lung metastasis was detected in 25% of SAHA-treated ThrbPV/PVPten+/− mice (Figure 3B-d). These results show SAHA promotes thyroid cancer progression in ThrbPV/PVPten+/− mice.
SAHA activates PI3K-AKT signaling in thyroid tumors of ThrbPV/PVPten+/− mice
To dissect the molecular events responsible for SAHA-induced aggressive thyroid cancer progression in ThrbPV/PVPten+/− mice, we examined altered cell signaling pathways involved in tumor growth and progression. We have previously shown that Pten haplodeficiency further over-activates the PI3K-AKT signaling in ThrbPV/PVPten+/− mice (Guigon et al. 2009). We therefore first evaluated whether protein levels of key regulators in the PI3K-AKT signaling were affected by SAHA treatment. As expected, the PTEN protein abundance was lower in Pten+/− mice (lane 3, Figure 4A-I-a) than in WT mice (lane 1) and ThrbPV/PV mice (lanes 5 & 6). SAHA treatment had no effect on the PTEN protein levels in these mice (compare lane 2 versus 1; 4 versus 3; 7 & 8 versus 5 & 6). Unexpectedly, we found that SAHA treatment led to a 60% decrease in the PTEN protein level in ThrbPV/PVPten+/− mice (compare lanes 11 & 12 versus 9 & 10) (also see the quantitative analysis, Figure 4A-II-a). Since PTEN is a negative regulator of PI3K-AKT signaling, p-AKT(S473) protein level, an indication of activated PI3K-AKT signaling, was highly elevated in thyroid tumors of the SAHA-treated ThrbPV/PVPten+/− mice (panel b, lanes 11 & 12 versus lanes 9 & 10) without any apparent altering of total AKT protein abundance (Figure 4A-I-c). The ratio of p-AKT(S473) to total AKT was increased by 6.5-fold in thyroid tumors of the SAHA-treated ThrbPV/PVPten+/− mice (Figure 4A-II-b). No significant changes in the p-AKT(S473) protein levels by SAHA treatment were detected in thyroids of wild-type mice (lane 2 versus 1), Pten+/− mice (lane 3 versus 4), or thyroid tumors of ThrbPV/PV mice examined (lanes 5 & 6 versus 7 & 8). In these mice, p-AKT(S473) protein levels were similarly relatively low. We further carried out immunohistochemistry to determine the expression of PTEN and p-AKT in thyroid cells of ThrbPV/PVPten+/− mice with or without SAHA treatment (Figure 4B). As shown in panel (ii) of Figure 4B-I-a, high levels of PTEN proteins were detected in vehicle-treated ThrbPV/PVPten+/− mice. After SAHA treatment, the PTEN protein signals were barely detectable [panel (iv) of Figure 4B-I-a; also see the quantitative data shown in 4B-II-a]. Consistently, the reduction of PTEN led an increased p-AKT in the thyroids of SAHA-treated ThrbPV/PVPten+/− mice [panel (iv) of Figure 4B-I-b; also see the quantitative data shown in Figure 4B-II-b]. These immunohistochemical findings are consistent with the western blot analysis that SAHA treatment lowered PTEN protein levels to activate PI3K-AKT signaling in thyroid tumors of ThrbPV/PVPten+/− mice.
Figure 4. SAHA altered the protein abundance of key cell cycle regulators in thyroid tumors of ThrbPV/PVPten+/− mice.
(A-I) Total protein extracts were prepared from thyroids of wild-type, Pten+/−, ThrbPV/PV, and ThrbPV/PVPten+/− mice treated with or without SAHA. Western blot analysis was carried out for PTEN (a), p-AKT (S473) (b), total-AKT (c), p-Rb (S748) (d), total Rb (e), CDK6 (f), p21Cip1 (g), and GAPDH (h) as described in Materials and Methods. Representative examples from thyroids of 2 mice for each genotype are shown. (A-II) Quantitative analysis of relative protein expression levels of PTEN (a), ratios of p-AKT (S473) to total AKT (b), ratios of p-Rb (S748) to total Rb (c), CDK6 (d), and p21Cip1 (d) on the basis of the intensities of the bands shown in (A), the p values are marked. The statistical analysis was carried using western blot data from 3–5 thyroids of vehicle-treated or SAHA-treated wild-type mice, Pten+/− mice, and ThrbPV/PV mice. For the analysis of western blot data of tumors of ThrbPV/PVPten+/− mice, we used 3–4 thyroid tumors of vehicle-treated ThrbPV/PVPten+/− mice and 6–8 thyroid tumors of SAHA-treated ThrbPV/PVPten+/− mice. The error bars show the standard error of the means. (B-I) Immunohistochemical analysis was carried out using antibody against PTEN (a) or p-AKT (b) for thyroid tumor tissues. Antibody IgG were used as a negative control. (B-II) PEN positive cells (a) or p-AKT positive cells (b) were quantified. The thyroid tumors treated with SAHA had significantly lower cell numbers stained for PTEN and higher cell numbers stained for p-AKT compared to those treated with vehicle (*p<0.0001).
To understand how activation of p-AKT led to increased tumor growth, we examined the expression of the PI3K-AKT signaling downstream effectors. Crosstalk between AKT signaling and the Rb pathway is critical in cell cycle regulation (Imai, et al. 2014). Phosphorylation of Rb drives cell cycle progression from the G1 to the S phase. Panel d (Figure 4A-I) shows that p-Rb(S748) protein levels were elevated in SAHA-treated ThrbPV/PVPten+/− mice (lanes 11 & 12 versus 9 & 10) without apparent changes in the total Rb protein level (panel d, Figure 4A-I). The ratio of p-Rb(S748) to total Rb was increased (Figure 4A-II, panel c). No marked effects of SAHA on the p-Rb(S748) levels in the wild-type mice (lane 2 versus 1), Pten+/− mice (lane 3 versus 4), thyroid tumors of ThrbPV/PV mice (lanes 5 & 6 versus 7 & 8). CDK6 protein levels were higher in SAHA-treated ThrbPV/PVPten+/− mice than untreated mice (lanes 11 & 12 versus 9 & 10, Figure 4A-I-f; see also quantitative data shown in Figure 4A-II-d), but no SAHA-induced changes were detected in CDK6 levels in wild-type (lane 2 versus 1), Pten+/− mice (lane 3 versus 4), thyroid tumors of ThrbPV/PV mice (lanes 5 & 6 versus 7 & 8).
We also examined the effect of SAHA on the protein abundance of p21Cip1, which is another effector downstream of the PI3K-AKT pathway (Warfel and El-Deiry 2013). p21Cip1 is a potent cyclin-dependent kinase inhibitor (CKI). It binds to and inhibits the activity of cyclin-CDK6 complexes and thus functions as a negative regulator of cell cycle progression from the G1 phase to the S phase (Gartel and Radhakrishnan 2005). The abundance of p21Cip1 protein was higher in the thyroid tumors of ThrbPV/PV mice than in wild-type and Pten+/− mice, but the p21Cip1 protein levels were not affected by SAHA treatment (lanes 5–8). The abundance of p21Cip1 protein was further elevated in the ThrbPV/PVPten+/− mice (lanes 9 & 10), but SAHA treatment lowered the p21Cip1 protein levels by 45% (lanes 11 & 12, Figure 4A-I-g; see also Figure 4A-II-e). The quantitative analyses of the band intensities are shown in Figure 4A-II (panel a for PTEN, panel b for the ratios of p–AKT versus total AKT; panel c, ratios of p-Rb versus total Rb; panel d, CDK6 and panel e, p21Cip1 proteins). The analysis was normalized to the loading controls using GAPDH (Figure 5A-h). These findings indicate that the decreased PTEN induced by SAHA led to activation of PI3K-AKT to drive cell cycle progression to promote thyroid tumor cell proliferation in ThrbPV/PVPten+/− mice.
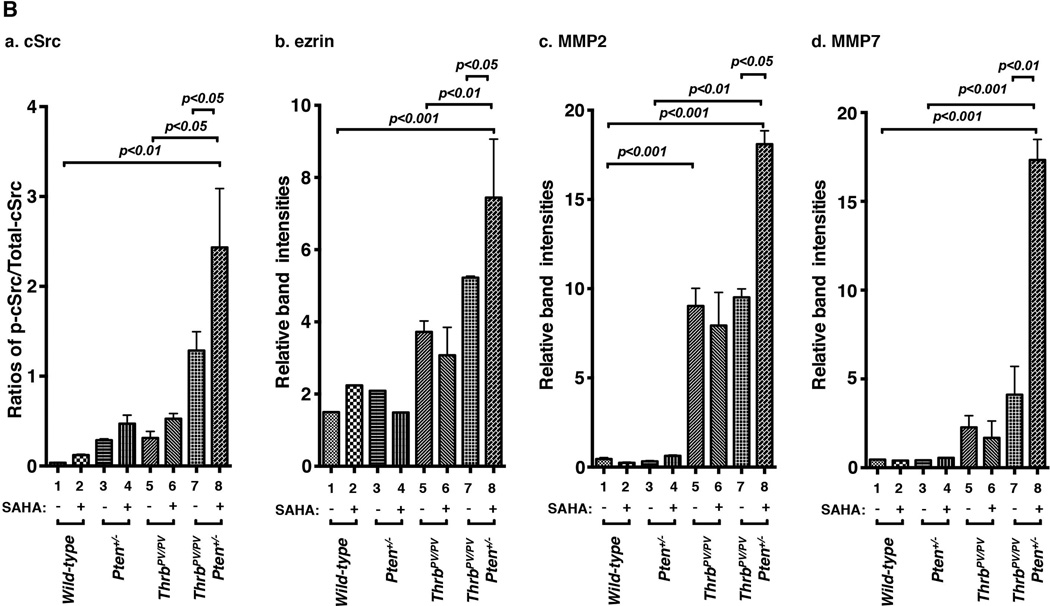
Figure 5. Increased abundance of key regulators of cSrc-ezrin-MMPs signaling in thyroid tumors of ThrbPV/PVPten+/− mice treated with SAHA.
(A) Total protein extracts were prepared from thyroids of wild-type, Pten+/−, ThrbPV/PV, and ThrbPV/PVPten+/− mice treated with or without SAHA. Western blot analysis was carried out for p-cSrc (a), total cSrc (b), ezrin (c), MMP2 (d), MMP7 (e), and GAPDH (f) as described in Materials and Methods. Representative examples from thyroids of 2 mice for each genotype are shown. (B) Quantitative analysis of relative protein expression levels of ratios p-cSrc versus total cSrc (a), ezrin (b), MMP2 (c), and MMP7 (d) on the basis of the intensities of the bands shown in (A); the p values are marked. The statistical analysis was carried using western blot data from 3–5 thyroids of vehicle-treated or SAHA-treated wild-type mice, Pten+/− mice, and ThrbPV/PV mice. For the analysis of western blot data of tumors of ThrbPV/PVPten+/− mice, we used 3–4 thyroid tumors of vehicle-treated ThrbPV/PVPten+/− mice and 6–8 thyroid tumors of SAHA-treated ThrbPV/PVPten+/− mice. The error bars show the standard error of the means.
It is known that AKT complexes with cSrc to link to cytoskeletal proteins such as ezrin to alter cell migration (Heiska, et al. 2011; Srivastava, et al. 2005). Moreover, AKT signaling mediates activation and secretion of matrix metalloproteinases (MMPs), which play key roles in tumor metastasis (Cho, et al. 2008). We found a higher level of activated p-cSrc (panel a, lanes 11 & 12, Figure 5A-a) without apparent effect of total c-Src (panel b, lanes 11 & 12; Figure 5A-b) in SAHA-treated ThrbPV/PVPten+/− mice. Moreover, ezrin protein levels were also higher in SAHA-treated ThrbPV/PVPten+/− mice (lanes 11 &12, panel c, Figure 5A-c). SAHA had no apparent changes in the ezrin levels in wild-type (lanes 1 & 2, Figure 5A-c), Pten+/− mice (lanes 3 & 4), and ThrbPV/PV mice (lanes 7 & 8 versus 5 & 6; Figure 5A-c).
MMP2 was detected in ThrbPV/PV mice (lanes 5–8; Figure 5A-d) than in wild-type (lanes 1 & 2, Figure 5A-d) and Pten+/− mice (lanes 3 & 4). But SAHA had no detectable effects on the MMP2 levels in thyroid tumors of ThrbPV/PV mice. However, MMP2 protein levels were clearly elevated in the thyroid tumors of SAHA-treated ThrbPV/PVPten+/− mice by 1.9-fold as compared with untreated mice (lanes 11 & 12 versus 9 & 10, see also Figure 5B-c). Similar patterns of changes were also found for MMP7. As shown in lanes 11 & 12 (Figure 5A-e), SAHA treatment led to markedly increased MMP7 in thyroid tumors of ThrbPV/PVPten+/− mice (4.2-fold), while no apparent effects were detected on the relatively low levels of MMP7 in wild-type (lanes 1 & 2, Figure 5A-e), Pten+/− mice (lanes 3 & 4), and ThrbPV/PV mice (lanes 7 & 8 versus 5 & 6; Figure 5A-e). The SAHA-induced changes in p-cSrc, ezrin, MMP2 and MMP7 can be seen more clearly in the quantitative analysis shown in Figure 5B-a, b, c, and d, respectively.
Loss of the wild-type Pten allele is responsible for reduced Pten expression in thyroid tumors of SAHA-treated ThrbPV/PVPten+/− mice
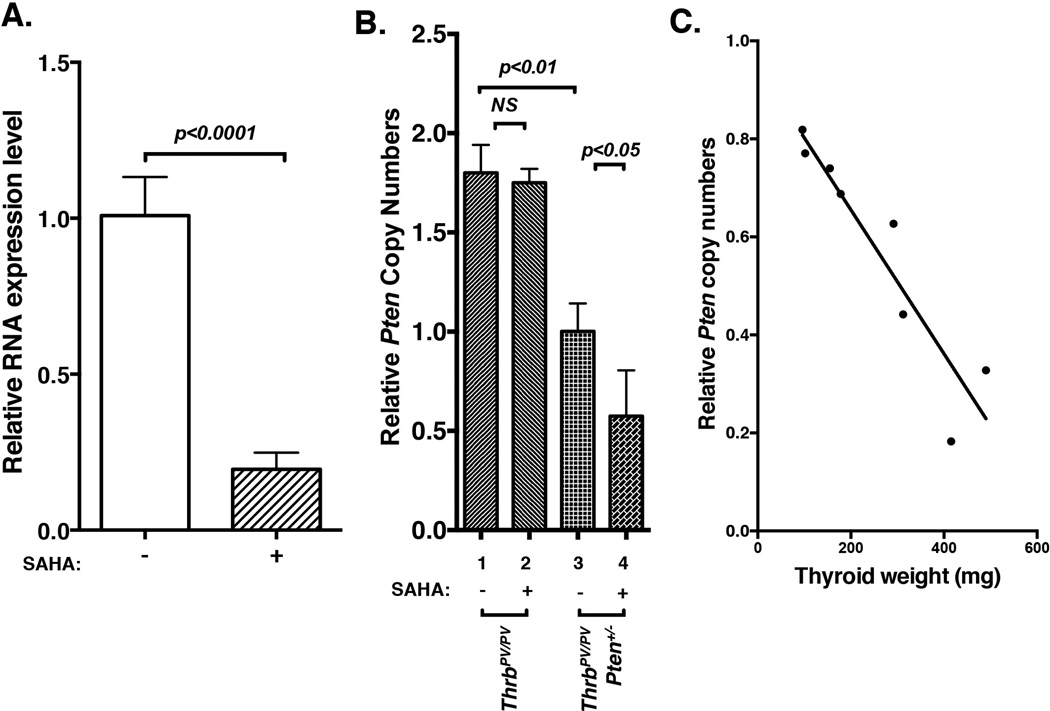
We further looked into the mechanism responsible for SAHA-induced PTEN downregulation in thyroid tumors of ThrbPV/PVPten+/− mice (see Figure 4A-I-a and 4A-II-a). We ascertained the effect of SAHA on the Pten mRNA expression in thyroid tumors of ThrbPV/PVPten+/− mice. Quantitative real-time PCR analysis indicated that Pten mRNA abundance was significantly reduced by 81% in thyroid tumors of SAHA-treated ThrbPV/PVPten+/− mice (Figure 6A). The results indicated that SAHA suppressed the Pten gene expression at the transcription level.
Figure 6. Decreased Pten mRNA expression and loss of wild-type Pten allele in thyroid tumors of ThrbPV/PVPten+/− mice treated with SAHA.
(A) Total RNAs prepared from thyroids of ThrbPV/PVPten+/− mice treated with or without SAHA were used for the analysis of the Pten mRNA expression by Q/PCR. (B) Relative copy numbers of the wild-type Pten allele. Forty ng of mouse genomic DNA was used for digital PCR to determine the copy numbers of the wild-type Pten and the control Tert genes, The copy number of the wild-type Pten gene per diploid genome was calculated as described in the Methods. (C) Correlation between relative copy numbers of the wild-type Pten allele and thyroid tumor weight of ThrbPV/PVPten+/− mice.
While there are multiple potential regulatory mechanisms that could lead to the decreased expression of the Pten mRNA, we focused on testing the possibility of the loss of the Pten gene copy due to selection pressure by the therapeutic agent SAHA. This phenomenon was recently observed in a patient with metastatic breast cancer carrying an activated PIK3CA mutation, resulting in constitutive over-activation of AKT signaling (Juric, et al. 2015). Treatment of the patient with the PI3Kα inhibitor BYL719 led to the copy loss of the PTEN gene. The patient developed resistance to the treatment and eventually succumbed to death (Juric et al. 2015). The ThrbPV/PVPten+/− mice used in our studies have only one copy of the functional wild-type Pten allele. We therefore examined whether SAHA treatment led to the loss of the wild-type Pten allele by using single molecule DNA analysis to determine the relative number of copies of the wild-type Pten allele. By using the genomic DNA isolated from thyroid tumors as PCR template, single molecule DNA ddPCR analysis was used to assess the genetic loss of the Pten gene. Compared with ThrbPV/PV mice without SAHA treatment, ThrbPV/PVPten+/− mice had only one copy of the Pten allele (bar 1 versus bar 3, Figure 6B). With SAHA treatment, no reduction in the relative copy number was observed in the thyroid of ThrbPV/PV mice (bar 2 versus 1). In contrast, 50% of thyroid tumor cells had lost one copy of the Pten allele in SAHA-treated ThrbPV/PVPten+/− mice (bar 4 versus 3). Interestingly, the extent of the Pten copy loss was positively correlated with the increased tumor growth. Figure 6C shows that in thyroid tumors with weight under 200 mg [average normal mouse thyroid weight= 3.24, (3.24±1.40; n=14], 30% of tumor cells had lost one wild type Pten allele; with weight over 400 mg, 70% of tumor cells had lost one wild type Pten allele. These results indicate that one mechanism by which the decreased expression of the Pten mRNA is due to the loss of the Pten allele in the thyroid tumor of SAHA-treated ThrbPV/PVPten+/− mice. The loss of the Pten gene, a PI3K-AKT negative regulator, resulted in the constitutive activation of AKT signaling, rendering the SAHA ineffective therapeutically to treat thyroid cancer.
Discussion
Since SAHA was approved to treat cutaneous T-cell lymphoma by the U.S. Food and Drug Administration (Duvic, et al. 2007; Grant, et al. 2010; Xu, et al. 2007), clinical trials have been carried out to treat many types of solid tumors such as lung, prostate, and colon cancers (Mann et al. 2007; Olsen et al. 2007). However, clinical trials in solid tumors have not had success because of the resistance of solid tumors to the treatment (Ramalingam, et al. 2010). Thyroid tumors often progress further even during the SAHA treatment (Woyach et al. 2009). The molecular basis of the resistance of SAHA treatment, however, has not been elucidated. Using two preclinical mouse models of thyroid cancer, we found that SAHA was ineffective as a therapeutic to treat thyroid cancer in ThrbPV/PV mice. In ThrbPV/PVPten+/− mice, SAHA not only is ineffective therapeutically, but paradoxically promotes thyroid cancer progression. These observations are reminiscent of the outcome of the clinical trial of 19 patients with advanced thyroid cancer (Woyach et al. 2009) in that no patient achieved a partial or complete response. Trials for some patients were terminated owing to clinical progression of the disease. The present findings are in contrast to the early observations from cell-based studies in which SAHA was effective in the inhibition of tumor cell proliferation and induction of apoptosis (Borbone et al. 2010; Luong et al. 2006; Mitsiades et al. 2005). The present results support the usefulness of these mouse models in testing potential therapeutic agents in vivo for treatment of thyroid cancer.
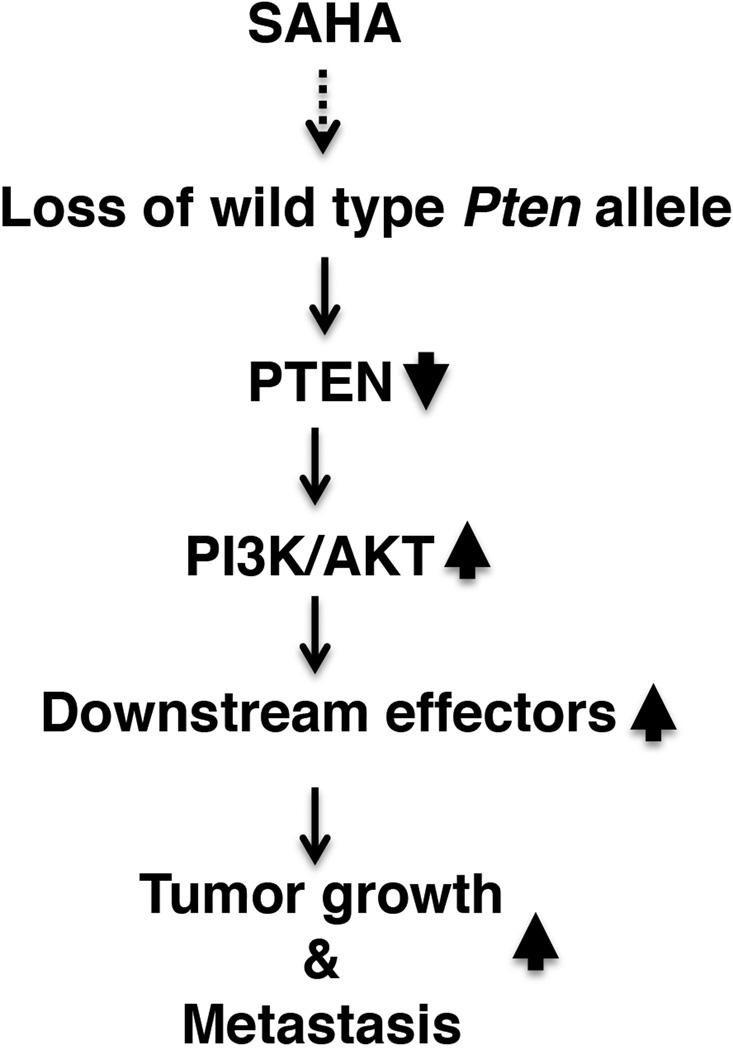
Therapeutic resistance is a major obstacle for effective treatment of cancers by HDAC inhibitors. HDAC inhibitors induce acetylation of histones and nonhistone proteins involved in the regulation of gene expression and in various cellular pathways including cell growth arrest, differentiation, DNA damage and repair, redox signaling, and apoptosis. Depending on the cellular context and genetic makeup of the cancer cells, the mechanisms of resistance to HDAC inhibitors vary. Resistance to HDAC inhibitors may involve aberrant expression and modifications of signaling molecules, causing intrinsic resistance to HDAC inhibitors in cancer cells, such as high level expression of thioredoxin, over-activation of pro-survival BCL-2, or constitutive activation of STAT proteins (Fantin, et al. 2008; Fedier, et al. 2007; Lee, et al. 2012; Shao, et al. 2010). In the aforementioned clinical trial of SAHA in 19 patients (Woyach et al. 2009), it was not clear what regulators were aberrantly induced and/or signaling molecules were aberrantly activated by SAHA treatment, leading to resistance and/or progression of the disease. In the present studies, we showed that SAHA treatment of ThrbPV/PVPten+/− mice led to the aberrant loss of the Pten gene, a negative regulator of the PI3K-AKT pathway. The loss of the PTEN tumor suppressor led to constitutive activation of the AKT and its downstream signaling to stimulate tumor growth and to promote cancer invasion and metastasis (see Figure 7). Thus, the present studies uncovered a novel mechanism by which SAHA not only lacks effectiveness as a therapeutic agent, but also detrimentally promotes cancer progression.
Figure 7. Proposed molecular pathways by which SAHA promotes thyroid carcinogenesis in ThrbPV/PVPten+/− mice.
The loss of the wild-type Pten allele in the thyroid tumors of SAHA-treated ThrbPV/PVPten+/− mice led to the further activation of PI3K-AKT signaling to increase thyroid growth and promote metastasis.
It is important to point out that ThrbPV/PV mice treated with SAHA only exhibited resistance without the progressive disease found in ThrbPV/PVPten+/− mice. These findings further highlight the importance of different sensitivities to SAHA treatment due to cellular context and genetic makeup of the cancer cells. The ThrbPV/PV mouse is model of FTC harboring a powerful dominant negative TRβPV mutant (Suzuki et al. 2002). The development and progression is partly driven by over-activation of PI3K-AKT signaling (Furuya et al. 2006; Furuya et al. 2007). The ThrbPV/PVPten+/− mouse is also a model of FTC, but in addition to harboring the TRβPV mutation, it has lost one allele of the Pten gene (Guigon et al. 2009). Thyroid carcinogenesis is driven further by PI3K-AKT signaling. At present, it is not clear how SAHA treatment led to the selective loss of the remaining the Pten gene in the thyroid tumors of ThrbPV/PVPten+/− mouse, but not in the thyroid tumors of the ThrbPV/PV mouse. Still, it is known that a reduced level of the PTEN gene due to methylation or other epigenetic silencing occurs in close association with activating genetic alterations of the PI3K-AKT pathway, constituting a unique self-enhancement mechanism in this pathway (Xing 2010). It is likely that loss of one Pten allele may render thyroid tumor cells more susceptible to further loss of the remaining functional allele through a PI3K-AKT self-enhancement mechanism. As a result, convergent bi-allelic loss of the Pten gene could cause enhanced AKT signaling to overcome antitumor effects of SAHA, resulting in elevated activity of the downstream effectors to drive aggressive thyroid carcinogenesis (see Figure 7).
That cancer cells could lose a tumor suppressor gene through selective pressure of anticancer agents is not without precedent. A recent report documented that treatment of a patient with metastatic breast cancer expressing an activating PIK3CA mutation with a PI3Kα inhibitor, BYL719, led to loss of the PTEN gene (Juric et al. 2015). While initially achieving a positive clinical response, the patient eventually progressed during treatment and died shortly thereafter. It is important to point out that in this study, while the patient had no loss of the PTEN gene in the primary lesions, the metastatic tumors resistant to the inhibitor treatment have a copy loss of the PTEN gene, suggesting that the loss of the PTEN gene is coupled to the resistance to inhibitor treatment. This clinical case together with our findings from the thyroid cancer mouse model highlighted the susceptibility of the loss of the PTEN gene to the selection pressure by anticancer agents targeting the PI3K-AKT pathway. On the basis of these findings, when cancer patients develop resistance to anticancer agents targeting the PI3K-AKT pathway, the loss of the PTEN gene should be suspected and explored such that other strategies and treatment modalities could be developed to improve patients’ outcome.
The above findings indicated that the selective pressure by a PI3Kα inhibitor could drive tumor cells to evolve mechanisms, resulting in the PTEN gene loss to overcome inhibitory effects. These studies also suggested that the loss of the PTEN gene could also be susceptible to other selective pressure such as SAHA as shown in the present studies. While it was not clear in the nature of genetic alterations in the thyroid cancer patients for whom vorinostat (SAHA) treatment was ineffective or even progressed (Woyach et al. 2009), on the basis findings by Juric et al (Juric et al. 2015) and the present studies, it is tempted to speculate the selective pressure by SAHA could also lead to the PTEN gene loss in those thyroid cancer patients to counter act the potential therapeutic effect of SAHA. The validation of this conjecture would await future studies.
Consistent with the clinical trial, the present findings clearly show that SAHA treatment was ineffective to improve the outcome of thyroid cancer in the two mouse models. Even so, our studies uncovered that SAHA led to the loss of the Pten gene, which resulted in the activation of the PI3K-AKT signaling. These findings raised the possibility that a combination treatment of SAHA with anticancer agents targeting PI3K-AKT downstream effectors could be beneficial. Moreover, since the U.S. Food and Drug Administration has approved two HDAC inhibitors, vorinostat and romidepsin, for the treatment of cutaneous T-cell lymphoma (Duvic et al. 2007; Grant et al. 2010; Marks and Breslow 2007), more than 20 chemically different HDAC inhibitors are in clinical trials for hematological cancer and solid tumors. In addition to the trial of vorinostat (SAHA), a phase I trial of romidepsin in patients with advanced thyroid cancer has been completed. The dosages used were tolerable by patients, but no objective responses were detected (Amiri-Kordestani, et al. 2013). Thus, it would be possible to use the same schedule to combine with other anticancer agents for the treatment of patients with advanced cancers. One of the possible treatments is to combine SAHA treatment with radioiodine since SAHA may enhance thyroid gene expression (Cheng, et al. 2016). The ThrbPV/PV and ThrbPV/PVPten+/− mice would be useful models to test the possibility of combined treatments of anticancer agents with SAHA or romidepsin in the future studies.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Sheue-yann Cheng conceived the project, analyzed the data, and wrote the paper. Xuguang Zhu designed and performed experiments, analyzed data and wrote the paper. Dong Wook Kim initiated SAHA treatment of the mice and performed experiments. Mark C. Willingham analyzed pathological features of tumors. Li Zhao performed IHC experiments.
References
- Amiri-Kordestani L, Luchenko V, Peer CJ, Ghafourian K, Reynolds J, Draper D, Frye R, Woo S, Venzon D, Wright J, et al. Phase I trial of a new schedule of romidepsin in patients with advanced cancers. Clin Cancer Res. 2013;19:4499–4507. doi: 10.1158/1078-0432.CCR-13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbone E, Berlingieri MT, De Bellis F, Nebbioso A, Chiappetta G, Mai A, Altucci L, Fusco A. Histone deacetylase inhibitors induce thyroid cancer-specific apoptosis through proteasome-dependent inhibition of TRAIL degradation. Oncogene. 2010;29:105–116. doi: 10.1038/onc.2009.306. [DOI] [PubMed] [Google Scholar]
- Cheng W, Liu R, Zhu G, Wang H, Xing M. Robust Thyroid Gene Expression and Radioiodine Uptake Induced by Simultaneous Suppression of BRAF V600E and Histone Deacetylase in Thyroid Cancer Cells. J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2015-3433. jc20153433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SJ, Chae MJ, Shin BK, Kim HK, Kim A. Akt- and MAPK-mediated activation and secretion of MMP-9 into stroma in breast cancer cells upon heregulin treatment. Mol Med Rep. 2008;1:83–88. [PubMed] [Google Scholar]
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin VR, Loboda A, Paweletz CP, Hendrickson RC, Pierce JW, Roth JA, Li L, Gooden F, Korenchuk S, Hou XS, et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer Res. 2008;68:3785–3794. doi: 10.1158/0008-5472.CAN-07-6091. [DOI] [PubMed] [Google Scholar]
- Fedier A, Dedes KJ, Imesch P, Von Bueren AO, Fink D. The histone deacetylase inhibitors suberoylanilide hydroxamic (Vorinostat) and valproic acid induce irreversible and MDR1-independent resistance in human colon cancer cells. Int J Oncol. 2007;31:633–641. [PubMed] [Google Scholar]
- Furuya F, Hanover JA, Cheng SY. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone beta receptor. Proc Natl Acad Sci U S A. 2006;103:1780–1785. doi: 10.1073/pnas.0510849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya F, Lu C, Willingham MC, Cheng SY. Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis. 2007;28:2451–2458. doi: 10.1093/carcin/bgm174. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- Grant C, Rahman F, Piekarz R, Peer C, Frye R, Robey RW, Gardner ER, Figg WD, Bates SE. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther. 2010;10:997–1008. doi: 10.1586/era.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng SY. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol Cell Biol. 2008;28:4598–4608. doi: 10.1128/MCB.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigon CJ, Zhao L, Willingham MC, Cheng SY. PTEN deficiency accelerates tumour progression in a mouse model of thyroid cancer. Oncogene. 2009;28:509–517. doi: 10.1038/onc.2008.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiska L, Melikova M, Zhao F, Saotome I, McClatchey AI, Carpen O. Ezrin is key regulator of Src-induced malignant phenotype in three-dimensional environment. Oncogene. 2011;30:4953–4962. doi: 10.1038/onc.2011.207. [DOI] [PubMed] [Google Scholar]
- Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab. 2010;95:820–828. doi: 10.1210/jc.2009-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Takahashi A, Hanyu A, Hori S, Sato S, Naka K, Hirao A, Ohtani N, Hara E. Crosstalk between the Rb pathway and AKT signaling forms a quiescence-senescence switch. Cell Rep. 2014;7:194–207. doi: 10.1016/j.celrep.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, Ebbesen SH, Ainscough BJ, Ramu A, Iyer G, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature. 2015;518:240–244. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, et al. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci U S A. 2000;97:13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- Lee JH, Choy ML, Marks PA. Mechanisms of resistance to histone deacetylase inhibitors. Adv Cancer Res. 2012;116:39–86. doi: 10.1016/B978-0-12-394387-3.00002-1. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Luong QT, O'Kelly J, Braunstein GD, Hershman JM, Koeffler HP. Antitumor activity of suberoylanilide hydroxamic acid against thyroid cancer cell lines in vitro and in vivo. Clin Cancer Res. 2006;12:5570–5577. doi: 10.1158/1078-0432.CCR-06-0367. [DOI] [PubMed] [Google Scholar]
- Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Poulaki V, McMullan C, Negri J, Fanourakis G, Goudopoulou A, Richon VM, Marks PA, Mitsiades N. Novel histone deacetylase inhibitors in the treatment of thyroid cancer. Clin Cancer Res. 2005;11:3958–3965. doi: 10.1158/1078-0432.CCR-03-0776. [DOI] [PubMed] [Google Scholar]
- Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- Ramalingam SS, Kummar S, Sarantopoulos J, Shibata S, LoRusso P, Yerk M, Holleran J, Lin Y, Beumer JH, Harvey RD, et al. Phase I study of vorinostat in patients with advanced solid tumors and hepatic dysfunction: a National Cancer Institute Organ Dysfunction Working Group study. J Clin Oncol. 2010;28:4507–4512. doi: 10.1200/JCO.2010.30.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners C, Hanscheid H, Luster M, Lassmann M, Verburg FA. Radioiodine for remnant ablation and therapy of metastatic disease. Nat Rev Endocrinol. 2011;7:589–595. doi: 10.1038/nrendo.2011.134. [DOI] [PubMed] [Google Scholar]
- Rivas M, Santisteban P. TSH-activated signaling pathways in thyroid tumorigenesis. Mol Cell Endocrinol. 2003;213:31–45. doi: 10.1016/j.mce.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Shao W, Growney JD, Feng Y, O'Connor G, Pu M, Zhu W, Yao YM, Kwon P, Fawell S, Atadja P. Activity of deacetylase inhibitor panobinostat (LBH589) in cutaneous T-cell lymphoma models: Defining molecular mechanisms of resistance. Int J Cancer. 2010;127:2199–2208. doi: 10.1002/ijc.25218. [DOI] [PubMed] [Google Scholar]
- Sherman EJ, Su YB, Lyall A, Schoder H, Fury MG, Ghossein RA, Haque S, Lisa D, Shaha AR, Tuttle RM, et al. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid. 2013;23:593–599. doi: 10.1089/thy.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava J, Elliott BE, Louvard D, Arpin M. Src-dependent ezrin phosphorylation in adhesion-mediated signaling. Mol Biol Cell. 2005;16:1481–1490. doi: 10.1091/mbc.E04-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- Warfel NA, El-Deiry WS. p21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol. 2013;25:52–58. doi: 10.1097/CCO.0b013e32835b639e. [DOI] [PubMed] [Google Scholar]
- Woyach JA, Kloos RT, Ringel MD, Arbogast D, Collamore M, Zwiebel JA, Grever M, Villalona-Calero M, Shah MH. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94:164–170. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- Ying H, Furuya F, Zhao L, Araki O, West BL, Hanover JA, Willingham MC, Cheng SY. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone beta receptor inhibits mitotic progression. J Clin Invest. 2006;116:2972–2984. doi: 10.1172/JCI28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhu X, Won Park J, Fozzatti L, Willingham M, Cheng SY. Role of TSH in the spontaneous development of asymmetrical thyroid carcinoma in mice with a targeted mutation in a single allele of the thyroid hormone-beta receptor. Endocrinology. 2012;153:5090–5100. doi: 10.1210/en.2012-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhao L, Park JW, Willingham MC, Cheng SY. Synergistic signaling of KRAS and thyroid hormone receptor beta mutants promotes undifferentiated thyroid cancer through MYC up-regulation. Neoplasia. 2014;16:757–769. doi: 10.1016/j.neo.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]